Abstract
In a model drinking water distribution system characterized by a low assimilable organic carbon content (<10 μg/liter) and no disinfection, the bacterial community was identified by a phylogenetic analysis of rRNA genes amplified from directly extracted DNA and colonies formed on R2A plates. Biofilms of defined periods of age (14 days to 3 years) and bulk water samples were investigated. Culturable bacteria were associated with Proteobacteria and Bacteriodetes, whereas independently of cultivation, bacteria from 12 phyla were detected in this system. These included Acidobacteria, Nitrospirae, Planctomycetes, and Verrucomicrobia, some of which have never been identified in drinking water previously. A cluster analysis of the population profiles from the individual samples divided biofilms and bulk water samples into separate clusters (P = 0.027). Bacteria associated with Nitrospira moscoviensis were found in all samples and encompassed 39% of the sequenced clones in the bulk water and 25% of the biofilm community. The close association with Nitrospira suggested that a large part of the population had an autotrophic metabolism using nitrite as an electron donor. To test this hypothesis, nitrite was added to biofilm and bulk water samples, and the utilization was monitored during 15 days. A first-order decrease in nitrite concentration was observed for all samples with a rate corresponding to 0.5 × 105 to 2 × 105 nitrifying cells/ml in the bulk water and 3 × 105 cells/cm2 on the pipe surface. The finding of an abundant nitrite-oxidizing microbial population suggests that nitrite is an important substrate in this system, potentially as a result of the low assimilable organic carbon concentration. This finding implies that microbial communities in water distribution systems may control against elevated nitrite concentrations but also contain large indigenous populations that are capable of assisting the depletion of disinfection agents like chloramines.
Drinking water is an important resource all around the globe. Nevertheless, little research has focused on identifying the bacteria in water distribution systems or how treatment techniques alter the composition. In contrast, the bacteria present in wastewater treatment plants are very well characterized by a suite of advanced molecular techniques (40). The monitoring and identification of bacteria present in drinking water have primarily been achieved using a plating and isolation strategy. The majority of these culturable bacteria described in drinking water networks are included in the phylum Proteobacteria and to a lesser extent in the phyla Actinobacteria, Firmicutes, and Bacteriodetes (18, 19, 29, 30, 32). Some of the commonly detected genera include Pseudomonas, Caulobacter, Aeromonas, Acinetobacter, and Bacillus. As a result, a common perception is that Pseudomonas is the most abundant bacterial organism in supply systems, regardless of the water source.
It is well known that the plate count technique significantly underestimates the total number of cells present in a given environment (1, 38), including drinking water distribution systems (3, 15), and often “overlooks” abundant lineages that could be hygienically relevant in potable water. Some studies have addressed this issue in drinking water. If chloramines are used for disinfection, ammonia (e.g., Nitrosomonas spp.) and nitrite oxidizers (Nitrospira spp.) constitute a large population in water supply systems (33). Using a system without chloramine addition, Kalmbach and coworkers were able to culture Aquabacterium on R2A medium under standard conditions and subsequently show the dominance of this species in a 14-day-old biofilm in a Berlin water distribution network using fluorescent in situ hybridization (FISH) (14, 16). This study suggested that R2A medium supported growth of the majority of the bacteria in the drinking water system. The discrepancy between total and colony counts was shown to be an effect of a physiological response of the bacteria in the system. Based on the Berlin study and other investigations of water distribution systems using molecular tools (35, 37), the consensus is that bacteria from the alpha-, beta-, and gamma-Proteobacteria constitute the majority of the cells in water distribution networks (15), although no studies have yet looked at older biofilms (e.g., >1 year).
We have recently shown that it takes more than 2 years before a mature biofilm is formed in our system (25). Based on this result, we wanted to identify abundant members of the microbial community from bulk water and biofilm samples independently of cultivation, including biofilms more than 3 years of age. The samples came from a model system directly connected into the distribution net and supplied with nonchlorinated groundwater with low assimilable organic carbon (AOC) content (4 to 6 μg AOC/liter [3]). We cloned and sequenced 16S rRNA fragments and compared the community profile with that of colonies forming bacteria on R2A medium. Bacteria associated with Nitrospira dominated the sequenced clones and suggested that a large part of the population had an autotrophic metabolism using nitrite as an electron donor. To test this hypothesis, nitrite was added to biofilm and bulk water samples, and the utilization was monitored over 15 days. In other distribution systems, nitrite oxidizers have primarily been associated with the depletion of chloramines, but this study shows that Nitrospira can be highly abundant in the absence of chlorinated nitrogen compounds.
MATERIALS AND METHODS
Description of system.
Bacteria living in the biofilm and bulk water were sampled from a model water distribution system operated under turbulent-flow conditions (described in detail by Boe-Hansen et al. [4] and Martiny et al. [25]). In brief, the model system was built as a completely mixed 12.2-m-long loop-shaped reactor aligned with the surface, and 72 stainless steel plugs (grade 316; area = 7.1 cm2) were inserted for biofilm samples. The model system was supplied with drinking water from a waterworks 1 km upstream. The drinking water was produced from aerated and filtered anaerobic groundwater containing no residual disinfectant.
Cultivation of bacteria on R2A medium.
