Abstract
Human and canine visceral leishmaniasis caused by Leishmania infantum emerged in central Israel after an absence of over 30 years. The origin of this outbreak was investigated by examining genetic polymorphisms in 37 strains isolated from dogs and patients with visceral leishmaniasis in the continuously active northern Israeli and West Bank foci and in a new Israeli focus using DNA fingerprinting with the human multilocus minisatellite probe 33.15. Analysis of the patterns obtained by DNA fingerprinting separated the strains geographically into northern (clade B) and central (clades A and C) genotypic groups. These results suggest that the emergence of visceral leishmaniasis in central Israel is due not to parasite spread from northern Israel to the new focus but rather to increased parasite transmission in central Israel and the West Bank coupled with changes in the ecoepidemiology of this region.
Human visceral leishmaniasis (VL) (HVL) caused by Leishmania infantum infection is a fatal disease if not rapidly diagnosed and treated (11, 18). In the Middle East and countries surrounding the Mediterranean Basin, the dog is the primary peridomestic reservoir host. In these regions, the seroprevalence of canine VL (CVL) varies from <1% to 24%, but other canids, such as jackals (Canis aureus) and red foxes (Vulpes vulpes), are potential reservoirs of this parasite (26). Until recently, HVL caused by L. infantum was primarily a pediatric disease; however, the epidemiology of this disease is changing. In southern Europe, 50% of the new cases are found in adults coinfected with human immunodeficiency virus (HIV), and in Israel, children accounted for only 58% of the cases diagnosed during the last decade (3, 38).
Northern Israel (Galilee) is an area where HVL is endemic, and 68 cases were reported in this region from 1960 to 1993, with no cases originating from other parts of the country (2, 15, 22, 38). Recent studies showed that active transmission still occurs in Galilee, even though the number of clinical cases is small (2). In 1994, after an absence of >30 years, index cases of HVL and CVL were diagnosed in central Israel (5). Since then, an additional 27 foci and numerous cases of CVL have been identified in this region by passive and active surveillance. Indeed, >50% of the HVL cases diagnosed between 1990 and 2002 originated from central Israel (38).
Molecular typing methods for characterizing strain polymorphisms have been useful in monitoring drug resistance, disease epidemiology, and spread of pathogens. While multilocus enzyme electrophoresis (MLEE) is still the “gold standard” for typing of Leishmania species, approximately 90% of Leishmania infantum strains analyzed by this method from HVL and CVL belong to one zymodeme, MON-1 (20). Other zymodemes for this parasite have been described, but they are rare and frequently associated with atypical clinical pathologies such as HIV-Leishmania coinfections or cutaneous leishmaniasis. Many Leishmania species, such as L. major, L. tropica, and L. (Viannia) braziliensis, show extensive polymorphism using several techniques, including MLEE, single-strand conformation polymorphism, DNA sequencing of the rRNA gene intergenic transcribed spacer region (ITS), and permissively primed intergenic polymorphic PCR; however, these techniques have shown limited usefulness in analyzing L. infantum (13, 33, 34). Randomly amplified polymorphic DNA PCR and microsatellite sequencing have been successfully used to examine genetic polymorphism in L. infantum (7, 12, 20, 37), and in at least two cases, an association between genotype and geographic distribution was noted (7, 37). While DNA fingerprinting using human multilocus minisatellite probes has been useful in genotyping many organisms (14, 17, 24, 27), including L. (Viannia) braziliensis, this technique has not been widely utilized to look at Leishmania polymorphism. L. infantum strains from the northern and central regions of endemicity in Israel and the adjacent West Bank were examined using DNA fingerprinting in an attempt to determine whether the reemergence of VL in central Israel is due to parasite spread southward from the northern focus or changes in the local ecoepidemiology, allowing parasite transmission from adjacent West Bank reservoirs to dogs and humans in central Israel. Our results suggest that this new outbreak of VL is probably caused by increased transmission of local parasite populations from preexisting reservoir hosts in the West Bank to dogs and people rather than by the introduction of the disease from northern Israel.
MATERIALS AND METHODS
Leishmania.
