Pleuromutilin was discovered in 1951 from an edible mushroom, Pleurotus mutilus, but it was not until the early 1970s that its potential for use as an antimicrobial was more fully recognized (2). Tiamulin and valnemulin were developed as semisynthetic pleuromutilins whose antimicrobial activity was traced to inhibition of bacterial protein synthesis following interaction with the peptidyltransferase center of the 50S ribosomal subunit (2, 3, 8, 9). Tiamulin has potent activity against anaerobes, intestinal spirochetes, and Mycoplasma spp., and it has been principally used in swine production to control gram-positive and -negative pathogens (3).
Retapamulin (formerly SB-275833; Fig. 1) is the first topical pleuromutilin derivative developed for human use with enhanced activity against gram-positive bacteria, including staphylococcal and streptococcal isolates (2). This report describes results from a multilaboratory trial designed to establish retapamulin quality control (QC) ranges for disk diffusion and broth microdilution MIC methods (1, 6, 7) and used study design criteria as published in the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) M23-A2 document (4).
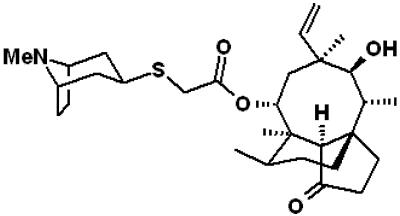
FIG. 1.
Chemical structure of retapamulin {mutilin 14-(exo-8-methyl-8-azabicyclo[3.2.1]oct-3-yl)-sulfanyl-acetate}.
An eight-laboratory study group was recruited for the development of MIC and disk diffusion zone diameter QC guidelines for retapamulin. The QC group consisted of laboratories at the University of Alberta (Edmonton, Alberta, Canada), The Cleveland Clinic Foundation (Cleveland, OH), University of Texas Medical Center (Houston, TX), University of Rochester Medical Center (Rochester, NY), Denver Health Medical Center (Denver, CO), University of Washington (Seattle, WA), TREK Diagnostics (Cleveland, OH), and JMI Laboratories (North Liberty, IA). Each laboratory followed a protocol based on CLSI document M23-A2 specifications (4), as well as the M7-A6 method for broth microdilution antimicrobial testing (7) and M2-A8 method for disk diffusion susceptibility testing (6).
The MIC portion of the study utilized frozen-form, reference broth microdilution panels prepared by TREK Diagnostics (Cleveland, OH). The panels contained four lots of cation-adjusted Mueller-Hinton broth (Oxoid, Hampshire, United Kingdom; BBL, Sparks, MD; Difco, Detroit, MI [two lots]) and the same four lots of Mueller-Hinton broth supplemented with 2 to 5% lysed horse blood. Ofloxacin and erythromycin were utilized as control agents. Each laboratory tested Staphylococcus aureus ATCC 29213 and Streptococcus pneumoniae ATCC 49619 and generated a total of 560 MIC results. Of the control agent results, 99.3% were within CLSI published guidelines (1). Colony counts were performed from the broth microdilution trays by subculturing in a quantitative manner onto drug-free solid media. The counts ranged from 1.1 × 105 to 8.5 × 105 CFU/ml, with an average of 3.8 × 105 CFU/ml.
The disk diffusion portion of the study utilized three different lots of commercially prepared Mueller-Hinton agar and three lots of Mueller-Hinton agar supplemented with 5% sheep blood (Remel, Lenexa, KS; BBL, Sparks, MD). Two different lots of retapamulin disks were utilized versus each QC strain (Oxoid lot 320100 and BBL lot 3336172). Single lots of ofloxacin, erythromycin, and ceftriaxone disks (Remel lots 290536, 308402, and 311176) were applied as internal control agents. A total of 1,350 control zone diameter results were produced, and 99.7% of reported results were within the CLSI QC ranges as published in document M100-S15 (1). Proposed retapamulin QC ranges were optimized to encompass ≥95.0% of all reported results, as recommended by the M23-A2 guideline (4). The MIC and disk diffusion zone diameter results were tabulated and compared by intra- and interlaboratory analyses to determine potentially unacceptable technical variations. Broth or agar medium and disk lots were also compared to determine variations among manufacturers. The S. pneumoniae ATCC 49619 internal QC zone diameter results from laboratory H were determined to be “out of control”; thus, all results from that participant were deleted from the final analysis.
