Abstract
Previous studies have demonstrated that the branched-chain fatty acid anteiso-C15:0 plays a critical role in the growth of Listeria monocytogenes at low temperatures by ensuring sufficient membrane fluidity. Studies utilizing a chemically defined minimal medium revealed that the anteiso fatty acid precursor isoleucine largely determined the fatty acid profile and fatty acid response of the organism to lowered growth temperature. When isoleucine was sufficient, the fatty acid profile was very uniform, with anteiso fatty acids comprising up to 95% of total fatty acid, and the major fatty acid adjustment to low temperature was fatty acid chain shortening, which resulted in an increase of anteiso-C15:0 solely at the expense of anteiso-C17:0. When isoleucine was not supplied, the fatty acid profile became more complex and was readily modified by leucine, which resulted in a significant increase of corresponding iso fatty acids and an inability to grow at 10°C. Under this condition, the increase of anteiso-C15:0 at low temperature resulted from the combined effect of increasing the anteiso:iso ratio and chain shortening. A branched-chain α-keto acid dehydrogenase-defective strain largely lost the ability to increase the anteiso:iso ratio. Cerulenin, an inhibitor of β-ketoacyl-acyl carrier protein synthase (FabF), induced a similar fatty acid chain shortening as low temperature did. We propose that the anteiso precursor preferences of enzymes in the branched-chain fatty acid biosynthesis pathway ensure a high production of anteiso fatty acids, and cold-regulated chain shortening results in a further increase of anteiso-C15:0 at the expense of anteiso-C17:0.
Contamination of refrigerated food with Listeria monocytogenes has led to a considerable number of outbreaks of food-borne listeriosis and to expensive food product recalls (22). A major factor in this is the relatively robust growth of Listeria at refrigeration temperatures and below, whereas the growth of other common food-borne pathogens such as Clostridium botulinum, Escherichia coli, Salmonella enterica serovar Typhimurium, and Staphylococcus aureus is inhibited (4, 17). However, the underlying mechanisms of L. monocytogenes psychrotolerance are incompletely understood.
Modulation of fatty acid composition to ensure sufficient membrane fluidity is an important bacterial strategy for growth at low temperatures (12, 29). At low temperatures, a more fluid membrane can usually be attained by increasing the degree of fatty acid unsaturation or branching and by decreasing the fatty acid chain length (12). These low-melting-point fatty acids lower the gel-liquid crystalline-phase transition temperature of the lipid and therefore maintain proper fluidity at low temperatures (14, 29). Homeoviscous adaptation proposes that phospholipids synthesized at different temperatures have similar viscosities or fluidity (13, 14, 28), which is primarily achieved through the modulation of the fatty acid content of the phospholipid. The strategy for many gram-negative bacteria is primarily to increase the content of straight-chain unsaturated fatty acids (29). In contrast, the strategy for the gram-positive bacterium L. monocytogenes appears to be solely dependent upon increasing the content of the branched-chain anteiso-C15:0 fatty acid (2, 15, 21, 24, 26). Bacillus subtilis, a close relative of L. monocytogenes, appears to regulate membrane fluidity by both unsaturated and branched-chain fatty acid (BCFA) strategies (9, 19, 31).
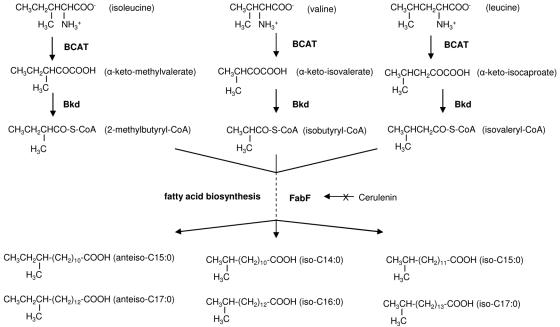
BCFAs in B. subtilis and straight-chain fatty acids (SCFAs) in Escherichia coli are biosynthesized by a homologous set of enzymes (7, 9). One major difference between the two systems is that B. subtilis, but not E. coli, possesses a branched-chain α-keto acid dehydrogenase (Bkd) producing branched-chain acyl coenzyme A (CoA) primers (18, 25). In addition, initiation enzyme β-ketoacyl-acyl carrier protein synthase III (FabH) from BCFA-producing bacteria prefers branched-chain acyl-CoAs over acetyl-CoA as substrates (5). BCFA biosynthesis starts with the branched-chain amino acids (BCAAs) isoleucine, valine, and leucine and usually ends with anteiso-C15:0 and anteiso-C17:0, iso-C14:0 and iso-C16:0, and iso-C15:0 and iso-C17:0, respectively (18). We hereafter refer to the pathway from BCAAs to BCFAs as the BCFA biosynthetic pathway (Fig. 1). Briefly, α-keto acids, produced by BCAA transaminase, are decarboxylated by Bkd to produce branched-chain acyl-CoAs, which are used by FabH to initiate fatty acid biosynthesis. β-Ketoacyl-acyl carrier protein synthase II (FabF) is responsible for the subsequent rounds of elongation until the acyl chain reaches 14 to 17 carbons (Fig. 1).
