Abstract
Purpose
To purify and characterize the glycoprotein lumican, isolated from human amniotic membrane (AM), and to examine its efficacy in treating corneal epithelium debridement.
Methods
An affinity-purified, anti-human lumican antibody–conjugated protein A Sepharose column was used to purify soluble lumican protein from human AM. The purified AM lumican was characterized by two-dimensional and SDS gel electrophoresis, plus Western blot analysis with anti-lumican antibody. The effects of lumican on corneal epithelial wound healing were examined in an organ culture mouse eye model.
Results
Lumican was found to be abundantly present in the stroma of human AM. It was extracted from the AM by isotonic, 1 M NaCl, and 4 M guanidine HCl solutions, suggesting that it is present in both the soluble and matrix-bound states. In two-dimensional gel electrophoresis, the 50-kDa human amniotic lumican purified by antibody-conjugated affinity chromatography migrated in a smear between pH 3.0 and 6.0. After endo-β-galactosidase digestion, it existed as a single core protein at pH 6.0, suggesting that native human amniotic lumican is a glycoprotein with short sugar moiety. Addition of purified human AM lumican to cultured medium promoted re-epithelialization and enhanced cell proliferation of wild-type mouse corneal epithelial cells in an organ culture. In lumican-knockout (lum−/−) mice, the effect of human lumican on promoting corneal epithelial wound healing was even more dramatic than in wild-type mice.
Conclusions
The diversified functions of lumican include modulation of epithelial cells in wound healing and serving as an extracellular matrix component. Administration of lumican may be beneficial for treating epithelial defects in the cornea and other tissues.
Lumican, a small leucine-rich proteoglycan (SLRP), is one of the major extracellular components in interstitial collagenous matrices of the corneal stroma, aorta, skin, skeletal muscle, lung, kidney, bone, cartilage, and intervertebral discs.1–8 In the cornea, lumican contains keratan sulfate chains. However, it is present as a low or nonsulfated glycoprotein (50 –57 kDa) in noncorneal tissues.1,2,9–11 Its wide distribution implies that it has multiple functions in tissue morphogenesis and maintenance of tissue homeostasis. This is best illustrated by the multiple clinical manifestations observed in Lumican-knockout (lum−/−) mice that exhibit corneal opacity, skin and tendon fragility, delayed wound healing, and low fertility. Indeed, lumican has been shown to play essential roles in corneal transparency by regulating collagen fibrillogenesis8 in wound healing, by modulating epithelial cell migration,12 and in the epithelium–mesenchyme transition of the injured lens.13 These results have led to the speculation that lumican may play an active role in corneal epithelial wound healing.
Transplantation of human AM as a temporary or permanent graft has become a popular surgical procedure for ocular surface reconstruction.14,15 Besides the observation that cryopreserved AM reduces inflammation,16–21 scarring,22,23 and neovascularization,24 many clinical studies25–28 have shown that human AM as a graft promotes corneal epithelial wound healing. Furthermore, human AM can help preserve corneal limbal epithelial progenitor cells and maintain the keratocyte phenotype during ex vivo expansion.29–32 Nevertheless, the exact molecular mechanism of the aforementioned effects remains unknown.
The present study revealed that human AM is a rich source of soluble lumican glycoprotein and that purified soluble lumican glycoprotein can facilitate proliferation and migration of corneal epithelial cells during wound healing in both wild-type and lum−/− mice.
Materials and Methods
Preparation of an Epitope-Specific, Polyclonal, Anti-Lumican Antibody
Rabbit anti-lumican peptide antiserum was obtained from EvoQuest Custom Antibody Services (Invitrogen Corp., Carlsbad, CA). In brief, an oligopeptide with the sequence of SLPPDMYECLRVANE, corresponding to the C-terminal amino acid residues deduced from human lumican cDNA (GenBank U18728; http://www.ncbi.nlm.nih.gov/Genbank; provided in the public domain by the National Center for Biotechnology Information, Bethesda, MD) was synthesized and coupled to a maleimide-activated carrier protein, KLH. The KLH-conjugated peptide was then used to raise antiserum in rabbit. To purify the anti-lumican antibody further, the SLPPDMYECLRVANE oligopeptide was immobilized by covalent reaction with iodoacetyl groups on gel (Sulfolink; Pierce, Rockford, IL). The rabbit antiserum was loaded onto a peptide-conjugated gel column, according to the manufacturer’s instruction. Fractions containing purified anti-lumican antibody were pooled and concentrated. The protein concentration was measured by spectrophotometry at OD280nm. The specificity of the anti-lumican antibody was evaluated by Western blot analysis.
Tissue Preparation and Immunohistochemistry
Human placenta tissues and AM were obtained from Bio-Tissue, Inc. (Miami, FL). In brief, the human AM was peeled from donated placental tissue after elective cesarean delivery and processed by a patented technology developed by one of the authors (SCGT; available at the Bio-Tissue Web site: http://www.biotissue.com). Human corneas without any recorded history of eye disease were obtained from the Florida Lions Eye Bank. The human fetal membrane tissue and mouse eyeballs were fixed with 4% paraformaldehyde in PBS and then embedded in paraffin. Deparaffinized sections (4 μm) were placed on slides and processed for immunohistochemistry. After they were blocked with hydrogen peroxide for 30 minutes, the samples were incubated with the primary anti-lumican antibody (0.1 μg/mL), washed in PBS, and incubated with a biotinylated secondary antibody (goat anti-rabbit IgG). After the sections were washed in PBS, they were incubated with streptavidin-horseradish peroxidase (HRP; Dako Corp., Carpinteria, CA), washed in PBS, and incubated with the 3′3-diaminobenzidine (DAB) chromogen for 5 to 10 minutes. Sections were counterstained with hematoxylin, dehydrated in ethanol, and mounted. Negative controls were obtained by omitting the primary antibody.
