Abstract
Biofilm cells differ phenotypically from their free-floating counterparts. Differential growth rates in biofilms are often referred to, particularly in response to limited diffusion of oxygen and nutrients. We observed growth rates of attached Pseudomonas sp. strain CT07 cells that were notably higher than the maximum specific growth rate measured in batch culture. Despite dilution rates in continuous flow cells that exceeded the maximum planktonic specific growth rate by 58 times, sampling of the effluent revealed >109 cells ml−1, suggesting that biofilms function as a source of planktonic cells through high cell yield and detachment. Further investigation demonstrated considerable planktonic cell yield from biofilms as young as 6 h, indicating that detachment is not limited to established biofilms. These biofilm-detached cells were more sensitive to a commercial biocide than associated biofilm- and chemostat-cultivated populations, implying that detached biofilm cells exhibit a character that is distinct from that of attached and planktonic cell populations.
In a minireview published in 1994, Costerton and coauthors asked, “What are the essential differences between a planktonic cell growing in the conventional batch culture and a cell of the same species growing in a natural multispecies biofilm?” (10). The inherent sessile and structurally heterogeneous character of natural biofilms come to mind, where bacteria and extracellular polymeric substances (EPS) are assembled into microcolonies interspersed with water-filled channels (9, 22). This sessile mode of growth generally allows greater numbers of bacteria to coexist than free-floating bacterial populations in bulk-liquid systems (13).
The metabolic activity of biofilms is theoretically controlled by environmental conditions at the surface and the expression of specific genes induced by adhesion. Investigations into the genetic basis of biofilm formation and development revealed several genes that are involved in biofilm formation (25). For example, genes encoding for flagella, type I and type IV pili, surface adhesins, homoserine lactones, and several others have been identified as being involved in the formation of biofilms under various environmental conditions (11, 26).
While numerous studies have investigated the changes in gene expression when microbes attach to surfaces, the process of detachment has received little attention and therefore affords an opportunity for future research (24). The traditional model of biofilm formation proposes development in discrete steps. These involve formation of a conditioning film, transport of microbes to the surface, and reversible initial adhesion through nonspecific van der Waals forces, followed by irreversible attachment and the production of EPS (8). However, once surface colonization has taken place, detachment of adherent cells is necessary to minimize starvation due to increasing numbers of cells at the surface and to allow colonization of new surfaces (1).
While it is thought that a lack of internal cohesive forces or unfavorable environmental conditions (i.e., high shear force) may result in the detachment of parts of the biofilm, the possible involvement of genetic determinants in the detachment process of individual cells has only recently been addressed. The active detachment of cells from biofilms can be mediated through the expression of extracellular enzymes that target the exopolysaccharide component of the biofilm matrix, as was shown when alginate lyase was expressed in alginate-producing Pseudomonas aeruginosa biofilms (6). The activity of an N-acetylglucosamonidase (dispersin B) produced by Actinobacillus actinomycetemcomitans was recently demonstrated against a component of the Staphylococcus epidermidis biofilm matrix. Enzymatic activity not only resulted in the detachment of existing S. epidermidis biofilms but also prevented initial colonization (17). Gilbert et al. noted that since changes in bacterial physiology occur after contact with a surface, one could argue that an alteration in the properties of an attached bacterium could result in detachment (14). Biofilm-detached Escherichia coli cells were found to be significantly more hydrophilic than the attached population, which Allison et al. suggested could reflect the involvement of the cell division cycle in detachment from biofilms (1).
Recent work by Sauer et al. showed that biofilm development by Pseudomonas aeruginosa proceeds through five physiologically distinct phases, the last phase involving the detachment of adherent bacteria. They observed motile cells exiting from within attached cell clusters, leaving behind a hollow shell of nonmotile cells embedded in an EPS matrix. Whole-cell protein profiles indicated that detached cells were more closely related to planktonic cells than mature biofilm cells. The authors suggested that these detached bacteria are in transition from an attached to a planktonic phenotype (28).
The contention that detachment is a discrete step in the process of biofilm development was further supported by theobservations of Kaplan et al. (18). They reported that the detachment of nonmotile A. actinomycetemcomitans from a biofilm is dependent on the synthesis of a lipopolysaccharide component, O-polysaccharide. While the attachment and development of the biofilm was not disrupted when the gene for O-polysaccharide was interrupted, the detachment of single cells and/or small clusters from the biofilm was abolished (18). These results generally did not mention the age of the detached cells, although it is clear from the discussions that these cells were previously immobilized in the biofilm.
