Abstract
Galliform and non-galliform birds express three immunoglobulin isotypes, IgM, IgA and IgY. Beyond this we should not generalize because differences in gene organization may have functional consequences reflected in the immune response. At present, studies on non-galliform birds are largely restricted to ducks. Ducks express an alternatively spliced form of their IgY heavy chain (ν) gene, the IgY(ΔFc), that lacks the Fc region and Fc-associated secondary effector functions. It is not known how common the expression of the IgY(ΔFc) is among birds, nor the functional consequences. It is also not known whether the unusual organization of the duck IgH locus, also shared with the chicken, having the gene order of μ, α and ν, with α inverted in the locus, is unique to the galloanseriform lineage. Ducks, like chickens, have a single immunoglobulin light chain of the lambda (λ) type. Evidence suggests that ducks, like chickens, generate their immunoglobulin repertoire through a single functional rearrangement of the variable (V) region, and generate diversity through gene conversion from a pool of pseudogenes. In Southern blots of germline and rearranged bursal DNA, both the heavy and light chain loci of ducks appear to each undergo one major rearrangement event. For both heavy and light chains, the functional V region element and the pseudogenes appear to consist of a single gene family. Further analysis of 26 heavy chain joining (JH) and 27 light chain JL segments shows there is use of a single J segment in ducks, which is diversified presumably through somatic mutations and gene conversion events. Despite this limitation on the rearrangement of immunoglobulin genes, analysis of 26 DH and 122 VL sequences suggests that extensive sequence diversity is generated.
1. Introduction
The birds are an enormously diverse group of vertebrates, comprised of around 9000 species. Outside the galliform birds almost nothing is known about the immunoglobulins. While it is clear that all birds have the typical vertebrate adaptive immune system and can produce antibodies, documentation of the structure, genetics and diversity of these antibodies is almost completely lacking in most species, although small islands of information, usually quite incomplete, do exist. Examples include very limited studies on the antibodies of psittaciform (parrots etc) [1] and struthioniform (ostriches, emus etc) species [2]. After the chicken, the birds about whose immune systems most is known are the ducks. In terms of the breadth of our knowledge, this is unfortunate, since the anseriform birds (ducks and their relatives) are the closest relatives of the chickens, the gallo-anserine lineage having split about 90 million years ago [3].
Ducks have the same hematopoietic tissues as chickens, including bone marrow, gut associated lymphoid tissue, spleen, thymus and the Bursa of Fabricius, a specialized organ for B lymphoid development. However, there is one notable difference. Ducks have lymph nodes, which are completely absent in chickens [4].
2. Duck antibody structure and function
Based on what we know, primarily from studies in ducks and chickens, it is possible to make some sweeping generalizations about the immune system of birds, while recognizing that future experimental findings may well require a major revision of our assumptions. Birds have three classes of antibody: IgM, IgY and IgA. Each of these has been identified in ducks and their structure is illustrated schematically in Fig. 1. In addition, ducks have a smaller form of IgY, called IgY (ΔFc). These antibodies are present in serum and secretions of ducks with a distinct tissue distribution (Table 1). IgM and IgY and IgY (ΔFc) are present in serum, while IgA is expressed in a variety of secretions [5,6]. The immunoglobulin heavy chain genes have been identified for each isotype [7,8] as well as the gene encoding the light chain [9] (Table 2). In the subsequent sections, we will focus on our knowledge of duck Ig structure and gene organization, noting where appropriate the distinctions between ducks and other species of non-anseriform birds that should probably be drawn.
Fig. 1.
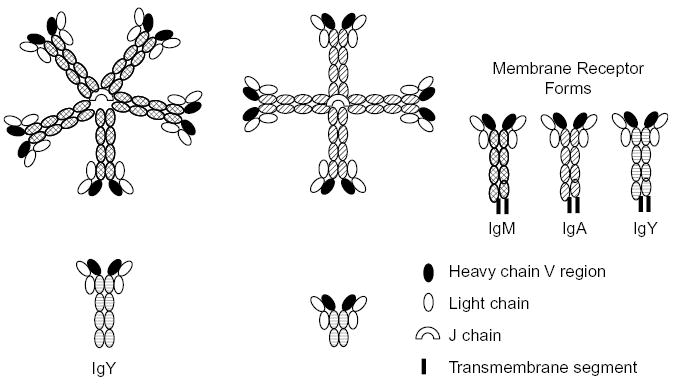
A schematic of the structures of duck antibodies. The polymeric structures of duck IgM and IgA have not been directly determined but inferred from size estimates of the intact molecules and their constituent polypeptide chains (see discussion in text).