For detection of BrdU-positive cells, sections were treated with 4 N HCl for 30 minutes and 0.5 mg/mL trypsin for 5 minutes at 37°C. After the reaction was blocked with hydrogen peroxide and PBS Tween containing 5% goat serum, the anti-BrdU antibody (1:50 dilution; Neo-Markers, Fremont, CA) was applied and incubated for 1 hour at room temperature. After the sections were washed with PBS, they were incubated for 30 minutes with a biotinylated secondary antibody (Dako Corp.). The sections were then incubated for 30 minutes with enzyme reagent (streptavidin-HRP), washed in PBS, and incubated with the DAB chromogen for 5 to 10 minutes. The sections were then counter-stained with hematoxylin and mounted. Negative control samples were obtained by omitting the primary antibody.
Western Blot Analysis
Chorion-free human AM (25 cm2) was first homogenized with 2 mL lysis buffer with 250 mM NaCl (lysis-250; also containing 50 mM Tris-HCl [pH 7.4], 5 mM EDTA, 0.1% NP-40, 25 mM NaF, and 1× protease inhibitor cocktail; Sigma-Aldrich, St. Louis, MO). The lysis-250-soluble fraction was separated by centrifugation at 13,000 rpm for 5 minutes at 4°C and extracted with 2 mL lysis buffer with 1000 mM NaCl (lysis-1000; containing the same remaining ingredients as lysis-250; Sigma-Aldrich). The lysis-1000-soluble fraction was separated by centrifugation at 13,000 rpm for 5 minutes at 4°C and extracted with 2 mL 4 M guanidine-HCl containing 10 mM sodium acetate, 10 mM sodium EDTA, 5 mM aminobenzamidine, and 0.1 M ε-amino-n-caproic acid. The guanidine extract was dialyzed exhaustively overnight against distilled water, and the insoluble pellet was dissolved in 6 M urea containing 0.1 M Tris-acetate solution (pH 6.0), 0.1% CHAPS (3-[3-cholamidopropyl]dimethylammonio-2-hydroxy-1-propanesulfonate), and 0.15 M NaCl. The protein concentrations from the lysis-250-soluble fraction, lysis-1000-soluble fraction, and 4 M guanidine HCl fraction were measured by spectrophotometry at OD280nm. To remove both keratan sulfate glycosaminoglycans and N-linked oligosaccharides, protein aliquots were incubated with 0.1 U/mL endo-β-galactosidase in PBS at 37°C overnight. Each sample was added with an equal volume of 2× SDS sample buffer, boiled for 10 minutes, and subjected to 10% SDS-PAGE and Western blot analysis with anti-lumican antibody (0.1 μg/mL). The immunocomplex was visualized by adding alkaline phosphatase-conjugated secondary goat anti-rabbit IgG (1:2500, Novagen, Madison, WI) and a stabilized substrate (Western Blue; Promega, Madison, WI).
Purification of Lumican from Human AM
A kit (IgG Plus Orientation kit; Pierce) was used to prepare an affinity column for purifying native lumican from human AM. In brief, the anti-lumican antibody was bound to immobilized recombinant protein A through the Fc region. Then, the homobifunctional NHS-ester cross-linker, disuccinimidyl suberate (DSS), was used to form the covalent bound between the antibody and protein A. A piece of human AM (5.75 g wet weight, ~100 cm2) was subjected to homogenization with 50 mL lysis-250. The soluble fraction was then dialyzed against PBS at 4°C for 16 hours, yielding 128.62 mg AM extract. Approximately 10 mg of the AM extract was loaded on the anti-lumican antibody column and incubated overnight at 4°C. Unbound AM proteins were washed from the affinity column by PBS until the eluant reached a baseline absorbance at OD280nm. The bound lumican was dissociated from the column by an elution buffer (0.1 M glycine HCl, pH 3.0) and neutralized with 1 M Tris-HCl (pH 9.5). After the absorbance was assayed at OD280nm, fractions, each of 100 μL volume, were pooled and concentrated. The affinity column can be regenerated and reused according to the manufacturer’s instructions (Pierce). In the affinity-column chromatography, 10 mg of lysis-250 AM-soluble extract that passed through the column constantly yielded 100 μg lumican (1.08% ± 0.03%, n = 11). Therefore, approximately 0.2 mg soluble lumican per human AM wet weight (g) was obtained.
Two-Dimensional Gel Electrophoresis
To evaluate the purity, samples were dialyzed against distilled water and dissolved in the rehydration buffer with 0.5% immobilized pH gradient (IPG) buffer, according to the manufacturer’s instructions (Amersham Pharmacia Biotech, Piscataway, NJ). First-dimensional electrophoresis was performed with precast immobile strips (pH 3–10, 7 cm; DryStrips; Amersham Pharmacia Biotech). Before use, the strips were rehydrated for 12 hours in a solution containing 0.5% IPG buffer (8 M urea, 2% CHAPS, 0.5% IPG buffer, bromophenol blue, and dithiothreitol). Running conditions were 50 μA per strip, with the voltage program set at 200 V for 1 hour, 500 V for 1 hour, and 1600 V for 8 hours (referred to as the step-’n-hold process). The strip gel was then equilibrated in SDS equilibration buffer (50 mM Tris-HCl [pH 8.8], 6 M urea 30% vol/vol glycerol, 2% SDS, bromophenol blue, and dithiothreitol) for 15 minutes. The second-dimension polyacrylamide electrophoresis was performed by inserting the strip gel into the precast gel cassette (Ready Gels containing 10% Tris-HCl, 2D/Prep; Bio-Rad, Hercules, CA). The resultant two-dimensional gel was subjected to silver staining, according to the instructions of the stain’s manufacturer (GelCode SilverSNAP Stain kit II; Pierce). The reaction was stopped by 5% acetic acid.
