Abstract
Objectives
To quantitatively compare the antibiotic susceptibility of biofilms formed by the coagulase-negative staphylococci (CoNS) Staphylococcus epidermidis and Staphylococcus haemolyticus with the susceptibility of planktonic cultures.
Methods
Several CoNS strains were grown planktonically or as biofilms to determine the effect of the mode of growth on the level of susceptibility to antibiotics with different mechanisms of action. The utility of a new, rapid colorimetric method that is based on the reduction of a tetrazolium salt (XTT) to measure cell viability was tested by comparison with standard bacterial enumeration techniques. A 6 h kinetic study was performed using dicloxacillin, cefazolin, vancomycin, tetracycline and rifampicin at the peak serum concentration of each antibiotic.
Results
In planktonic cells, inhibitors of cell wall synthesis were highly effective over a 3 h period. Biofilms were much less susceptible than planktonic cultures to all antibiotics tested, particularly inhibitors of cell wall synthesis. The susceptibility to inhibitors of protein and RNA synthesis was affected by the biofilm phenotype to a lesser degree. Standard bacterial enumeration techniques and the XTT method produced equivalent results both in biofilms and planktonic assays.
Conclusions
This study provides a more accurate comparison between the antibiotic susceptibilities of planktonic versus biofilm populations, because the cell densities in the two populations were similar and because we measured the concentration required to inhibit bacterial metabolism rather than to eradicate the entire bacterial population. While the biofilm phenotype is highly resistant to antibiotics that target cell wall synthesis, it is fairly susceptible to antibiotics that target RNA and protein synthesis.
Keywords: nosocomial infections, pathogens, biofilms, antibiotic resistance, CoNS
Introduction
Staphylococcus epidermidis and related coagulase-negative staphylococci (CoNS) are now well established as major nosocomial pathogens associated with infections of indwelling medical devices. Biofilm formation is one of the major virulence factors of these organisms,1 often leading to persistent infections.2
The fact that biofilm bacteria are able to tolerate significantly higher levels of antibiotics than planktonic bacteria has been well established in susceptibility assays, and the clinical relevance of this phenomenon is underscored by the occurrence of medical device-related infections that are refractory to antibiotic therapy.3,4 Despite concerted efforts to treat biofilm infections with antibiotic therapy, the physical removal of an infected medical device is often necessary,5 which carries an additional economic and health cost. The resistance of bacterial cells in a biofilm to antibiotics does not seem to depend on traditional mechanisms of antibiotic resistance.6,7 Although it is not yet clear how biofilms resist antimicrobial agents, a possible explanation has been suggested by several authors who assume that biofilms present a diffusional barrier to antibiotics.8–10 However, it seems that this mechanism can only partially explain the increased resistance phenotype generally present in clinically relevant biofilms.11 Other mechanisms have been suggested, including slow growth of the cells within the biofilm,12 activation of the general stress response,13 emergence of a biofilm-specific phenotype14 and persister cells.15 Resistance is reportedly up to 1000-fold greater in bacterial cells in biofilms, but a reliable method to compare the antibiotic susceptibilities of planktonic bacteria with cells in biofilms is lacking.16
The goal of this study was to compare the antibiotic susceptibilities of planktonic versus biofilm bacterial cells, using an adequate and reliable methodology. We evaluated the resistance of CoNS cells in biofilms to antibiotics with different molecular weights and different mechanisms of action: inhibitors of cell wall synthesis (cefazolin, vancomycin and dicloxacillin), inhibitors of protein synthesis (tetracycline) and inhibitors of RNA synthesis (rifampicin). We also compared the susceptibility of CoNS biofilms with that of planktonic cells, using the classic cfu plating assay and also a new rapid colorimetric method that measures cellular metabolic activity, based on the reduction of tetrazolium salt (XTT), in an attempt to correlate viability assays and activity assays with the effects of specific antibiotics on cells present in biofilms.
Material and methods
Antibiotics
The antibiotics and respective concentrations used in this study were cefazolin 63 mg/L, vancomycin 40 mg/L, dicloxacillin 59 mg/L, tetracycline 16 mg/L and rifampicin 10 mg/L. The main characteristics of these antibiotics are described in Table 1. The antibiotic concentration used in all assays was the peak concentration in human serum (PS).
