Abstract
Background.
In patients with cystic fibrosis (CF), lung infection with mucoid Pseudomonas aeruginosa strains overexpressing the exopolysaccaride alginate is preceded by colonization with nonmucoid strains. We investigated the kinetics, impact of environmental signals, and genetics of P. aeruginosa alginate expression in a mouse model and in patients with CF.
Methods.
Using indirect immunofluorescence, microarray technology and real-time reverse-transcriptionpolymerase chain reaction, we assessed alginate gene expression during aerobic and anaerobic growth of the nonmucoid strain PAO1 in vitro, in a mouse lung-infection model and in sputum specimens from patients with CF infected with nonmucoid or mucoid P. aeruginosa strains.
Results.
Anaerobic conditions increased the transcription of alginate genes in vitro and in murine lungs within 24 h. Alginate production by PAO1 in murine lungs and by nonmucoid P. aeruginosa strains in patients with CF was reversible after in vitro culture under aerobic conditions. A subpopulation of P. aeruginosa clones revealing stable alginate production was detected in murine lungs 2 weeks after infection.
Conclusions.
Anaerobiosis and lung infection rapidly induce alginate production by gene regulation in nonmucoid P. aeruginosa. This trait may contribute to early persistence, leading to chronic P. aeruginosa infection once stable mucoid strains are generated.
Optimal survival of organisms under changing environmental conditions demands adaptation, which results in the emergence of new phenotypes [1]. In bacteria, signal-transduction systems by which the organisms sense the environment and change their phenotype accordingly have been described [2]. The presence of intricate global gene-regulation mechanisms that preserve broad cell versatility under many environmental conditions is thought to be one reason for the exceptional environmental and evolutionary success of microorganisms. Indeed, organisms with larger genomes, such as Pseudomonas aeruginosa, have a wealth of genes involved in transcriptional regulation [3]. The emergence of mutated sub-populations is thought to permit the persistence of bacterial species in a given ecological niche [4–7].
The airways of patients with the hereditary disease cystic fibrosis (CF) [8] may be regarded as an ecosystem in which P. aeruginosa must adapt to challenges from factors of the innate immune system, antibiotics, and growth in an microanaerobic/anaerobic environment [5, 9]. In response to anaerobic growth, P. aeruginosa can change its phenotype [9]; this process is characterized by the production of increased amounts of the extra-cellular polysaccharide alginate [10–13]. The overexpression of alginate by P. aeruginosa leads to mucoid colony formation, which is a key factor in the organisms’ persistence in the respiratory tracts of patients with CF.
Mucoid, alginate-overexpressing P. aeruginosa variants have recently been detected, 10.9 years after the acquisition of nonmucoid P. aeruginosa in patients with CF [12]; however, this may also occur earlier. Alginate overproduction has been linked to mutations in a gene cluster designated as the mucABCD genes, which encode proteins that inhibit the activity of the alternative σ-factor AlgU [14]. AlgU acts on the key alginate biosynthesis gene algD, which encodes a guanosine diphosphate mannose dehydrogenase, and on algR, a response regulator gene that increases alginate production [15]. Mutations in the negative regulators mucA, mucB, and mucD lead to alginate overproduction and conversion to a stable mucoid phenotype in P. aeruginosa [14, 16–18]. However, the contribution of alginate gene regulation, as opposed to adaptive mutations, as a key factor in controlling the ability of P. aeruginosa to both establish and chronically infect the lungs of patients with CF in the infection process is still unclear, and this has not been investigated in vivo.
The objective of the present study was to investigate whether changes in gene regulation lead to an initial increase in alginate production that precedes the adaptive mutation causing full-fledged alginate overproduction in mucoid P. aeruginosa. To achieve this aim, we assessed alginate gene expression during aerobic and anaerobic growth of the nonmucoid strain PAO1 in vitro, in a mouse lung-infection model and in sputum specimens from patients with CF infected with nonmucoid or mucoid P. aeruginosa strains. Our data suggest that alginate gene regulation is a key factor in the process of establishing infection shortly after colonization by P. aeruginosa nonmucoid strains and before the mucoid phenotype becomes fully detectable on bacterial cells cultured in vitro.
PATIENTS, MATERIALS, AND METHODS
Bacterial strains, growth conditions, and patients with CF.
The nonmucoid laboratory strain P. aeruginosa PAO1, its mucoid isogenic variant PDO300 [3, 19], and Bacteroides fragilis (ATCC 25285) were used for in vitro experiments and mouse infection studies. Twenty-five environmental P. aeruginosa strains were isolated from water and salad sources, and 40 clinical strains were obtained from sputum or throat swabs from patients with CF. P. aeruginosa was cultured in trypticase soy broth (TSB) or was plated onto Columbia blood agar plates or Pseudomonas isolation agar (PIA; Heipha) for 24 h at 37° C. B. fragilis was grown in chopped meat carbohydrate medium [20]. For anaerobic growth, strain PAO1 was cultivated aerobically on solid medium for 12 h and then incubated anaerobically for an additional 12 h in anaerobic jars with Anaerocult A (Merck). For microarray experiments, strain PAO1 was grown in TSB/PBS medium (pH 7.4) overnight, adjusted to a starting OD600 of 0.05, and grown aerobically and anaerobically. For fermenter experiments, overnight cultures of PAO1 were adjusted to an OD600 of 0.1 in modified basic medium (10 g of casein hydrolysate, 5 g of yeast extract, 2.5 g of NaCl, 10 g of disodium hydrogenphosphate–trihydrate, and 10 μL of antifoam solution [pH 7.25], supplemented with 0.5% glucose) and cultured to the midlog (4 h) and stationary (8 h) phases in a fermenter (Biostat Q-Fermenter; Braun-Biotech) under aerobic and anaerobic conditions. Anaerobiosis was created by bubbling filtered helium gas (150 mL/min) through the medium, and aerobiosis was created by use of filtered oxygen gas (150 mL/min).