Mouse Model of Corneal Wound Healing
Animals were handled according to the guidelines in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol was approved by the Animal Care and Use Committee of Bascom Palmer Eye Institute, University of Miami School of Medicine. C57BL/6 mice, age 6 to 8 weeks, obtained from Jackson Laboratories (Bar Harbor, ME), were anesthetized by intraperitoneal injections of ketamine hydrochloride (2 mg/g body weight) and xylazine (0.4 mg/g body weight). After topical application of 1 drop of proparacaine (Alcaine; Alcon Laboratories, Inc., Fort Worth, TX) to each eye, the central cornea was marked by a trephine 2 mm in diameter, and the epithelium was debrided by a corneal rust ring remover with a 0.5-mm burr (Algerbrush IITM; Alger Equipment Co., Inc., Lago Vista, TX) under a stereomicroscope (SV11; Carl Zeiss Meditec, Dublin, CA). The animal was killed, and the eyeballs were enucleated and cultured in Dulbecco’s modified Eagle’s medium (Invitrogen-Gibco, Grand Island, NY) containing 1% fetal bovine serum (FBS) and 50 μg/mL gentamicin, with or without 10 μg/mL purified lumican, in a humidified atmosphere of 5% CO2 at 37°C. To label the proliferating cells, 40 μg/mL of BrdU (Sigma-Aldrich) was added to the cultured eyeballs 1 hour before 6, 12, 18, and 24 hours of cultivation, respectively. After incubation, the extent of corneal wound closure was examined by fluorescein staining and photographed with a digital camera (Axiovision4; Carl Zeiss Meditec). The circumference of the wound margin of each mouse eye, as projected onto the photograph, was traced on a digitizer, and the remaining defect area was determined by image-analysis software (Image Beta 4.02; Scion Corp., Frederick, MD). All measurements were counted in a masked fashion, and the size of epithelial defect was expressed as a percentage of the total corneal area. The eyeballs were then fixed in 4% paraformaldehyde in PBS and embedded in paraffin.
Statistical Analysis
The difference within each group in the remaining epithelial defect after debridement and the difference between the two groups in the amount of BrdU-positive cells in each zone at the same time course were calculated by using Student’s t-test. P < 0.05 was considered statistically significant.
Results
Lumican in Human AM
Human AM contains decorin and biglycan, members of the SLRP family.33 To examine whether human AM also contains lumican, human placental tissues containing AM, chorion, and an underlying decidual layer were prepared for immunohistochemistry by using the newly generated anti-lumican peptide antibody for localization. As shown in Figure 1, immunoreactivity to lumican was present throughout the entire fetal membrane containing AM, chorion, and placenta (Fig. 1A). In the decidual layer of the placenta, lumican immunoreactivity was particularly found in areas surrounding the blood vessels (Figs. 1A, 1B). In the AM, lumican protein was detected in a compact layer (CL; Fig. 1C). The negative control using preimmune rabbit IgG is shown in Figure 1D. Because the oligopeptide used to raise anti-lumican antibody is identical in sequence between the human and the mouse lumican, Figure 2A shows that, indeed, the anti-lumican antibody did not detect lumican protein in the lum−/− mice (Fig. 2A; lanes 1 and 2) but recognized keratan sulfated lumican as a smear pattern (lane 3) and a ~50-kDa lumican core protein after treatment with β-endogalactosidase (lane 4) in the wild-type mouse cornea. Western blot analysis demonstrated that human lumican appeared as glycoprotein in the AM extracted by 4 M guanidine HCl, but as a keratan sulfate-containing proteoglycans in the human corneal stroma similarly extracted (Fig. 2B). To determine whether lumican glycoprotein is also present in a soluble form, a piece of human AM (1.25 g, 25 cm2) was subjected to sequential homogenization by the same amount of lysis-250, lysis-1000, and 4 M guanidine HCl. Human AM indeed contained both soluble and insoluble forms of lumican, with the latter being much more abundant than the former (Fig. 2C).
Figure 1.
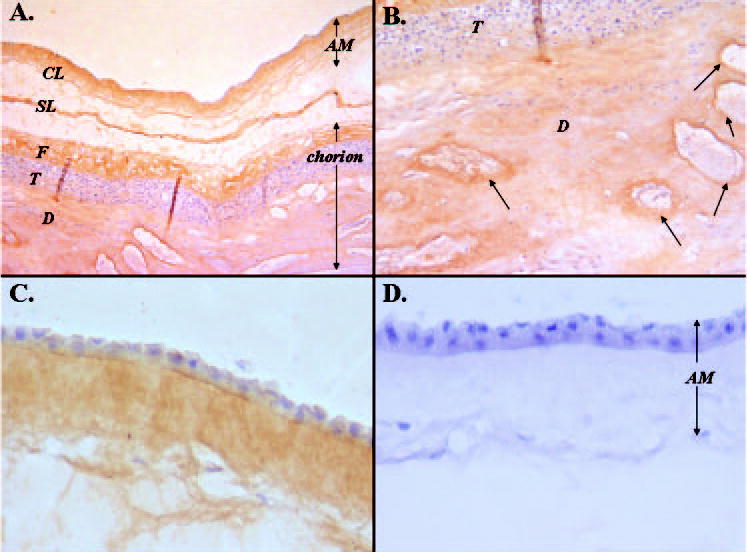
Localization of lumican expression in human placenta. (A) A 5-μm section of placental tissue including amniotic membrane (AM), chorion consisting of fibroblastic (F) and trophoblastic (T) parts, and decidual cell layers (D) immunostained with anti-lumican antibody. Note that the AM and chorion should be fused. The space between them that appears in this image may have been generated in the AM spongy layer (SL) during tissue processing. Lumican protein was detected in the compact layer (CL) of the AM, chorion, and placenta. (B) Higher magnification of (A). (C) A 5-μm tissue section of the AM only, peeled from the placenta, and immunostained with anti-lumican antibody. (D) No immunoreactivity was detected in the negative control sample. Arrows: blood vessels that were also lumican positive.
Figure 2.