Continuous flow cells have found wide application to study biofilms. The associated planktonic populations in flow cell effluent have received comparatively little attention. In our studies, we observed cell numbers in flow cell effluent that could not be attributed solely to planktonic growth in the flow cell chambers under prevailing conditions of high dilution. This led us to hypothesize that, in contrast to the general assumption that attached cells replicate at lower rates than planktonic populations, biofilms indeed maintain high growth rates with concomitant yield of cells to the planktonic phase. We therefore conducted a series of experiments to directly and indirectly ascertain growth rates in biofilms. This was followed by studies applying viability and culturability determinations to compare biofilm-detached cells with biofilm and planktonically grown cells when challenged with an antimicrobial substance.
MATERIALS AND METHODS
Bacterial strain and cultivation.
A pseudomonad strain was isolated from industrial cooling water. Sequencing in both directions to completion of the 16S rDNA of the isolate revealed a 99% nucleotide identity (1,489 bp/1,495 bp) to Pseudomonas sp. strain AEBL3 (AY 247063) using the gapped-BLAST program at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/) (2). This strain, designated Pseudomonas sp. strain CT07, had a maximum planktonic specific growth rate (μmax planktonic) of 0.62 h−1 in batch culture. Freeze cultures were made in 40% glycerol and kept at −80°C. Cultivation for all subsequent biofilm and planktonic studies was done at room temperature (22 ± 1°C) on 3 g liter−1 tryptone soy broth (TSB) and 3 g liter−1 tryptone soy agar plates, respectively, unless stated otherwise.
Biofilm development and planktonic cell yield.
Perspex flow cells (32) covered with glass coverslips (no. 1 thickness, 75 mm by 50 mm) were used to cultivate biofilms. Each flow cell contained eight recessed flow chambers with the dimensions 31 mm by 4.0 mm by 2.2 mm. Effluent silicone tubes with an inner diameter of 1 mm and either 10 or 150 mm in length were used. The total available attachment area (Plexiglas flow chamber plus glass coverslip plus 10- or 150-mm silicone tubing) was thus 500 or 1,400 mm2. The flow chambers were sterilized with 3.5% (vol/vol) sodium hypochlorite for 20 min and rinsed with sterile growth medium for a minimum of 20 min prior to inoculation. A flow rate of 14.5 ml h−1 was maintained with a Watson Marlow 205S multichannel peristaltic pump. This flow rate resulted in a dilution rate in the flow cell chamber of 53 h−1 and a shear rate of 1.24 s−1.
Duplicate flow chambers were inoculated with Pseudomonas sp. strain CT07 with the pump turned off, and flow resumed after 30 min. After an additional 12 h, a Nikon Eclipse E400 microscope at 600-fold magnification and a COHU high-performance charge-coupled device camera (model 4912-5010/0000) were used to record biofilm development at 30-min intervals. Isolated attached cells or small microcolonies were selected at random and the time of first measurement recorded as time zero. The initial growth rate of attached cells at the substratum (i.e., planktonic cell attachment, growth, and cell division) and subsequent microcolony formation could be assessed with this approach. The variation in biofilm biomass over extended periods of time was measured photometrically in an adapted parallel plate flow cell. For this, a large-area photometer (S. Saftic, July 2003, German patent 19947651) was used to quantify the accumulation of biomass at the inner surfaces of the flow cell with an internal volume of 12 ml. The optical large-area photometer measures visible light scattered by small particles that are distributed across a rectangular plane of comparatively large size. The data were captured and analyzed over a 6-day period using LabView-based software (27).
The number of biofilm-detached cells (i.e., planktonic cell yield) was determined from the flow channel effluent using conventional plate counts. The effect of nutrient type and concentration on planktonic cell yield was evaluated in a parallel study. This involved the substitution of 3.0 g liter−1 TSB with 0.03 and 0.3 g liter−1 TSB, as well as a minimal salt solution with either 0.1 or 1.0 g liter−1 glucose. For the present study, we conducted a single series of experiments to determine whether our observations using a rich nutrient at relatively high concentration (i.e., 3.0 g liter−1 TSB) was representative of a variety of nutrient conditions.
Cultivation of planktonic populations.
Planktonic populations were cultivated either in 50-ml batch cultures or an aerated chemostat with a total volume of 200 ml. The chemostat was inoculated with 1 ml of an overnight culture and incubated for 12 h with continuous stirring and aeration before introduction of sterile growth medium at a dilution rate of either 0.3 or 0.5 h−1. The batch flasks were inoculated with overnight cultures and incubated with moderate shaking.