Parasites were isolated by tissue aspiration from dogs (lymph nodes and/or spleen) or humans (bone marrow) and cultured on NNN semisolid medium or M199 plus 10% fetal calf serum and antibiotics (21, 25). All isolates were characterized as L. infantum by excreted-factor serotyping and intergenic transcribed spacer 1 region (ITS1) PCR (31, 33). PCR products were analyzed by restriction fragment length polymorphism following digestion with the restriction enzyme HaeIII. Randomly selected L. infantum strains were further characterized by DNA sequencing of the ITS1 rRNA gene region, permissively primed intergenic polymorphic PCR, microsatellite markers, and isoenzyme analysis (7, 13, 28).
DNA fingerprinting.
Genomic DNA was purified from each of the parasite strains by phenol-chloroform extraction. DNA (10 μg) was digested with HaeIII overnight. Complete digestion was ensured by further digestion with additional enzyme for 6 h. The digestion products were separated by 0.8% agarose gel electrophoresis and Southern blotting, carried out essentially as previously described (30), using a digoxigenin (DIG)-labeled human multilocus probe, 33.15 (23). pBluescript containing the 33.15 probe was a gift from A. Jeffreys, University of Leicester, Leicester, United Kingdom. In brief, the genomic DNA was UV cross-linked (UV Stratalinker 1800; Stratagene, CA) after transfer to a positively charged nylon membrane (Roche Applied Science, Mannheim, Germany) and then incubated in DIG Easy Hyb (Roche Applied Science) for 45 min at 42°C. DIG-labeled 33.15 probe was heated to 100°C for 15 min, chilled on ice, diluted in DIG Easy Hyb, and hybridized to the membrane overnight at 42°C. The membrane was washed three times: once with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate at room temperature (RT), a second time with 1× SSC-0.1% sodium dodecyl sulfate at 42°C, and finally with blocking solution (Roche Applied Science) for 45 min at RT. The blot was incubated with the sheep anti-DIG antibody (1/10,000 dilution in blocking solution; Roche Applied Science) for 30 min (RT) and then washed three times. Hybridization of the probe to genomic DNA bands was detected by adding the substrate CDP-Star (Roche Applied Science) in detection buffer, removing excess solution, and exposing the membrane to X-ray film. The film was scanned, and the hybridization pattern was analyzed as described below.
The 33.15 multilocus probe was labeled with DIG-dUTP by PCR using specific primers (upper [5′-CCTGCCTTTCCTAACATTTGGGTAC-3′] and lower [5′-GTACCCAAATGTTAGGAAAGGCAGG-3′]) in a PTC-100 Thermocycler (MJ Research, Inc., MA) as follows. Plasmid DNA (1 to 2 ng) was added to a reaction mixture (50-μl final volume) containing primers (2 μM), Taq polymerase (2.5 U), PCR DIG labeling mix (0.2 mM), and 5 μl Taq polymerase 1× buffer (Sigma). The DNA was initially denatured at 94°C for 4 min, followed by 40 cycles of denaturation at 90°C for 20 s, annealing at 65°C for 30 s, and elongation at 72°C for 60 s. A final elongation step at 72°C for 6 min was carried out after the last cycle. Purity of the DIG-labeled probe was examined by agarose gel electrophoresis (1%), and the probe was stored at −20°C until use.
Data analysis.
DNA fingerprints for the leishmanial strains were analyzed using the RAPDistance Package version 1.04 (http://www.anu.edu.au/BoZo/software/). All bands obtained were numbered and scored for their absence or presence. Similarities representing the ratio of shared bands among the total bands were compared for all the L. infantum strains tested. Distance matrices based on pairwise comparisons were calculated using Pearson's phi coefficient, and trees were constructed by the neighbor-joining method.
RESULTS
A total of 37 Leishmania strains (Table 1) were isolated from dogs and humans with VL in different regions of Israel and the West Bank over the period of 1994 to 2003 and examined by various molecular techniques. All isolates were characterized by excreted-factor serotyping and ITS1-PCR and showed serotype B2 and restriction fragment length polymorphism patterns typical of the Leishmania donovani complex (data not shown). All of the dog and human strains (13) analyzed by MLEE were MON-1 (7, 22), except for one human strain from Jenin that differed in the electrophoretic mobility of malate dehydrogenase (MON-281) (4). MON-1 is the most common and widespread zymodeme for L. infantum.