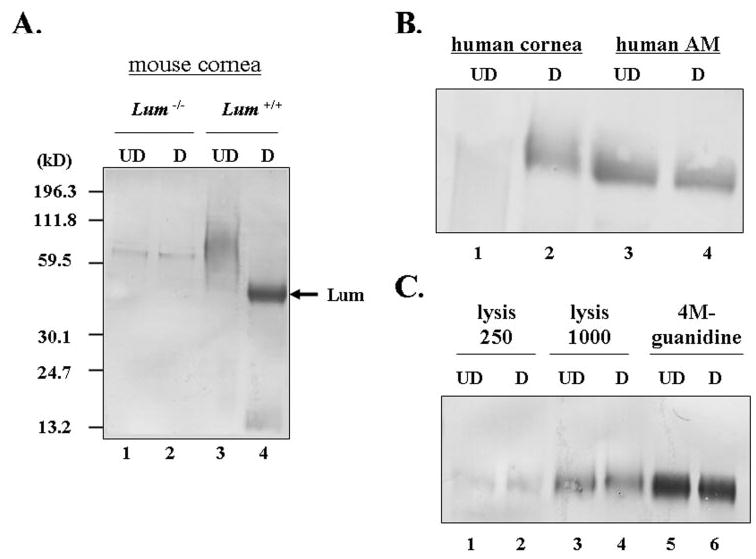
Western blot analysis of the anti-lumican antibody. (A) Corneas from lum−/− (lanes 1 and 2) and wild-type (lanes 3 and 4) mice were extracted with 4 M guanidine-HCl, and the extracts were treated with or without endo-β-galactosidase and subjected to 10% SDS-PAGE followed by Western blot analysis. The blots were probed with anti-lumican antiserum (1:500). (B) With affinity-purified anti-lumican antibody, human corneal stromal lumican showed a smearing pattern (lane 1) and a ~50-kDa band when treated with endo-β-galactosidase (lane 2). However, the lumican from human AM showed an approximately 50-kDa single band, with (lane 4) or without (lane 3) digestion. (C) Human AM extracts from lysis-250 (lanes 1 and 2), lysis-1000 (lanes 3 and 4), and 4 M guanidine-HCl (lanes 5 and 6). UD, undigested sample; D, endo-β-galactosidase-digested sample.
Affinity Purification of Human AM Lumican
Human lumican was eluted as a single peak, measured by spectrophotometry at OD280nm (Fig. 3A). The purified lumican appeared as a single band in an SDS-polyacrylamide gel followed by silver staining (Fig. 3B, lane 4). Two-dimensional SDS-PAGE revealed that purified AM lumican was moderately glycosylated, as evidenced by a band smearing between pH 3.0 and 6.0 (Fig. 3C) and by its conversion to a single spot at pH 6.0 (molecular mass of 50 kDA), after preincubation with endo-β-galactosidase to remove the sugar moiety (Fig. 3D).
Figure 3.
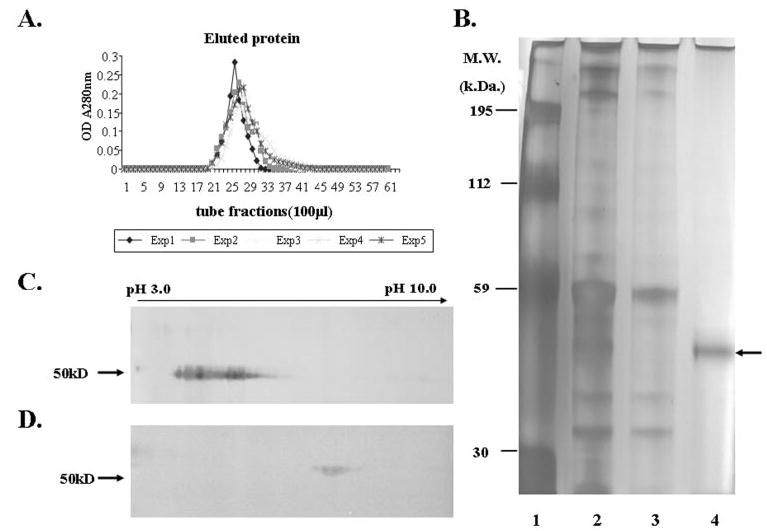
Purification of human AM lumican by affinity-column chromatography. (A) Elution profile of five independent AM extracts showed that one major peak was eluted from an anti-lumican antibody column. (B) Silver-stained gel of AM lysis-250 extract (lane 2); flow-through, unbound AM extract (lane 3); and eluted lumican protein (lane 4, arrow). Lane 1: molecular weight standards. (C) Two-dimensional gel electrophoresis of lumican (10 μg) showed the smearing (pH 3.0–6.0) pattern before endo-β-galactosidase digestion. (D) Two-dimensional gel electrophoresis of lumican (3 μg) showed a single ~50-kDa spot at ~pH 6.0 after endo-β-galactosidase digestion.
Effect of Exogenous Human AM Lumican on Corneal Epithelial Wound Healing in Wild-Type and lum−/− Eyes
Lumican has been shown to be ectopically and transiently expressed by the corneal epithelium after a 2-mm-diameter central corneal epithelial debridement in adult mice.12 Exogenous anti-mouse lumican antibody retards corneal epithelial wound healing in cultured mouse eyes. Mice without lumican have shown a delayed corneal epithelial healing rate in an organ culture model.12 These results strongly suggest that lumican may be directly involved in wound healing. To test this hypothesis, we administered exogenous purified human AM lumican in cultured wild-type mouse eyes to see whether it might facilitate corneal re-epithelialization. One milligram per milliliter and 10 μg/mL purified AM lumican were used. The dosage of AM lumican of 10 μg/mL showed a better effect than 1 μg/mL (data not shown). Exogenous human AM lumican (10 μg/mL) accelerated healing of the epithelium when compared with the control eyes receiving the vehicle (Fig. 4). Twelve and 24 hours after corneal epithelial lesions, the lumican-treated eyes showed a reduction of the wounded area: 30% ± 6% and 9% ± 8% of the originally debrided area, respectively, whereas the control eyes still had a larger wounded area (45% ± 17% and 20% ± 11%, respectively). Forty-eight hours after debridement, both lumican-treated and control eyes were completely healed. Furthermore, the effect of exogenous lumican was even more dramatic in the lum−/− eye (Fig. 5). Twenty-four hours after the corneal epithelial lesion, lumican-treated eyes showed a 3.4-fold reduction in the wound area compared with the control eyes (17% ± 11% vs. 61% ± 19%). The statistical difference was confirmed by Student’s t-test (P < 0.05).
