Abstract
Previous work has shown that microbial communities in As-mobilizing sediments from West Bengal were dominated by Geobacter species. Thus, the potential of Geobacter sulfurreducens to mobilize arsenic via direct enzymatic reduction and indirect mechanisms linked to Fe(III) reduction was analyzed. G. sulfurreducens was unable to conserve energy for growth via the dissimilatory reduction of As(V), although it was able to grow in medium containing fumarate as the terminal electron acceptor in the presence of 500 μM As(V). There was also no evidence of As(III) in culture supernatants, suggesting that resistance to 500 μM As(V) was not mediated by a classical arsenic resistance operon, which would rely on the intracellular reduction of As(V) and the efflux of As(III). When the cells were grown using soluble Fe(III) as an electron acceptor in the presence of As(V), the Fe(II)-bearing mineral vivianite was formed. This was accompanied by the removal of As, predominantly as As(V), from solution. Biogenic siderite (ferrous carbonate) was also able to remove As from solution. When the organism was grown using insoluble ferrihydrite as an electron acceptor, Fe(III) reduction resulted in the formation of magnetite, again accompanied by the nearly quantitative sorption of As(V). These results demonstrate that G. sulfurreducens, a model Fe(III)-reducing bacterium, did not reduce As(V) enzymatically, despite the apparent genetic potential to mediate this transformation. However, the reduction of Fe(III) led to the formation of Fe(II)-bearing phases that are able to capture arsenic species and could act as sinks for arsenic in sediments.
The mobilization of arsenic from sediments to drinking water constitutes a major toxic hazard to millions in Bangladesh and West Bengal. A number of mechanisms have been proposed for the release of arsenic into the groundwater in Bengal shallow alluvial sedimentary aquifers (1, 3, 8, 11, 12, 18, 21, 33-35, 39), including the oxidation of arsenic-rich pyrite in aquifer sediments, driven by lowering of the water level by abstraction, and then penetration of the aquifer by oxygen (8, 11, 12), or the reductive dissolution of arsenic-rich iron-oxy-hydroxides, driven by the microbial consumption of sedimentary organic matter in anoxic groundwater (33, 34, 39). The latter mechanism has received recent support as the dominant mechanism for groundwater arsenic contamination (3, 20, 21, 39).
In a recent microcosm-based study (21), we provided the first direct evidence of the role of indigenous metal-reducing bacteria in the formation of toxic, mobile As(III) in sediment from the Ganges Delta. The study showed that addition of acetate to anaerobic sediments, as a proxy for organic matter and a potential electron donor for metal reduction, resulted in stimulation of the microbial reduction of Fe(III), followed by As(V) reduction and release of As(III). Culture-dependent techniques confirmed a role for Fe(III)-reducing bacteria in As release, while PCR studies showed that the microbial communities in these sediments were dominated by Fe(III)-reducing bacteria most closely related to known Geobacter species. These results suggest that either direct enzymatic microbial reduction of As(V) by Fe(III)-reducing bacteria or indirect mechanisms associated with the reduction of Fe(III) oxides [for example, the reductive dissolution of host Fe(III) oxy-hydroxides or reduction of As(V) via microbially generated Fe(II)] could be important mechanisms for arsenic release in these sediments, with the involvement of Geobacter species implicated in these transformations.
Multiple studies have shown that members of the family Geobacteraceae predominate in aquifers where Fe(III) reduction is a significant terminal electron-accepting process, especially when sediments are stimulated with acetate as an electron donor (for examples, see references 19, 21, and 41). In these environments, they play a dominant role in the degradation of organic matter and also control the mobility of toxic metals and radionuclides (23). Given the environmental importance of this group of bacteria, there have been considerable research activities focused on the physiology and biochemistry of Geobacter species (26), underpinned by the recently published genome sequence for Geobacter sulfurreducens (30) and the development of a genetic system for this organism (9). Although Geobacter species have not been reported to reduce As(V), these organisms do have the physiological capacity to reduce a wide range of metals and metalloids (7, 23) via a battery of c-type cytochromes (30), while the existence of an arsenic resistance operon, including a gene for a putative arsenate reductase (arsC), has also been reported for G. sulfurreducens (30). Thus, the aim of this study was to determine if this model Geobacter species is able to mobilize arsenic via three potential mechanisms: (i) the direct enzymatic reduction of As(V) to potentially more soluble As(III), (ii) release of As(V) or As(III) via the reductive dissolution of host Fe(III) oxy-hydroxides, or (iii) indirect reduction and mobilization by biogenic Fe(II).
MATERIALS AND METHODS
Maintenance and growth of the organism.
G. sulfurreducens (strain ATCC 51573) was grown at 20°C under anaerobic conditions in a modified freshwater medium as described previously (24). Acetate (20 mM) and fumarate (40 mM) were supplied as the electron donor and the electron acceptor, respectively, unless otherwise stated. Cells were manipulated under an atmosphere of N2-CO2 (80:20).
Metal reduction experiments with actively growing cells of Geobacter sulfurreducens.