Table 1.
Characteristics of the antibiotics used in this study
| Antibiotic | Mechanism of actiona | PSb | MWa |
|---|---|---|---|
| Cefazolin | Cell wall synthesis inhibitor | 63 | 477 |
| Vancomycin | Cell wall synthesis inhibitor | 40 | 1485 |
| Dicloxacillin | Cell wall synthesis inhibitor | 59 | 481 |
| Tetracycline | Protein synthesis inhibitor | 16 | 823 |
| Rifampicin | RNA synthesis inhibitor | 10 | 492 |
The mechanism of action and the molecular weight (MW) in g/mol of the antibiotics were provided by the manufacturer.
Bacterial strains and growth conditions
A total of six CoNS biofilm-producing strains were used in this study: S. epidermidis 9142, S. epidermidis IE186, S. epidermidis M129, S. epidermidis M18717,18 and Staphylococcus haemolyticus IE246, S. haemolyticus M176.19 Tryptic soy broth (TSB) and tryptic soy agar (TSA) were prepared according to the manufacturer’s instructions. All strains were incubated in 15 mL of TSB inoculated with bacterial cultures <2 days old and grown on TSA plates, for 24 (±2) h at 37°C in a shaker rotator at 130 rpm. Cells were harvested by centrifugation (for 5 min at 10 500g and 4°C), and resuspended in a saline solution (0.9% NaCl prepared in distilled water) adjusted to an optical density (620 nm) equivalent to 1 × 109 cells/mL, and then used in the subsequent assays. MICs were determined according to NCCLS standards, with some minor modifications, using TSB as a growth medium, with at least three replicates for each determination (Table 2).
Table 2.
Determination of the MIC ranges in mg/L for six coagulase-negative staphylococci and five antibiotics
| Strain | CFZ | VAN | DCX | TET | RIF |
|---|---|---|---|---|---|
| S. epidermidis 9142 | 64–128 | 8–16 | 64–128 | 0.5–2 | 0.03–0.12 |
| S. epidermidis IE186 | 2–16 | 8 | 0.5–4 | 2–4 | 0.03–0.06 |
| S. epidermidis M129 | 4–32 | 8 | 4–16 | 16–32 | 0.03–0.06 |
| S. epidermidis M187 | 64–128 | 8 | 16–64 | 2–8 | 0.03–0.06 |
| S. haemolyticus IE246 | 0.5–2 | 2–4 | 0.25–2 | 0.25–1 | 0.03–0.12 |
| S. haemolyticus M176 | 32–128 | 2–4 | 16–128 | 2–8 | 0.03–0.06 |
CFZ, cefazolin; VAN, vancomycin; DCX, dicloxacillin; TET, tetracycline; RIF, rifampicin.
Biofilm formation
Biofilms were produced as described previously.18 Briefly, for each strain, 10 μL of a cell suspension adjusted to 1 × 109 cells/mL in 0.9% NaCl was added to a 96-well microtitre plate containing 240 μL of TSB supplemented with 0.25% of glucose per well to promote biofilm formation. Plates were incubated at 37°C with shaking at 150 rpm, for 24 (±4) h. The planktonic cells were removed carefully, and the biofilm was washed twice with 200 μL of 0.9% NaCl. This procedure yielded a biofilm containing ~2 × 108 cells/mL. This was determined as previously described20 by disrupting the biofilm and resuspending the cells in TSB + 0.05% Tween, followed by 20 s of sonication at 20 W to homogenize the suspension. This procedure disrupted the cell clumps without impairing cell viability.20 This was determined by comparing sonicated and non-sonicated suspensions by Gram staining and cell viability tests.
Serial dilutions were made and plated on TSA plates that were incubated overnight at 37°C.
Antibiotic susceptibility of planktonic cultures assessed by cfu plating
For each strain, 200 μL of a cell suspension adjusted to 1 × 109 cells/mL in 0.9% NaCl was added to 30 mL of TSB, and incubated at 37°C with shaking at 130 rpm, until a cell density of 1 × 109 cells/mL was reached. Then, a fivefold dilution was made in TSB containing each antibiotic at the respective PS concentration in order to obtain a cell suspension of 2 × 108 cells/mL, and growth was allowed to occur over 6 h. A control was obtained by diluting the suspension in fresh TSB without adding antibiotic. Each hour a 1 mL sample was collected and centrifuged for 8 min at 9000g, and the pellet resuspended in 0.9% NaCl. Two washing steps were performed, followed by 20 s of sonication at 20 W. The viable cells were determined by performing 10-fold serial dilutions of this suspension, which were plated on TSA plates that were then incubated for 20 h at 3 7 °C. This experiment was repeated three times, in triplicate.