Figure 4.
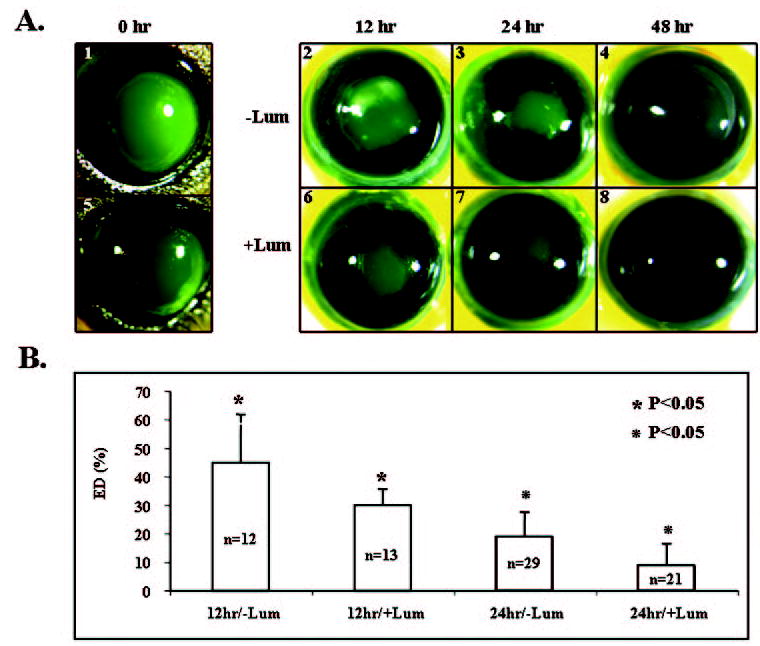
Purified lumican from human AM promoted corneal epithelial wound healing in wild-type eyes. (A) Representative photographs of fluorescein-stained eyes (green). An epithelial defect (2 mm in diameter) was created at the center of the corneas (A1, A5). Mouse eyes were enucleated and cultured in DMEM with 1% FBS (A2–A4) or in DMEM with 1% FBS plus purified AM lumican (10 μg/mL; A6–A8), to allow re-epithelialization for the different periods indicated. (B) Percentage of the remaining epithelial defect (ED). Note that the lumican-treated (+Lum) eyes healed better than the non–lumican-treated ones (−Lum) at 12 and 24 hours after debridement.
Figure 5.
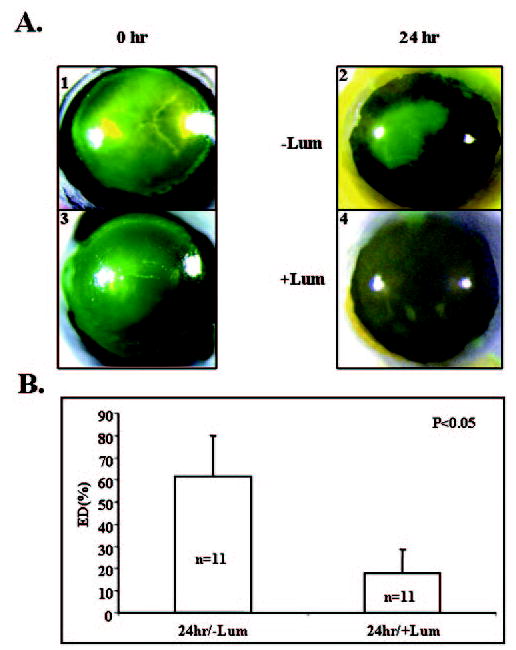
Purified lumican from human AM promoted corneal epithelial wound healing in lum−/− eyes. (A) Representative photographs of fluorescein-stained eyes (green). An epithelial defect (2 mm in diameter) was created at the center of the corneas (A1, A3). Mouse eyes were enucleated and cultured in DMEM with 1% FBS (A2) or in DMEM with 1% FBS plus purified AM lumican (10 μg/mL; A4) to allow re-epithelialization for 24 hours. (B) Percentage of the remaining epithelial defect (ED). Note that the lumican-treated (+Lum) eyes healed three times better than the non–lumican-treated ones (−Lum).