Table 1.
Immunoglobulin concentrations in sera and bile
| Concentration (mg/ml) | IgM | IgY* | IgA |
|---|---|---|---|
| Serum | 2–4 | 2–5 | ND |
| Bile | ND | ND | 5–12 |
The proportion of IgY to IgY(ΔFc) is variable, but approximately 3:5.
Table 2.
Duck immunoglobulin nomenclature
IgM is the only class of antibody that is found in all vertebrates, and it exists primarily in two forms: a secreted, soluble, circulating polymeric form, and a monomeric form (illustrated in Fig. 1) that serves B lymphocytes as a membrane receptor for antigens. Ducks have a circulating polymeric IgM, having a molecular weight of approximately 800 kDa [10]. The heavy chains are 86 kDa and the light chains 23–25 kDa [10]. This has been previously interpreted as a tetrameric structure for IgM in ducks [6]. However, size determinations of chicken IgM, which was inferred to have a pentameric structure, yielded values ranging from 823 to 954 kDa, depending on solvent conditions [11]. The size of both chicken [11] and duck [10] IgM is larger than that of a true tetrameric IgM, such as occurs in teleost fish [11] and thus both are likely to be pentameric.
IgY is the major low-molecular weight form of antibody found circulating in birds, where it has sometimes been referred to as IgG. However, IgY differs from IgG in possessing an additional constant (C) region domain, there are 4 in IgY versus 3 C region domains in IgG. An IgY-like molecule is likely to have been the evolutionary precursor of both IgG and IgE immunoglobulins [12,13]. Genes encoding IgY from amphibian and birds group together in a phylogenetic distance tree, and are basal to the branches of independent groups carrying γ and ɛ genes [14]. The similarity of the terminal secretory tail of two amino acids beyond the cryptic splice site used for the transmembrane exons in the γ, ɛ and ν genes suggests their common origin [12,13,15–17], while the secretory tail for α and μ is 20 amino acids. The adjacent position of γ and ɛ genes in mammalian IgH chain loci is consistent with their recent duplication. Most importantly, the function of IgY appears to combine the two functions that are typically served separately by IgG and IgE. Like IgG, IgY is the major systemic antibody, involved in complement fixation and opsonization and maternally transferred to the developing offspring in egg yolk. Like IgE, IgY is a tissue sensitizing antibody, and can be involved in anaphylaxis [18].
Ducks possess three forms of IgY (see Fig. 1); a secreted form, with four constant region (C) domains, a receptor form with a hydrophobic membrane-spanning C-terminus, and a truncated form termed IgY(ΔFc) that has only 2 C region domains [7,13,19]. The genetic basis of the structural diversity seen in the duck IgY heavy (-upsilon or ν) chain is now understood [13]. There is a single ν gene, and the C regions of the 3 ν isoforms are encoded by 7 exons, which undergo alternative pathways of RNA processing to generate the 3 species of ν mRNA (Fig. 2). The ratio of full-length IgY to truncated IgY (ΔFc) in serum is approximately 3–5, however, the truncated form appears to predominate later in the immune response. While both IgY and IgY(ΔFc) appear to be passed to offspring through yolk sac transmission, the full-length form is predominantly transmitted [20]. An obvious question that arises from these observations in the duck, is how common is it for birds to express three isoforms of IgY? This intriguing question cannot be answered, but certainly not all birds have three isoforms. Chickens express only the full-length and membrane-receptor forms of IgY [12], and whether they possess the alternate exon for production of the truncated form has not been investigated.
Fig. 2.