Antibiotic susceptibility of biofilms assessed by cfu plating
Biofilms were prepared in 96-well microtitre plates, as described above, yielding an initial cell concentration of about 2 × 108 cells/mL. To each well containing the biofilm, 200 μL of TSB supplemented with 0.25% of glucose and one of the antibiotics at the PS concentration were added. Growth was allowed to occur over 6 h. A control was obtained by adding TSB with 0.25% of glucose but without any antibiotic to the biofilm cultures. At 2 h time intervals, the planktonic cells of four random wells were removed carefully and the biofilm was washed twice with 200 μL of 0.9% NaCl. The four wells were thoroughly scraped until >93% (±5%) of the biofilm was removed (as determined by crystal violet spectrophotometric readings)21 and resuspended in 1 mL of 0.9% NaCl, followed by centrifugation for 8 min at 9000g. The pellet was resuspended in 0.9% NaCl and washed twice, followed by 20 s of sonication at 20 W. The viable cells were determined by performing 10-fold serial dilutions of this suspension and plating 100 μL of the dilutions in triplicate on TSA plates that were then incubated for 20 h at 37°C. This experiment was repeated three times, with individual samples evaluated in triplicate.
Antibiotic susceptibility of planktonic cultures assessed by XTT
The XTT colorimetric method was applied to determine antibiotic susceptibility as described previously,22 with some modifications. Briefly, after 3 h of exposure of 200 μL of a bacterial suspension to the antibiotics, cells were washed twice with 200 μL of 0.9% NaCl and transferred to individual wells of a 96-well microtitre plate. Then, 50 μL of a solution containing 200 mg of XTT ({2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenyalamino)carbonyl]-2H-tetrazolium hydroxide}) (Sigma, MO, USA) per L and 20 mg PMS (phenazine methosulphate) (Sigma, MO, USA) per L was added to each well. The microtitre plates were incubated for 3 h at 37 °C in the dark. After that, 200 μL of the liquid medium in each well was transferred to a 1.5 mL tube and centrifuged for 5 min at 9500g. For the spectrophotometric readings, 100 μL of the supernatant was transferred to a new microtitre plate, and the absorbance was measured at 490 nm. A control was obtained with a cell suspension not exposed to the antibiotics. All samples were conducted in quadruplicate, and each experiment was repeated three times.
Antibiotic susceptibility of biofilms assessed by XTT
The XTT colorimetric method was applied to determine the antibiotic susceptibility as described previously,22 with some modifications. Briefly, biofilms exposed to the antibiotics were gently washed twice with 200 μL of 0.9% NaCl, then 250 μL of a solution containing 200 mg XTT/L and 20 mg PMS/L was added to each well. Microtitre plates were incubated for 3 h at 37°C in the dark. Then, 200μL from each well was transferred to a 1.5 mL tube, and centrifuged for 5 min at 9500g. For the spectrophotometric readings, 100 μL of the supernatant was transferred to a new microtitre plate, and the absorbance was measured at 490 nm. Controls were biofilms not exposed to the antibiotics. All samples were carried out in quadruplicate, and the experiment was repeated three times.
Statistical analysis
All the assays were compared using one-way analysis of variance (ANOVA) by applying the Levene’s test of homogeneity of variances, and the Tukey multiple-comparisons test, or by paired samples t-tests, using the Statistical Package for the Social Sciences software. Differences were considered significant when non-overlapping confidence levels of ≥95% were achieved.
Results
Kinetics study
Figure 1 presents the time–kill curves for the tested antibiotics (see Table 1) at the PS concentration against both planktonic and biofilm cells of S. epidermidis 9142. The growth profiles of S. epidermidis 9142 cells not exposed to antibiotics in planktonic cultures versus biofilm cultures were significantly different (P < 0.05, ANOVA and Tukey multiple-comparisons test).
Figure 1.