A biofilm (319 days old) and an inlet bulk water sample were plated on standard R2A medium, incubated for 10 days at room temperature (approximately 23°C), and finally transferred twice to obtain clonal cultures. One hundred colonies were isolated from each sample, but 29 strains could not be recultured. To avoid the redundant sequencing of isolates, restriction fragment length polymorphism (RFLP) on PCR-amplified 16S rRNA genes was applied to screen for unique isolates using RsaI and MspI as restriction enzymes. All genotypes represented by more than one isolate were selected for 16S rRNA sequencing and phylogenetic analysis to avoid redundant sequencing (see below).
Total DNA extraction.
Biofilm and bulk water samples were taken from the system on three dates, in March (outlet, biofilm 818 days old), May (inlet and outlet, biofilms 14, 94, 256, 571, 1,093 days old), and December 2002 (outlet, biofilm 956 days old). The samples were treated as previously described (11, 25). In brief, 2-liter samples of water from inlet and outlet were filtered onto a 0.22-μm Durapore filter (hydrophilic polyvinylidene difluoride; Millipore, Bedford, MA), whereas cells from the plugs were removed from the surface with a wet cotton swab. Biofilm and bulk water samples were then added to 1% sodium dodecyl sulfate in TEN buffer (50 mM Tris [pH 8], 5 mM EDTA, 50 mM NaCl) and boiled for 2 min, and DNA was precipitated with 96% ethanol at −20°C. The DNA was further purified with phenol extraction and a QIAprep spin column (QIAGEN, Hilden, Germany) to remove any inhibitory agents (including ferric oxides) that interfered with PCR amplification. Finally, DNA was quantified by determining the absorbance at 260 nm.
Construction of 16S rRNA gene libraries.
Amplification of DNA was performed by PCR with Bacteria primers 9F (5′-GAG TTT GAT CCT GGC TCA G-3′) and 1512R (5′-ACG GCT ACC TTG TTA CGA CTT-3′) (17). Next, 16.5 ng of genomic DNA was used as a template in five parallel PCRs (50-μl reaction volume) and pooled afterwards. The following thermal cycling program was applied: hot start at 94°C for 5 min, followed by 30 cycles at 94°C for 45 s, 55°C for 45 s, and 72°C for 2 min, and a final extension step at 72°C for 10 min. Amplified DNA was gel purified to select for the correct size of the fragments and cloned using a TOPO TA cloning kit (Invitrogen, Carlsbad, CA). Next, 220 clones (approximately 20 from each sample) were sequenced on an ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA) at the Genomic Technology Support Facility (Michigan State University, East Lansing, MI) using M13F as the sequence primer (and M13R for sequences used in the presented phylogenetic trees).
Phylogeny.
To identify bacteria in the samples, 16S rRNA gene sequences were aligned using the fast aligner tool implemented in the ARB software package (23) and manually corrected for alignment errors. The new sequence was analyzed against the phylogenetic tree containing all sequences in the ARB database (version 6spring2001) using the maximum-parsimony “quick add” tool to get a first estimate of the association. Additionally, closely associated sequences were found using BLAST and imported to the database. For a more detailed phylogenetic analysis of Nitrospirae, Planctomycetes, and Acidobacteria, a base frequency filter excluding all positions (including gaps) different in more than 50% of the sequences was generated from selected sequences from each phylum. This was done to ensure a comparison of homologous positions. The selected sequences were exported to PAUP* v. 4b10 for a detailed phylogenetic analysis using neighbor joining (minimum evolution as the criterion and maximum-likelihood distance correction), maximum parsimony, and maximum likelihood (HKY+G model parameters, gamma shape optimized by iterative-likelihood searches) (39). Bootstrap analysis (100 resamplings) was done with heuristic searches utilizing random addition and tree bisection reconnection branch-swapping methods. The outgroup consisted of Escherichia coli (strain X80724), Flavobacterium johnsonii (strain M59051), Acidobacterium capsulatum (strain D26171), Verrucomicrobium spinosum (strain X90515), Planctomyces maris (strain AJ231190), and Nitrospira moscoviensis (strain X82558). In the case where some of these sequences were included in the analyzed phyla, they were omitted from the outgroup. Finally, the tree was exported through Treeview (31).
OTU analysis.
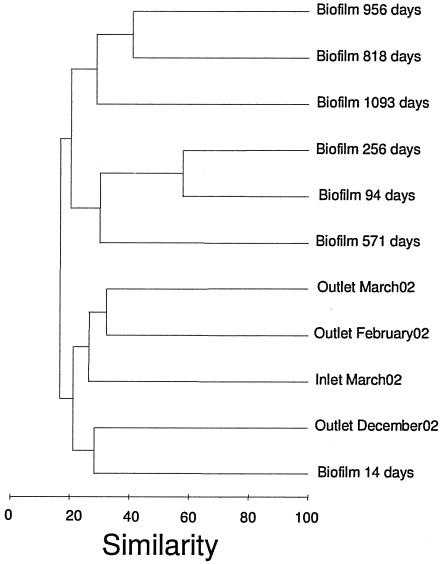
An uncorrected distance matrix was calculated from the alignment of all sequences retrieved from the supply system, and a >97% similarity cutoff was applied to assign operational taxonomic units (OTUs) for the individual sequences. A dendrogram of the biofilm and bulk water samples was generated with primer v.5 (Primer-E Ltd., Plymouth, Great Britain) based on Bray-Curtis indices with square root transformation. The richness estimator Chao1 was calculated using EstimateS (7).
Nitrite conversion.