FIG. 1.
Proposed BCFA biosynthetic pathway from branched-chain amino acids in L. monocytogenes. BCAT, branched-chain amino acid transaminase; Bkd, branched-chain α-keto acid dehydrogenase; FabF, β-ketoacyl-acyl carrier protein synthase II.
B. subtilis membrane fatty acids are dominated by BCFAs, and a characteristic response of B. subtilis to low temperatures is an increase in the ratio of anteiso:iso fatty acid (19). In addition, a cold-induced lipid acyl desaturase controlled by a sensor-regulator system directly modifies lipids producing unsaturated fatty acids when the temperature falls (9). This quick response is an alternative strategy in B. subtilis to increase membrane fluidity upon cold shock (8, 31). In contrast, the cold regulation of BCFA synthesis is poorly studied. It has been shown that in B. subtilis, isoleucine increased anteiso fatty acid content and showed a cold-protective function, whereas leucine was not protective (8, 19). Moreover, isoleucine significantly depressed the synthesis of unsaturated fatty acid and abolished cold sensitivity in an unsaturated fatty acid-defective B. subtilis strain (8, 31).
The membrane fatty acid composition of L. monocytogenes is dominated to an unusual extent by BCFAs. The six common BCFAs have different roles in cold adaptation. Phase transition temperatures of diacylphosphatidycholine with these fatty acids are as follows: −16.5°C for anteiso-C15:0, 7.6°C for anteiso-C17:0, 6.5°C for iso-C14:0, 22°C for iso-C16:0, 6.5°C for iso-C15:0, and 27.0°C for iso-C17:0 (18). Anteiso-C15:0 has a significantly greater ability to lower lipid phase transition temperature than other fatty acids, and it typically increases to constitute more than 60% of the total fatty acids when Listeria is grown at temperatures of 10°C or below (2, 15, 21, 24, 26). A similar high content of anteiso-C15:0 (90%) was observed in a psychrotolerant Pseudomonas species when it was grown at 5°C (11).
We have described two L. monocytogenes transposon-induced cold-sensitive mutants, cld-1 and cld-2. The mutants are severely growth impaired at low temperature, and their fatty acid compositions are significantly deficient in odd-numbered BCFAs and increased in even-numbered SCFAs and BCFAs (2). Transposon Tn917 is inserted in the bkd gene cluster in both mutants (32). Electron paramagnetic resonance spectroscopic studies of the membrane fluidity of BCFA-deficient cld-1 showed that the mutant had a significantly less-fluid membrane than its parent strain (10, 16). 2-Methylbutyric acid, a precursor of anteiso fatty acids, restored the anteiso-C15:0 content, membrane fluidity, and growth of the mutants at low temperatures, whereas isobutyrate and isovalerate, precursors of iso fatty acids, did not (10, 32). Hence, as far as cold adaptation of the membrane is concerned, the central strategy of L. monocytogenes is to increase the proportion of anteiso-C15:0.
The cellular pool of branched-chain acyl-CoAs, starter units for BCFAs, plays a critical role in determining the L. monocytogenes fatty acid profile (18, 32). These acyl-CoAs are primarily derived from BCAAs (Fig. 1). However, the effect of individual BCAAs on the Listeria fatty acid profile has not been studied. The vast majority of previous studies of the fatty acid composition of L. monocytogenes have employed complex media for growth. Recently, Tsai and Hodgson described a minimal medium that does not require BCAA supplements (30). We have used this medium to gain insights into how L. monocytogenes ensures its high content of anteiso-C15:0. We found that isoleucine had a profound influence on the fatty acid profile and the fatty acid response to cold temperatures.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. monocytogenes strain 10403S was grown in brain heart infusion broth (BHI; Difco Laboratories, Detroit, MI) at 37°C and in the chemically defined minimal medium HTM (30) at 30°C, unless specified otherwise, with shaking at 150 rpm. 10403S Bkd mutant cld-2 (32) was grown in BHI with supplements of three short BCFAs, 2-methylbutyrate, isobutyrate, and isovalerate (0.1 mM each). E. coli strain Top10 (Invitrogen, Carlsbad, CA) was grown in Luria-Bertani (LB) medium at 37°C. When necessary, ampicillin and chloramphenicol were added to final concentrations of 100 μg ml−1 and 10 μg ml−1, respectively. For preparation of L. monocytogenes cultures for growth studies and fatty acid analysis, 2 ml of overnight starter culture in BHI or HTM was used to inoculate 100 ml of BHI or HTM in a 250-ml Erlenmeyer flask. Growth was monitored by measuring the optical density at 600 nm with a Beckman DU-65 spectrophotometer.