Microbiological cultures of biological specimens from 24 patients revealed only nonmucoid strains of P. aeruginosa, whereas 16 patients carried at least 1 mucoid colony, as detected by use of a specific anti–alginate antibody (see below). The nonmucoid phenotype of P. aeruginosa was defined by the appearance of single colonies on agar plates (figure 1A). The mucoid phenotype of P. aeruginosa was defined as a viscous, slimy colony appearing on agar plates in which single cells could not be distinguished because of the production of copious amounts of alginate (figure 1B). Nonmucoid phenotypes may be alginate negative (figure 1D) or alginate positive (figure 1F), as detected by use of a specific anti–alginate antibody.
Figure 1.
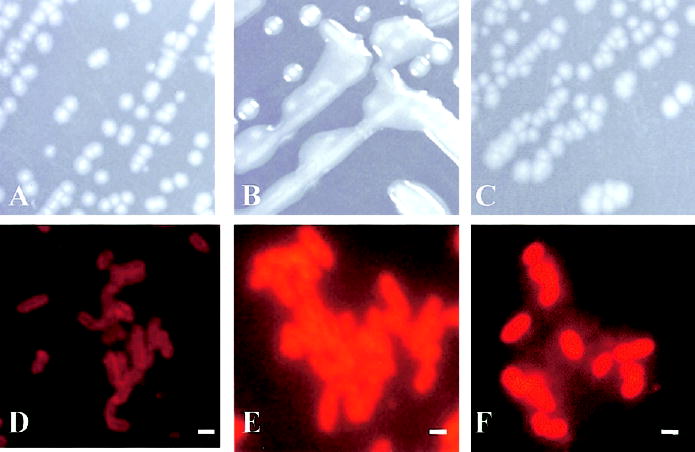
Production of nonmucoid Pseudomonas aeruginosa alginate grown anaerobically in vitro that does not change its phenotype. On Pseudomonas isolation agar, P. aeruginosa strain PAO1 revealed a nonmucoid phenotype when cultivated aerobically (A) or anaerobically (C), whereas its isogenic mutant PDO300 showed only a mucoid phenotype (B). When indirect immunofluorescence was used, alginate production of PAO1 was detectable under anaerobic growth conditions (F) but not under aerobic growth conditions (D), whereas PDO300 was alginate-positive under aerobic growth conditions (E). Bars in D–F, 1 μm.
Agar bead preparation and mouse model.
Agar beads containing P. aeruginosa or B. fragilis were prepared as described elsewhere [21]. Sodium deoxycholic acid was omitted in washings of B. fragilis beads. C57Bl/6 male mice (Charles-River; weight, 24–26 g) were infected as described elsewhere [21, 22]. After 1, 3, 7, 14, and 28 days, mice were killed, and lungs were excised, homogenized in PBS, and plated onto PIA. Additionally, 1 loop of PAO1, grown on blood agar, was processed for immunostaining. For histopathological analysis, lungs were removed en bloc and fixed in 4% paraformaldehyde/PBS for 24 h at 4° C and processed for paraffin embedding. Longitudinal sections of 5 μm, collected at regular intervals, were obtained by use of a microtome from the proximal, medial, and distal lung regions. Sections were stained with hematoxylineosin (HE).
P. aeruginosa alginate gene expression.
To assess how alginate gene expression responds to a change from an aerobic to an anaerobic environment, P. aeruginosa Affymetrix Gene-Chips and LightCycler reverse-transcription polymerase chain reaction (RT-PCR) were used. For microarray experiments, RNA was isolated at 2 h from aerobically grown cultures and on days 1, 2, 3, and 4 for anaerobically grown cultures by use of Trizol (Gibco). Cell walls were disrupted with FastRNA tubes blue and FastPrepFP120 (Bio101). Contaminating DNA was digested by use of DNaseI (Ambion). Purified RNA was used for microarray experiments according to the manufacturer’s procedure for use of the P. aeruginosa GeneChip (Affymetrix). Data analysis was performed by use of the Affymetrix Microarray Suite (version 5.0; Affymetrix UK) and Data Mining Tool (version 3.0; Affymetrix UK) software. Student’s t test was applied for statistical analysis.