Nitrite was measured fluorometically using diaminonaphthalene (DAN) according to the method of Misko et al. (26). Three 100-ml water samples were taken from the inlet and two from the outlet, and 10 μM nitrite (final concentration) was added. Additionally, cells from a biofilm sample (1,396 days old) were scraped off and added to outlet water samples including nitrite to see if bacteria from the biofilm utilized nitrite. This procedure was necessary due to difficulties in growing Nitrospira cells in sterile medium (autoclaved inlet water [6] and 0.9% NaCl). Autoclaved bulk water samples acted as abiotic controls. Each sample was split into three portions and incubated at room temperature. Nitrite was measured by adding 100 μl of DAN at 5 mg/liter in 0.62 N HCl to 500-μl volumes from the batch experiments and incubating them in the dark for 10 min at 23°C. Then, 500 μl 0.28 N NaOH was added and incubated for 10 min in the dark. Fluorescence was measured with a fluorometer (model RF-1501; Shimadzu, Tokyo, Japan) set at an excitation wavelength of 363 nm and an emission detection wavelength of 426 nm. Abiotic nitrite oxidation rates were subtracted to calculate the nitrite activity in the samples.
Nucleotide sequence accession numbers.
Sequences from this study were submitted to GenBank under accession numbers DQ058673 to DQ058686.
RESULTS AND DISCUSSION
Which fraction of the diversity is culturable on R2A medium?
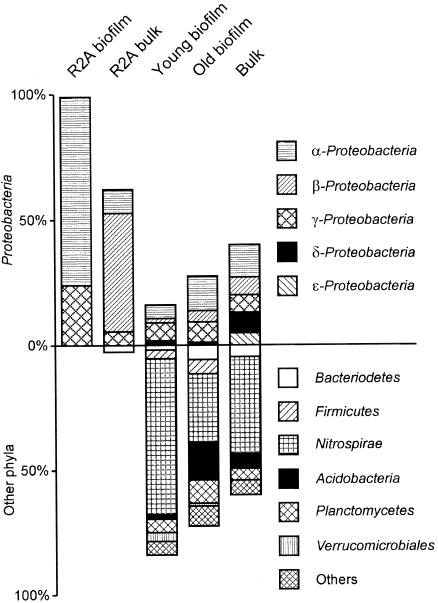
A total of 97 colonies from the biofilm and 74 colonies from the bulk water were isolated on R2A medium (23°C, 10 days). Based on restriction patterns of the 16S rRNA gene from the isolates, three unique genotypes were found in the biofilm, whereas 35 were found in the bulk water. As shown in Table 1, the most dominant isolate in the biofilm was related to the genera Devosia and Rhodobium of the α-Proteobacteria. Also, many colonies were associated with the genus Luteimonas. In the bulk water, Hydrogenophaga, Brevundimonas, and Aquabacterium spp. constituted more than one-third of the isolated colonies, but many other lineages were also detected. Cloning and sequencing methods detected bacteria from 12 different phyla or candidate divisions throughout the model system, with Nitrospirae, Proteobacteria, Acidobacteria, and Planctomycetes as the most abundant groups (Fig. 1). The relative abundance of culturable strains and clones detected in our model system are listed in Table 1. Similar to findings from other systems, the most abundant isolates were rarely found independent of cultivation. However, Pseudomonas was identified from the clone libraries and constituted 6% of the amplified sequences in the old biofilm. The relatively high occurrence of Pseudomonas in both the biofilm and bulk water suggests that there is an ecological niche for a fast-growing subpopulation. In contrast to the study by Manz and coworkers (24), Aquabacterium was not found to dominate the community in our system and was detected only in the 14-day-old biofilm DNA sample. Overall, 83 different OTUs were found in the distribution system and 6 (7%) of these could be cultured on R2A medium. Using a Chao1 richness estimator, at least 167 species are present in this system, but it is highly likely that the true richness is much higher (7). None of the frequently detected lineages of bacteria (e.g., Nitrospira, Acidobacterium, etc.) were seen among the culturable isolates on standard R2A medium.
TABLE 1.
Relative abundance of strains isolated on R2A for 10 days at 23°C and cloned 16S rRNA sequences from young biofilm, old biofilm, and bulk watera
| Phylum and genus | % of sequences from:
|
||||
|---|---|---|---|---|---|
|
|
Direct extraction
|
||||
| R2A medium
|
Biofilm
|
Bulk water (n = 85) | |||
| Biofilm (n = 97) | Bulk water (n = 74) | Young (n = 56) | Old (n = 87) | ||
| Proteobacteria | |||||
| Brevundimonas | -b | 6.8 | - | - | - |
| Devosia/Rhodobium | 75.0 | - | - | - | 1.2 |
| Hydrogenophaga | - | 18.9 | - | - | - |
| Aquabacterium | - | 10.8 | 1.8 | - | - |
| Luteimonas | 24.0 | - | - | - | - |
| Legionella | - | - | - | - | 3.5 |
| Methylomonas | - | - | - | - | 2.4 |
| Pseudomonas | - | 1.4 | 3.6 | 5.7 | 1.2 |
| Thiobacillus | - | - | - | 1.1 | 2.4 |
| Acidobacteria | |||||
| Acidobacterium | - | - | - | 13.8 | 5.9 |
| Planctomycetes | |||||
| Planctomyces | - | - | 5.4 | 9.2 | 4.7 |
| Nitrospirae | |||||
| Nitrospira | - | - | 25.0 | 25.3 | 38.8 |
| Unknown | - | - | 39.3 | 3.4 | - |
| Verrucomicrobia | |||||
| Verrucomicrobium | - | - | 5.4 | - | - |
| Not identified | 1.0 | 47.3 | 0 | 0 | 0 |
| Genera not listed | 0 | 14.8 | 19.5 | 41.5 | 39.9 |
Young biofilm, 1 to 256 days; old biofilm, 571 to 1,093 days; bulk water, inlet and outlet. n values in parentheses indicate total numbers of clones or isolates from each source. Percentages in the body of the table are out of these totals.