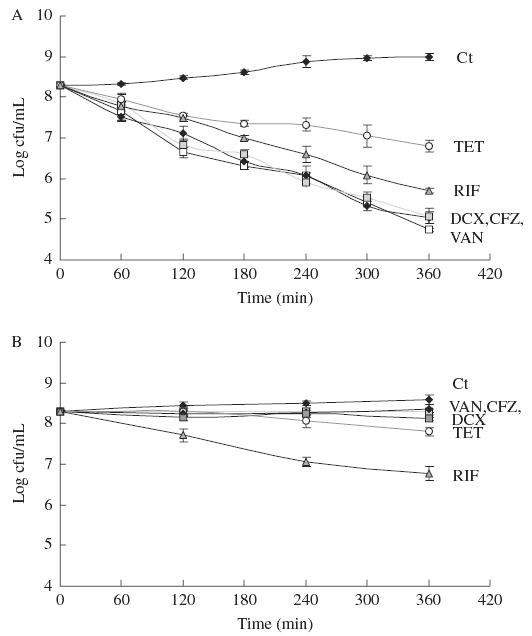
Control growth curves (Ct) and time–kill plots of the effect of cefazolin (CFZ), vancomycin (VAN), dicloxacillin (DCX), tetracycline (TET) and rifampicin (RIF) on S. epidermidis 9142 planktonic cells (a) and biofilm (b). Points represent means and error bars the sem.
Non-antibiotic-treated planktonic cells had significant growth over 6 h , whereas only a slight increase in cfu/mL was noted in cells growing in biofilms. All three cell-wall synthesis inhibitors (cefazolin, vancomycin, and dicloxacillin) were highly effective against planktonic cells, showing nearly a 1 log decrease in viable cells in the first hour, and a near 3 log difference after 6 h of exposure. Tetracycline was the least effective antibiotic on planktonic cells, whereas rifampicin exhibited intermediate efficacy. The activity of cell-wall synthesis inhibitors on cells growing in biofilms was markedly less than their activity on planktonic cells: the decrease in viable bacterial cells was <0.5 log even after 6 h of exposure to these antibiotics. It is interesting to note that in contrast to planktonic cells, tetracycline and rifampicin were the most effective antibiotics in terms of reducing viability of cells in biofilms.
Antibiotic susceptibility assessed by cfu plating
Figure 2 shows the mean reduction in bacterial cell viability for six different CoNS strains after exposure of planktonic cultures or cells in biofilms to antibiotics. As expected, for all S. epidermidis and S. haemolyticus strains, planktonic cells were more susceptible to all cell wall synthesis inhibitors used, compared with cells in biofilms. The differences in cellular survival detected between planktonic cells and biofilm cells with protein and RNA synthesis inhibitors were lower. In fact, for 50% of the strains there was no significant difference in the susceptibility of planktonic cells and biofilms to rifampicin (P > 0.05, paired t-test). The same was observed for S. epidermidis with tetracycline but not for S. haemolyticus since cells in biofilms or planktonic cultures had significantly different susceptibilities.
Figure 2.
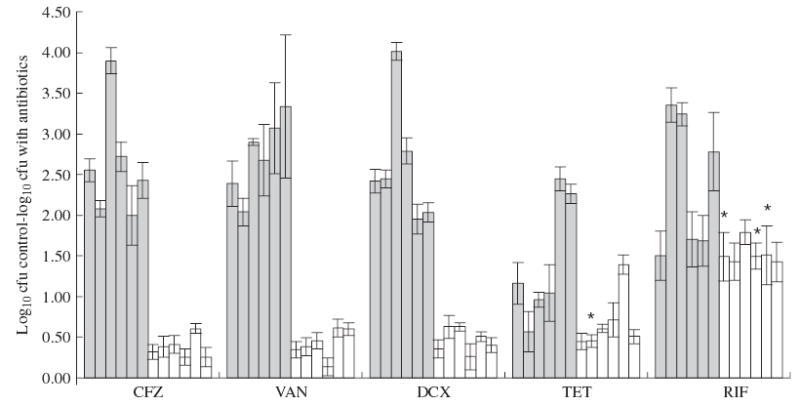
Mean fold reduction of cellular viability after 3 h of exposure to antibiotics. The y-axis indicates the difference in the log10 cfu/mL between strains without antibiotic (controls) and strains treated with antibiotics (CFZ, cefazolin; VAN, vancomycin; DCX, dicloxacillin; TET, tetracycline; RIF, rifampicin), in planktonic cells (grey bars) and biofilm (white bars). Different bars represent different strains, from left to right: S. epidermidis 9142, IE186, M129, M187, S. haemolyticus IE246 and M176. *Indicates values that are not significantly different (P > 0.05, paired t-test).