For real-time RT-PCR, fermenter-cultured PAO1 cells were incubated with RNA Protect Bacteria Reagent (Qiagen) and disrupted with lysozyme (1 mg/mL). RNA was isolated by use of the RNeasy Mini kit (Qiagen) and treated with DNaseI. This method was also used for the isolation of RNA from homogenized murine lungs and sputum specimens from patients with CF. Real-time RT-PCR was performed by use of a LightCycler (Roche) and the LightCycler-RNA amplification kit SYBR Green I (Roche). The following primers were used: algD, 5′-TGTCGCG-CTACTACATGCGTC-3′ and 5′ -GTGTCGTGGCTGGTGATG-AGA-3′; and gyrA, 5′ -TGTGCTTTATGCCATGAGCGA-3′ and 5′ -TCCACCGAACCGAAGTTGC-3′. After RT for 20 min at 50° C, the following temperature profile was used: denaturation for 1 cycle at 95° C for 30 s; 45 cycles at 95° C for 1 s, 62° C–58° C for 10 s, and 72° C for 13 s; and fluorescence acquisition at 62° C– 58° C in single mode. Melting curves were obtained from 45° C to 96° C by stepwise fluorescence acquisition.
Alginate determination.
P. aeruginosa alginate gene expression was quantified by use of the carbazole assay [23] and was determined by indirect immunofluorescence that used a rabbit antiserum specific for P. aeruginosa alginate [24]. The secondary antibody was indocarbocyanin-3 (Cy3) or Texas Red–labeled goat anti–rabbit IgG (Molecular Probes). For the in vivo experiments, 38 single clones and 4 mixtures of different clones from mice infected for 7 days and 101 single clones and 10 mixtures of different clones from mice infected for 14 days were randomly picked and analyzed for alginate production by use of the alginate-specific antiserum. Positive clones were subcultured twice for analysis of the stability of alginate gene expression. Because of the presence of a heterogeneous population of bacterial cells, clones were considered to be positive when at least 50% of the cells revealed positive staining with the alginate antiserum.
RESULTS
Rapid and persistent increase in alginate biosynthesis and regulatory gene transcription during anaerobic growth of P. aeruginosa in vitro.
Previous in vitro experiments that used the nonmucoid P. aeruginosa strain PAO1 grown anaerobically demonstrated a significant increase in alginate production [9] without the phenotypic change typical of highly mucoid strains. Figure 1 shows the nonmucoid strain PAO1 (figure 1A) and its mucoid, alginate-overexpressing isogenic variant PDO300 (figure 1B). To determine how fast the production of alginate could be detected when nonmucoid strain PAO1 was grown anaerobically and which P. aeruginosa genes involved in alginate production were differentially regulated, we measured alginate production and transcriptional changes during 4 days of anaerobic growth. Alginate production was detectable after 12 h under anaerobiosis that used specific antibodies (figure 1F). Aerobic PAO1 cultures were alginate negative (figure 1D). As was expected, PDO300 grown aerobically stained positive for alginate (figure 1E). Thus, the phenotypically nonmucoid strain PAO1 produced detectable levels of alginate when it was grown anaerobically, without the phenotypic change (figure 1C) typical of highly mucoid strains (figure 1B).
To determine whether such a phenotypical change is a general phenomenon, we tested 25 environmental P. aeruginosa strains for alginate production using the carbazol assay. Only traces of alginate were produced by the strains grown aerobically (mean ± SD, 0.022 ± 0.004 μg of alginate/μg of protein), whereas a mean (±SD) of 0.191 ± 0.037 μg of alginate/μg of protein was produced under anaerobic growth conditions. Similarly, under aerobic growth conditions, these strains were all found to be negative for alginate by immunofluorescence, whereas, under anaerobic growth conditions, they were found to be positive (data not shown). We therefore conclude that increased alginate production during anaerobic growth is a general phenomenon of P. aeruginosa strains.
When we used P. aeruginosa Affymetrix GeneChips to assess differential gene expression under anaerobic versus aerobic conditions, the expression of the positive alginate regulator algU increased ~10-fold during days 1–4 of anaerobic growth, compared with that during aerobic growth (figure 2). Additionally, algR and algD transcription was also increased under anaerobic conditions. We also noted increased transcription of negative regulatory genes in the muc cluster, which may have served as a counterbalance to the increased transcription of algR and algD, resulting in increased alginate production without the transition to the full mucoid phenotype. Transcription of the napEFDABC and arcDABC clusters was increased, reflecting anaerobically increased nitrate and arginine turnover, whereas transcription of the housekeeping gene gyrA was not affected (figure 2).
Figure 2.

Increase in Pseudomonas aeruginosa alginate gene expression under anaerobic growth conditions. Differential gene expression by strain PAO1, grown for 1–4 days under anaerobic growth conditions (−O2) or aerobically for 2 h (+O2), was assessed by use of the microarray technique. Values are given as means ± SDs. Dotted lines indicate cutoff levels for differential transcription that is increased or decreased. napC and arcC genes represent positive controls for anaerobiosis. gyrA is a constitutively transcribed P. aeruginosa gene.
These results were confirmed by real-time RT-PCR when strain PAO1 was grown in a fermenter under anaerobic and aerobic growth conditions for 4 and 8 h. Although, at 4 h, no difference was observed in algD expression, a 4-fold increase in algD expression was detected at 8 h under anaerobic versus aerobic growth conditions (figure 3).
Figure 3.