Biocide sensitivity.
A stock solution of a commercial biocide consisting of glutaraldehyde (12%) and a blend of isothiazolones (4%) was used to compare biofilm-detached cells with associated biofilm- and chemostat-cultivated populations. The biocide was applied at a dilution of 1:100 in TSB (3.0 g liter−1 TSB) for 5 h. This concentration was determined empirically and reduced the viability of planktonic cultures of Pseudomonas sp. strain CT07 by 90% when grown in a rich growth medium (3.0 g liter−1 TSB). The BacLight Live/Dead bacterial viability probe (Molecular Probes, Invitrogen), in combination with epifluorescence microscopy and appropriate excitation/barrier filter sets, was used for viability determinations. Images (n = 30) were analyzed with ScionImage software (Scion Corporation). The total percentage area of the microscope field covered by viable or nonviable bacteria was determined using manually entered threshold values, and the viable cell numbers were expressed as a percentage of all the cells present in each field (21). Conventional plate counts with 3.0 g liter−1 TSA were used to determine culturable cell numbers.
Biofilms and biofilm-derived planktonic cells.
Two-day-old biofilms (n = 4) were treated with the biocide, and the viability of biofilms, as well as the viability and culturability of the biofilm-derived cells, was determined for the treated and untreated flow chambers. An estimation of the amount of attached and biofilm-derived biomass before and after treatment could also be made with image analysis of BacLight-stained samples.
Chemostat populations.
Replicate (n = 4) chemostats were maintained with continuous stirring and aeration at a dilution rate of either 0.3 or 0.5 h−1. After 2 days, the biocide was added to the inflowing growth medium for a period of 5 h. Samples were collected before and after biocide treatment for viability and culturability determinations.
Statistical analyses were performed to determine whether the difference in decreased percentage viability among the three populations was statistically significant. The arc sine square root transformation was used to normalize the percentage of data using the difference in viability before and after biocide treatment. Single-factor analysis of variance and paired t tests were performed, and a 95% confidence level was specified.
RESULTS
Biofilm formation rate and planktonic cell yield.
A primary objective of this study was to evaluate the growth of adherent cells and subsequent planktonic cell yield to the bulk liquid phase. Therefore, to minimize the impact of planktonic growth on the cell numbers in flow cell effluent, the dilution rate in the flow cells was maintained at 58 times the μmax planktonic (0.62 h−1).
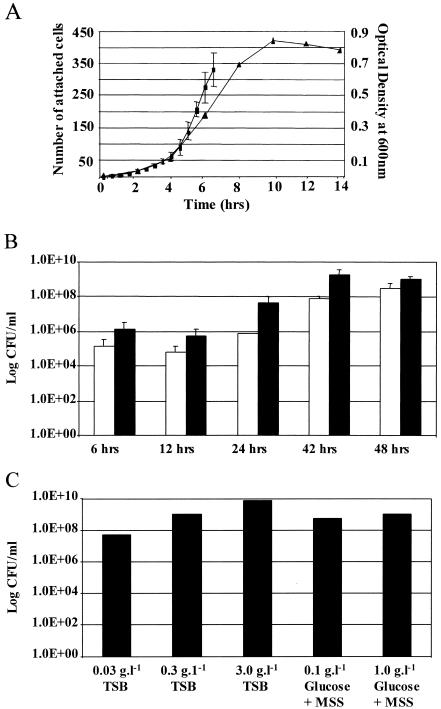
The rate of Pseudomonas sp. strain CT07 biofilm development during the early stages is shown in Fig. 1A. The increase in cell numbers at the glass attachment surface was determined until three-dimensional microcolony formation prevented accurate enumeration. Based on these counts, the initial biofilm growth rate (i.e., attachment and detachment, as well as growth and cell division of attached cells) was calculated as 1.2 h−1, which is notably higher than the specific planktonic growth rate (0.62 h−1) calculated in batch cultures. Figure 1B shows the numbers of culturable cells in the flow cell effluent. The effect of nutrient type and concentration on the number of culturable biofilm-detached cells is shown in Fig. 1C.
FIG. 1.