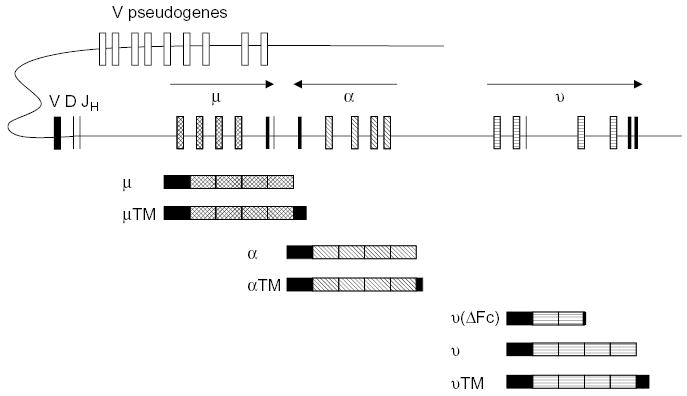
A schematic of the duck immunoglobulin heavy chain locus. The D region through the three C region genes has been completely sequenced on overlapping lambda phage clones. The number of functional V and D elements is inferred and the number of pseudo V region genes is unknown. Below the locus are schematics of the heavy chain transcripts to illustrate the alternative transcripts arising from each gene, giving rise to the heavy chains for the secreted and membrane bound immunoglobulins.
There has been no systematic study of bird immunoglobulins. A small form of IgY (5.7S) is produced by some species of turtles [21–23] suggesting that its production may not be restricted to ducks. Whether this truncated form is functionally important has not been rigorously studied. However, some have termed the duck immune response inept. Its antibodies are produced in good quantity and bind ligand well, and thus can participate in neutralization. However, they are lacking in secondary biological effector functions including precipitation, agglutination, complement fixation and opsonization [24,25]. An obvious reason for the lack of these functions is the predominance of a circulating antibody that lacks an Fc region [6,7]. Interestingly, despite this apparent handicap ducks typically survive quite well in their wet and dirty environment. Either this truncated antibody plays a role in immunity that has not yet been elucidated, or perhaps ducks rely on innate immune defenses.
IgA is the antibody that has evolved to perform defense functions at (or beyond) the outer surface of the body [26], and it has been described to date only in mammals and birds [27]. IgA is found in the secretions of the gut, respiratory and reproductive tracts, as well as in tears, bile and (in mammals) the milk. The secreted IgA of mammals is typically a dimer, complexed with J chain and with secretory component [26]. Bile immunoglobulin in ducks (which was at one stage termed IgX, [10] appears to exist as a polymer with a Mr of 890 kDa [10]. Molecular cloning of the secretory Ig suggested that it is a true homologue of IgA [8]. The exact structure of this polymer is unknown, although a tetrameric structure, as drawn in Fig. 1, is plausible [8,10]. The genes encoding polymeric Ig receptor (the precursor for secretory component) and J chain have been identified in ducks (K. Magor, unpublished observations). The post-hatching expression of IgA in ducks is delayed in ontogeny compared to chickens, and this may also have an impact on the immune defense in ducks [8].
The overwhelming evidence is that all birds express only a single class of immunoglobulin light (L) chain [9,28] most closely related to the λ chain of the mammals [29,30]. The suggestion of additional classes of L chain in the birds [31] has not been substantiated at the amino acid sequence or genetic level.
3. Antigen recognition and generation of antibody diversity
Birds have very limited numbers of functional V region encoding elements in their germline, and utilize extensive gene-conversion mechanisms from suites of pseudogenes as a major mechanism for the generation of diversity. The generation of antibody binding-site diversity is very well understood for the chicken [12,32–34] but much less well understood for the duck [35]. Duck antibodies undergo affinity maturation [36] and protective antibodies can be elicited to several duck pathogens [37–39].
The structure of the IgH locus of the mallard duck (Anas platyrhynchos) has been reconstructed from recombinant genomic phage clones, and the region containing the D element to the three constant (C) region genes (μ, ν, α) completely sequenced (Fig. 2). The most interesting aspect of the arrangement of the C genes in the duck is that the α gene has apparently been translocated to the middle of the locus, as the order of the genes is μ/α/ν [40]. This translocation event also involved the inversion of the α gene, which is in opposite transcriptional orientation to the μ and ν genes [40,41]. This translocation and inversion event predated the split between the anseriform and galliform birds, as it is also found in the chicken [42]. It is not known, however, if this unusual structure of the IgH locus is unique to the gallo-anserine lineage, as the IgH locus of no other birds has been elucidated. The potential impact of the translocation/inversion of the α gene on the immune system of the duck (especially the expression of IgA) has been speculated upon. Because class switching to α involves an inversion within the locus, rather than a deletion of μ, the switching may be cumbersome and a possible cause for the delayed expression of IgA in duck ontogeny [40,41]. Subtle differences in the way the genes are regulated may account for the observed difference in IgA expression between chickens and ducks.