Pseudomonas aeruginosa algD transcription in murine lungs and in sputum specimens from patients with cystic fibrosis (CF), as measured by real-time reverse-transcription polymerase chain reaction. Total RNA from lung homogenates of mice infected with P. aeruginosa strain PAO1 in agar beads or from sputum specimens from patients with CF was isolated. algD expression was quantified in relation to the gyrA expression in each specimen. Data are presented as the fold change vs. the referent strain, PAO1. In vitro data showed increased algD expression after 8 h of anaerobic growth (−O2) vs. aerobic growth (+O2). Infected mice showed low algD expression 1 h after infection, whereas a significant increase was detected 24 h after infection. Similarly, algD expression was detected in sputum specimens from patients with CF whose respiratory cultures grew only nonmucoid (NM) P. aeruginosa and in sputum specimens from patients with CF whose respiratory cultures grew only mucoid (M) P. aeruginosa colonies. Control experiments were performed by growing M and NM strains in vitro. In vitro, M strains expressed high levels of algD transcripts, whereas only low expression was measured for NM strains.
Induction of anaerobic growth conditions for bacteria by agar beads leading to alginate gene expression in a mouse lung-infection model of P. aeruginosa.
We next monitored the kinetics of alginate production in vitro using the agar bead model of chronic P. aeruginosa lung infection [21]. We asked to what extent does this model mimic the microanaerobic growth conditions of P. aeruginosa in the CF lung [9]. The obligate anaerobe B. fragilis, embedded into agar beads, was incubated aerobically and anaerobically in vitro for 24 h at 37° C, and survival was quantified by plating serial dilutions on blood agar plates under anaerobic conditions. No statistical difference was observed in the survival of B. fragilis in beads incubated either aerobically or anaerobically (mean ± SD; aerobic, 0.92 × 109 ± 6.8 × 108 cfu/mL; anaerobic, 1.3 × 109 ± 9.5 × 108 cfu/mL; P = 455, Student’s t test). When B. fragilis was grown in the absence of beads, growth was observed under only anaerobic conditions. HE staining of freshly prepared bead sections revealed low numbers of B. fragilis cells, whereas, 24 h after incubation, in the absence or presence of oxygen, there was bacterial growth inside the beads (data not shown). When B. fragilis, embedded in beads that contained 2 ×106 bacteria, was inoculated into the lungs of C57Bl/6 mice, 7.5 × 105 cfu of B. fragilis/lung were recovered after 24 h. The decrease in counts in vivo was not unexpected, because B. fragilis may escape from the anaerobic bead niche and subsequently be cleared by host defenses.
Next, we infected C57Bl/6 mice intratracheally with strain PAO1 embedded in beads. A total dose of 106–107 cfu/mouse was found to cause only low mortality during the first 3 days of infection (figure 4), whereas higher doses resulted in 100% mortality, and lower doses did not cause a chronic infection (data not shown). Numbers of bacteria increased 1 day after infection, from 106–107 to 6.3 × 109 cfu/lung, and decreased thereafter, to a mean of 2.3 × 104 cfu/lung at day 7 (figure 4). In parallel with the decrease in numbers of bacteria, the number of infected mice decreased after day 3, to 27.5% ± 3.5% (mean ± SD) on day 7. Thereafter, there were no significant differences in the percentage of infected mice (P = .75, χ2 test) or the bacterial number of colony-forming units per lung during the next month (P = .55, Kruskal-Wallis test). The data suggest that a subpopulation of PAO1 that persists for at least 7 days is selected in vivo in approximately one-fourth of the mice.
Figure 4.
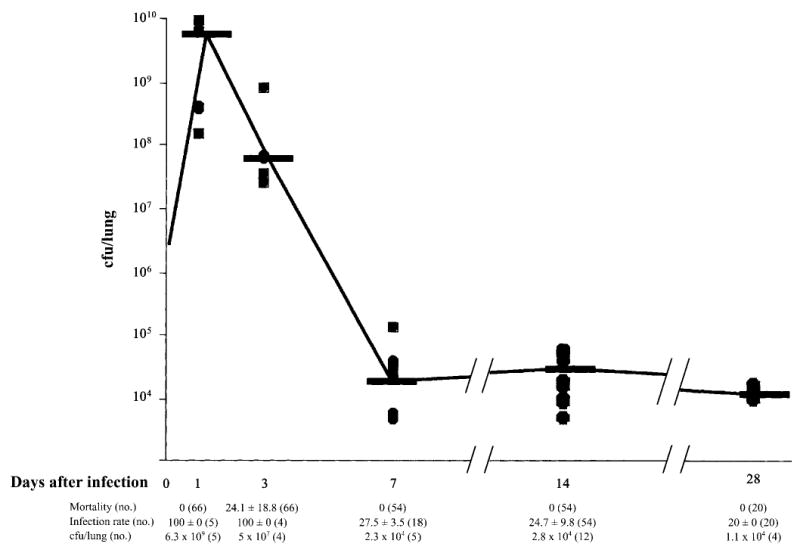
Subpopulation of Pseudomonas aeruginosa selected in the lungs of agar bead–infected C57BL/6 mice. C57Bl/6 mice were infected with 106–107 cfu of P. aeruginosa strain PAO1/lung, and mortality was determined. Other groups of mice were killed at the indicated time points, for the determination of the no. of colony forming units per lung and the percentage of infected mice. Shown is the growth curve of strain PAO1 in murine lungs. Dots represent individual measurements of the no. of colony forming units per lung, and horizontal lines represent median values. Mortality (mean ± SD) and infection rates (mean ± SD) of PAO1-infected mice and no. of colony forming units per lung (median) are also shown. Values were selected from 2–6 different experiments. n, no. of pooled mice analyzed for each condition.