Cells were grown in freshwater medium containing acetate (20 mM) as the electron donor and soluble Fe(III)-citrate (56 mM) or fumarate (40 mM) as the electron acceptor. Sodium arsenate (500 μM) was added from an anaerobic stock solution and in some experiments was used as the terminal electron acceptor for growth in lieu of Fe(III)-citrate. A 10% inoculum was used throughout, and the cultures were incubated in the dark at 20°C. All the manipulations were done under a modified atmosphere of 5% H2 + 95% N2 in an anaerobic cabinet (Coy Laboratory Products Inc., Michigan).
Metal reduction experiments with resting cells of Geobacter sulfurreducens.
Late-log-phase cultures were harvested by centrifugation at 3,000 rpm (Centaur 2 centrifuge; MSE, Kent, United Kingdom) for 10 min and washed twice in carbonate buffer (NaHCO3 at 30 mM, pH 7.1) in N2-CO2 (80:20). Aliquots of the washed cell suspension (1.5 ml) were added to the anaerobic bottles containing 18.5 ml carbonate buffer and sealed with butyl rubber stoppers. The final biomass concentration was about 0.7 mg/ml protein. The following additions were made from anaerobic stock solutions as required: poorly crystalline Fe(III) oxide (10 mM) (27), sodium arsenate (100 μM), and sodium acetate (20 mM). All of these preparations were done using strict anoxic techniques in N2-CO2 at 80:20. Bottles were incubated in the dark at 20°C.
Analytical techniques.
Approximately 1 ml of sample was removed from experimental cultures with a sterile syringe and needle in an anaerobic cabinet, and 100 μl of sample was used to measure Fe(II) concentrations spectrophotometrically after reaction with ferrozine (27). The rest of the sample was centrifuged for 10 min at a maximum speed of 14,000 rpm in a microcentrifuge (Spectrafuge 16 M; National Labnet Co., Woodbridge, NJ). An aliquot of the supernatant (100 μl) was used for the quantification of soluble Fe(II), where appropriate, using ferrozine, and 700 μl of the supernatant was filtered (0.1- to 0.2-μm Anotop 10 inorganic membrane filter; Whatman, England) into an Eppendorf tube (1.5 ml) to determine the total arsenic concentration. The pellet was washed twice with phosphate buffer (30 mM, pH 7.0), and the protein concentrations were determined by using bicinchoninic acid with bovine serum albumin as a standard (40).
Total arsenic in solution was assayed by inductively coupled plasma (ICP)-atomic emission spectrometry. Samples (500 μl) were removed from the bottles in an anaerobic cabinet and digested with 2% HNO3 (2.5 ml). Calibration standards were prepared with 2% subdistilled HNO3 prior to analysis by dilution of the concentrated reference element stock solutions. Calibration blocks were placed at even intervals throughout each analytical run in order to correct the instrument drift. Samples (1 ml) for arsenic speciation were removed from the bottles in an anaerobic cabinet and passed through a 0.45-μm filter prior to analysis by ion chromatography (IC)-ICP-mass spectrometry (MS) using the method of Gault et al. (16). Standard reference materials were included in each analytical run; the arsenic concentrations determined were found to agree well with those certified.
XRD.
X-ray diffraction (XRD) was used to determine the mineralogy of the precipitates that had been formed in the reduction experiments. The settled mineral residue was removed from the reduction experiments and dried under anaerobic conditions. The dried solid was smeared on a glass slide and was ground into a fine slurry with the addition of a few drops of amyl acetate, and the sample was then analyzed using a model 1730 Diffractometer with CuKα radiation (Phillips, Eindhoven, The Netherlands). Slides were kept under an anoxic atmosphere until analysis.
ESEM.
An environmental scanning electron microscope (model XL30; Phillips, Eindhoven, The Netherlands) was used to image G. sulfurreducens, and an energy-dispersive spectroscopy (EDS) system (PGT-PRISM-detector; Princeton-Gamma-Tech., NJ) was used to identify the major elements present in the associated biominerals. Aliquots (2 ml) of the cell suspensions were collected in a Spectrafuge 16 M microcentrifuge (6,000 rpm) for 5 min. The supernatant was discarded, the pellet was washed three times in carbonate buffer, and 25 μl of the washed suspension was pipetted onto an aluminum sample holder for viewing using environmental scanning electron microscopy (ESEM).
XAS.
The oxidation state and coordination environment of arsenic associated with the precipitates that had been formed in the reduction experiments were probed using X-ray absorption spectroscopy (XAS). All samples analyzed in these experiments were taken from cultures that were 40 to 45 days old. X-ray absorption spectra at the arsenic K-edge were collected on station 16.5 at the United Kingdom CCLRC Daresbury Synchrotron Radiation Source Laboratory operating at 2 GeV with a beam current of between 130 and 240 mA. A Si (220) double-crystal monochromator was used, with harmonic contamination of the beam minimized by a vertically focusing mirror in addition to detuning to 70%. Sample data were collected at liquid-nitrogen temperature with the station operating in fluorescence mode using an Ortec 30 element solid-state Ge detector. Standards of sodium arsenite, disodium arsenate heptahydrate, arsenopyrite (FeAsS), and As(III)-glutathione were collected at room temperature in transmission mode. The As(III)-glutathione was synthesized by mixing a 10-fold molar excess of glutathione with sodium arsenite.