When analysing the average differences in susceptibilities between planktonic cells and biofilms, for each antibiotic, a ratio can be calculated between the susceptibility of planktonic cells and biofilm cells: 7.0 for cefazolin, 6.4 for vancomycin, 5.6 for dicloxacillin, 2.0 for tetracycline and 1.6 for rifampicin. This shows that rifampicin and tetracycline are less affected by the biofilm phenotype, since the ratios of their activities are close to 1 when comparing effects in planktonic and biofilm cells. In contrast, all three cell wall inhibitors were highly affected by the biofilm phenotype, as demonstrated by the high ratios of the activities.
Antibiotic susceptibility assessed by XTT
Figure 3 presents the mean reduction in metabolic activity after exposure of planktonic cultures and cells in biofilms to antibiotics, as measured by the decrease in metabolic activity using the XTT reagent. The findings were very similar to the results obtained by measuring the decrease in viability by cfu plating: the major reductions in metabolic activity were primarily found with planktonic cells, and the differences between cells in biofilms and planktonic cells were greater for cell wall synthesis inhibitors.
Figure 3.
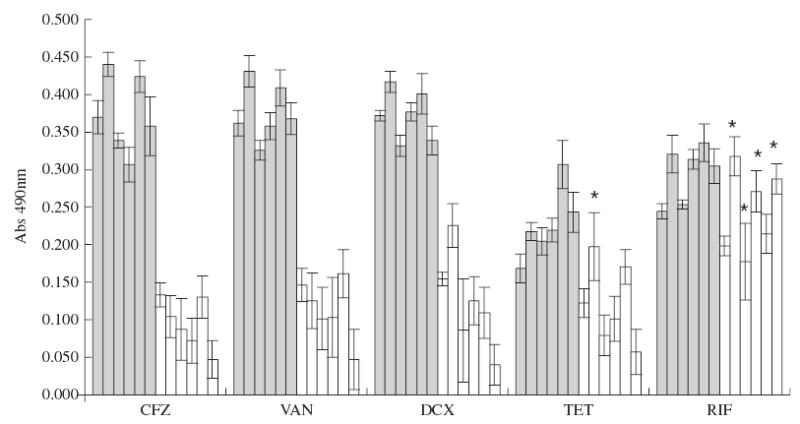
Mean reduction of cellular activity, measured by XTT, after 3 h of exposure to the antibiotics, expressed as the difference in absorbance readings at 490 nm between controls without antibiotic and antibiotic treated strains (CFZ, cefazolin; VAN, vancomycin; DCX, dicloxacillin; TET, tetracycline; RIF, rifampicin) per μg of antibiotic used (Δ/PS), in planktonic cells (grey bars) and biofilm (white bars). Different bars represent different strains, from left to right: S. epidermidis 9142, IE186, M129, M187, S. haemolyticus IE246 and M176. *Indicates values that are not significantly different (P > 0.05, paired t-test).
Discussion
Antibiotic resistance is a serious problem encountered with many human pathogens,23 and is particularly notable with S. epidermidis, since many clinical isolates of this organism are resistant to up to eight different antibiotics.24 Adding to this problem is the fact that the major virulence factor of S. epidermidis and other CoNS is biofilm formation25 and that cells in biofilms are normally more resistant to antibiotics than planktonic cells,6 making drug resistance in a CoNS infection an even more serious problem.