Fatty acid analysis.
Fatty acid analysis was carried out as previously described (32). Cells were harvested in the mid-exponential phase of growth, and the pellet was washed three times with distilled water. The fatty acids in the cells (20 to 40 mg [wet weight]) were saponified, methylated, and extracted. The resulting methyl ester mixtures were separated by an Agilent 5890 dual-tower gas chromatograph. Fatty acids were identified by the MIDI microbial identification system (Sherlock 4.5 Microbial Identification System). This analysis was performed at Microbial ID Inc. (Newark, DE). Minor fatty acids were not shown in the fatty acid profile.
MIC determination.
A stock solution of cerulenin (10 mg ml−1) was prepared in acetone. BHI plates containing different concentrations of cerulenin were inoculated with a 10-μl drop of a 10−2 dilution of a culture grown overnight. The MIC was recorded after 24 h of incubation at 37°C or 7 days at 10°C.
Construction of fabF overexpression strain.
L. monocytogenes genomic DNA was isolated using a Wizard Genomic DNA purification kit (Promega, Madison, WI). Intact fabF plus a 130-bp upstream sequence were amplified with two oligonucleotides, 5′-GGTGGATCCTAATGGAGCAGGCTTTTGGT-3′ and 5′-GCTGAATTCTTAGGCGAATATAAACGC-3′, and cloned into the shuttle vector pCU1 (3). Purified plasmid DNA from ampicillin-resistant E. coli transformants was used to transform strain 10403S as described previously (32). FabF overexpression strain F29 was obtained through chloramphenicol selection on BHI plates.
RESULTS
Isoleucine determines the fatty acid profile.
L. monocytogenes typically has a relatively simple fatty acid profile, and anteiso-C15:0, anteiso-C17:0, iso-C15:0, iso-C17:0, iso-C14:0, and iso-C16:0 comprise 95 to 100% of the total fatty acids in cells grown in complex medium (2, 15, 24). The HTM medium does not contain any BCAAs (30), and the cells grown in this medium are strictly dependent on the biosynthesis of isoleucine, valine, and leucine for the biosynthesis of BCFAs. L. monocytogenes grown in HTM had considerable amounts of iso fatty acids (42%) and some straight-chain fatty acids (4%), although anteiso fatty acids were still the most abundant fatty acids (54%) (Table 1). No unsaturated fatty acids were detected, whereas B. subtilis produced a considerable amount of unsaturated fatty acid when no exogenous isoleucine was available (31). The complex medium BHI provides considerable amounts of isoleucine, valine, and leucine to cells, either as free amino acids or in the form of peptides that are hydrolyzed within the cell (1). Anteiso fatty acids rose to 82% of the total fatty acids when L. monocytogenes was grown in BHI medium (Table 2). When HTM medium was supplemented with equal amounts of isoleucine, valine, and leucine, anteiso fatty acids increased to more than 92%, and the fatty acid profile was very simple (Table 1). As a result, when isoleucine was sufficient, the fatty acid profile was quite uniform, whereas when no exogenous isoleucine was provided, the fatty acid profile was more complex. The remarkably high content of anteiso fatty acids indicated that the enzymes in the BCFA biosynthetic pathway preferred anteiso precursors over iso or straight-chain precursors.
TABLE 1.