The results of retapamulin MIC distributions among the eight participant laboratories for both QC strains are listed in Table 1. For S. aureus ATCC 29213, all eight laboratories had a modal MIC of 0.12 μg/ml, with results for each laboratory ranging from this by only two log2 dilution steps. The proposed MIC QC range was 0.06 to 0.25 μg/ml, which would include all reported results reported in this study. Although 99% of the reported values range from 0.06 to 0.12 μg/ml, a three-dilution range is preferred. For S. pneumoniae ATCC 49619, four of the eight laboratories had a modal MIC of 0.12 μg/ml and four had a modal MIC of 0.25 μg/ml. The MIC results for each laboratory ranged from two to three log2 dilution steps. The proposed MIC QC range was determined to be 0.06 to 0.5 μg/ml (four log2 dilutions), which would include all generated values. When MICs are evenly distributed between two dilutions, a four-dilution range is recommended (4).
TABLE 1.
Inter- and intralaboratory comparisons of retapamulin MIC resultsa
| MIC (μg/ml) | No. of occurrences of organism in laboratory:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | Total | |
| S. aureus ATCC 29213 | |||||||||
| 0.03 | 0 | ||||||||
| 0.06 | 14 | 2 | 16 | 7 | 1 | 40b | |||
| 0.12 | 26 | 40 | 40 | 38 | 24 | 33 | 38 | 39 | 278b |
| 0.25 | 2 | 2b | |||||||
| 0.5 | 0 | ||||||||
| S. pneumoniae ATCC 49619 | |||||||||
| 0.03 | 0 | ||||||||
| 0.06 | 1 | 1c | |||||||
| 0.12 | 31 | 1 | 35 | 10 | 19 | 20 | 9 | 30 | 155c |
| 0.25 | 8 | 32 | 5 | 30 | 21 | 1 | 30 | 10 | 137c |
| 0.5 | 7 | 19 | 1 | 27c | |||||
| 1 | 0 | ||||||||
Results represent testing of two QC strains for an eight-medical-center protocol meeting the study design guidelines found in CLSI M23-A2 (4).
One hundred percent of qualifying laboratory results were within the proposed QC range (0.06 to 0.25 μg/ml).
One hundred percent of qualifying laboratory results were within the proposed QC range (0.06 to 0.5 μg/ml).
Table 2 shows the retapamulin disk diffusion zone diameter distributions among the eight laboratories testing S. aureus ATCC 25923, with an overall median value of 26 mm, and seven laboratories testing S. pneumoniae ATCC 49619, with an overall median value of 16 mm. The proposed QC range for S. aureus ATCC 25923 calculated by the CLSI median statistical method was 26 ± 3 mm (4). However, only 94.6% of reported results were within this range; therefore, 1 mm was added to the range upper limit (30 mm) to achieve ≥95.0% of the results within the proposed range (4). Internal quality control results were within range for S. aureus for all eight laboratories; therefore, all laboratories were included in the analysis. With laboratory H omitted from the S. pneumoniae ATCC 49619 analysis, the CLSI median statistical method was also applied without adjustment to the remaining minimum seven laboratories to propose a QC range at 16 ± 3 mm, resulting in 97.4% of results within the suggested range (4). Although laboratories A and G had no overlap of results, both were included to meet the CLSI statistical requirements.
TABLE 2.