A number of assays have been developed to quantify the level of susceptibility of cells in biofilms, but such assays do not provide a fair comparison with the standard MIC assay for planktonic bacteria.16,26,27 This is because NCCLS standards stipulate the use of low inocula of the planktonic bacteria,28 whereas bacteria are at a high cell density in established biofilms. This difference affects antibiotic susceptibility assays in a number of ways. First, certain antibiotics such as vancomycin exhibit a cell density-dependent effect and are much less effective against high bacterial inocula. Second, most antibiotic susceptibility assays for cells in biofilms measure the minimum biofilm eradication concentration (MBEC) rather than the MIC.16 It has been established that in any bacterial population, be it planktonic or sessile cells in biofilms, there exists a certain percentage of persister cells, which are extremely tolerant to antibiotics.15,29 For this reason it takes a high concentration of a given antibiotic to completely eradicate even a planktonic population of bacteria. Overall, comparing the MICs of planktonic bacteria with the MBEC of biofilm bacteria may result in exaggerated differences between the susceptibility levels. Indeed, while comparisons between MICs for planktonic cells and MBECs for cells in biofilms suggests there may be a 1000-fold difference in the susceptibility of the biofilm cells to certain antibiotics, we found in this study that the killing efficacy of achievable peak serum concentrations of cefazolin, vancomycin, dicloxacillin, tetracycline and rifampicin was less than 10-fold higher against S. epidermidis cells in biofilms. This decrease in efficacy is still quite significant, however, and could account for frequent therapeutic failure of these drugs against biofilm infections.
A critical first step in understanding mechanisms of antibiotic resistance of cells in biofilms is to have a method to accurately determine the level of resistance relative to planktonic populations. Besides determination of viable cells by classic cfu plating, we evaluated the effects of antibiotics on metabolic activity by colorimetrically measuring XTT reduction.30 This method has started to become widely used and several comparisons with NCCLS standard susceptibility tests have already demonstrated its reliability.22,31 One of the major advantages of this method is the short time necessary to obtain results. Measuring antibiotic susceptibilities of cells in biofilms can be laborious and the application of a reliable and rapid method is desirable in a clinical laboratory. Kuhn et al. described the lower sensitivity as one disadvantage of the XTT method.32 Our results also demonstrated that the cfu plating method has a higher sensitivity, when compared with XTT.
The assay described here provides a more consistent comparison between the antibiotic susceptibilities of planktonic versus biofilm populations. Furthermore, since the antibiotics were used at the PS concentration, the relative differences in the effects of antibiotics on planktonic versus biofilm cells can be considered to have potential clinical relevance. The present results demonstrate that rifampicin was the most effective antibiotic against S. epidermidis or S. haemolyticus cells in biofilms, as assessed by both cfu plating and XTT measurements. As the pathogenesis of CoNS is often dependent upon colonization of abiotic, implanted surfaces and subsequent biofilm formation, it is of utmost importance to assess the susceptibility of cells in biofilms to antibiotics rather than the susceptibility of planktonic cells. Many clinical practices still rely on MIC determinations performed with microbial suspensions;33–35 however, as the present results demonstrate, the susceptibility levels of planktonic cells do not reflect the correspondent susceptibility of the cells in biofilms.
The differences in cfu following antibiotic treatment demonstrated that, on average, for all strains tested, the susceptibility to tetracycline and rifampicin was less affected by the biofilm phenotype. In fact, for some strains there was no significant difference in the cfu achieved comparing planktonic cells and biofilm cells in regard to their susceptibility to antibiotics (the ratio was almost 1).
Low growth rates have been considered as an antibiotic resistance mechanism that has been observed in Escherichia coli biofilms.36 In the present analysis, growth rates of cells in bio-films were lower than that of planktonic cells (Figure 1), which could partially explain the higher resistance of biofilms to antibiotics, particularly for cell wall synthesis inhibitors whose efficiency is very much dependent of the growth rate of the cells. However, RNA synthesis inhibitors are also dependent on the growth rate of the cell and our results showed that both planktonic cells and cells in biofilms were comparably susceptible to these agents. It has also been suggested that the biofilm matrix itself could pose a barrier to the penetration of the antibiotics.8–10 However, other studies indicate that diffusion through the biofilm matrix is roughly equivalent to water 37 and our results indicate that antibiotics with the same mechanism of action but having different molecular weights did not differ in their efficacy against cells in biofilms.