-, not found.
FIG. 1.
Bacterial phyla present and their proportional abundances (percentages of total) among isolates cultured on R2A medium and sequences in the clone library. Identification of strains and clones is based on 16S rRNA sequence analysis. The upper part represents subclasses of Proteobacteria, whereas the lower part represents other phyla detected. Some isolates could not be identified, which is the reason why some bars sum to less than 100%. The young biofilm category includes ages from 1 to 256 days, and the old biofilm category includes ages from 571 to 1,093 days. Bulk samples are from the inlet and outlet.
The high occurrence of sequences in the clone library associated with a described nitrite oxidizer, Nitrospira, suggests that a considerable part of the population requires substrates not provided on R2A plates (10).
Major phyla present in the distribution system. (i) Acidobacteria, Planctomycetes, and Verrucomicrobia.
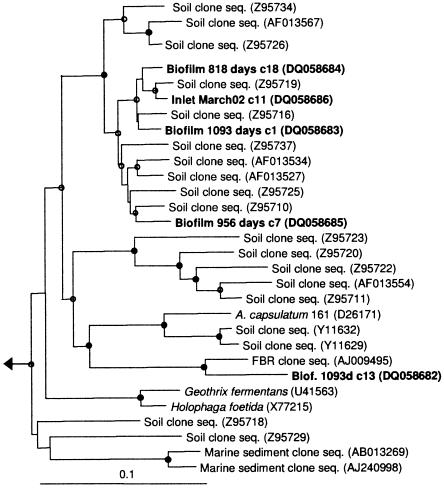
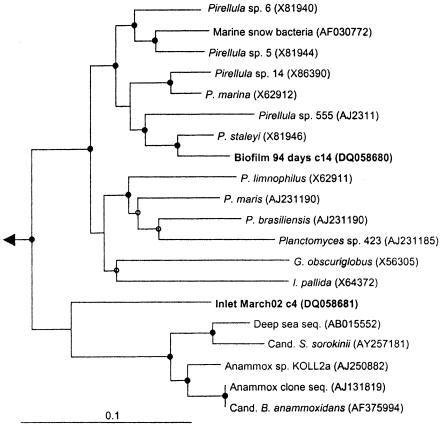
Between 11 and 23% of the retrieved sequences were associated with the phylum Acidobacteria, Planctomycetes, or Verrucomicrobia. These phyla were most abundant in the biofilms older than 600 days. The sequences formed several distinct lineages supported by high bootstrap values and probably represent independent species (Fig. 2 and 3). A few clones (e.g., Inlet March02 c4) grouped with bacteria known for anaerobic ammonium oxidation, but there was no bootstrap support for this clustering. Therefore, it is not clear if bacteria capable of this process are present in the distribution system. Bacteria included in these three phyla have rarely been observed in drinking water. Manz and coworkers observed cells with a morphology resembling that of Planctomyces (24), and clones with sequences associated with Acidobacteria have recently been found on rubber gaskets in a distribution network (35). In general, these lineages are detected in most examinations of terrestrial environments and often at high abundance but can inhabit a variety of other locations (2, 5, 8, 13, 20, 27, 28). This suggests that microorganisms from the aquifer supplying the distribution network are an important source of bacteria in the analyzed system. Therefore, the community composition appears not to be a result of the proliferation of a small group of cells specialized for drinking water distribution systems. All three phyla are broad phylogenetic groups and could potentially harbor as much physiological diversity as other phyla, such as Proteobacteria. Unfortunately, these phyla are poorly represented by isolated bacteria, making it difficult to interpret their presence and function in water supply systems.
FIG. 2.
Phylogenetic reconstruction of selected cloned rRNA sequences (1,202 bases) associated with Acidobacteria. Entries generated from this study are in boldface, and biofilm samples are indicated by age and bulk water samples by inlet/outlet and month of sampling. c_ represents a unique clone number from a given sample. The tree shown was inferred by neighbor joining to accurately reproduce the branch lengths, but the topology was supported by maximum-parsimony and maximum-likelihood inference. Solid circles signify clades with bootstrap support of >90 from all algorithms. Open circles signify clades with support from one or two algorithms. The scale bar represents 10 substitutions per 100 base pairs. seq., sequence. See Materials and Methods for description of outgroup.
FIG. 3.
Phylogenetic reconstruction of selected cloned rRNA sequences (1,192 bases) associated with Planctomycetes. Entries generated from this study are in boldface, and biofilm samples are indicated by age and bulk water samples by inlet/outlet and month of sampling. c_ represents a unique clone number from a given sample. The tree shown was inferred by neighbor joining to accurately reproduce the branch lengths, but the topology was supported by maximum-parsimony and maximum-likelihood inference. Solid circles signify clades with bootstrap support of >90 from all algorithms. Open circles signify clades with support from one or two algorithms. The scale bar represents 10 substitutions per 100 base pairs. See Materials and Methods for description of outgroup. Among others, Pirellula species include P. marina and P. staleyi; Planctomyces species include P. limnophilus, P. maris, and P. brasiliensis. Gemmata obscuriglobus, Isosphaera pallida, “Candidatus Scalindua sorokinii,” and “Candidatus Brocadia anammoxidans” are also shown.