(A) Growth rate of Pseudomonas sp. strain CT07 in batch culture (▴) compared to the rate of attachment and growth typically observed on a glass surface (▪) during the early stages of biofilm formation. After inoculation and a 30-min period without flow, flow was resumed for 12 h, after which the accumulation at the glass surface was quantified at 30-min intervals (time zero and onward on the x axis). (B) Numbers of culturable biofilm-detached cells in the effluent of flow cells with total attachment areas of 500 (□) and 1,400 (▪) mm2, respectively. (C) Effect of carbon source and nutrient type on the number of culturable biofilm-detached cells in the effluent associated with 3-day-old biofilms. MSS, minimal salt solution.
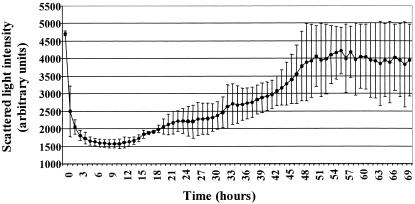
It was possible to record biofilm accumulation beyond the development of a monolayer by using a large-area photometer (Fig. 2). Data obtained by both direct microscopic quantification of sessile cells (Fig. 1A) and indirect photometrical quantification of three-dimensional biofilm formation with the photometer (Fig. 2) showed an exponential increase in biomass. Direct observations commenced 12.5 h after the inoculation of the flow cells (Fig. 1A) (time zero) because notable growth occurred only after this time. Indirect monitoring showed an initial decrease in biomass in the flow cell as the unattached inoculum washed out (Fig. 2). This was followed by a phase of no increase that lasted approximately 15 h and a rapid increase in biofilm biomass over the ensuing 33 h. This approach provided evidence that the initial establishment and subsequent biofilm development spanned 48 h. This was followed by a relatively stable maintenance of the biofilm biomass, with variation presumably due to the detachment of parts of the biofilm and regrowth to the original thickness.
FIG. 2.
Attachment and biofilm accumulation by Pseudomonas sp. strain CT07 at a Plexiglas surface over a period of 70 h. This plot shows the average of the two replicate experiments.
Figure 2 shows a typical profile of biofilm development obtained under identical experimental conditions, indicating the variability in behavior of microbes at surfaces, even when grown in pure culture. Lewandowski et al. reported that, despite efforts to follow precise methodology, the cultivation of structurally comparable biofilms over long periods was generally not successful. They observed that duplicate biofilms exhibited similar structures only until the first sloughing of the biofilm occurred, after which the pattern of biofilm formation varied (23).
Since the dilution rate applied to the flow cells was much greater than the maximum specific planktonic growth rate, it was assumed that the bacterial cells in the flow cell effluent were predominantly released from the biofilm. Despite the high dilution rate, the number of cells in the effluent that detached from steady-state (≥48 h) biofilms often exceeded 108 ml−1, suggesting a high yield of cells to the bulk-liquid phase. Indeed, there was a notable number of cells in the flow cell effluent after only 6 h of biofilm growth (Fig. 1B). This raised the question whether the biofilm-detached cells exhibited a biofilm character. If indeed so, it may contribute to our understanding of the variable response of microbes to environmental perturbations. We used sensitivity to an antimicrobial compound to investigate this question.
Response of biofilm, biofilm-detached, and planktonic cells to biocide treatment.
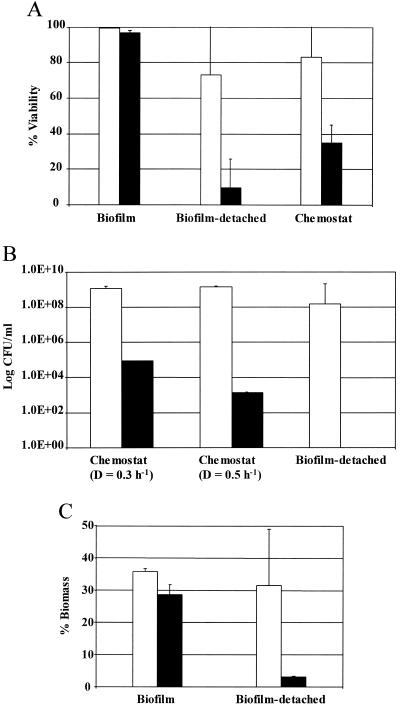
Comparison of the effect of the biocide on the viability of chemostat-grown and biofilm cells suggested that the biofilm population was more resistant to the antimicrobial compound (Fig. 3A). Analysis of variance indicated a significant difference between the reduction in percent viability among the biofilm, chemostat, and biofilm-detached populations (P = 0.004; df between groups = 2, df within groups = 9). Paired t tests revealed a statistically significant difference between the biofilm and chemostat (P = 0.049; df = 3) and the biofilm and biofilm-detached populations (P = 0.018; df = 3). No statistically significant difference in viability was indicated between the chemostat and biofilm-detached populations (P = 0.246; df = 3). However, Fig. 3B shows that while the culturable number of cells in the chemostat with dilution rates of 0.3 h−1 and 0.5 h−1 decreased from 1.2 × 109 to 6.6 × 104 ml−1 and 1.4 × 109 to 1.4 × 103 ml−1, respectively, no biofilm-detached cells could be cultivated from the flow cell effluent following biocide treatment.