The generation of diversity in the VH regions of duck antibodies is not definitively understood, but partial information is available that can help us deduce a likely model. First, analyses of the available cDNA sequences of duck H chains indicate that there is a single family of VH sequences. An analysis of the distribution of sequence identities within 26 duck JH sequences (Fig. 3a) confirms that there is a single expressed JH element. The genomic sequence analysis identified a single JH element, which was found immediately downstream of a D segment [40]. It is not known whether there is more than one D segment. A similar pairwise comparison of D segments indicated widely divergent sequences are generated (Fig. 3b). Genomic Southern blot analysis [7] suggests that there is a diversified family of VH sequences in the duck, but does not allow any conclusion to be drawn as to whether or not the detected sequences represent functional or pseudo VH genes.
Fig. 3.
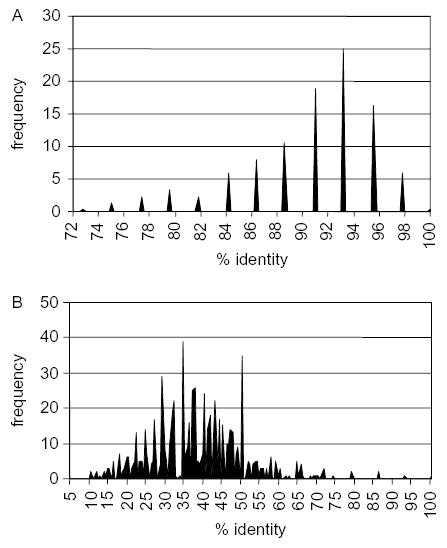
Frequency of percent sequence identity in pairwise comparisons of 26 duck JH segments (A) and 26 duck DH segments (B). The data were derived from a multiple alignment of the sequences of 26 cDNA clones isolated from a single duck. Sequences were aligned using Clustal W (Megalign, DNAStar, Madison WI) and the J regions and D regions identified by comparison. Individual JH region sequences and individual DH region sequences were compared to each other generating 325 pairwise comparisons.
To examine the rearrangement of the duck heavy chain locus we carried out a Southern blot analysis as originally described by Reynaud et al., [32] in defining the limited immunoglobulin gene rearrangements in the chicken. In Fig. 4a, germline DNA from nucleated erythrocytes is compared to that of rearranged DNA from bursa in a Southern blot hybridized with the JH region probe. There is a single major rearrangement product obvious as one hybridizing band in the Pvu II digested bursal DNA, and two hybridizing bands evident in the Eco RI digestion of bursal DNA. Since, there is a single J element in the duck heavy chain locus [40], most likely this can be attributed to one major rearrangement event at two allelic forms of the heavy chain locus in a heterozygous individual. The rearrangement event combines the J region with a single D and single V region. Alternatively, to account for the two hybridizing bands in the Eco RI digestion, there are two major rearrangement events involving two separate V region genes in the locus. Thus, the available evidence for the IgH locus of the mallard duck is compatible with (but does not definitively demonstrate) the model for V region diversification established in the chicken. That is, the use of a restricted number of functional VH, D and JH germline elements, with diversification being generated by mechanisms of gene conversion from a large suite of donor germline sequences.
Fig. 4.
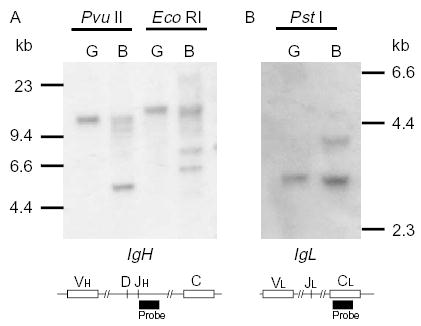
Restricted rearrangements of the IgH (A) and IgL (B) loci of the duck. Approximately 10 μg of DNA in germline organization extracted from nucleated erythrocytes (G) and DNA in rearranged organization extracted from Bursa of Fabricius (B) was digested with the restriction enzymes shown and subjected to Southern Blot analysis. Schematics of the IgH and IgL loci below the Southern blots indicate the locations of the probes used. The number of functional VH and DH segments, and the number of functional V and J segments are not known, but are inferred from the Southern blot analysis.