TABLE 1.
Sources and origins of L. infantum strains examined
| Strain | WHO code | Source(s)a | Origin | Regionb | 33.15c |
|---|---|---|---|---|---|
| LRC-L741 | MCAN/IL/98/Siko A | D | Alei Zahav | WB | C |
| LRC-L742 | MHOM/IL/98/L742 | H | Barkan | WB | C |
| LRC-L921 | MCAN/IL/02/Naila | D | Beit Arif | CI | A |
| LRC-L772 | MCAN/IL/99/L772 | D | Har Adar | CI | A |
| LRC-L808 | MCAN/PS/00/SawalhaS | D | Jenin | WB | C |
| LRC-L807 | MCAN/PS/00/SawalhaK | D | Jenin | WB | C |
| LRC-L773 | MHOM/PS/99/L773 | H | Jenin | WB | C |
| LRC-L745 | MHOM/IL/98/L745 | H | Kiryat Sefer | WB | C |
| LRC-L709 | MCAN/IL/96/L709 | D | Klil | NI | B |
| LRC-L705 | MCAN/IL/96/8308 | D | Klil | NI | B |
| LRC-L706 | MCAN/IL/96/8294 | D | Klil | NI | B |
| LRC-L708 | MCAN/IL/96/8402 | D | Klil | NI | B |
| LRC-L699 | MCAN/IL/96/Vit Y | D | Klil | NI | B |
| LRC-L911 | MCAN/IL/02/L911 | D | Modi'in | CI | C |
| LRC-L894 | MCAN/IL/02/Yukon | D | Modi'in | CI | C |
| LRC-L639 | MCAN/IL/94/Robi | D | Nataf | CI | A |
| LRC-L718 | MCAN/IL/97/8635 | D | Nataf | CI | A |
| LRC-L700 | MCAN/IL/96/Borschtein | D | Nataf | CI | A |
| LRC-L716 | MCAN/IL/97/8639 | D | Nataf | CI | A |
| LRC-L717 | MCAN/IL/97/8632 | D | Nataf | CI | C |
| LRC-L792 | MCAN/IL/00/Shiraz | D | Nataf | CI | A |
| LRC-L966 | MCAN/IL/03/L966 | D | Nataf | CI | A |
| LRC-L880 | MCAN/IL/02/L880 | D | Neve Ellan | CI | C |
| LRC-L695 | MCAN/IL/96/8041 | D | Nili | WB | C |
| LRC-L697 | MCAN/IL/96/Chen | D | Nili | WB | C |
| LRC-L690 | MCAN/IL/96/L690 | D | Nili | WB | C |
| LRC-L692 | MCAN/IL/96/Khoumeini | D | Nili | WB | C |
| LRC-L693 | MCAN/IL/96/Donna A | D | Nili | WB | C |
| LRC-L696 | MCAN/IL/96/Miara | D | Nili | WB | A |
| LRC-L783 | MCAN/IL/00/Freddie | D | Reshafim | NI | B |
| LRC-L842 | MCAN/IL/00/L842 | D | Reut | CI | C |
| LRC-L760 | MCAN/IL/99/Shelly | D, S | Rosh Ha'ayin | CI | A |
| LRC-L689 | MCAN/IL/96/Bashan | D | Sataf | CI | C |
| LRC-L847 | MCAN/IL/02/Skoshi S | D | Sataf | CI | C |
| LRC-L869 | MCAN/IL/02/Tequilla | D | Shilat | WB | C |
| LRC-L905 | MCAN/IL/02/Leiboy | D | Shoham | CI | C |
| LRC-L720 | MCAN/IL/97/Rex | D | Tzur Natan | CI | C |
D, dog; H, human; S, sand fly.
NI, northern Israel; CI, central Israel; WB, West Bank.
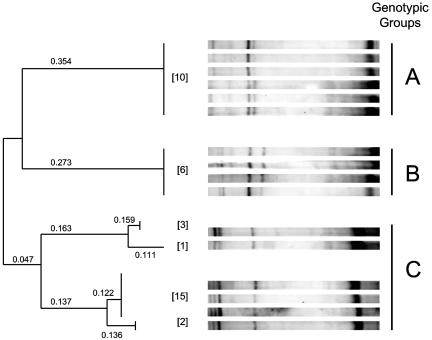
DNA fingerprinting with the human multilocus probe 33.15, clades A to C.