(ii) Nitrospirae.
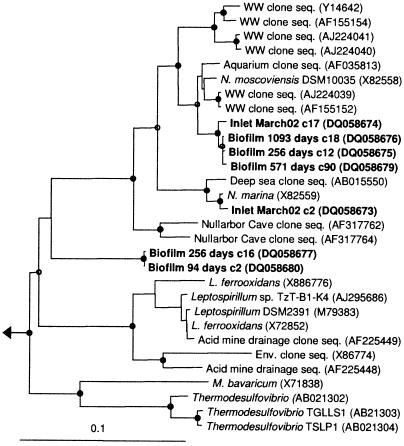
The most abundant sequence in the clone libraries from the younger biofilm branched deeply in the Nitrospirae phylum and was only distantly related to any previously described species (Fig. 4; e.g., Biofilm 256 days c16) and may constitute a novel taxa. This bacterium was absent in the 14-day-old biofilm but dominated the community in 94- and 256-day-old biofilms and could be detected in a 571-day-old sample. The phylogeny of this strain does not provide a suggestion for the type of metabolism due to the deep branching. To quantify the abundance of Nitrospira, we attempted FISH. Several FISH approaches, including multilabeled polyribonucleotides, were used to visualize the cells, but these trials were unsuccessful. The outcome may have been due to low cell activity and therefore low ribosome levels. Our results agree with observations by other investigators. Loy and coworkers recently reported that less than 3% of the total population gave a positive signal with a bacterial probe (Fig. 4) (at 0 days) in bottled mineral water (22). Similarly, Kalmbach and coworkers observed a positive probe signal from a small fraction (<23%) of planktonic bacteria in a German water distribution system (15). These observations suggest that FISH may not always be appropriate for quantifying bacteria in drinking water and that other approaches (e.g., quantitative PCR assays) should be further developed for these systems.
FIG. 4.
Phylogenetic reconstruction of selected cloned rRNA sequences (1,140 bases) associated with Nitrospirae. Entries generated from this study are in boldface, biofilm samples are indicated by age, and bulk water samples by inlet/outlet and month of sampling. c_ represents a unique clone number from a given sample. The tree shown was inferred by neighbor joining to accurately reproduce the branch lengths, but the topology was supported by maximum-parsimony and maximum-likelihood inference. Solid circles signify clades with bootstrap support of >90 from all algorithms. Open circles signify clades with support from one or two algorithms. The scale bar represents 10 substitutions per 100 base pairs. WW, wastewater treatment plant; Env., environmental. Among others, Leptospirillum species include L. ferrooxidans; Magnetobacterium bavarium is also shown. See Materials and Methods for description of outgroup.
The most abundant cloned sequence from the older biofilm (5 to 27%) as well as bulk water (30 to 40%) was associated with Nitrospira group II (see Daims et al. [9]), which includes N. moscoviensis (Table 1 and Fig. 4). Importantly, the nitrifiers from this system formed an independent lineage and may contain novel ecological properties. N. moscoviensis is known as an autotrophic nitrite-oxidizing bacterium (10), although a recent in situ study of populations of Nitrospira demonstrated uptake of pyruvate (9). Nevertheless, to our knowledge, group II Nitrospira has been found only in environments where nitrite oxidation was possible. Therefore, it was unexpected to find a large Nitrospira population in this system, since nitrite oxidizers have been observed mostly in significant numbers in distribution networks where chloramines have been used as a disinfection agent (34), although one study found nitrite oxidizers regardless of the addition of chloramines (21). We have recently examined the microbial community in a sand filter in a nearby waterworks and found Nitrospira to be highly abundant (A. C. Martiny, C. B. Corfitzen, E. Arvin, S. Molin, and H.-J. Albrechtsen, unpublished data). This result suggests that the source of this bacterium in the water phase is detachment from a sand filter at the waterworks upstream of the model system, where nitrification is known to occur. The functional role of Nitrospira in this distribution is still to be determined. The nitrite concentration was often below the detection limit, and no net drop in concentration across the model system was observed. Nitrospira is described to survive during long periods of starvation and could be dormant, so our only indication for a functional role of Nitrospira was the frequent detection as well as temporal dynamics in this system.
The bacterial community of the Danish water supply system is significantly different from that of a Berlin waterworks where Aquabacterium was found to dominate (16). In Danish aquifers, most readily utilized organic compounds as well as oxygen are consumed due to the long retention time of the groundwater, whereas the Berlin waterworks distribute bank infiltrated and posttreated water. This difference in water source may explain the observed communities.
Comparing the biofilm and bulk water populations.