Our results demonstrated that antibiotics that target cell wall synthesis have a reduced activity in biofilms, independent of the size of the antibiotic molecule, but antibiotics that target RNA and protein synthesis have similar activities on planktonic cells as they do on cells in biofilms, suggesting that the phenotypic resistance of cells in biofilms to antibiotics is affected primarily by the mechanism of action of the antibiotic. Several different resistance mechanisms in biofilms have been suggested, and although each one can partially explain some cases of antibiotic resistance of cells in biofilms, an overall understanding of antibiotic resistance of this phenotype is still yet to be elucidated. Considering that biofilm formation is one of the major virulence factors involved in CoNS infections,1 susceptibility testing of CoNS should not rely on MIC determinations. XTT activity measurements could provide a quick and reliable methodology to assess the susceptibility of CoNS cells growing in biofilms to antibiotics.
Acknowledgments
We acknowledge the financial support of FCT, through the project FCT POCTI/ESP/42688/2001 and also the grant SFRH/BD/8676/ 2002. GBP was supported by NIH grant AI 46706.
References
- 1.Voung C, Otto M. Staphylococcus epidermidis infections. Microbes Infect. 2002;4:481–9. doi: 10.1016/s1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 2.Costerton J, Stewart P, Greenberg P. Bacterial Biofilms: a commom cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 3.Arciola C, Campoccia D, Montanaro L. Effects on antibiotic resistance of Staphylococcus epidermidis following adhesion to polymethyl-methacrylate and to silicone surfaces. Biomaterials. 2002;23:1495–1502. doi: 10.1016/s0142-9612(01)00275-7. [DOI] [PubMed] [Google Scholar]
- 4.Monzón M, Oteiza C, Leiva J, et al. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn Microbiol Infect Dis. 2002;44:319–24. doi: 10.1016/s0732-8893(02)00464-9. [DOI] [PubMed] [Google Scholar]
- 5.Jansen B, Kristinsson K, Jansen S, et al. In-vitro efficacy of a central venous catheter complexed with iodine to prevent bacterial colonization. J Antimicrob Chemother. 1992;30:135–9. doi: 10.1093/jac/30.2.135. [DOI] [PubMed] [Google Scholar]
- 6.Stewart P, Costerton J. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 7.Anderl J, Franklin M, Stewart P. Role of antibiotic penetration limitation in Klebsiella pmeumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1818–24. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Beer D, Srinivasan R, Stewart PS. Direct measurement of chlorine penetration into biofilms during disinfection. Appl Environ Microbiol. 1994;60:4339–44. doi: 10.1128/aem.60.12.4339-4344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suci PA, Mittelman MW, Yu FP, et al. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 1994;38:2125–33. doi: 10.1128/aac.38.9.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoyle BD, Alcantara J, Costerton JW. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob Agents Chemother. 1992;36:2054–6. doi: 10.1128/aac.36.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne WM, Jr, Mason EO, Jr, Kaplan SL. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother. 1993;37:2522–6. doi: 10.1128/aac.37.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuomanen E, Cozens R, Tosch W, et al. The rate of killing of Escherichia coli by β-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986;132:1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- 13.Brown MR, Barker J. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 1999;7:46–50. doi: 10.1016/s0966-842x(98)01425-5. [DOI] [PubMed] [Google Scholar]
- 14.Mah T, O’Toole G. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–9. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 15.Spoering A, Lewis K. Biofilms and planktonic cells of Pseudomonas aeroginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183:6744–51. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceri H, Olson M, Stremick C, et al. The calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37:1771–6. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerca N, Pier G, Oliveira R, et al. Comparative evaluation of coagulase-negative staphylococci (CoNS) adherence to acrylic by a static method and a parallel-plate flow dynamic method. Res Microbiol. 2004;155:755–60. doi: 10.1016/j.resmic.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Cerca N, Pier G, Vilanova M, et al. Influence of batch or fed-batch growth on Staphylococcus epidermidis biofilm formation. Lett Appl Microbiol. 2004;39:420–4. doi: 10.1111/j.1472-765X.2004.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerca N, Martins S, Pier G, et al. The relationship between inhibition of bacterial adhesion to a solid surface by sub-mic concentrations of antibiotics and the subsequent development of a biofilm. Res Microbiol. 