In terms of the generation of diversity in the VL domains of duck antibodies, the situation is very similar to that described above for the VH domains; there are few functional coding VL and JL elements in the germline, and diversification most likely arises in large measure from gene conversion events from an extensive suite of germline VL-related sequences. Genomic Southern blot analysis showed that there is a large family of germline VL-related sequences in the mallard duck [9]. Southern blot analysis showed that a single major rearrangement was evident at the light chain locus of mallard duck, yielding a single hybridizing band in germline DNA compared to two bands in rearranged bursal DNA (Fig. 4b). It is not known whether there is a single functional V and J element in the duck L chain locus, however, that is the simplest explanation of the results. This mechanism for the generation of diversity at the light chain may be true for the majority of birds, including mallard duck, quail, pigeon and turkey, with the one exception being the muscovy duck (Cairina moschata) [35]. In the muscovy duck, the genomic analyses showed that there were at least two functional VL genes, although only one of them contributed to the final repertoire [35]. Thus, there is certainly the possibility that recombinatorial processes contribute to the generation of diversity in the antibodies of non-galliform birds.
Analysis of sequence identities within a collection of 121 light chain cDNA sequences is compatible with the conclusion that there is a single family of VL sequences (Fig. 5a). Pairwise comparisons of the V region sequences yielded a single peak, suggesting that all sequences are related to each other. The vast majority of sequence pairs differ by about 10–20% suggesting the pseudogenes and functional V region can all be considered one family. What emerges from this analysis is that despite this limitation to the diversification of a single VL region, there is extensive diversity in the light chain sequences. Only two pairs of identical VL sequences were observed in 122 randomly selected sequences. The 27 JL sequences that were compared mainly differ by single nucleotide point substitutions, although one sequence carried an insertion (Fig. 5b). These differences suggest that a single J sequence is being diversified through gene conversion and somatic mutations.
Fig. 5.
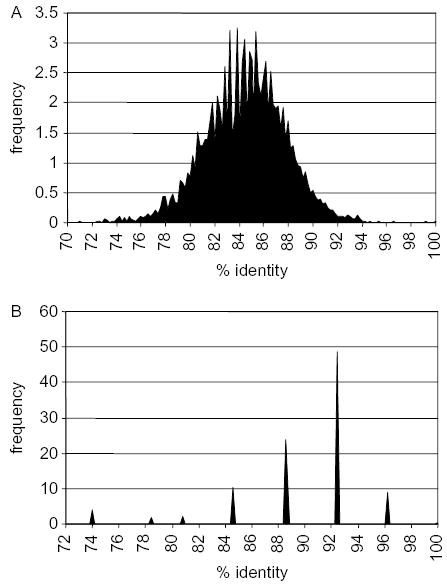
Frequency of percent identity in pairwise comparisons of 121 VL (A) and 27 JL (B) segments. The sequences were aligned using Clustal W (Megalign, DNAStar, Madison WI), and sequences corresponding to individual VL and JL regions were separated. Comparison of individual sequences was done for 121 VL and 27 JL sequences yielding 7260 and 351 pairwise comparisons, respectively. A cDNA library constructed from duck spleen [45] was once amplified, plasmids excised from lambda ZAP in bulk, and individual bacterial colonies randomly selected. Single-pass sequences of cDNA clones used in the analysis of heavy and light chains are deposited in the Genbank dbEST database under accession numbers CX95445-581 and DN161074-85.