The community compositions of biofilm and bulk water bacteria differed in our samples. A cluster analysis showed that the attached and planktonic communities form separate clades (Fig. 5). A one-way analysis of similarities of bulk water and biofilm samples supported the dendrogram, demonstrating a significant difference between these two environments (P = 0.027). We do not expect these community similarities to change with further sampling, given the number of independent samples, the sample sizes being approximately equivalent, and the clustering being supported by a previous terminal RFLP analysis (25). Based on a Chao1 estimator, no significant difference in overall OTU richness was observed between the environments. Several OTUs were found only in the bulk water population and not identified in any of the biofilm samples, including a Legionella and a Methylomonas species (Table 1). Similarly, deeply rooted Nitrospira and Verrucomicrobia cells were not found in the bulk water. This separation of biofilm and bulk water populations is supported by the observation that no strains isolated on R2A medium from the biofilm were found in the bulk water. Culturable strains associated with Devosia or Rhodobium and Luteimonas were detected in the biofilm, whereas Brevundimonas, Hydrogenophaga, and Aquabacterium cells were found in high numbers in the water.
FIG. 5.
Cluster analysis of the population profiles from biofilm and bulk water samples (inlet/outlet) assembled from the distribution of OTUs defined as >97% similarity at the 16S rRNA locus. The dendrogram was generated using Bray-Curtis indices with square root transformation and group average assembly.
The biofilm community could be divided into two distinct populations depending on age, an observation supported by a previous analysis using terminal restriction fragment length analysis (25). This is partly due to the proliferation of a bacterium deeply rooted in Nitrospirae which is not observed in the bulk water and seems to be specialized for the conditions in the young biofilm. After 2 years, a more mature biofilm consisting of many species is formed. Nitrospira is abundant in the community, but Pseudomonas and Planctomyces are also detected at this stage.
The only discrepancy between the clone analysis and terminal RFLP was that the 2-week-old biofilm sample showed higher similarity to the bulk water cluster and shared OTUs associated with Nitrospira, Bacteriodetes, and Rhodobacter. The combination of certain bacteria being unique for the bulk water and the clustering of the initially attached and bulk water population shows that part of the bacteria in the water phase are potential initial colonizers. This result suggests that the bacteria colonizing the biofilm are not a strongly selected subpopulation but rather common bacteria in the water phase. This finding is supported by the study of Kalmbach and coworkers where Aquabacterium was found commonly in both the water phase and the young biofilm (16).
It is noteworthy that the bulk water community showed little temporal variation, although it had been sampled on three separate occasions. Also, the inlet water community was inseparable from the outlet water in the cluster analysis, suggesting a relatively limited contribution of bacteria from the biofilm to the bulk water during the 2-h recirculation in the model system.
The high diversity in both the old biofilm and the bulk water resulted in a number of OTUs being detected only once, which complicated a deduction of the similarity between these two communities. Nevertheless, the old biofilm and the bulk water formed separate clusters, and several OTUs were detected primarily in the old biofilm but could occasionally be found in bulk water. Also, Nitrospira was present in both communities. The finding of several populations predominantly in either the biofilm or bulk water, but occasionally in the opposite environment, suggests that a dynamic exchange occurs between the adjacent communities but that many lineages have a primary habitat.
Detection of nitrification activity in batch tests.
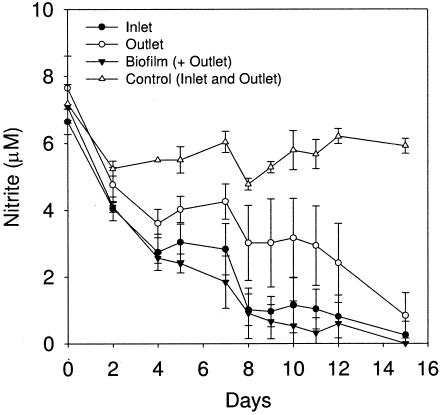
To test if the prediction from the phylogenetic analysis of a large nitrifying population in this model system was in agreement with measurements of nitrite removal, 10 μM nitrite was added to samples from the inlet, the outlet, and the biofilm. Figure 6 shows the metabolism of nitrite in the inlet, outlet, and biofilm samples. An autoclaved abiotic control had an average of 5.6 μM nitrite (compared to 10 μM added), demonstrating some initial chemical oxidation of nitrite. In addition, variation in nitrite concentration in the abiotic control was observed, reflecting some uncertainty in determining the concentration at this low nitrite level. Nevertheless, clear utilization was seen in both bulk water and biofilm samples in comparison to the abiotic control. A first-order nitrite oxidation constant, k1, was calculated as 0.03 to 0.19 day−1 for inlet water, 0.07 to 0.08 day−1 for outlet water, and 0.15 day−1 for the biofilm (when the outlet contribution was subtracted). Based on published bacterial nitrite oxidation rates in wastewater treatment plants (12), we calculated the cell number using a first-order model and assuming no growth. This corresponds to a concentration of nitrifiers of 0.5 × 105 to 2 × 105 cells/ml in the bulk water and 3.1 × 105 cells/cm2 in the biofilm. From previous studies, we have found that the average total cell number is approximately 1.3 × 105 cells/ml in the bulk water (R. Boe-Hansen, unpublished results) and 2.6 × 106 cells/cm2 in the biofilm (3) using direct cell counts. The calculated number of nitrifiers is somewhat uncertain, since it is directly dependent on the chosen cell-specific nitrite oxidation rate, but the calculation confirms the findings from the direct sequencing of rRNA fragments that nitrifiers constitute a significant fraction of the community in this water supply system.
FIG. 6.
Nitrite utilization in batch samples from the model distribution system during the course of 15 days. Inlet and outlet, 100-ml water samples; biofilm, outlet water samples plus bacteria scraped off steel plugs due to complications from growth of Nitrospira in artificial medium; controls, autoclaved samples. The initial concentration of nitrite was 10 μM.