The Effect of Exogenous Lumican on Cell Proliferation during Corneal Epithelial Wound Healing
Corneal epithelial wound healing includes cell proliferation and migration. To monitor the cell proliferation index, proliferating cells were labeled with the thymidine analogue BrdU at different times during wound healing. Figure 6 shows the temporal spatial patterns of cell proliferation during wound healing. Six hours after epithelial debridement, very few BrdU-labeled epithelial cells were detected in either the control (Figs. 6A1– 6A3) or the lumican-treated group (Figs. 6B1– 6B3). At 12 hours after debridement, the number of BrdU-labeled cells was significantly more at central and peripheral zones in the lumican-treated group (3.5 ± 2.9 and 5.5 ± 2.1 cells, respectively, n = 6) compared with the control group (0.4 ± 0.9 and 0.8 ± 1.1 cells, respectively, n = 5; P < 0.05; Figs. 6A4 – 6A6, 6B4 – 6B6, 6C). There was no difference between both groups in the midperipheral zone (Figs. 6A5, 6B5, 6C). We found many more BrdU-labeled cells in the central area of the lumican-treated group at 18 hours after debridement than in the control group, which had very few BrdU-labeled cells (18.9 ± 5.6 vs. 0.3 ± 0.8 cells, n = 7 P < 0.05; Figs. 6A9, 6B9, 6C). Twenty-four hours after debridement, many BrdU-labeled cells were still present in the central (16.4 ± 2.0 cells, n = 5) and midperipheral zones (10.2 ± 5.4 cells, n = 5) of the lumican-treated group (Figs. 6B11– 6B12, 6C). However, fewer BrdU-labeled cells were found in these two zones of the control group: 2.3 ± 2.0 cells in the central (n = 7) and 2 ± 1.6 cells in the midperipheral zone (n = 7; Figs. 6A11– 6A12, 6C). It was obvious that lumican-treated eyes contained more BrdU-labeled cells than did the control eyes at 12, 18, and 24 hours after corneal debridement. A similar stimulation of cell proliferation by lumican was also seen when lumican was added to the lum−/− eyes (Fig. 7A). Twenty-four hours after debridement, many more BrdU-labeled cells were found in all three zones of the lumican-treated lum−/− eyes than in those zones in the control eyes: 12.6 ± 2.8, central; 14.6 ± 2.6, midperipheral; and 15.8 ± 1.92, peripheral versus 2.8 ± 0.84, central; 5.2 ± 0.8, midperipheral; and 8.2 ± 3.0, peripheral (P < 0.05; n = 21; Fig. 7B).
Figure 6.
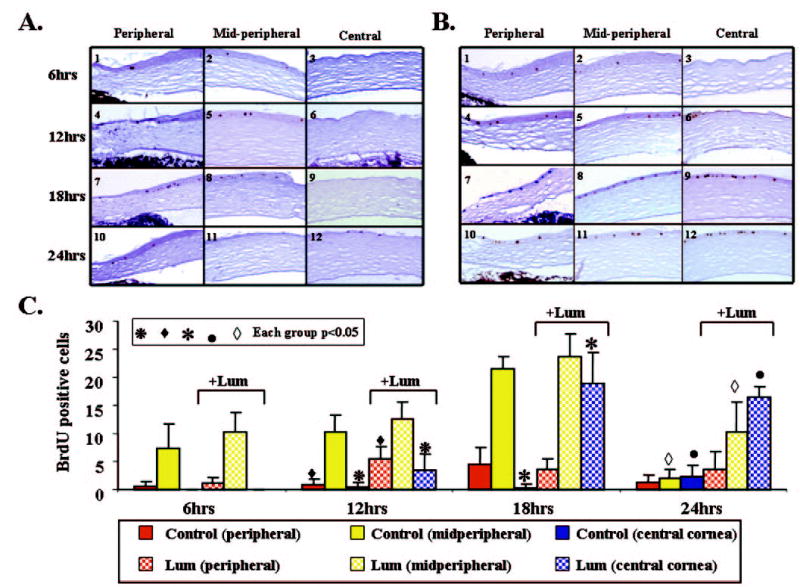
Distribution of BrdU-positive cells in wild-type eyes during corneal debridement in DMEM with 1% fetal bovine serum (A) or in DMEM with 1% fetal bovine serum plus 10 μg/mL lumican (B). Representative slides show that the immunoreactivity of BrdU-incorporated cells was detected by anti-BrdU antibody (dark brown). (C) Histogram presenting the accumulated BrdU-positive cells distributed in the peripheral, midperipheral, and central corneal regions of mouse corneas at 6, 12, 18, and 24 hours after injury. Statistical analysis shows that the lumican-treated corneas (+Lum) had significantly more BrdU-positive cells than did non–lumican-treated ones (−Lum) in most regions at different times, as indicated.
Figure 7.
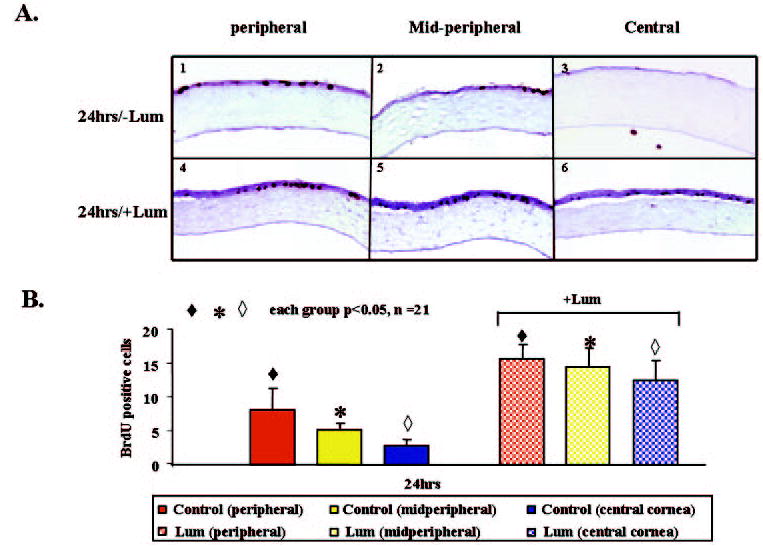
Distribution of BrdU-positive cells in lum−/− eyes at 24 hours after corneal debridement in DMEM with 1% fetal bovine serum (A1–A3) or in DMEM with 1% fetal bovine serum plus 10 μg/mL lumican (A4–A6). (B) Summary of the sum of BrdU-positive cells in each region. There were many more BrdU-positive cells in the lumican-treated samples (+Lum) than in the non–lumican-treated ones (−Lum).