Inter- and intralaboratory comparisons of retapamulin zone diameter resultsa
| Zone diam (mm) | No. of occurrences of organism in laboratory:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | Total | |
| S. aureus ATCC 25923 | |||||||||
| 21 | 0 | ||||||||
| 22 | 1 | 1 | |||||||
| 23 | 6 | 2 | 12 | 7 | 27b | ||||
| 24 | 3 | 9 | 11 | 28 | 24 | 75b | |||
| 25 | 21 | 4 | 21 | 3 | 17 | 20 | 8 | 94b | |
| 26 | 36 | 8 | 21 | 20 | 18 | 16 | 119b | ||
| 27 | 20 | 3 | 1 | 32 | 6 | 4 | 66b | ||
| 28 | 17 | 14 | 5 | 5 | 1 | 42b | |||
| 29 | 11 | 19 | 30b | ||||||
| 30 | 21 | 21b | |||||||
| 31 | 5 | 5 | |||||||
| 32 | 0 | ||||||||
| S. pneumoniae ATCC 49619 | |||||||||
| 11 | 0 | ||||||||
| 12 | 4 | 4 | |||||||
| 13 | 28 | 28c | |||||||
| 14 | 26 | 9 | 13 | 19 | 67c | ||||
| 15 | 2 | 19 | 29 | 30 | 1 | 21 | 102c | ||
| 16 | 18 | 6 | 13 | 21 | 10 | 3 | 71c | ||
| 17 | 6 | 11 | 12 | 14 | 6 | 13 | 62c | ||
| 18 | 8 | 1 | 5 | 15 | 4 | 18 | 51c | ||
| 19 | 9 | 19 | 28c | ||||||
| 20 | 6 | 6 | |||||||
| 21 | 1 | 1 | |||||||
| 22 | 0 | ||||||||
Results represent testing of two QC strains for an eight-medical-center protocol meeting the study design guidelines found in CLSI M23-A2 (4).
Proposed QC range (23 to 30 mm) that included 98.8% of reported zone diameters from eight laboratories.
Proposed QC range (13 to 19 mm) that included 97.4% of reported zone diameters from seven laboratories (laboratory H results were omitted from analysis).
The veterinary drug tiamulin, which had QC ranges published recently by the CLSI (5, 8), was used for comparison. The disk diffusion QC range published for tiamulin against S. aureus ATCC 25923 was 25 to 32 mm. The MIC QC ranges for tiamulin were 0.5 to 2 μg/ml for S. aureus ATCC 29213 and 0.5 to 4 μg/ml for S. pneumoniae ATCC 49619. The proposed MIC QC ranges for retapamulin presented here were significantly lower (eightfold) than those for tiamulin, reflecting the greater potency of retapamulin compared to tiamulin (5, 8) (Table 1).
The results from this collaborative study provide the initial retapamulin broth microdilution MIC and disk diffusion QC ranges for S. aureus ATCC 29213 or 25923 and S. pneumoniae ATCC 49619. As this novel topical pleuromutilin agent progresses through human clinical trials, the susceptibility testing results can be accurately validated by concurrent quality assurance procedures.
Acknowledgments
We thank the following participating members of the Quality Control Working Group: R. Rennie, University of Alberta, Edmonton, Alberta, Canada; G. Hall, The Cleveland Clinic Foundation, Cleveland, Ohio; A. Wagner, University of Texas Medical Center, Houston; D. Hardy, University of Rochester Medical Center, Rochester, N.Y.; M. Wilson, Denver Health Medical Center, Denver, Colo.; A. Limaye, University of Washington, Seattle; C. Knapp, TREK Diagnostics, Cleveland, Ohio; and T. Fritsche, JMI Laboratories, North Liberty, Iowa.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2005. Performance standard for antimicrobial susceptibility testing. Document M100-S15. CLSI, Wayne, Pa.
- 2.Hunt, E. 2000. Pleuromutilins antibiotics. Drugs Future 25:1163-1168. [Google Scholar]
- 3.Jones, R. N., M. A. Pfaller, P. R. Rhomberg, and D. H. Walter. 2002. Tiamulin activity against fastidious and nonfastidious veterinary and human bacterial isolates: initial development of in vitro susceptibility test methods. J. Clin. Microbiol. 40:461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. 2001. Development of in vitro susceptibility testing criteria and quality control parameters, 2nd ed. Approved standard M23-A2. NCCLS, Wayne, Pa.
- 5.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolates from animals. Approved standard M31-A2. NCCLS, Wayne, Pa.
- 6.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests. Approved standard, 8th ed. Document M2-A8. NCCLS, Wayne, Pa.
- 7.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Document M7-A6. NCCLS, Wayne, Pa.
- 8.Pfaller, M. A., R. N. Jones, D. H. Walter, et al. 2001. Proposed quality control guidelines for National Committee for Clinical Laboratory Standards standard susceptibility tests using the veterinary antimicrobial agent tiamulin. Diagn. Microbiol. Infect. Dis. 40:67-70. [DOI] [PubMed] [Google Scholar]
- 9.Walsh, C. 2003. Antibiotics: actions, origins, resistance, p. 237-270. ASM Press, Washington, D.C.