Fatty acid profile of strain 10403S in HTM with various supplements at 30°C and 10°C
| HTM supplement, growth temp | % (wt/wt) of total fatty acida
|
Anteiso: iso ratio | ACCLc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anteiso-C13:0 | Iso-C14:0 | Iso-C15:0 | Anteiso-C15:0 | C16:0 | Iso-C16:0 | Iso-C17:0 | Anteiso-C17:0 | |||
| None, 30°C | NDb | 10.1 | 2.4 | 36.4 | 2.1 | 28.8 | 0.7 | 17.7 | 1.3 | 15.6 |
| None, 10°C | 1.0 | 8.0 | 1.3 | 65.6 | ND | 11.1 | ND | 12.6 | 3.9 | 15.3 |
| 0.1 mM Leu, 30°C | ND | 7.4 | 17.9 | 33.2 | 1.7 | 19.5 | 3.5 | 14.5 | 1.0 | 15.5 |
| 0.5 mM Leu, 30°C | ND | 5.4 | 58.5 | 14.5 | 0.7 | 8.2 | 8.6 | 3.6 | 0.2 | 15.3 |
| 1.5 mM Ile, Leu, and Val, 30°C | ND | ND | 2.0 | 50.7 | ND | 1.0 | 0.7 | 44.9 | 25.8 | 15.9 |
| 1.5 mM Ile, Leu, and Val, 10°C | 1.0 | 1.2 | 2.0 | 74.0 | ND | 3.3 | ND | 18.2 | 14.3 | 15.4 |
Some minor fatty acid components are not shown in this table.
ND, not detected.
ACCL, average carbon chain length [∑CFA×C)/100, where FA is the percentage of each fatty acid and C is the number of carbon atoms in the chain].
TABLE 2.
Fatty acid profile of strain 10403S and Bkd mutant cld-2 in BHI with various supplements at 37°C and 10°C
| Strain, supplement, and temp | % (wt/wt) of total fatty acida
|
Anteiso: iso ratio | ACCLd | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anteiso-C13:0 | Iso-C14:0 | Iso-C15:0 | Anteiso-C15:0 | Iso-C16:0 | Iso-C17:0 | Anteiso-C17:0 | |||
| 10403S, none, 37°C | NDb | 0.8 | 10.1 | 46.8 | 2.4 | 3.2 | 35.7 | 5 | 15.8 |
| 10403S, none, 10°C | 0.3 | 1.1 | 12.9 | 67.7 | 1.5 | 0.8 | 14.9 | 5.1 | 15.3 |
| 10403S, cerulenin, 37°C | 1.3 | ND | 9.4 | 70.1 | 1.3 | ND | 16.7 | 8.2 | 15.3 |
| cld-2, short BCFAsc, 37°C | ND | 8.5 | 5.8 | 38.0 | 22.6 | 1.3 | 17.4 | 1.5 | 15.6 |
| cld-2, short BCFAs, 10°C | ND | 16.3 | 7.1 | 52.7 | 11.9 | ND | 6.2 | 1.7 | 15.1 |
Some minor fatty acid components are not shown in this table.
ND, not detected.
Short BCFAs 2-methylbutyrate, isobutyrate, and isovalerate were added at 0.1 mM each.
ACCL, average carbon chain length (calculated as described for Table 1).
Leucine increases iso fatty acids and inhibits growth in HTM at low temperatures.
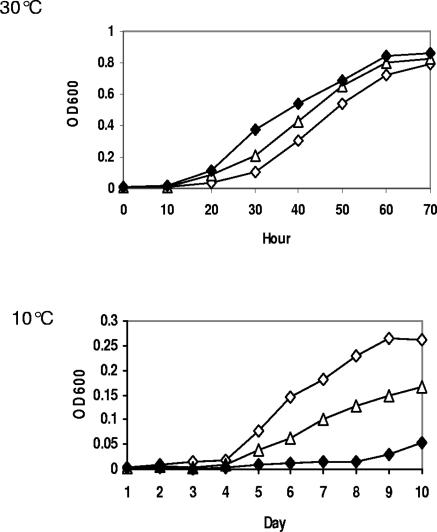
As described above, the dominant effect of isoleucine largely limited the influence of the iso fatty acid precursors leucine and valine. However, when isoleucine was not provided, leucine was able to greatly increase the corresponding iso-C15:0 and iso-C17:0 content and concomitantly decrease the anteiso-C15:0 and anteiso-C17:0 content. (Table 1). The influence of leucine on growth at low temperatures was investigated. At 30°C, leucine stimulated the growth of strain 10403S, whereas it strongly inhibited growth at 10°C (Fig. 2). Previously, we have shown that the odd-numbered iso fatty acid precursor isovalerate stimulated growth of L. monocytogenes Bkd mutants at 37°C but not at 10°C (32). Taken together, these finding indicate that a fatty acid profile characterized by high levels of odd-numbered iso fatty acids could provide appropriate fluidity at higher growth temperatures but could not provide sufficient fluidity for growth at low temperatures.