FIG. 3.
(A) Viability of control (□) and biocide-treated (▪) cells as determined with BacLight viability staining. There was a significant difference between the sensitivity (percent reduction in viable numbers) of sessile and biofilm-detached cells and sessile and planktonically grown (chemostat) populations. (B) Culturability of control (□) and biocide-treated (▪) cells. The cells derived from biofilms were more sensitive than chemostat-derived cells; the effect on viability was similar to that shown in panel A (D; dilution rate). (C) Percent area coverage by biofilms and released biofilm biomass before (□) and after (▪) biocide treatment. To determine the extent of detached biofilm biomass, flow cell effluent was stained with BacLight and a standardized volume was immobilized on filters, followed by measuring the area coverage using image analysis. The difference in percent reduction in area coverage between the attached and flow cell effluent cells was significant (P = 0.026).
While the amount of biofilm biomass at the surface was not significantly affected by the biocide, the number of biofilm-detached cells yielded into the effluent declined substantially (Fig. 3C). When determining the difference in the responses to biocide treatment between the biofilm and biofilm-derived cells, it was found to be statistically significant (P = 0.026; df between groups = 1, df within groups = 6). These results suggest that the outer regions of biofilms are responsible mainly for the yield of planktonic cells to the effluent at high dilution rates. This implies that the cells at the biofilm-liquid interface maintain a high growth rate, as suggested by others.
The observed trend in the loss of culturability of cells in the flow cell effluent corroborated the reduction in viability as determined with the BacLight probe, but the difference in the percentages of decrease was a cause for concern. We further evaluated this by determining the ability of biofilm-derived planktonic cells to grow in liquid cultures containing a range of nutrient concentrations (0.3, 3.0 and 30 g liter−1 TSB) after incubation with the biocide by using the three-tube most-probable-number (MPN) technique. Furthermore, untreated and treated biofilms were removed from glass surfaces and the surviving culturable cell numbers (using plate counts) and the percent viability (using BacLight) were determined. No regrowth was observed for any of the liquid-based MPN cultures, while a small decrease was noted in the viability of physically removed biofilm cells compared to that of cultured cells. This corroborates results obtained for the chemostat samples (data not shown) which strongly suggest that the biofilm-derived cells were indeed more susceptible to antimicrobial treatment.
Differential susceptibility to antimicrobial treatment in biofilms will then likely be correlated to growth rate. It was therefore necessary to assess whether growth phase and, by inference, metabolic activity influenced the survival of our test strain when challenged with the antimicrobial agent. This was done in batch cultures, where it was found that no bacteria could be cultured from the lag and early exponential phase after incubation with the biocide. In contrast, ∼1.1 × 102 and 2.3 × 102 ml−1 cells survived treatment during the late exponential and stationary phases, respectively. Comparable data were obtained with BacLight.
DISCUSSION
Although reference is often made to differential growth rates in biofilms, discussions in this regard are primarily focused on the slower growth associated with the biofilm phenotype. However, the data in Fig. 1A show that the growth rate of Pseudomonas sp. strain CT07 during early biofilm formation is notably higher than the maximum specific growth rate measured for planktonic cells in batch culture. A shorter generation time in biofilms than that of planktonic populations has been reported elsewhere (15), although the opposite has also been observed (5).
We were interested in determining whether the observed initially high growth rates are maintained in older, multilayered biofilms. A reasonable assumption is that once the cells become entrapped in the biofilm matrix, their growth rates decrease, or even cease, due to substrate and spatial limitations, and subsequently, other properties, such as increased resistance to outside disturbances, emerge. Higher growth rates will thus be restricted to the outer regions of the biofilm. Because of their fixed position, we could observe cells near the attachment surface that, although not dividing, remained viable over extended periods of time. The irregular and often changing shape of the outer biofilm layers did not allow temporal studies at the biofilm-liquid interface. The measured biofilm-detached cell numbers in the flow cell effluent could therefore serve as an indirect indicator of the growth rate in the biofilms.