To assess whether microaerobic or anaerobic growth conditions in agar beads would lead to a switch from a non–alginate-expressing to an alginate-expressing P. aeruginosa phenotype, as occurs in the airways of patients with CF [10, 12, 13], we analyzed infected murine lungs histologically and in situ bacteria by indirect immuofluorescence. One hour after infection, beads deposited in the bronchial lumen contained bacterial cells negative for alginate (figure 5A and 5B). However, 1 day after infection, bacteria formed alginate-expressing microcolonies (figure 5D and 5E). Real-time RT-PCR was used to measure algD gene expression in murine lungs. One day after infection, algD gene expression of strain PAO1 increased 4-fold, compared with that 1 h after infection (P < .05, Student’s t test) (figure 3). Three days after infection, microscopic examination of HE-stained lung-tissue sections revealed that the number of beads per lung had decreased significantly (mean ± SD; day 1, 7.1 ± 3.5 beads/lobe; day 3, 0.3 ± 0.5 beads/lobe; P < .01, Student’s t test), which suggests that the persisting alginate-positive PAO1 cells were protected from the murine respiratory defense system by the alginate coat and not by the beads.
Figure 5.
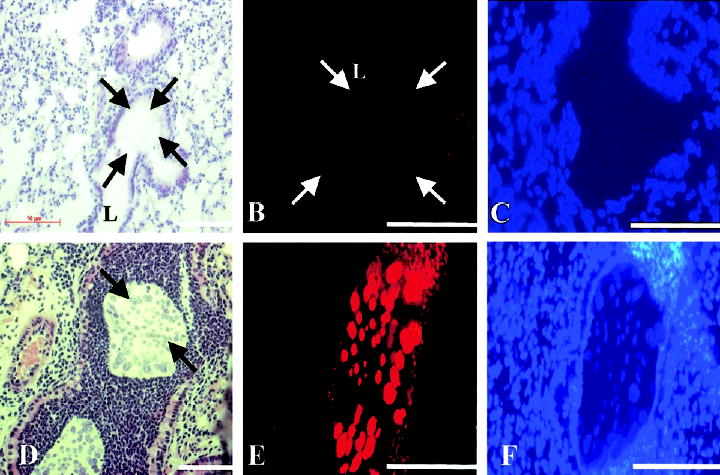
Expression of alginate by Pseudomonas aeruginosa within agar beads in murine lungs. Mice were infected with PAO1 embedded in agar beads, and lungs were investigated histologically and for alginate by indirect immunofluorescence. A, Agar bead (arrows), deposited in the bronchial lumen (L) 1 h after intratracheal injection. B, No alginate detectable by indirect immunofluorescence in an agar bead (arrows). C, 4′,6′-Diamidino-2-phenylindole dihydrochloride (DAPI) staining of the same tissue section as that in panel B, 1 h after infection. D, Bacterial macrocolonies in an agar bead (arrows) 1 day after infection. E, Expression of alginate by bacterial macrocolonies 1 day after infection. F, DAPI staining of the same tissue section as that in panel E. Bars in A–F, 100 μm.
Stability of alginate gene expression during chronic lung infection.
We next monitored the phenotype of the selected population of P. aeruginosa during the establishment of chronic airway colonization. PAO1 was positive for alginate in all lung homogenates obtained without subculturing from mice infected for 1–14 days (figure 6A–C). P. aeruginosa recovered from murine lungs up to 7 days after infection reverted to a non–alginate-producing phenotype when they were cultured aerobically in vitro (figure 6D and 6E). However, later (weeks 2–4), many of the recovered colonies stably produced alginate aerobically in vitro (figure 6F). After 14 days of infection, 30.6% of the clones stably produced alginate in vitro. When selected alginate-positive clones were further subcultured aerobically (either once or twice), 67% and 60% of the clones, respectively, stained positive for alginate, which suggests a heterogeneous population among which the majority were stably producing alginate after 14 days in murine lungs. However, none of the colonies revealed the classic alginate-overexpressing mucoid CF phenotype.
Figure 6.

Pseudomonas aeruginosa alginate gene expression in mouse lungs and in vitro under aerobic growth conditions. P. aeruginosa strain PAO1 expressed alginate as detected by indirect immunofluorescence in lung homogenates of C57Bl/6 mice, infected for 1 (A), 7 (B), and 14 (C) days. Bacterial colonies analyzed after 1 (D) or 7 (E) days of infection lost this phenotypic trait when they were subcultured in vitro under aerobic conditions; after 14 days of infection (F), colonies remained positive for alginate gene expression after aerobic subculture. Bars in A–F, 1 μm.
Alginate gene expression of phenotypically nonmucoid and mucoid P. aeruginosa in sputum specimens from patients with CF.