FIG. 2.
Effect of leucine on growth of strain 10403S in HTM at 30°C and 10°C. Cultures were unsupplemented (⋄) or supplemented with 0.1 mM (▵) or 0.5 mM (⧫) leucine; OD600, optical density at 600 nm.
Isoleucine alters the fatty acid response to low temperatures.
The fatty acid response to a lowered growth temperature was determined in HTM medium without BCAA supplements. As the temperature dropped from 30°C to 10°C, the anteiso:iso ratio was significantly increased from 1.3 to 3.9, which resulted in an increase in anteiso content from 54% to 78% (Table 1). This ratio increase has not been clearly seen in previous fatty acid studies due to the presence of exogenous isoleucine. Another major fatty acid response was chain shortening. The average chain length was shortened from 15.6 at 30°C to 15.3 at 10°C (Table 1). Thus, L. monocytogenes increased anteiso-C15:0 at low temperatures through a combined effect of increasing the anteiso:iso ratio and chain shortening, i.e., at the expense of iso fatty acids and anteiso-C17:0. When isoleucine was sufficient, the content of iso fatty acids was very low, and the only significant change in response to a lowered temperature was fatty acid chain shortening. The average chain length was shortened from 15.9 at 30°C to 15.4 at 10°C (Table 1). Anteiso-C15:0 rose from 50% at 30°C to 74% at 10°C solely at the expense of anteiso-C17:0. The anteiso:iso ratio was not significantly changed, since anteiso content remained at 93 to 95% when the growth temperature fell from 30°C to 10°C (Table 1). Therefore, the mode of the anteiso:iso ratio increase was abolished by exogenous isoleucine, because isoleucine resulted in an extremely high anteiso fatty acid content regardless of the temperature. Similarly, when the complex medium BHI was used, chain shortening was the major fatty acid response to cold, and the anteiso:iso ratio was not significantly changed (Table 2). Hence, the mode of cold-regulated BCFA biosynthesis was affected by exogenous isoleucine, and chain shortening was the major adjustment when isoleucine was sufficient.
Bkd is involved in the anteiso:iso ratio increase.
A key enzyme in the BCFA biosynthetic pathway, Bkd, is responsible for the production of branched-chain acyl-CoAs, starter units for BCFA biosynthesis. It has been proposed that Bkd might play a role in cold-regulated BCFA biosynthesis (20). A previous study showed that short BCFAs bypassed the Bkd defect and restored the normal BCFA content and growth of Bkd mutant cld-2 (32). In the presence of equal amounts of three short BCFAs, 2-methylbutyrate, isobutyrate, and isovalerate (precursors of odd-numbered anteiso, even-numbered iso, and odd-numbered iso fatty acids, respectively), the total anteiso fatty acid content (55%) in Bkd mutant cld-2 was significantly less than that of strain 10403S grown in BHI (82%) (Table 2), suggesting that Bkd is one player in the pathway ensuring a high anteiso fatty acid content. Moreover, when the growth temperature was lowered from 37°C to 10°C, mutant cld-2 was able to shorten the fatty acid chain length but was not able to significantly increase the anteiso:iso ratio, indicating that Bkd plays an important role in increasing the anteiso:iso ratio when the growth temperature is lowered.
Fatty acid biosynthesis inhibitor cerulenin induces fatty acid chain shortening.