As stated previously, a dilution rate that greatly exceeded the maximum planktonic growth rate was maintained in the flow cells to minimize the contribution of planktonic growth to the biofilm-detached cell numbers in the effluent. The flow cell channels each had a volume of 0.27 ml, and the silicon effluent tubes had a volume of 0.13 ml (total attachment area of 1,400 mm2). For comparison, shorter tubes that contributed 0.04 ml to the total volume (total attachment area of 500 mm2) were also included (Fig. 1B). However, to facilitate efficient routine sampling and to minimize possible contamination of the flow cell channels, the longer effluent tubes were used in routine experimentation. When the internal volume of the flow cell channels and longer silicone effluent tube were taken into account, the dilution rate was 36.1 h−1, which is 58 times theμmax planktonic. Despite this high dilution rate, there were >109 cells ml−1 in the effluent originating from biofilms older than 42 h (Fig. 1B), suggesting high rates of cell yield and detachment from the biofilms. Indeed, this value translates in a cell yield per attachment surface area of 1.14 × 109 cm−2 h−1. In comparison, the cell yield per attachment area in the chemostat was 2.7 × 108 cm−2 h−1. However, even if it is assumed that biofilm growth occurred on the sides and bottom of the chemostat flask at a dilution rate of 0.48 times the μmax planktonic,planktonic growth probably yielded the majority of cells in the chemostat effluent.
The cell size of Pseudomonas sp. strain CT07 in biofilms was typically 4 to 5 μm by 1 μm. Hypothetically, if the cells were tightly packed in a monolayer (50% polarly attached and 50% laterally), there would be 6.25 × 107 cells cm−2. A doubling time of 1.2 h−1 would therefore result in a yield of 7.5 × 107 cells cm−2 h−1. The cell yield measured in the flow cells was notably higher than this theoretical value, demonstrating the capacity of biofilms to serve as a planktonic “cell factory.” Our experimental approach did not enable the measurement of cell growth in multilayered biofilms; therefore, the nature of factors primarily responsible for the measured high yield of biofilm-detached cells can only be speculated on. We hypothesize that the outer biofilm layers in mature biofilms maintain the high growth rate observed during the early stages and that this, together with the increase in the area at the biofilm-liquid interface resulting from the development of a three-dimensional biofilm architecture, ensures a high yield of detached cells. Indeed, analysis that would allow accurate measurement of the surface area of the biofilm-liquid interface will most probably demonstrate this contention and thus support our hypothesis. Various factors such as nutrient flux, type of organism, shear, and predation will have an effect on this yield. Furthermore, the presence of EPS may restrict the release of cells into the planktonic phase. Our previous observations on biofilm structure showed a degree of feedback in response to the chemical environment (20, 32). In fact, this could be a contributing mechanism determining the relative position of the biofilm population on the r-K gradient (r strategists are adapted to rapid reproduction, while K strategists are better adapted to environmental limitations) (3, 4). Interestingly, biofilms growing on 0.3 g liter−1 TSB (100-fold dilute) or a minimal salts solution plus 0.1 g liter−1 glucose still yielded 7 × 107 and 7.6 × 108 cells ml−1, respectively (Fig. 1C). Furthermore, the effluent numbers obtained in this study are in the same order as those that were reported earlier (19) when a mixed community was cultivated in a similar flow cell system, indicating that the measured high yield is not an isolated case.
An interesting observation made by Hendrickx et al. was that most transformation events in an Acinetobacter sp. biofilm took place near the substratum despite their demonstration of increased competency among exponentially growing cells in batch and young biofilms (16). It is possible that the lack of observable transformation events in cell layers closer to the bulk liquid was due to the continuous detachment of the young, actively growing cells from the biofilm.
Figure 1B shows a notable number of biofilm-detached cells in suspension even at the early stages (6 h) of biofilm formation, suggesting that our classical view that biofilms develop in discrete steps, with detachment typically occurring after reaching a (semi-) steady state, may need reevaluation. It is indeed possible that detachment occurs whenever nutritional and other environmental conditions allow cell growth and is not solely related to biofilm age or stage. Cells are thus released from the primary attachment surface during early biofilm formation and from the biofilm-liquid interface after the development of multilayered biofilms.