4. Critical areas for research
The similarities and differences between duck and chicken antibodies and immune responses may help develop a framework for avian immunology, from which we can pose interesting research questions. Ducks, like chickens, generate their immunoglobulin variable region for both heavy and light chains by a single rearrangement event, and diversify it through gene conversion from a pool of pseudogenes. Despite this limitation, ducks and chickens do generate an extensive antibody repertoire. Would comparison of their pseudogene sequences help identify sequence elements necessary for targeting gene conversion events? Ducks and chickens have an unusual organization of constant region genes in the IgH locus, of mu-alpha-upsilon, with the alpha gene in opposite transcriptional orientation. How widespread is this organization among birds, and what consequences does it have on isotype switching and immune memory? Ducks have lymph nodes, which are absent in chickens. What genes are responsible for this difference, and what is the impact on the immune response? Ducks have a truncated IgY, the IgY (ΔFc), as a major serum antibody, while chickens do not. What role does this antibody play in the duck, and how widespread is it among birds? Finally, when one can identify obvious anomalies in the immunoglobulin genes of ducks, how is it that they survive so well in their wet and dirty environment? In particular, how do they remain largely unharmed by avian influenza, while chickens are so susceptible? With the genetic resources now available for chickens [43] and ducks [44], we hope to answer some of these questions.
Acknowledgments
The authors would like to thank Andrea Oko for editing sequences. We thank two anonymous reviewers for helpful comments and editing of an earlier version of this manuscript. Duck genomic DNA for the Southern blot was provided by Dr D. Higgins, University of Hong Kong. We acknowledge financial support from NIH award R01 AI45111 to GWW, and an NSERC Strategic grant and the Alberta Heritage Foundation for Medical Research to KEM.
References
- 1.Baghian A, Reyes CV, Mendoza A, Tully TN, Jr, Kousoulas KG. Production of a rabbit anti-cockatiel immunoglobulin G and characterization of its cross-reactivities with immunoglobulin G of other psittacine species. Avian Dis. 1999;43:48–54. [PubMed] [Google Scholar]
- 2.Cadman HF, Kelly PJ, Dikanifura M, Carter SD, Azwai SM, Wright EP. Isolation and characterization of serum immunoglobulin classes of the ostrich (Struthio camelus) Avian Dis. 1994;38:616–20. [PubMed] [Google Scholar]
- 3.van Tuinen MSBH. Calibration of avian molecular clocks. Mol Biol Evol. 2001;18:206–13. doi: 10.1093/oxfordjournals.molbev.a003794. [DOI] [PubMed] [Google Scholar]
- 4.Flajnik MF, Miller KM, Du Pasquier L. Evolution of the immune system. In: Paul WE, editor. Fundamental immunology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2003. p. 519–70.
- 5.Higgins DA, Shortridge KF, Ng PL. Bile immunoglobulin of the duck (Anas platyrhynchos). II. Antibody response in influenza A virus infections. Immunology. 1987;62:499–504. [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins DA, Warr GW. Duck immunoglobulins: structure, functions and molecular genetics. Avian Path. 1993;22:211–36. doi: 10.1080/03079459308418916. [DOI] [PubMed] [Google Scholar]
- 7.Magor KE, Warr GW, Middleton D, Wilson MR, Higgins DA. Structural relationship between the two IgY of the duck, Anas platyrhynchos: molecular genetic evidence. J Immonol. 1992;149:2627–33. [PubMed] [Google Scholar]
- 8.Magor KE, Warr GW, Bando Y, Middleton DL, Higgins DA. Secretory immune system of the duck (Anas platyrhynchos), Identification and expression of the genes encoding IgA and IgM heavy chains. Eur J Immunol. 1998;28:1063–8. doi: 10.1002/(SICI)1521-4141(199803)28:03<1063::AID-IMMU1063>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Magor KE, Higgins DA, Middleton DL, Warr GW. cDNA sequence and organization of the immunoglobulin light chain gene of the duck, Anas platyrhynchos. Dev Comp Immunol. 1994;18:523–31. doi: 10.1016/s0145-305x(06)80006-6. [DOI] [PubMed] [Google Scholar]