To determine whether the increased production of alginate early after infection of mice with P. aeruginosa was representative of CF, we analyzed sputum specimens from patients with CF. As expected, sputum specimens from patients whose respiratory cultures grew mucoid P. aeruginosa when they were cultured aerobically (figure 7A) stained positive for alginate (figure 7B). However, sputum specimens from a patient with CF whose P. aeruginosa strain grew exclusively nonmucoid colonies on blood agar (figure 7D) also stained positive for alginate (figure 7E), which suggests a gene-regulation mechanism for alginate gene expression in the lungs of patients with CF similar to that in murine lungs. We used real-time RT-PCR to confirm the immunofluorescence data by measuring algD gene expression directly in sputum specimens from patients with CF. Compared with strain PAO1 expression, algD expression was increased by 5-fold in the sputum from a patient with CF whose respiratory cultures grew only nonmucoid P. aeruginosa (figure 3). A 9-fold increase in algD expression was detectable in sputum from patients with CF whose respiratory cultures grew mucoid P. aeruginosa (figure 3). No significant differences were detected in algD expression between the 2 patient groups (P = .3, Student’s t test). The data demonstrate, for the first time, that P. aeruginosa that reveals a nonmucoid, alginate-negative phenotype when subcultured in vitro produces alginate within the CF lung habitat.
Figure 7.
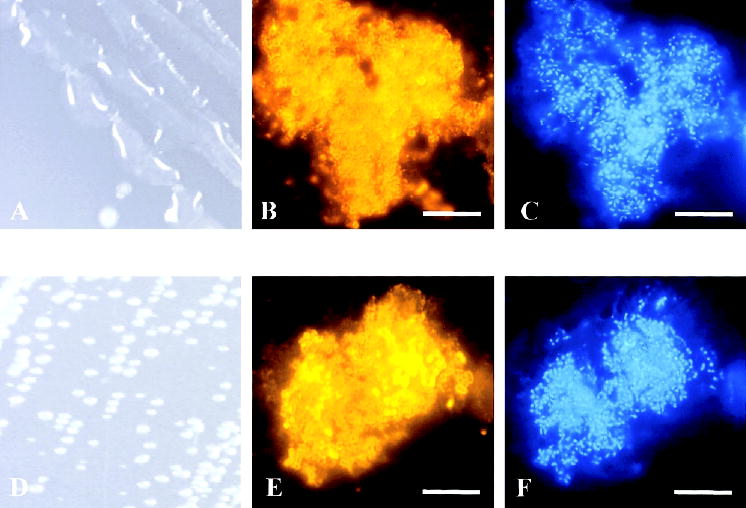
Pseudomonas aeruginosa alginate gene expression in sputum specimens from patients with cystic fibrosis (CF) and its phenotype on agar plates. P. aeruginosa cultured from sputum specimens from 2 different patients with CF showed a mucoid (A) or a nonmucoid (D) phenotype when they were plated on Pseudomonas isolation agar for 24 h. However, P. aeruginosa bacterial cells in the sputum from the patient with CF whose respiratory cultures grew only mucoid (B) or only nonmucoid P. aeruginosa (E) expressed alginate when they were stained by indirect immunofluorescence. C and F, 4′,6′-Diamidino-2-phenylindole dihydrochloride staining of the same sputum as that in panels B and E. Bar in B, C, E, and F, 10 μm.
DISCUSSION
The exopolysaccharide alginate is regarded as an essential component of the mucoid P. aeruginosa phenotype, and it has been observed primarily on strains recovered from chronically infected airways of patients with CF [10–13]. Also, phenotypically nonmucoid strains of P. aeruginosa from patients with CF have been reported to produce low levels of alginate [25, 26]. It appears that the elaboration of some alginate, even in the absence of a switch to a mucoid morphology, is critical for bacterial virulence in the setting of CF, given that an alginate-negative mutant of a phenotypically nonmucoid strain was unable to establish chronic colonization in mice with CF [27]. The isolation of mucoid strains from the airways of patients with CF indicates the onset of an increased rate of deterioration in pulmonary function [12, 28, 29]. Overall, it appears that there is a clear pathophysiological importance for alginate in chronic P. aeruginosa lung infection in patients with CF, but it is still not clear how fast alginate is produced when nonmucoid, alginate-negative P. aeruginosa strains colonize the airways of patients with CF. Our results show that non–alginate-producing P. aeruginosa rapidly expresses alginate when it is exposed to anaerobic conditions or is inoculated into murine lungs embedded in agar beads. Alginate gene expression is initially transient but becomes stable among a subset of clones after ~2 weeks of animal infection.
Anaerobic conditions may be one stimulus for initiation of alginate production by P. aeruginosa. We have confirmed previous findings [9] that the microaerobic/anaerobic growth conditions present in agar beads rapidly induce a switch from a completely alginate-negative to an alginate-positive but nonmucoid phenotype in strain PAO1. As a biological marker for the degree of anaerobiosis, we used the obligate anaerobic microorganism B. fragilis. Inside beads, this bacterium grew well in the presence of air, which suggests that the beads decrease oxygen diffusion sufficiently to allow growth. The switch from a non–alginate-producing to an alginate-producing phenotype after the infection of mice with strain PAO1 was observed within 24 h. Although this model has been used by others [21, 22, 30–32], changes in alginate elaboration by a nonmucoid P. aeruginosa strain has not been reported previously. Additionally, we found that, in sputum from patients with CF whose bacterial cultures grew only phenotypically nonmucoid colonies, there was detectable alginate production on a large number of bacterial cells. Our results suggest that, in agar bead–infected murine lungs as in the airways of patients with CF, alginate is elaborated by many P. aeruginosa strains shortly after infection.