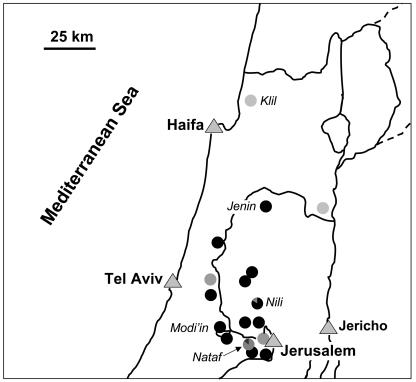
All the strains were examined by DNA fingerprinting using the human multilocus minisatellite probe 33.15. This probe has been used to examine polymorphism and genetic relatedness between South American Leishmania strains as well as higher eukaryotes (14, 17, 24, 27). A total of 15 different bands could be identified. Polymorphism among the L. infantum strains was demonstrated using this technique for all the fragments. Analysis of the hybridization patterns allowed the strains to be separated into three genetic clusters (clusters A to C) (Fig. 1 and Table 1). A correlation with geographic distribution was readily apparent using this genotyping method, since all the strains examined from northern Israel (n = 6) grouped together in clade B, while those from the central regions, Israel and the West Bank, grouped into clades A and C (Fig. 1 and 2 and Table 1). Interestingly, 6/7 strains from Nataf and 5/6 strains from Nili in central Israel and the West Bank, respectively, clustered together in separate clades, clades A and C, respectively. The remaining strains from the central region also clustered with these isolates in either clade A or C (Fig. 1 and 2). All of the strains analyzed from Klil (clade B, northern Israel) and Nili (the West Bank) were isolated from dogs during a single visit in 1996. However, the seven strains examined from Nataf (central Israel) were collected over a period of 10 years, from 1994 to 2003, yet 86% (6/7) still showed identical hybridization patterns. The lone Nataf strain that belongs to a different clade (clade C) was collected in 1997. However, a strain belonging to clade A was also isolated from this village during that same year. These results suggest that the hybridization patterns, i.e., sequences recognized by probe 33.15, are relatively stable and probably change slowly over time. Therefore, this method should be useful for monitoring the origin of the L. infantum strains and their spread into new areas. Strains belonging to clade A may have a more limited geographical distribution than those belonging to clade C. To date, they have been only found within a 20-km radius of Modi'in (Fig. 2), while strains belonging to clade C are more widely distributed and appear to be the predominant genotype in central Israel and the West Bank (21/31 strains).
FIG. 1.
DNA fingerprinting patterns and genotypes of Israeli Leishmania infantum obtained with the human multilocus probe 33.15. The dendrogram was calculated using the RAPDistance Package (version 1.04) based on pairwise comparison of the Southern blot hybridization patterns. The number of strains belonging to each genotypic branch is given in square brackets.
FIG. 2.
Distribution of Leishmania infantum strains in Israel and the West Bank. The origin of each parasite strain, its genotype, and the percentage of parasites belonging to each genotype (pie graph) are shown. Genotypes: A, ; B, ; C, .
Interestingly, all of the parasites isolated from HVL cases to date and examined by DNA fingerprinting fall into clade C. Dog strains, isolated from the same focus as the human cases, also belong to clade C. Additional Israeli and Palestinian HVL strains are needed to determine if a correlation exists between parasites clustering in clade C and the ability to cause human visceral disease.
DISCUSSION
Human and canine VL reemerged in central Israel after an absence of more than 30 years (5, 22). Even though VL cases were not reported from this region during the intervening period of 1960 to 1993, sporadic cases of infantile HVL continued to occur in northern Israel (2, 15, 22). In the Mediterranean region, zoonotic VL caused by L. infantum occurs when environmental and ecological conditions favor domestic and peridomestic transmission among pet and stray dogs by sand fly vectors (26). Conditions leading to human disease are still unknown, but most likely, humans are accidental hosts.