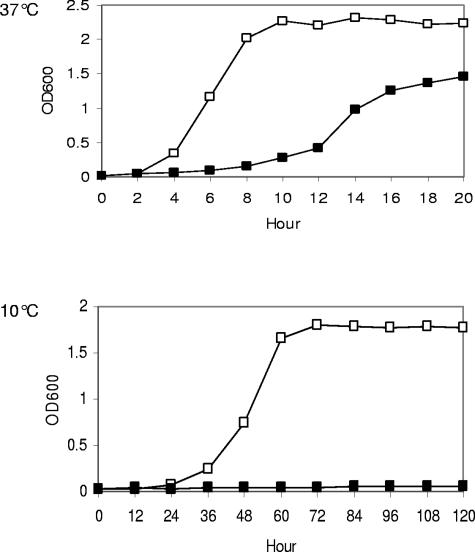
A search of the L. monocytogenes genome revealed that one condensation enzyme, FabF, was responsible for fatty acyl chain elongation in the fatty acid biosynthesis pathway. The fatty acid biosynthetic inhibitor cerulenin covalently attaches to the active site and irreversibly inhibits FabF (23). Sublethal concentrations of cerulenin induced significant fatty acid chain shortening at 37°C. The average fatty acid chain length was shortened from 15.8 to 15.3 with 2 μg ml−1 cerulenin (Table 2). As a result, anteiso-C15:0 was increased from 46% to 70% at the expense of anteiso-C17:0. In addition, the shorter BCFA anteiso-C13:0 was detected in cerulenin-treated cells. Previously, anteiso-C13:0 was only observed in cells grown at low temperatures. The fatty acid profile resulting from cerulenin treatment was remarkably similar to the profile of cells grown at low temperatures (Table 2). Furthermore, overexpression of FabF in strain 10403S increased MICs of cerulenin at 37°C from 25 μg ml−1 to 40 μg ml−1, confirming that FabF is the drug target in L. monocytogenes. More importantly, at 10°C, the MIC of cerulenin was decreased to 10 μg ml−1 for strain 10403S. A similar temperature-dependent increase in susceptibility was also observed in liquid culture, where 10 μg ml−1 cerulenin completely inhibited growth at 10°C (Fig. 3). Strain 10403S did not show increased susceptibility to the antibiotics tetracycline and ampicillin at low temperatures (data not shown), which provides evidence that the temperature-dependent change in susceptibility to cerulenin was specific to this antibiotic. This observation suggested that FabF in L. monocytogenes was likely to be less active at low temperatures, and the low activity of FabF might play a role in accomplishing fatty acid chain shortening in L. monocytogenes.
FIG. 3.
Increased susceptibility of strain 10403S to cerulenin in BHI broth at 10°C compared to that at 37°C. □, BHI; ▪, BHI with 10 μg ml−1 cerulenin; OD600, optical density at 600 nm.
DISCUSSION
Most bacteria can maintain similar membrane fluidities at different growth temperatures, and this ability is termed homeoviscous adaptation (13, 14, 28). This adaptation is largely accomplished by changing membrane fatty acid chain length, unsaturation degree, and branching pattern, although lipid head groups also play roles in this process (13). Proper membrane fluidity at low temperatures can be achieved by increasing unsaturated fatty acid, BCFA, and shorter-chain fatty acids, because these low-melting-point fatty acids lower the gel-to-liquid crystalline-phase transition temperature of lipids (29). Bacterial psychrotolerance depends to a significant extent on a high proportion of the above-described fatty acids in the membrane. L. monocytogenes increased its anteiso-C15:0 content to more than 70% when grown at low temperatures because lipids containing anteiso-C15:0 have significantly lower phase transition temperatures than those containing iso fatty acids and/or anteiso-C17:0 (18). This high content of anteiso-C15:0, with its effects on membrane fluidity (2, 10, 16), enables this organism to grow at refrigeration temperatures, temperatures at which most food-borne pathogens cannot grow. How L. monocytogenes produces its characteristically high content of anteiso-C15:0 was the main focus of this study.
The Bkd complex, which converts branched-chain keto acids to branched-chain acyl-CoAs, plays an important role in determining the ultimate BCFA produced (18, 20, 32). Bkd in B. subtilis has a higher relative activity with isoleucine-derived substrate (100%) than with valine (75%)- and leucine (31%)-derived substrates (25). As a result, anteiso fatty acids are typically the most abundant fatty acids in B. subtilis. L. monocytogenes contained as high as 95% anteiso fatty acid when equal amounts of exogenous isoleucine, valine, and leucine were present. The dominant effect of isoleucine in L. monocytogenes implies that Bkd has a significantly higher preference for anteiso precursors over iso precursors and SCFA precursors. This was supported by the somewhat low anteiso content in Bkd mutant cld-2 in the presence of a mixture of BCFA precursors (Table 2). Other enzymes downstream of Bkd in the BCFA pathway probably also have a preference for isoleucine-derived substrates (Fig. 1). This appears likely because a considerable excess amount of the iso fatty acid precursor isobutyrate or isovalerate over the anteiso precursor 2-methylbutyrate was required to significantly decrease anteiso fatty acid content in L. monocytogenes Bkd mutant cld-2 (32).
When the temperature was lowered, the enzymatic preferences seem to be further enhanced, because the anteiso:iso ratio was greatly increased in cells grown in HTM (Table 1). The ratio change could involve endogenous isoleucine synthesis and a change in substrate specificity of enzymes in the BCFA biosynthetic pathway (Fig. 1). L. monocytogenes Bkd mutant cld-2 could not efficiently increase the anteiso:iso ratio when the growth temperature was lowered in the presence of a mixture of anteiso and iso precursors, indicating that Bkd played an import role in increasing the anteiso:iso ratio.