2005;156:650–5. doi: 10.1016/j.resmic.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerca N, Pier G, Vilanova M, et al. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcusepidermidis. Res Microbiol. 2005;156:506–14. doi: 10.1016/j.resmic.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramton S, Ulrich M, Gotz F, et al. Anerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun. 2001;69:4079–85. doi: 10.1128/IAI.69.6.4079-4085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logu A, Pellerano M, Sanna A, et al. Comparison of the susceptibility testing of clinical isolates of Mycobacterium tuberculosis by the XTT colorimetric method and the NCCLS standards method. Int J Antimicrob Agents. 2003;21:244–50. doi: 10.1016/s0924-8579(02)00350-3. [DOI] [PubMed] [Google Scholar]
- 23.Hoban D, Bouchillon S, Johnson J, et al. Comparative in vitropotency of amoxycillin-clavulanic acid and four oral agents against recent North American clinical isolates from a global surveillance study. Int J Antimicrob Agents. 2001;21:425–53. doi: 10.1016/s0924-8579(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 24.Fluit A, Schmitz F, Verhoef J. Multi-resistance to antimicrobial agenst for the ten most frequently isolated bacterial pathogens. Int J Antimicrob Agents. 2001;18:147–60. doi: 10.1016/s0924-8579(01)00357-0. [DOI] [PubMed] [Google Scholar]
- 25.O’Gara J, Humphreys H. Staphylococcus epidermidis biofilms: importance and implications. J Med Microbiol. 2001;50:582–7. doi: 10.1099/0022-1317-50-7-582. [DOI] [PubMed] [Google Scholar]
- 26.Amorena B, Gracia E, Monzón M, et al. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother. 1999;44:43–55. doi: 10.1093/jac/44.1.43. [DOI] [PubMed] [Google Scholar]
- 27.Knobloch J, von Osten H, Horstkotte M, et al. Minimal attachment killin (MAK): a versatile method for susceptibility testing of attached biofilm-positive and -negative Staphylococcus epidermidis. Med Microbiol Immunol. 2002;191:107–14. doi: 10.1007/s00430-002-0125-2. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically—Fifth Edition: Approved Standard M7-A5. NCCLS, Wayne, PA, USA, 1997.
- 29.Keren I, Kaldalu N, Al S, et al. Persister cells and tolerence to antimicrobials. FEMS Microbiol Lett. 2004;230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 30.Tunney M, Ramage G, Field T, et al. Rapid colorimetric assay for antimicrobial susceptibility testing of Pseudomonas aeroginosa. Antimicrob Agents Chemother. 2004;48:1879–81. doi: 10.1128/AAC.48.5.1879-1881.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawser S, Norris H, Jessup C, et al. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5[(phenyl-amino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized national committee for clinical laboratory standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–2. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn DM, Balkis M, Chandra J, et al. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin Microbiol. 2003;41:506–8. doi: 10.1128/JCM.41.1.506-508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung-Tomc J, Clark J, Minassian B, et al. In vitro and in vivo activities of a novel cephalosporin, BMS-247243, against methicillin-resistant and susceptible Staphylococci. Antimicrob Agents Chemother. 2002;46:971–6. doi: 10.1128/AAC.46.4.971-976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermsen E, Hovde L, Hotchkiss K, et al. Increased killing of staphylococci and streptococci by daptomycin compared with cefazolin and vancomycin in an in vitro peritoneal dialysate model. Antimicrob Agents Chemother. 2003;47:3764–7. doi: 10.1128/AAC.47.12.3764-3767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoellman D, Lin G, Ednie L, et al. Antipneumococcal and antista-phylococcal activities of ranbezolid (RBX 7644), a new oxazolidinone, compared to those of other agents. Antimicrob Agents Chemother. 2003;47:1148–50. doi: 10.1128/AAC.47.3.1148-1150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans DJ, Allison DG, Brown MR, et al. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: effect of specific growth rate. J Antimicrob Chemother. 1991;27:177–84. doi: 10.1093/jac/27.2.177. [DOI] [PubMed] [Google Scholar]
- 37.Stewart PS. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng. 1998;59:261–72. doi: 10.1002/(sici)1097-0290(19980805)59:3<261::aid-bit1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Friberg O, Jones I, Sjoberg L, et al. Antibiotic concentrations in serum and wound fluid after local gentamicin or intravenous dicloxacillin prophylaxis in cardiac surgery. Scand J Infect Dis. 2004;35:251–4. doi: 10.1080/003655400310000184. [DOI] [PubMed] [Google Scholar]