In the late 1950s and early 1960s, widespread poisoning of foxes and jackals was carried out as part of a rabies control campaign in Israel. This program led to a drastic reduction in canid reservoirs and the near eradication of jackals (5, 22). Over the last two decades, there has been a dramatic increase in these sympatric wild-canid populations throughout the region. In parallel, an exponential increase has been seen in the number of rabies cases in foxes and jackals from 1985 to 1997 (9, 39). While wild canids are thought to be involved in the spread of VL via a sylvatic cycle, the exact role they play in parasite transmission to sand flies in the Mediterranean region is unclear (26). The phylogenetic relationship between jackals and domestic dogs is considered to be very close and contributes to the transmission of infectious diseases between them (35). However, to our knowledge, little or no information is available on the pathology of VL in foxes (Vulpes vulpes) or jackals (Canis aureus) (19) infected with L. infantum or on the susceptibility of putative vectors for VL in the eastern Mediterranean to parasite infection when allowed to feed on these animals. Preliminary serological surveys carried out during the last decade in central Israel and the West Bank showed that jackals and foxes are positive for antileishmanial antibodies (7.6 and 5%, respectively) (5). In addition, putative vectors of L. infantum in the eastern Mediterranean region, including Phlebotomus tobbi, Phlebotomus syriacus, Phlebotomus neglectus, and Phlebotomus perfiliewi, have been identified in Nataf and Al-Jdideh, two foci of VL in central Israel and the West Bank, respectively (reference 29 and unpublished data). One study carried out with colony-reared Lutzomyia longipalpis, and xenodiagnosis on naturally infected crab-eating foxes (Cerdocyon thous), a potential reservoir host in Latin America, suggested that foxes belonging to this genus are unable to maintain a transmission cycle independently of domestic dogs. The contribution of crab-eating foxes to parasite transmission was calculated to be only 9%, compared to 91% by dogs, and unlike dogs, these foxes showed no clinical signs of progressive disease over a 15-month period (8). It is unclear, however, whether this finding will be applicable to parasite transmission in the Middle East and Mediterranean regions.
Recent urbanization and agricultural development along the 1967 green line separating central Israel and the West Bank, coupled with an increase in the wild-canid populations and stray dogs and changes in insecticide usage, have generated appropriate conditions to bring pet dogs and humans into contact with a peridomestic and sylvatic cycle of transmission. A substantial increase in the number of stray dogs, a trend which appears to be continuing (16), occurred in 1991 during the Gulf War, when pets were abandoned by residents fleeing the bombing in central Israel (36). Even though no cases of HVL were reported from central Israel during the period preceding 1994, 50 cases of HVL were diagnosed in the Jenin district, the West Bank, from 1989 to 1998, with a peak of 21 cases seen in 1994 (1). Several cases of HVL were also reported by the Palestinian Ministry of Health in other districts of the West Bank during this time period (1). This suggests that sylvatic and urban transmissions were present in the West Bank prior to the reemergence of VL in central Israel. The emergence of the disease in central Israel coincided with a peak of HVL in adjacent areas of the West Bank.
Previous studies of the genetic evolution of Leishmania indicate a clonal population structure with only occasional genetic exchange (6, 32). L. infantum stocks isolated from immunocompromised people (HIV/VL coinfections), atypical clinical presentations, and sand flies show more polymorphism by MLEE than parasites isolated from dogs or immunocompetent humans with VL. The latter isolates primarily belong to the MON-1 zymodeme (20). Analysis using techniques with faster molecular clocks, such as randomly amplified polymorphic DNA or microsatellite markers, is more discriminatory than MLEE. Strains from areas of endemicity in southern France and Madrid, monomorphic by MLEE (MON-1), were shown to be polymorphic, with nine and four genotypes, respectively, when examined using five microsatellite markers for L. infantum. A limited number of L. infantum stocks (n = 10) from central and northern Israel were also examined with these microsatellite markers and showed low polymorphism. However, similar to DNA fingerprinting with probe 33.15, the Israeli strains were grouped together by geographic origin and split into northern and central clades (7).
Interestingly, phylogenetic analysis using the virus nucleoprotein DNA sequence of 226 rabies virus isolates from Israel and the West Bank showed that they could be grouped by geographical region rather than host species (10). The five viral variants identified were distributed in four geographical regions, with rabies isolates from northern Israel (Galilee) grouped separately from those in central Israel and the West Bank. Geographic barriers, such as the Gilboa mountain range separating Galilee from the Central Region, were postulated to act as a barrier, interrupting transmission by separating fox populations, the main reservoir host of the virus in Israel. A similar mechanism involving geographic barriers may be operating in the case of L. infantum in Israel.