Fatty acid chain shortening was a critical response to further increase the anteiso-C15:0 level when the total anteiso content was already high. Lipids containing anteiso-C17:0 have significantly higher phase transition temperatures than those containing anteiso-C15:0 (18). Therefore, a high proportion of anteiso-C17:0 probably could not support growth at low temperatures. L. monocytogenes Bkd mutant cld-2 could accomplish fatty acid chain shortening in the presence of 2-methylbutyrate, indicating that Bkd was not responsible for cold-induced chain shortening, and the regulation of the fatty acid chain length probably occurred downstream of Bkd in the BCFA pathway (32). The cerulenin-induced fatty acid chain shortening indicated that the inhibition of fatty acid biosynthesis could achieve a shorter fatty acid profile, similar to that which usually occurs only at low temperatures. Ultimately, competition between fatty acid synthesis and phospholipid synthesis determines fatty acid chain length in bacteria (7, 27). A less expressed or less active FabF in cold conditions, or its inhibition by cerulenin, may slow the fatty acid chain elongation reaction, thereby resulting in shorter fatty acids in the membrane phospholipids. Overproduction of a key enzyme for initiation of phospholipid synthesis, glycerol 3-phosphate acyltransferase, also resulted in a decreased average fatty acid chain length in E. coli (6). Further biochemical studies are necessary to completely understand cold-regulated fatty acid chain shortening in L. monocytogenes.
It is noteworthy that B. subtilis could increase its anteiso:iso fatty acid ratio but could not efficiently shorten fatty acid chain length at low temperatures (19). As a result, anteiso-C15:0 content in B. subtilis (45%) was significantly less than that in L. monocytogenes when grown at low temperatures. A lower anteiso-C15:0 content could be one explanation for the inability of B. subtilis to grow at refrigeration temperatures. These studies highlight the primary role of the fatty acid anteiso-C15:0 in membrane fluidity. Indeed, it is possible that the low minimum growth temperature of L. monocytogenes is mainly due to the fatty acid anteiso-C15:0. The characteristic high content of this fatty acid appears to be accomplished in large measure by a combination of anteiso precursor preferences by biosynthetic enzymes and fatty acid chain shortening.
Acknowledgments
This work was supported by award 2002-35201-12791 from the National Research Initiative Competitive Grants Program of the U.S. Department of Agriculture and by grants from the Graduate School at Illinois State University and the Beta Lambda Chapter of the Phi Sigma Biological Honor Society.
REFERENCES
- 1.Amezaga, M. R., L. Davidson, D. McLaggan, A. Verheul, T. Abee, and I. R. Booth. 1995. The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology 141:41-49. [DOI] [PubMed] [Google Scholar]
- 2.Annous, B. A., L. A. Becker, D. O. Bayles, D. P. Labeda, and B. J. Wilkinson. 1997. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustin, J., R. Rosenstein, B. Wieland, U. Schneider, N. Schnell, G. Engelke, K. Entian, and F. Gotz. 1992. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur. J. Biochem. 204:1149-1154. [DOI] [PubMed] [Google Scholar]
- 4.Bryan, F. L. 2004. The “danger zone” reevaluated. Food Safety Mag. 10:55-69. [Google Scholar]
- 5.Choi, K., R. J. Heath, and C. O. Rock. 2000. β-Ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in BCFA biosynthesis. J. Bacteriol. 182:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper, C. L., S. Jackowski, and C. O. Rock. 1987. Fatty acid metabolism in sn-glycerol-3-phosphate acyltransferase (plsB) mutants. J. Bacteriol. 169:605-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cronan, J. E., Jr., and C. O. Rock. 1996. Biosynthesis of membrane lipids, p. 612-636. In F. C. Neidhardt, R. Curtiss III, C. A. Gross, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 8.Cybulski, L. E., M. C. Albanesi, M. C. Mansilla, S. Altabe, P. S. Aguilar, and D. de Mendoza. 2002. Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol. Microbiol. 45:1379-1388. [DOI] [PubMed] [Google Scholar]