The rapid switch of PAO1 to an alginate-positive, nonmucoid phenotype in the murine lung and under anaerobic growth conditions in vitro was reversible up to ~7 days after infection when cells were cultured in an aerobic environment, which suggests that the initial increase in alginate production was due to changes in regulatory gene activity and not to newly acquired, stable mutations (data not shown). A similar finding has been obtained in an acute lung-infection model with strain PAO1, wherein no alginate was detectable on the surface of the infecting strain but, within 1 h of infection, bacteria in lung sections stained strongly positive for alginate gene expression [33]. Here, we have shown that specific genes involved in alginate biosynthesis and its regulation have increased transcription under anaerobic versus aerobic growth conditions, according to the results of DNA arrays and real-time RT-PCR, which corroborate data derived from indirect immunofluorescence with anti–alginate antibodies. These results are of clinical importance, because alginate-producing, nonmucoid P. aeruginosa variants may be present in the airways of patients with CF (but this phenotype may not by expressed by organisms cultured on agar plates in vitro) within days after colonization and may contribute to bacterial persistence [26]. Consistent with this conclusion are the results of a previous study that demonstrated the presence of serum antibodies to P. aeruginosa alginate in patients with CF harboring only nonmucoid strains, as determined by in vitro cultures from respiratory secretions [34]. Conversion to the stable, mucoid P. aeruginosa phenotype that produces high levels of alginate has been linked to mutations in the alginate repressor gene cluster mucABCD [14, 18, 35, 36]; however, whether such mutations would occur in alginate-producing, nonmucoid strains is not known and is subject to further investigations.
In summary, we found that anaerobic conditions induce a rapid production of alginate in P. aeruginosa and that this response is initially reversible but can become stable after a fairly short period (2 weeks) of chronic lung infection in mice. These findings corroborate those of an analysis of nonmucoid P. aeruginosa isolates from patients with CF, which showed that these phenotypes are also alginate positive. Because alginate gene expression by nonmucoid strains is key in the establishment and maintenance of chronic lung infection in transgenic mice with CF [27], it appears that this exopolysaccharide has to be regarded as one of the most important virulence factors of this opportunistic pathogen, at least in patients with CF.
Acknowledgments
We thank Lisa Cariani, for assistance in collecting the specimens from patients with CF; and Giliola Calori, for statistical analysis.
Footnotes
Presented in part: 26th Congress of the European Cystic Fibrosis Society, Belfast, Northern Ireland, 4–7 June 2003; 17th Annual North American Cystic Fibrosis Conference, Anaheim, California, 16–19 October 2003.
Financial support: Marie Curie Fellowship (grant MCFI-2001-51061); Mukoviszidose; Cystic Fibrosis Foundation; National Institutes of Health (grant AI48917); Associazione Lombarda Fibrosi Cistica.
Potential conflicts of interest: none reported.
References
- 1.Darwin C. The origin of species. Available at: http://www.literature.org/authors/darwin-charles/the-origin-of-species/ Accessed 20 June 2005.
- 2.Vicente M, Chater KF, De Lorenzo V. Bacterial transcription factors involved in global regulation. Mol Microbiol. 1999;33:8–17. doi: 10.1046/j.1365-2958.1999.01445.x. [DOI] [PubMed] [Google Scholar]
- 3.Stover CK, Pham XQ, Erwin AC, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–64. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 4.LeClerc JE, Li B, Payne WL, Cebula TA. High mutation frequencies among Escherichia coli and Salmonella pathogens. Science. 1996;274:1208–11. doi: 10.1126/science.274.5290.1208. [DOI] [PubMed] [Google Scholar]
- 5.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–4. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 6.Oliver A, Baquero F, Blazquez J. The mismatch repair system (mutS, mutL, and uvrD genes) in Pseudomonas aeruginosa: molecular characterization of naturally occurring mutants. Mol Microbiol. 2002;43:1641–50. doi: 10.1046/j.1365-2958.2002.02855.x. [DOI] [PubMed] [Google Scholar]
- 7.Taddei F, Radman M, Maynard-Smith J, Toupance B, Gouyon PH, Godelle B. Role of mutator alleles in adaptive evolution. Nature. 1997;387:700–2. doi: 10.1038/42696. [DOI] [PubMed] [Google Scholar]
- 8.Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003;361:681–9. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 9.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infection of cystic fibrosis patients. J Clin Invest. 2002;109:317–25. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doggett RG, Harrison GM, Stillwell RN, Wallis ES. An atypical Pseudomonas aeruginosa with cystic fibrosis of the pancreas. J Pediatr. 1966;68:215–21. [Google Scholar]
- 11.Høiby N. Prevalence of mucoid strains of Pseudomonas aeruginosa in bacteriological specimens from patients with cystic fibrosis and patients with other diseases. Acta Pathol Microbiol Scand Suppl. 1975;83:549–52. [PubMed] [Google Scholar]