- 10.Ng PL, Higgins DA. Bile immunoglobulin of the duck (Anas platyrhynchos). I. Preliminary characterization and ontogeny. Immunology. 1986;58:323–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie GA, Clem LW. Phylogeny of immunoglobulin structure and function. 3. Immunoglobulins of the chicken. J Exp Med. 1969;130:1337–52. doi: 10.1084/jem.130.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parvari R, Avivi A, Lentner F, Ziv E, Tel-Or S, Burstein Y, et al. Chicken immunoglobulin gamma-heavy chains: limited VH gene repertoire, combinatorial diversification by D gene segments and evolution of the heavy chain locus. EMBO J. 1988;7:739–44. doi: 10.1002/j.1460-2075.1988.tb02870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magor KE, Higgins DA, Middleton DL, Warr GW. One gene encodes the heavy chains for three different forms of IgY in the duck. J Immonol. 1994;153:5549–55. [PubMed] [Google Scholar]
- 14.Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunol Today. 1995;16:392–8. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 15.Fellah JS, Kerfourn F, Wiles MV, Schwager J, Charlemagne J. Phylogeny of immunoglobulin heavy chain isotypes: structure of the constant region of Ambystoma mexicanum upsilon chain deduced from cDNA sequence. Immunogenetics. 1993;38:311–7. doi: 10.1007/BF00210471. [DOI] [PubMed] [Google Scholar]
- 16.Bensmana M, Lefranc MP. Gene segments encoding membrane domains of the human immunoglobulin gamma 3 and alpha chains. Immunogenetics. 1990;32:321–30. doi: 10.1007/BF00211646. [DOI] [PubMed] [Google Scholar]
- 17.Hellman L. Characterization of four novel epsilon chain mRNA and a comparative analysis of genes for immunoglobulin E in rodents and man. Eur J Immunol. 1993;23:159–67. doi: 10.1002/eji.1830230126. [DOI] [PubMed] [Google Scholar]
- 18.Faith RE, Clem LW. Passive cutaneous anaphylaxis in the chicken. Biological fractionation of the mediating antibody population. Immunology. 1973;25:151–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Parham P. Antibody structure. The duck’s dilemma. Nature. 1995;374:16–17. doi: 10.1038/374016a0. [DOI] [PubMed] [Google Scholar]
- 20.Liu SS, Higgins DA. Yolk-sac transmission and post-hatching ontogeny of serum immunoglobulins in the duck (Anas platyrhynchos) Comp Biochem Physiol. 1990;97B:637–44. doi: 10.1016/0305-0491(90)90100-8. [DOI] [PubMed] [Google Scholar]
- 21.Chartrand SL, Litman GW, Lapointe N, Good RA, Frommel D. The evolution of the immune response. XII. The immunoglobulins of the turtle. Molecular requirements for biologic activity of 5.7S immunoglobulin. J Immonol. 1971;107:1–11. [PubMed] [Google Scholar]
- 22.Benedict AA, Pollard LW. Three classes of immunoglobulins found in the sea turtle, Chelonia mydas. Folia Microbiol. 1972;17:75–8. doi: 10.1007/BF02872256. [DOI] [PubMed] [Google Scholar]
- 23.Leslie GA, Clem LW. Phylogeny of immunoglobulin structure and function, VI. 17S, 7.5S and 5.7S anti-DNP of the turtle, Pseudamys scripta. J Immunol. 1972;108:1656–64. [PubMed] [Google Scholar]
- 24.Grey HM. Duck immunoglobulins. II. Biologic and immunochemical studies. J Imunol. 1967;98:820–6. [PubMed] [Google Scholar]
- 25.Humphrey BD, Calvert CC, Klasing KC. The ratio of full length IgY to truncated IgY in immune complexes affects macrophage phagocytosis and the acute phase response of mallard ducks (Anas platyrhynchos) Dev Comp Immunol. 2004;28:665–72. doi: 10.1016/j.dci.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Corthesy B, Kraehenbuhl JP. Antibody-mediated protection of mucosal surfaces. Curr Top Microbiol Immunol. 1999;236:93–111. doi: 10.1007/978-3-642-59951-4_6. [DOI] [PubMed] [Google Scholar]
- 27.Bengten E, Wilson M, Miller N, Clem LW, Pilstrom L, Warr GW. Immunoglobulin isotypes: structure, function, and genetics. Curr Top Microbiol Immunol. 2000;248:189–219. doi: 10.1007/978-3-642-59674-2_9. [DOI] [PubMed] [Google Scholar]