- 9.de Mendoza, D., G. E. Schujman, and P. S. Aguilar. 2002. Biosynthesis and function of membrane lipids, p. 43-55. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 10.Edgcomb, M. R., S. Sirimanne, B. J. Wilkinson, P. Drouin, and R. P. D. Morse II. 2000. Electron paramagnetic resonance studies of the membrane fluidity of the foodborne pathogenic psychrotroph Listeria monocytogenes. Biochim. Biophys. Acta 1463:31-42. [DOI] [PubMed] [Google Scholar]
- 11.Fukunaga, N., and N. J. Russell. 1990. Membrane lipid composition and glucose uptake in two psychrotolerant bacteria from Antarctica. J. Gen. Microbiol. 136:1669-1673. [Google Scholar]
- 12.Fulco, A. J. 1983. Fatty acid metabolism in bacteria. Prog. Lipid Res. 22:133-160. [DOI] [PubMed] [Google Scholar]
- 13.Hazel, J. R. 1995. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 57:19-42. [DOI] [PubMed] [Google Scholar]
- 14.Hazel, J. R., and E. E. Williams. 1990. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid Res. 29:167-227. [DOI] [PubMed] [Google Scholar]
- 15.Jones, C. E., G. Sharma, D. Jones, I. S. Roberts, and P. W. Andrew. 1997. Physiological and biochemical studies on psychrotolerance in Listeria monocytogenes. J. Appl. Microbiol. 83:31-35. [DOI] [PubMed] [Google Scholar]
- 16.Jones, S. L., P. Drouin, B. J. Wilkinson, and P. D. Morse II. 2002. Correlation of long-range membrane order with temperature-dependent growth characteristics of parent and a cold-sensitive, branched-chain-fatty-acid-deficient mutant of Listeria monocytogenes. Arch. Microbiol. 177:217-222. [DOI] [PubMed] [Google Scholar]
- 17.Junttila, J. R., S. I. Niemala, and J. Hirn. 1988. Minimum growth temperature of Listeria monocytogenes and non-haemolytic Listeria. J. Appl. Bacteriol. 65:321-327. [DOI] [PubMed] [Google Scholar]
- 18.Kaneda, T. 1991. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55:288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu, Y. J., Y. M. Zhang, and C. O. Rock. 2004. Product diversity and regulation of type II fatty acid synthases. Biochem. Cell Biol. 82:145-155. [DOI] [PubMed] [Google Scholar]
- 21.Mastronicolis, S. K., N. Arvanitis, A. Karaliota, C. Litos, G. Stavroulakis, H. Moustaka, A. Tsakirakis, and G. Heropoulos. 2005. Cold dependence of fatty acid profile of different lipid structures of Listeria monocytogenes. Food Microbiol. 22:213-219. [Google Scholar]
- 22.Mead, P. S., L. Slutsker, V. Dietz, F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moche, M., G. Schneider, P. Edwards, K. Dehesh, and Y. Lindqvist. 1999. Structure of the complex between the antibiotic cerulenin and its target, β-ketoacyl-acyl carrier protein synthase. J. Biol. Chem. 274:6031-6034. [DOI] [PubMed] [Google Scholar]
- 24.Nichols, D. S., K. A. Presser, J. Olley, T. Ross, and T. A. McMeekin. 2002. Variation of branched-chain fatty acids marks the normal physiological range for growth in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2809-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oku, H., and T. Kaneda. 1988. Biosynthesis of branched-chain fatty acids in Bacillus subtilis. J. Biol. Chem. 263:18386-18396. [PubMed] [Google Scholar]
- 26.Puttman, M., N. Ade, and H. Hof. 1993. Dependence of fatty acid composition of Listeria spp. on growth temperature. Res. Microbiol. 144:279-283. [DOI] [PubMed] [Google Scholar]
- 27.Rock, C. O., and S. Jackowski. 2002. Forty years of bacterial fatty acid synthesis. Biochem. Biophys. Res. Commun. 292:1155-1166. [DOI] [PubMed] [Google Scholar]
- 28.Sinensky, M. 1974. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 71:522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suutari, M., and S. Laakso. 1994. Microbial fatty acids and thermal adaptation. Crit. Rev. Microbiol. 20:285-328. [DOI] [PubMed] [Google Scholar]
- 30.Tsai, H. N., and D. A. Hodgson. 2003. Development of a synthetic minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 69:6943-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber, M. H. W., W. Klein, L. Muller, U. M. Niess, and M. Marahiel. 2001. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol. Microbiol. 39:1321-1329. [DOI] [PubMed] [Google Scholar]
- 32.Zhu, K., D. O. Bayles, A. Xiong, R. K. Jayaswal, and B. J. Wilkinson. 2005. Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon interrupted branched-chain α-keto acid dehydrogenase. Microbiology 151:615-623. [DOI] [PubMed] [Google Scholar]