Work by Spoering and Lewis showed that P. aeruginosa biofilms and planktonic cells in the stationary phase have similar abilities to resist killing by antimicrobial agents. Indeed, in their examination of the effects of four antimicrobial agents with diverse modes of action, they found that stationary-phase cells were moderately more resistant to killing than biofilm bacteria. They attributed this ability to survive antimicrobial treatment to slow growth and the presence of persister cells (29). The varied responses of batch-grown Pseudomonas sp. strain CT07 to antimicrobial treatment in the different growth phases also showed that actively dividing bacteria in the exponential growth phase were more susceptible to antimicrobial treatment than cells in the stationary phase. By inference, a rapidly dividing, metabolically active layer of the biofilm should similarly be more sensitive to treatment with an antimicrobial agent. The result of this would be cessation of cell division and, consequently, a decline in the number of cells yielded by the biofilm to the planktonic phase, as illustrated in Fig. 3C. The presence of a highly metabolically active region within biofilms has been documented previously in an investigation into the spatial distribution of specific growth rates within Klebsiella pneumoniae biofilms when acridine orange was used to show that the outer 10- to 30-μm layer of cells was more active than those in the interior of the biofilm (31). A higher ratio of RNA to DNA content, which correlates to a high specific growth rate, was visualized in the layer closest to the biofilm bulk-liquid interface, and this was attributed to greater access to nutrients and oxygen.
The high yields of detached cells as reported here indicate rapid growth by the cells positioned at the outer regions of the biofilm, supporting the view that differential growth rates occur in biofilms. The formation of an extensive multilayered architecture ensures that the outer regions of the biofilm are optimally positioned to benefit from easy access to nutrients and oxygen from the bulk fluid. In fact, the irregular surface of biofilms was shown to increase the diffusion of oxygen into the biofilm from the bulk liquid (12). The biofilm mode of growth thus allows cells from the same microbial population to optimize reproductive capacity (fast growers at the biofilm-liquid interface) as well as the conservation of resources (slow growers in deeper regions), which is in contrast with the r-K scheme of population selection. The latter assumes that populations optimize either reproductive ability or resource conservation, but not both (3, 4).
A question that needs to be addressed is whether these recently released biofilm-derived planktonic cells maintain their biofilm character. There has been considerable discussion on the switch in gene expression that occurs when planktonic cells attach to a surface, but comparatively little has been mentioned in this regard when cells detach. It can be expected that when older biofilm cells are released from the biofilm as a result of shear, or other forces, these released cells should exhibit a biofilm phenotype. However, Fig. 3A and B show that the viability and culturability of biofilm-detached Pseudomonas sp. strain CT07 cells differ significantly from those of associated biofilm- and chemostat-grown populations when challenged with an antimicrobial agent. There is a wealth of literature suggesting that the biofilm mode of growth is more resistant to killing by antimicrobial agents than the planktonic phase (7, 30). In this study, attached cells also retained greater viability after biocide treatment than that of the chemostat-derived cells.
In conclusion, this study demonstrated that biofilms act as a “cell factory” under conditions of high nutrient flux. It remains to be confirmed whether these released cells exhibit a biofilm or planktonic pattern of gene expression or whether a third phenotype exists. If the released cells exhibit a biofilm-related or a third phenotype, it needs to be established how long this trait is retained when the cells are subsequently restricted to a planktonic existence. This phenomenon will be especially relevant in systems with high dilution rates and large surface-to-volume ratios such as intertidal zones and shallow rivers in the natural environment, as well as engineered systems such as water cooling plants, biofilm reactors, and hydroponic installations.
Acknowledgments
Murray N. Gardner is thanked for technical advice.
Sasol Technology (Pty) Limited, the South African National Research Foundation, the South African Medical Research Council, and the Claude Harris Leon Foundation are acknowledged for financial support.
REFERENCES
- 1.Allison, D. G., D. J. Evans, M. R. W. Brown, and P. Gilbert. 1990. Possible involvement of the division cycle in dispersal of Escherichia coli from biofilms. J. Bacteriol. 172:1667-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, J. H., and R. F. Harris. 1986. r- and K-selection and microbial ecology. In K. C. Marshall (ed.), Advances in microbial ecology, vol. 9. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 4.Atlas, R. M., and R. Bartha. 1998. Microbial ecology: fundamentals and applications, 4th ed. Benjamin/Cummins Science Publishing, Menlo Park, Calif.
- 5.Barton, A. J., R. D. Sagers, and W. G. Pitt. 1996. Measurement of bacterial growth rates on polymers. J. Biomed. Mat. Res. 32:271-278. [DOI] [PubMed] [Google Scholar]
- 6.Boyd, A., and A. M. Chakrabarty. 1994. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 60:2355-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broon, A., S. Liu, and K. Lewis. 2000. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 44:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busscher, H. J., and H. C. Van der Mei. 2000. Initial microbial adhesion events: mechanisms and implications. Community structure and co-operation in biofilms. Cambridge University Press, Cambridge, United Kingdom.