Discussion
This study demonstrated in a mouse cornea model that soluble lumican glycoprotein from human AM can promote epithelial proliferation and migration, resulting in faster wound healing. Previously, human AM stroma was found to contain major extracellular matrix (ECM) components, such as collagen-I, -III, -IV, -V, and -VII; fibronectin; laminin-5; biglycan; decorin; and hyaluronan.33,34 Grover et al.9 showed that lumican mRNA is highly expressed in the adult placenta, but whether it is distributed in the AM is still not known. We found that the human and mouse placentas had abundant lumican (data not shown). The avascular amniotic membrane was attached loosely to vascularized chorion tissue and could be peeled easily from the placental tissue. In the present study, we showed for the first time that human AM also contains abundant lumican. This was demonstrated by immunohistochemistry and Western blot analysis with an affinity-purified rabbit polyclonal antibody against an oligopeptide (SLPPDMYECLRVANE) corresponding to the C-terminal domain of human lumican. This 15-aminoacid sequence is identical for both mouse and human lumican. As expected, the anti-lumican antibody can recognize both human and mouse lumican. The absence of lumican-positive reaction in the lum−/− cornea suggests that this antibody can specifically recognize lumican but not keratocan or other SLRPs (Fig. 2A). We further demonstrated that the lumican extracted by 4 M guanidine HCl from the human corneal stroma was indeed a keratan sulfate– containing proteoglycan, whereas that extracted from human AM was a glycoprotein (Fig. 2B), a finding similar to that reported in nonocular tissues.1–8 The human AM contained a soluble form of lumican in addition to the aforementioned insoluble form (Fig. 2C) and was a rich source for purifying soluble lumican. Because 4 M guanidine HCl may denature AM lumican and extract other types of proteoglycans and high salt (lysis-1000) may change biological and pharmacological effect, we purified soluble lumican from the human AM through lysis-250 extraction, using affinity-column chromatography with the aforementioned antibody. Using two-dimensional electrophoresis with and without digestion of endo-β-galactosidase, we further confirmed that the soluble lumican purified from human AM was indeed a glycoprotein containing sugar moieties resulting in a wide range of isoelectric points from pH 3.0 to 6.0. Moreover, the phenomenon of converting to a higher pH form after endo-β-galactosidase implies that the glycosylation contains a negative charge such as a sialic acid terminal sugar, which may be physiologically important for binding to growth factors that may enhance the biological activity of the soluble lumican purified from human AM. Western blot analysis using AP-conjugated anti-human or anti-rabbit IgG was performed to rule out the possibility of contamination of human IgG from AM preparation or rabbit IgG from the rabbit anti-serum (data not shown), indicating that the human AM lumican preparation was nearly homogeneous.
The role of lumican in collagen fibrillogenesis has been implicated in lum−/− mice8 and in vitro.35 Nevertheless, the possibility that lumican may function as a soluble ligand to modulate cell behavior has been explored recently. Funderburgh et al.36 also purified lumican from bovine cornea and aorta and showed that the soluble lumican facilitates macrophage adhesion to the substrate. In addition, Vuillermoz et al.37 demonstrated that recombinant human lumican protein purified from Escherichia coli exerts an inhibitory effect on melanoma cell migration in vitro. In this study, we used purified soluble lumican to demonstrate its action in promoting corneal epithelial wound healing in an organ culture model, using both the wild-type and lum−/− mice. We also detected more BrdU-positive cells in the lumican-treated group than in the control group at any time point examined within 24 hours after epithelial debridement. These results showed that the effect of exogenously added soluble lumican glycoprotein in facilitating re-epithelialization during wound healing was mediated in part by the stimulation of cell proliferation. These findings also corroborated with a previous report12 showing that injured mouse corneal epithelium ectopically and transiently expresses lumican during the early phase of wound healing and that anti-lumican antibody retards corneal epithelial wound healing in cultured mouse eyes. Collectively, these findings strongly support the notion that soluble lumican glycoprotein plays an important role in modulating epithelial cell proliferation and migration during the wound healing. However, we do not know whether the pharmacologic dose-dependent effect of the purified lumican was dependent on the rate of wound healing or of BrdU incorporation. These questions should be further investigated.
The possibility that lumican may function as a soluble ligand to elicit cell signaling by its direct binding to the cell has not been demonstrated. The exact mechanism whereby soluble lumican glycoprotein exerts the aforementioned actions remains to be determined. It is possible that exogenously added soluble lumican works indirectly by presenting growth factors or cytokines to their receptors in a manner similar to the binding of transforming growth factor-β to decorin, biglycan, and fibromodulin.38,39 The other possibility may be that extra-cellular lumican works directly on an as yet unknown surface receptor to trigger the signal transduction that is essential in cell proliferation and migration.
Collectively, these findings support the idea that soluble lumican glycoprotein in human AM is partly responsible for the clinical efficacy of human cryopreserved AM in treating patients with persistent corneal epithelial defects. Furthermore, this study also revealed an as yet unknown role of a non–keratan sulfate lumican glycoprotein as a soluble ligand in epithelial wound healing. Further probing its signaling mechanism may shed light on a novel therapeutic potential of lumican in corneal diseases.
Acknowledgments
The authors thank the reviewers for constructive suggestions.
Footnotes
Supported in part by Grants EY12486 (C-YL), EY11845 (WW-YK), and EY06819 (SCGT); a P30 center grant to University of Miami (Research to Prevent Blindness) from the National Institutes of Health; the Ohio Lions Eye Research Foundation; and a fellowship grant from Chang-Gung Memorial Hospital, Taiwan (L-KY). WW-YK is a Research to Prevent Blindness (RPB) senior investigator, and C-YL is a recipient of the Olga Weiss Scholarship from RPB.