Taken together, our data suggest that VL reemerged in central Israel due to changing ecoepidemiological conditions resulting in increased human presence and numbers of canids and modification of habitats along the green line between Israel and the West Bank, where parasite transmission was taking place. These results suggest that VL did not spread south from the old northern Israeli focus in Galilee, but rather, changing conditions and increased mobility of infected canids in the central region allowed an epidemic clonal expansion of existing isolates previously circulating at low levels in the local canid population. The presence of several potential sand fly vectors, expanding jackal and fox populations, and the movement of pet, stray, or feral dogs between the West Bank and central Israel may complicate the analysis of the epidemiology of this emerging disease. However, DNA fingerprinting of additional L. infantum strains from all locations will enlighten our understanding of the molecular epidemiology of this disease.
Acknowledgments
We thank Lee Schnur for culturing and maintaining the parasite strains used for this study at the WHO Leishmania Reference Center, Department of Parasitology, Hebrew University of Jerusalem.
This research was supported in part by grants from the Deutsche Forschungsgemeinschaft (grant SO 220/5-1) as part of a German-Israeli-Palestinian Co-operative Project on “Leishmaniasis in Israel and the West Bank,” the Center for the Study of Emerging Diseases (C.L.J. and G.B.), and the USAID CDR Program, grant number TA-MOU-00-C20-025PH (G.B.).
REFERENCES
- 1.Abdeen, Z. A., S. S. Sawalha, C. L. Eisenberger, H. M. Khanfar, C. L. Greenblatt, O. Yousef, L. F. Schnur, K. Azmi, A. Warburg, K. A. Bader, C. L. Jaffe, and G. Baneth. 2002. Epidemiology of visceral leishmaniasis in the Jenin District, West Bank: 1989-1998. Am. J. Trop. Med. Hyg. 66:329-333. [DOI] [PubMed] [Google Scholar]
- 2.Adini, I., M. Ephros, J. Chen, and C. L. Jaffe. 2003. Asymptomatic visceral leishmaniasis, northern Israel. Emerg. Infect. Dis. 9:397-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar, J., B. Gutierrez-Solar, I. Pachon, E. Calbacho, M. Ramirez, R. Valles, J. L. Guillen, C. Canavate, and C. Amela. 1996. AIDS and Leishmania infantum. New approaches for a new epidemiological problem. Clin. Dermatol. 14:541-546. [DOI] [PubMed] [Google Scholar]
- 4.Bader, K. A., L. F. Schnur, A. Nasereddin, F. Pratlong, J.-P. Dedet, L. Shaheen, O. Yousef, and C. L. Greenblatt. 2005. Palestinian infantile visceral leishmaniasis caused by a genetic variant of Leishmania infantum belonging to a new zymodeme. Trop. Med. Int. Health 10:618-620. [DOI] [PubMed] [Google Scholar]
- 5.Baneth, G., G. Dank, E. Keren-Kornblatt, E. Sekeles, I. Adini, C. L. Eisenberger, L. F. Schnur, R. King, and C. L. Jaffe. 1998. Emergence of visceral leishmaniasis in central Israel. Am. J. Trop. Med. Hyg. 59:722-725. [DOI] [PubMed] [Google Scholar]
- 6.Banuls, A. L., M. Hide, and M. Tibayrenc. 1999. Molecular epidemiology and evolutionary genetics of Leishmania parasites. Int. J. Parasitol. 29:1137-1147. [DOI] [PubMed] [Google Scholar]
- 7.Bulle, B., L. Millon, J. M. Bart, M. Gallego, F. Gambarelli, M. Portus, L. Schnur, C. L. Jaffe, S. Fernandez-Barredo, J. M. Alunda, and R. Piarroux. 2002. Practical approach for typing strains of Leishmania infantum by microsatellite analysis. J. Clin. Microbiol. 40:3391-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtenay, O., R. J. Quinnell, L. M. Garcez, and C. Dye. 2002. Low infectiousness of a wildlife host of Leishmania infantum: the crab-eating fox is not important for transmission. Parasitology 125:407-414. [DOI] [PubMed] [Google Scholar]