- 12.Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581–8. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 13.Lam J, Chan R, Lam K, Costerton JW. Production of mucoid micro-colonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980;28:546–56. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin DW, Schurr MJ, Mudd MH, Govan JRW, Holloway BW, Deretic V. Mechanisms of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci USA. 1993;90:8377–81. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schurr MJ, Yu H, Martinez-Salazar JM, Boucher JC, Deretic V. Control of AlgU, a member of the σE-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J Bacteriol. 1996;178:4997–5004. doi: 10.1128/jb.178.16.4997-5004.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boucher JC, Martinez-Salaza J, Schurr MJ, Mudd MH, Yu H, Deretic V. Two distinct loci affecting conversion to mucoidy in Pseudomonas aeruginosa in cystic fibrosis encode homologs of the serine protease HtlA. J Bacteriol. 1996;178:511–23. doi: 10.1128/jb.178.2.511-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher JC, Schurr MJ, Yu H, Rowen, Deretic V. Pseudomonas aeruginosa in cystic fibrosis: role of mucC in the regulation of alginate production and stress sensitivity. Microbiology. 1997;143:3473–80. doi: 10.1099/00221287-143-11-3473. [DOI] [PubMed] [Google Scholar]
- 18.Martin DW, Shurr MJ, Mudd MH, Deretic V. Differentiation of Pseudomonas aeruginosa into the alginate-producing form: inactivation of mucB causes conversion to mucoidy. Mol Microbiol. 1993;9:497–506. doi: 10.1111/j.1365-2958.1993.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 19.Mathee K, Ciofu O, Sternberg C, et al. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology. 1999;145:1349–57. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 20.Holdeman LV, Cato EP, Moore WEC. Anaerobe laboratory manual. 4th ed. Blacksburg, VA: Anaerobe Laboratory, Virginia Polytechnic Institute and State University, 1972
- 21.Cash HA, Woods DE, McCullough B, Johanson WE, Bass JA. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119:453–9. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- 22.Starke JR, Edwards MS, Langston C, Bacer CJ. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987;22:698–702. doi: 10.1203/00006450-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 23.May TB, Chakrabarty AM. Isolation and assay of Pseudomonas aeruginosa alginate. Methods Enzymol. 1994;235:295–304. doi: 10.1016/0076-6879(94)35148-1. [DOI] [PubMed] [Google Scholar]
- 24.Theilacker C, Coleman FT, Mueschenborn S, Llosa N, Grout M, Pier GB. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect Immun. 2003;71:3875–84. doi: 10.1128/IAI.71.7.3875-3884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pier GB, DesJardins D, Aguilar T, Barnard M, Speert DP. Polysaccharide surface antigens expressed by nonmucoid isolates of Pseudomonas aeruginosa from cystic fibrosis patients. J Clin Microbiol. 1986;24:189–96. doi: 10.1128/jcm.24.2.189-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anastassiou ED, Mintzas AC, Kounavis C, Dimitracopoulos G. Alginate production by clinical nonmucoid Pseudomonas aeruginosa strains. J Clin Microbiol. 1987;25:656–9. doi: 10.1128/jcm.25.4.656-659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman FT, Mueschenborn S, Meluleni G, et al. Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection. Proc Natl Acad Sci USA. 2003;100:1949–54. doi: 10.1073/pnas.0437901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48:1041–9. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen SS, Espersen F, Høiby N, Jensen T. Immunoglobulin A and immunoglobulin G antibody responses to alginate from Pseudomonas aeruginosa in patients with cystic fibrosis. J Clin Microbiol. 1990;28:747–55. doi: 10.1128/jcm.28.4.747-755.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Heeckeren AM, Tscheikuna J, Walenga RW, et al. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med. 2000;161:271–9. doi: 10.1164/ajrccm.161.1.9903019. [DOI] [PubMed] [Google Scholar]
- 31.O’Reilly T. Relevance of animal models for chronic bacterial airway infections in humans. Am J Respir Crit Care Med. 1995;151:2101–8. doi: 10.1164/ajrccm.151.6.7767564. [DOI] [PubMed] [Google Scholar]
- 32.Morissette C, Skamene E, Gervais F. Endobronchial inflammation following Pseudomonas aeruginosa infection in resistant and susceptible strains of mice. Infect Immun. 1995;63:1718–24. doi: 10.1128/iai.63.5.1718-1724.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pier GB, Boyer D, Preston M, et al. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J Immunol. 2004;173:5671–8. doi: 10.4049/jimmunol.173.9.5671. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen SS, Hoiby N, Espersen F, Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax. 1992;47:6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boucher JC, Yu H, Mudd MH, Deretic V. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect Immun. 1997;65:3838–46. doi: 10.1128/iai.65.9.3838-3846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthony M, Rose B, Pegler MB, et al. Genetic analysis of Pseudomonas aeruginosa isolates from the sputa of Australian adult cystic fibrosis patients. J Clin Microbiol. 2002;40:2772–8. doi: 10.1128/JCM.40.8.2772-2778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