Disclosure: L.-K. Yeh, None; W.-L. Chen, None; W. Li, Tissue-Tech, Inc. (E); E.M. Espana, TissueTech, Inc. (E); J. Ouyang, None; T. Kawakita, TissueTech, Inc. (E); W.W.-Y. Kao, None; S.C.G. Tseng, TissueTech, Inc. (E); C.-Y. Liu, None
References
- 1.Funderburgh JL, Funderburgh ML, Mann MM, Conrad GW. Arterial lumican: properties of a corneal-type keratan sulfate proteoglycan from bovine aorta. J Biol Chem. 1991;266:24773–24777. [PubMed] [Google Scholar]
- 2.Funderburgh JL, Caterson B, Conrad GW. Distribution of proteoglycans antigenically related to corneal keratan sulfate proteoglycan. J Biol Chem. 1987;262:11634–11640. [PubMed] [Google Scholar]
- 3.Iozzo RV, Murdoch AD. Proteoglycan of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- 4.Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 5.Ying S, Shiraishi A, Kao CW, et al. Characterization and expression of the mouse lumican gene. J Biol Chem. 1997;272:30306–30313. doi: 10.1074/jbc.272.48.30306. [DOI] [PubMed] [Google Scholar]
- 6.Raouf A, Ganss B, McMahon C, Vary C, Roughley PJ, Seth A. Lumican is a major proteoglycan component of the bone matrix. Matrix Biol. 2002;21:361–367. doi: 10.1016/s0945-053x(02)00027-6. [DOI] [PubMed] [Google Scholar]
- 7.Iozzo RV, Danielson KG. Transcriptional and posttranscriptional regulation of proteoglycan gene expression. Prog Nucleic Acids Res Mol Biol. 1999;62:19–53. doi: 10.1016/s0079-6603(08)60504-8. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Biol Chem. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover J, Chen XN, Korenberg JR, Roughley PJ. The human lumican gene: organization, chromosomal location, and expression in articular cartilage. J Biol Chem. 1995;270:21942–21949. doi: 10.1074/jbc.270.37.21942. [DOI] [PubMed] [Google Scholar]
- 10.Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW. Molecular cloning and tissue distribution of keratan. Bovine corneal keratan sulfate proteoglycan 37A. J Bio Chem. 1996;271:9759–9763. doi: 10.1074/jbc.271.16.9759. [DOI] [PubMed] [Google Scholar]
- 11.Funderburgh JL, Funderburgh ML, Mann MM, Conrad GW. Physical and biological properties of keratan sulphate proteoglycan. Biochem Soc Trans. 1991;19:871–876. doi: 10.1042/bst0190871. [DOI] [PubMed] [Google Scholar]
- 12.Saika S, Shiraishi A, Saika S, Liu CY, Funderburgh JL, Kao WW. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275:2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saika S, Miyamoto T, Tanaka SI, et al. Response of lens epithelial cells to injury: role of lumican in epithelial-mesenchymal transition. Invest Ophthalmol Vis Sci. 2003;44:2094–2102. doi: 10.1167/iovs.02-1059. [DOI] [PubMed] [Google Scholar]
- 14.Kim JC, Tseng SCG. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–484. [PubMed] [Google Scholar]
- 15.Tseng SCG, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf. 2004;2:10–20. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 16.Na BK, Hwang JH, Kim JC, et al. Analysis of human amniotic membrane components as proteinase inhibitors for development of therapeutic agent of recalcitrant keratitis. Trophoblast Res. 1999;13:459–466. [Google Scholar]
- 17.Soloman A, Rosenblatt M, Monroy DC, Ji Z, Pflugfelder SC, Tseng SCG. Suppression of interleukin-1α and interleukin-1β in the human corneal epithelial cells cultured on the amniotic membrane matrix. Br J Ophthalmol. 2001;85:444–449. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000;70:329–337. doi: 10.1006/exer.1999.0794. [DOI] [PubMed] [Google Scholar]
- 19.Wang MX, Gray TB, Parks WC, et al. Corneal haze and apoptosis is reduced by amniotic membrane matrix in excimer laser photoablation in rabbits. J Cat Refract Surg. 2001;27:310–319. doi: 10.1016/s0886-3350(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 20.Park WC, Tseng SCG. Modulation of acute inflammation and keratocyte death by suturing, blood and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000;41:2906–2914. [PubMed] [Google Scholar]
- 21.Heiligenhaus A, Meller D, Steuhl K-P, Tseng SCG. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 2001;42:1969–1974. [PubMed] [Google Scholar]
- 22.Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179:325–335. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–334. [PubMed] [Google Scholar]
- 24.Hao Y, Ma DH-K, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and anti-inflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18:73–79. [PubMed] [Google Scholar]
- 26.Sonnenberg A, Calafat J, Janssen H, et al. Intergrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khodadoust AA, Silverstein AM, Kenyon DR, Dowling JE. Adhesion of regenerating corneal epithelium. The role of basement membrane. Am J Ophthalmol. 1968;65:339–348. doi: 10.1016/0002-9394(68)93082-1. [DOI] [PubMed] [Google Scholar]
- 28.Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–886. [PubMed] [Google Scholar]
- 29.Espana EM, He H, Kawakita T, et al. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci. 2003;44:5136–5141. doi: 10.1167/iovs.03-0484. [DOI] [PubMed] [Google Scholar]
- 30.Meller D, Pires RT, Tseng SC. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–471. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002;120:783–790. doi: 10.1001/archopht.120.6.783. [DOI] [PubMed] [Google Scholar]
- 32.Grueterich M, Espana EM, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci. 2002;43:63–71. [PubMed] [Google Scholar]
- 33.Meinert M, Eriksen GV, Petersen AC, et al. Proteoglycans and hyaluronan in human fetal membranes. Am J Obstet Gynecol. 2001;184:679–685. doi: 10.1067/mob.2001.110294. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Yoshitani M, Rigby H, et al. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45:93–99. doi: 10.1167/iovs.03-0752. [DOI] [PubMed] [Google Scholar]
- 35.Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core protein. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- 36.Funderburgh JL, Mitschler RR, Funderburgh ML, Roth MR, Chapes SK, Conrad GW. Macrophage receptors for lumican. Invest Ophthalmol Vis Sci. 1997;38:1159–1167. [PubMed] [Google Scholar]
- 37.Vuillermoz B, Khoruzhenko A, D’Onofrio M-F, et al. The small leucine-rich proteoglycan lumican inhibits melanoma progression. Exp Cell Res. 2004;296:294–306. doi: 10.1016/j.yexcr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor B by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 39.Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]