- 9.David, D., C. E. Rupprecht, J. Smith, I. Samina, S. Perl, and Y. Shram. 1999. Human rabies in Israel. Emerg. Infect. Dis. 5:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David, D., B. Yakobson, J. S. Smith, and Y. Stram. 2000. Molecular epidemiology of rabies virus isolates from Israel and other Middle- and Near-Eastern countries. J. Clin. Microbiol. 38:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desjeux, P. 2001. Worldwide increasing risk factors for leishmaniasis. Med. Microbiol. Immunol. (Berlin) 190:77-79. [DOI] [PubMed] [Google Scholar]
- 12.Diakou, A., and C. I. Dovas. 2001. Optimization of random-amplified polymorphic DNA producing amplicons up to 8500 bp and revealing intraspecies polymorphism in Leishmania infantum isolates. Anal. Biochem. 288:195-200. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberger, C. L., and C. L. Jaffe. 1999. Leishmania: identification of Old World species using a permissively primed intergenic polymorphic-polymerase chain reaction. Exp. Parasitol. 91:70-77. [DOI] [PubMed] [Google Scholar]
- 14.Elder, J. F., Jr., and I. J. Schlosser. 1995. Extreme clonal uniformity of Phoxinus eos/neogaeus gynogens (Pisces: Cyprinidae) among variable habitats in northern Minnesota beaver ponds. Proc. Natl. Acad. Sci. USA 92:5001-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ephros, M., A. Paz, and C. L. Jaffe. 1994. Asymptomatic visceral leishmaniasis in Israel. Trans. R. Soc. Trop. Med. Hyg. 88:651-652. [DOI] [PubMed] [Google Scholar]
- 16.Gdalevich, M., D. Mimouni, I. Ashkenazi, and J. Shemer. 2000. Rabies in Israel: decades of prevention and a human case. Public Health 114:484-487. [DOI] [PubMed] [Google Scholar]
- 17.Gomes, R. F., A. M. Macedo, S. D. Pena, and M. N. Melo. 1995. Leishmania (Viannia) braziliensis: genetic relationships between strains isolated from different areas of Brazil as revealed by DNA fingerprinting and RAPD. Exp. Parasitol. 80:681-687. [DOI] [PubMed] [Google Scholar]
- 18.Guerin, P. J., P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 19.Hervas, J., A. Mendez, L. Carrasco, and J. C. Gomez-Villamandos. 1996. Pathological study of visceral leishmaniasis in a jackal (Canis aureus). Vet. Rec. 139:293-295. [DOI] [PubMed] [Google Scholar]
- 20.Hide, M., A. L. Banuls, and M. Tibayrenc. 2001. Genetic heterogeneity and phylogenetic status of Leishmania (Leishmania) infantum zymodeme MON-1: epidemiological implications. Parasitology 123:425-432. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson, R. L. 2003. Leishmania tropica (Kinetoplastida: Trypanosomatidae)—a perplexing parasite. Folia Parasitol. 50:241-250. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe, C. L., G. Baneth, Z. A. Abdeen, Y. Schlein, and A. Warburg. 2004. Leishmaniasis in Israel and the Palestinian Authority. Trends Parasitol. 20:328-332. [DOI] [PubMed] [Google Scholar]
- 23.Jeffreys, A. J., V. Wilson, and S. L. Thein. 1985. Hypervariable ‘minisatellite’ regions in human DNA. Nature 314:67-73. [DOI] [PubMed] [Google Scholar]
- 24.Macedo, A. M., M. N. Melo, R. F. Gomes, and S. D. Pena. 1992. DNA fingerprints: a tool for identification and determination of the relationships between species and strains of Leishmania. Mol. Biochem. Parasitol. 53:63-70. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy-Burke, C., P. A. Bates, and D. M. Dwyer. 1991. Leishmania donovani: use of two different, commercially available, chemically defined media for the continuous in vitro cultivation of promastigotes. Exp. Parasitol. 73:385-387. [DOI] [PubMed] [Google Scholar]