A low assimilable organic carbon content (4 to 6 μg/liter) in the inlet water (3) seems to have established a condition where reduced nitrogen compounds become a considerable energy source influencing the community composition. Nevertheless, the observed concentrations of ammonia and nitrite in the bulk water were very low and often below the detection limits of 3 μM and 0.1 μM, respectively (25) (data not shown). The source of nitrite to support this population is therefore unknown. One possible source is nitrite generated from biological ammonium oxidation, although this concentration is low and often below the detection limit. Nitrospira is usually found in association with ammonium-oxidizing strains, but bacteria associated with known ammonium-oxidizing bacteria (e.g., Nitrosomonas and Nitrosospira spp.) were detected in only two samples and at a low frequency. This could be explained by an ammonium oxidation rate per cell that is much higher than the nitrite oxidation rate, which results in nitrite oxidizers being present in greater abundance, as seen in other systems (36). Alternatively, nitrite may be present or generated in sufficient quantities to support the Nitrospira population, either from large volumes of water flowing through daily or by a chemical reduction of nitrate mediated by reduced iron activated in corrosion processes on the pipe surface. The latter process has been described as a technique to remove nitrate from groundwater, despite the presence of oxygen (41).
In summary, it appears that detachment of Nitrospira from the sand filter at the waterworks upstream from the model system “seeds” our system. The frequency of Nitrospira clones shows dependency on the age of the biofilm and is not constant as a reflection of the density of Nitrospira in the water phase. Therefore, nitrite oxidation could be an important process for the function of the microbial ecosystem and a key fraction of the community in this water supply system.
The use of chloramines as a disinfectant is rapidly expanding as an alternative to chlorine. In conjunction with ammonia oxidizers, nitrifiers in water supply systems can assist the depletion of residual chloramines while generating biomass. Therefore, their presence poses a significant problem to water utilities (33, 34). The occurrence of a large population of bacteria potentially capable of depleting chloramines could be a factor in deciding the type of disinfection residual to use in Danish water supplies. Also, nitrite is not commonly incorporated into calculations and measurement of regrowth potential, but autotrophic growth would increase the amount of biomass formed in the system. On the positive side, a large population of nitrite oxidizers will control against elevated nitrite concentrations that could reduce the quality of the water. The findings of bacteria present in the biofilter of the waterworks (Nitrospira) and in the aquifer (Acidobacteria and Planctomycetes) indicate that these environments may be important sources of bacteria in this type of water distribution system, a hypothesis we currently are testing.
Acknowledgments
We thank Rasmus Boe-Hansen for providing biofilm samples from the Danish model system and Per Halkjær Nielsen from Aalborg University and Jennifer Hughes from Brown University for critically reviewing the manuscript.
This work was supported by a grant from the Technical University of Denmark.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barns, S. M., S. L. Takala, and C. R. Kuske. 1999. Wide distribution and diversity of members of the bacterial kingdom Acidobacterium in the environment. Appl. Environ. Microbiol. 65:1731-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boe-Hansen, R., H.-J. Albrechtsen, E. Arvin, and C. Jørgensen. 2002. Dynamics of biofilm formation in a model drinking water distribution system. J. Water Supply Res. Technol. Aqua 51:399-406. [Google Scholar]
- 4.Boe-Hansen, R., A. C. Martiny, E. Arvin, and H.-J. Albrechtsen. 2003. Monitoring biofilm formation and activity in drinking water distribution networks under oligotrophic conditions. Water Sci. Technol. 47:91-97. [PubMed] [Google Scholar]
- 5.Buckley, D. H., and T. M. Schmidt. 2003. Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ. Microbiol. 5:441-452. [DOI] [PubMed] [Google Scholar]
- 6.Clark, J. D., and O. Maaløe. 1967. DNA replication and the cell cycle in Escherichia coli cells. J. Mol. Biol. 23:293-300. [Google Scholar]
- 7.Colwell, R. K., and J. A. Coddington. 1994. Estimating terrestrial biodiversity through extrapolation. Phil. Trans. R. Soc. Lond. B 345:101-118. [DOI] [PubMed] [Google Scholar]
- 8.Crump, B. C., E. V. Armbrust, and J. A. Baross. 1999. Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia River, its estuary, and the adjacent coastal ocean. Appl. Environ. Microbiol. 65:3192-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daims, H., J. L. Nielsen, P. H. Nielsen, K.-H. Schleifer, and M. Wagner. 2001. In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl. Environ. Microbiol. 67:5273-5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrich, S., D. Behrens, E. Lebedeva, W. Ludwig, and E. Bock. 1995. A new obligately chemolithoautotrophic, nitrite-oxidizing bacterium, Nitrospira moscoviensis sp. nov. and its phylogenetic relationship. Arch. Microbiol. 164:16-23. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrman, J. A., D. E. Comeau, Å. Hagström, and A. M. Chan. 1988. Extraction from natural planktonic microorganisms of DNA suitable for molecular biological studies. Appl. Environ. Microbiol. 54:1426-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henze, M., P. Harremöes, J. L. C. Jansen, and E. Arvin. 2002. Wastewater treatment. Biological and chemical processes. Springer, Berlin, Germany.