- 28.Reynaud CA, Dahan A, Weill JC. Complete sequence of a chicken lambda light chain immunoglobulin derived from the nucleotide sequence of its mRNA. Proc Natl Acad Sci USA. 1983;80:4099–103. doi: 10.1073/pnas.80.13.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant JA, Sanders B, Hood L. Partial amino acid sequences of chicken and turkey immunoglobulin light chains. Homology with mammalian lambda chains. Biochemistry. 1971;10:3123–32. doi: 10.1021/bi00792a022. [DOI] [PubMed] [Google Scholar]
- 30.Kubo RT, Rosenblum IY, Benedict AA. The unblocked N-terminal sequence of chicken IgG lambda-like light chains. J Immunol. 1970;105:534–6. [PubMed] [Google Scholar]
- 31.Leslie GA. Evidence for a second avian light chain isotype. Immunochemistry. 1977;14:149–51. doi: 10.1016/0019-2791(77)90294-4. [DOI] [PubMed] [Google Scholar]
- 32.Reynaud CA, Anquez V, Dahan A, Weill JC. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985;40:283–91. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- 33.Reynaud CA, Anquez V, Grimal H, Weill JC. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987;48:379–88. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- 34.Reynaud CA, Dahan A, Anquez V, Weill JC. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989;59:171–83. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- 35.McCormack WT, Carlson LM, Tjoelker LW, Thompson CB. Evolutionary comparison of the avian IgL locus: combinatorial diversity plays a role in the generation of the antibody repertoire in some avian species. Int Immunol. 1989;1:332–41. doi: 10.1093/intimm/1.4.332. [DOI] [PubMed] [Google Scholar]
- 36.Higgins DA, Ko OKH, Chan SWS. Duck antibody responses to keyhole limpet haemocyanin, human immunoglobulin G and the trinitrophenyl hapten. Evidence of affinity maturation. Avian Pathol. 2001;30:381–90. doi: 10.1080/03079450120066386. [DOI] [PubMed] [Google Scholar]
- 37.Lin WQ, Lam KM, Clark WE. Active and passive immunization of ducks against duck viral enteritis. Avian Dis. 1984;28:968–73. [PubMed] [Google Scholar]
- 38.Thermet A, Robaczewska M, Rollier C, Hantz O, Trepo C, Deleage G, et al. Identification of antigenic regions of duck hepatitis B virus core protein with antibodies elicited by DNA immunization and chronic infection. J Virol. 2004;78:1945–53. doi: 10.1128/JVI.78.4.1945-1953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins DA, Henry RR, Kounev ZV. Duck immune responses to Riemerella anatipestifer vaccines. Dev Comp Immunol. 2000;24:153–67. doi: 10.1016/s0145-305x(99)00070-1. [DOI] [PubMed] [Google Scholar]
- 40.Lundqvist ML, Middleton DL, Hazard S, Warr GW. The immunoglobulin heavy chain locus of the duck. Genomic organization and expression of D, J, and C region genes. J Biol Chem. 2001;276:46729–36. doi: 10.1074/jbc.M106221200. [DOI] [PubMed] [Google Scholar]
- 41.Magor KE, Higgins DA, Middleton DL, Warr GW. Opposite orientation of the alpha- and upsilon-chain constant region genes in the immunoglobulin heavy chain locus of the duck. Immunogenetics. 1999;49:692–5. doi: 10.1007/s002510050666. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Rabbani H, Shimizu A, Hammarstrom L. Mapping of the chicken immunoglobulin heavy-chain constant region gene locus reveals an inverted alpha gene upstream of a condensed upsilon gene. Immunology. 2000;101:348–53. doi: 10.1046/j.1365-2567.2000.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Consortium ICGS. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 44.Moon DA, Magor KE. Construction and characterization of a fosmid library for comparative analysis of the duck genome. Anim Genet. 2004;35:417–8. doi: 10.1111/j.1365-2052.2004.01177.x. [DOI] [PubMed] [Google Scholar]
- 45.Mesa CM, Thulien KT, Moon DA, Veniamin SM, Magor KE. The dominant MHC class I gene is adjacent to the polymorphic TAP2 gene in the duck, Anas platyrhynchos. Immunogenetics. 2004;56:192–203. doi: 10.1007/s00251-004-0672-3. [DOI] [PubMed] [Google Scholar]