- 9.Caldwell, D. E., D. R. Korber, and J. R. Lawrence. 1992. Confocal laser microscopy and computer image analysis. Adv. Microb. Ecol. 12:1-67. [Google Scholar]
- 10.Costerton, J. W., Z. Lewandowski, D. DeBeer, D. Caldwell, D. Korber, and G. James. 1994. Biofilms, the customized microniche. J. Bacteriol. 176:2137-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 12.DeBeer, D., P. Stoodley, F. Roe, and Z. Lewandowski. 1994. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 43:1131-1138. [DOI] [PubMed] [Google Scholar]
- 13.Geesey, G. G., W. T. Richardson, H. G. Yeomans, R. T. Irvin, and J. W. Costerton. 1977. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can. J. Microbiol. 23:1733-1736. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert, P., D. J. Evans, and M. R. W. Brown. 1993. Formation and dispersal of bacterial biofilms in vivo and in situ. J. Appl. Bacteriol. Symp. Suppl. 74:67S-78S. [DOI] [PubMed] [Google Scholar]
- 15.Gottenbos, B., H. C. van der Mei, and H. J. Busscher. 2000. Initial adhesion and surface growth of Staphylococcus epidermidis and Pseudomonas aeruginosa on biomedical polymers. J. Biomed. Mat. Res. 50:208-214. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickx, L., M. Hausner, and S. Wuertz. 2003. Natural genetic transformation in monoculture Acinetobacter sp. strain BD143 biofilms. Appl. Environ. Microbiol. 69:1721-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan, J. B., C. Ragunath, K. Velliyagounder, D. H. Fine, and N. Ramasubbu. 2004. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 48:2633-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan, J. B., M. F. Meyenhofer, and D. H. Fine. 2003. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J. Bacteriol. 185:1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karthikeyan, S., D. R. Korber, G. M. Wolfaardt, and D. E. Caldwell. 2000. Monitoring the organization of microbial biofilms, p. 171-188. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods, and applications. Humana Press, Totowa, N.J.
- 20.Karthikeyan, S., G. M. Wolfaardt, D. R. Korber, and D. E. Caldwell. 1999. Functional and structural responses of a degradative microbial community to substrates with varying degrees of complexity in chemical structure. Microb. Ecol. 38:215-224. [DOI] [PubMed] [Google Scholar]
- 21.Korber, D. R., A. Choi, G. M. Wolfaardt, and D. E. Caldwell. 1996. Bacterial plasmolysis as a physical indicator of viability. Appl. Environ. Microbiol. 62:3939-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence, J. R., D. R. Korber, and G. M. Wolfaardt. 1996. Heterogeneity of natural biofilm communities. Cells Mater. 6:175-191. [Google Scholar]
- 23.Lewandowski, Z., H. Beyenal, and D. Stookey. 2004. Reproducibility of biofilm processes and the meaning of steady state in biofilm reactors. Water Sci. Technol. 49:359-364. [PubMed] [Google Scholar]
- 24.O'Toole, G. A., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 25.O'Toole, G. A., and R. Kolter. 1998. The initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 26.Pratt, L. A., and R. Kolter. 1999. Genetic analysis of bacterial biofilm formation. Curr. Opin. Microbiol. 2:598-603. [DOI] [PubMed] [Google Scholar]
- 27.Saftic, S., L.-M. Joubert, E. Bester, and G. M. Wolfaardt. 2005. A biofilm apparatus for the teaching lab. ASM Focus Microbiol. Educ. 11: 12-14. [Google Scholar]
- 28.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spoering, A. L., and K. Lewis. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walters, M. C., F. Roe, A. Bugnicourt, M. J. Franklin, and P. S. Stewart. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wentland, E. J., P. S. Stewart, C.-T. Huang, and G. A. McFeters. 1999. Spatial variations in growth rate within Klebsiella pneumonia colonies and biofilms. Biotechnol. Prog. 12:316-321. [DOI] [PubMed] [Google Scholar]
- 32.Wolfaardt, G. M., J. R. Lawrence, R. D. Robarts, S. J. Caldwell, and D. E. Caldwell. 1994. Multicellular organization in a degradative biofilm community. Appl. Environ. Microbiol. 60:434-446. [DOI] [PMC free article] [PubMed] [Google Scholar]