- 13.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalmbach, S., W. Manz, B. Bendinger, and U. Szewzyk. 1999. In situ probing reveals Aquabacerium commune as a widespread and highly abundant bacterial species in drinking water biofilms. Water Res. 34:575-581. [Google Scholar]
- 15.Kalmbach, S., W. Manz, and U. Szewzyk. 1997. Dynamics of biofilm formation in drinking water: phylogenetic affiliation and metabolic potential of single cells assessed by formazan reduction and in situ hybridization. FEMS Microbiol. Ecol. 22:265-279. [Google Scholar]
- 16.Kalmbach, S., W. Manz, and U. Szewzyk. 1997. Isolation of new bacterial species from drinking water biofilms and proof of their in situ dominance with highly specific 16S rRNA probes. Appl. Environ. Microbiol. 63:4164-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, England.
- 18.LeChevallier, M. W., T. M. Babcock, and R. G. Lee. 1987. Examination and characterization of distribution system biofilms. Appl. Environ. Microbiol. 53:2714-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeChevallier, M. W., R. J. Seidler, and T. M. Evans. 1980. Enumeration and characterization of standard plate count bacteria in chlorinated and raw water supplies. Appl. Environ. Microbiol. 40:922-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liles, M. R., B. F. Manske, S. B. Bintrim, J. Handelsman, and R. M. Goodman. 2003. A census of rRNA genes and linked genomic sequences within a soil metagenomic library. Appl. Environ. Microbiol. 69:2684-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipponen, M. T., M. H. Suutari, and P. J. Martikainen. 2002. Occurrence of nitrifying bacteria and nitrification in Finnish drinking water distribution systems. Water Res. 36:4319-4329. [DOI] [PubMed] [Google Scholar]
- 22.Loy, A., W. Beisker, and H. Meier. 2005. Diversity of bacteria growing in natural mineral water after bottling. Appl. Environ. Microbiol. 71:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manz, W., U. Szewzyk, P. Ericsson, R. Amann, K.-H. Schleifer, and T.-A. Stenström. 1993. In situ identification of bacteria in drinking water and adjoining biofilms by hybridization with 16S and 23S rRNA-directed fluorescent oligonucleotide probes. Appl. Environ. Microbiol. 59:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martiny, A. C., T. M. Jørgensen, H.-J. Albrechtsen, E. Arvin, and S. Molin. 2003. Long-term succession in structure and diversity of a biofilm formed in a model drinking water distribution system. Appl. Environ. Microbiol. 69:6899-6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misko, T. P., R. J. Schilling, D. Salvemini, W. M. Moore, and M. G. Currie. 1993. A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 214:11-16. [DOI] [PubMed] [Google Scholar]
- 27.Neef, A., R. Amann, H. Schlesner, and K. H. Schleifer. 1998. Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 144:3257-3266. [DOI] [PubMed] [Google Scholar]
- 28.Norris, T. B., J. M. Wraith, R. W. Castenholz, and T. R. McDermott. 2002. Soil microbial community structure across a thermal gradient following a geothermal heating event. Appl. Environ. Microbiol. 68:6300-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norton, C. D., and M. W. LeChevallier. 2000. A pilot study of bacteriological population changes through potable water treatment and distribution. Appl. Environ. Microbiol. 66:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson, B. H., and L. A. Nagy. 1984. Microbiology of potable water. Adv. Appl. Microbiol. 30:73-132. [DOI] [PubMed] [Google Scholar]
- 31.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biol. Sci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 32.Payment, P., F. Gamache, and G. Paquette. 1988. Microbiological and virological analysis of water from two water filtration plants and their distribution systems. Can. J. Microbiol. 34:1304-1309. [DOI] [PubMed] [Google Scholar]
- 33.Regan, J. M., G. W. Harrington, H. Baribeau, R. De Leon, and D. R. Noguera. 2003. Diversity of nitrifying bacteria in full-scale chloraminated distribution systems. Water Res. 37:197-205. [DOI] [PubMed] [Google Scholar]
- 34.Regan, J. M., G. W. Harrington, and D. R. Noguera. 2002. Ammonia- and nitrite-oxidizing bacterial communities in a pilot-scale chloraminated drinking water distribution system. Appl. Environ. Microbiol. 68:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmeisser, C., C. Stockigt, C. Raasch, J. Wingender, K. N. Timmis, D. F. Wenderoth, H. C. Flemming, H. Liesegang, R. A. Schmitz, K. E. Jaeger, and W. R. Streit. 2003. Metagenome survey of biofilms in drinking-water networks. Appl. Environ. Microbiol. 69:7298-7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schramm, A., D. de Beer, J. C. van den Heuvel, S. Ottengraf, and R. Amann. 1999. Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl. Environ. Microbiol. 65:3690-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz, T., S. Hoffmann, and U. Obst. 1998. Formation and bacterial composition of young, natural biofilms obtained from public bank-filtered drinking water systems. Water Res. 32:2787-2797. [Google Scholar]
- 38.Staley, J. T., and A. Konopka. 1985. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu. Rev. Microbiol. 39:321-346. [DOI] [PubMed] [Google Scholar]
- 39.Swofford, D. L. 1999. PAUP*: phylogenetic analysis using parsimony (* and other methods). Sinauer Associates, Sunderland, Mass.
- 40.Wagner, M., and A. Loy. 2002. Bacterial community composition and function in sewage treatment systems. Curr. Opin. Biotechnol. 13:218-227. [DOI] [PubMed] [Google Scholar]
- 41.Westerhoff, P., and J. James. 2003. Nitrate removal in zero-valent iron packed columns. Water Res. 37:1818-1830. [DOI] [PubMed] [Google Scholar]