- 26.Moreno, J., and J. Alvar. 2002. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 18:399-405. [DOI] [PubMed] [Google Scholar]
- 27.Perez, T., J. Albornoz, E. Garcia-Vazques, and A. Dominguez. 1996. Application of DNA fingerprinting to population study of chamois (Rupicapra rupicapra). Biochem. Genet. 34:313-320. [PubMed] [Google Scholar]
- 28.Rioux, J. A., J. Dereure, A. Khiami, F. Pratlong, K. Sirdar, and M. Lambert. 1990. Ecoepidemiology of leishmaniasis in Syria. 1. Leishmania major Yakimoff and Schokhor (Kinetoplastida-Trypanosomatidae) infestation of Psammomys obesus Cretzschmar (Rodentia-Gerbillidae). Ann. Parasitol. Hum. Comp. 65:203-207. (In French.) [DOI] [PubMed] [Google Scholar]
- 29.Sawalha, S. S., M. S. Shtayeh, H. M. Khanfar, A. Warburg, and Z. A. Abdeen. 2003. Phlebotomine sand flies (Diptera: Psychodidae) of the Palestinian West Bank: potential vectors of leishmaniasis. J. Med. Entomol. 40:321-328. [DOI] [PubMed] [Google Scholar]
- 30.Schnur, L., A. Nasereddin, C. L. Eisenberger, C. L. Jaffe, M. El Fari, K. Azmi, G. Anders, M. Killick-Kendrick, R. Killick-Kendrick, J. P. Dedet, F. Pratlong, M. Kanaan, T. Grossman, R. L. Jacobson, G. Schonian, and A. Warburg. 2004. Diversity of Leishmania tropica isolates from Phlebotomus sergenti sand flies and humans in a Judean Desert focus. Am. J. Trop. Med. Hyg. 70:364-372. [PubMed] [Google Scholar]
- 31.Schnur, L. F., and A. Zuckerman. 1977. Leishmanial excreted factor (EF) serotypes in Sudan, Kenya and Ethiopia. Ann. Trop. Med. Parasitol. 71:273-294. [DOI] [PubMed] [Google Scholar]
- 32.Schonian, G., M. El Fari, S. Lewin, C. Schweynoch, and W. Presber. 2001. Molecular epidemiology and population genetics in Leishmania. Med. Microbiol. Immunol. (Berlin) 190:61-63. [DOI] [PubMed] [Google Scholar]
- 33.Schonian, G., A. Nasereddin, N. Dinse, C. Schweynoch, H. D. Schallig, W. Presber, and C. L. Jaffe. 2003. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn. Microbiol. Infect. Dis. 47:349-358. [DOI] [PubMed] [Google Scholar]
- 34.Schonian, G., L. Schnur, M. el Fari, L. Oskam, A. A. Kolesnikov, W. Sokolowska-Kohler, and W. Presber. 2001. Genetic heterogeneity in the species Leishmania tropica revealed by different PCR-based methods. Trans. R. Soc. Trop. Med. Hyg. 95:217-224. [DOI] [PubMed] [Google Scholar]
- 35.Shamir, M., B. Yakobson, G. Baneth, R. King, S. Dar-Verker, A. Markovics, and I. Aroch. 2001. Antibodies to selected canine pathogens and infestation with intestinal helminths in golden jackals (Canis aureus) in Israel. Vet. J. 162:66-72. [DOI] [PubMed] [Google Scholar]
- 36.Shimshony, A. 1997. Epidemiology of emerging zoonoses in Israel. Emerg. Infect. Dis. 3:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toledo, A., J. Martin-Sanchez, B. Pesson, C. Sanchiz-Marin, and F. Morillas-Marquez. 2002. Genetic variability within the species Leishmania infantum by RAPD. A lack of correlation with zymodeme structure. Mol. Biochem. Parasitol. 119:257-264. [DOI] [PubMed] [Google Scholar]
- 38.Ya'ari, A., C. L. Jaffe, and B. Z. Garty. 2004. Visceral leishmaniasis in Israel, 1960-2000. Isr. Med. Assoc. J. 6:205-208. [PubMed] [Google Scholar]
- 39.Yom-Tov, Y., S. Ashkenazi, and O. Viner. 1995. Cattle predation by the golden jackal Canis aureus in the Golan Heights, Israel. Biol. Conserv. 73:19-22. [Google Scholar]