X-ray absorption near-edge structure (XANES) spectra were analyzed using the Daresbury Laboratory program LINCOM. The summed-sample XANES spectra were fitted by adjusting the relative proportion (and hence contribution) of each of the three end member standard spectra until a least-squares residual was minimized. Further details regarding the analysis of the background-subtracted extended X-ray absorption fine-structure (EXAFS) spectra can be found in the supplemental material accompanying reference 21.
Bioinformatics.
The amino acid sequences for Staphylococcus aureus plasmid pI258 and Escherichia coli plasmid R773 arsenate reductases (ArsC) were retrieved from the National Center for Biotechnology Information (NCBI) database (accession numbers AAA25638.1 and C25937, respectively). These sequences were used to search the Geobacter sulfurreducens genome, also accessed through the NCBI database (accession number NC 002939.4) using the NCBI Genomic TBLASTN v2.2.9 program (4, 10). Open reading frames were detected using the NCBI ORF Finder (44) with the bacterial genetic coding option and Glimmer 2.13 from The Institute for Genomic Research (13). Promoter regions (σ70 dependent) were detected using the Sequence Alignment Kernel method embedded in the Dual Support Vector machine (17). The amino acid sequence of the putative G. sulfurreducens ArsC (accession number AAR36345.1) was used to search the NCBI nucleotide database using TBLASTN v2.2.9. Amino acid sequence alignments were made using CLUSTAL X (42).
RESULTS AND DISCUSSION
Potential for enzymatic reduction of As(V) by Geobacter sulfurreducens.
Given the wide range of high-oxidation-state metals utilized as electron acceptors for anaerobic respiration by G. sulfurreducens (7), coupled with the association of Geobacter species with sediments supporting the reduction of As(V) to As(III) (21), initial tests were conducted to determine if G. sulfurreducens was able to couple anaerobic growth to the reduction of As(V). However, attempts to subculture G. sulfurreducens in a defined medium containing 500 μM to 10 mM As(V) as the sole electron acceptor were unsuccessful; there was no increase in turbidity with prolonged incubation of cells in the growth medium and no evidence of As(V) reduction in the inoculated cultures. This was initially surprising, as parallel PCR experiments using primers designed to amplify a conserved region of the arrA (arsenate respiratory reductase) gene (28) amplified a 170-bp product consistent with partial arrA sequences amplified from other As(V)-respiring bacteria (G. Lear and J. R. Lloyd, unpublished data). However, when this PCR product was sequenced, it was not a close match to known arrA genes, and we could find no evidence of an arrA gene in the published genome of G. sulfurreducens. These results, therefore, support the hypothesis that G. sulfurreducens cannot respire through the dissimilatory reduction of As(V). It also suggests that existing primers designed against arrA (28) should be used with care when using genomic DNA from subsurface sediments.
Another explanation for the nongrowth of the strain in these experiments was toxicity of the arsenate anion to the cells. However, the presence of 500 μM As(V) added to medium containing 40 mM fumarate as an alternative terminal electron acceptor had no impact on the anaerobic growth of the organism. These results suggest that although it is not able to utilize As(V) as an electron acceptor, G. sulfurreducens does have an intrinsic level of resistance to As(V). Indeed, initial annotation of the genome of G. sulfurreducens suggested the presence of genes that may encode resistance of the organism to arsenic (30). Thus, we made a detailed analysis of potential arsenic resistance genes in this organism to verify whether G. sulfurreducens has the genetic potential to reduce As(V) to As(III) via a detoxification pathway, which could potentially result in the formation of As(III) when the organism is grown using other terminal electron acceptors [e.g., fumarate or Fe(III)]. It should be noted that the concentrations of arsenic used in this study are much higher than those reported for soluble arsenic in aquifer sediments (21, 39).
Analysis of the genome showed the presence of a cluster of three genes potentially coding for a transcriptional regulator of the ArsR family (arsR), an arsenate reductase (arsC), and an inorganic ion efflux protein homologous to the arsenite-transporting protein (acr3) in Saccharomyces cerevisiae. Furthermore, the putative As(V) reductase showed considerable homology with the many other prokaryotic ArsC proteins from the thioredoxin-dependent low-molecular-weight protein tyrosine phosphatase family, including that from Staphylococcus aureus pI258. Alignment of the amino acid sequence of the reductase with the ArsC from pI258 showed that many of the residues shown to be essential for the catalytic reduction of As(V) by S. aureus pI258, including all three of the active-site cysteine residues involved in the nucleophilic attack on the arsenic substrate, plus arginine-16 and aspartate-105 (29), are conserved in the putative ArsC of G. sulfurreducens (data not shown).
Further analysis showed that the three genes lie contiguously and are potentially transcribed in the same direction. There was strong evidence for the presence of σ70-dependent promoter sequences at positions −35 and −10 upstream of the arsC transcription start site, indicating that the three genes do not form a traditional operon and that arsC and acr-3 are potentially transcribed into a single polycistronic mRNA sequence independently of the putative arsR gene. The presence of eight contiguous thymine residues between the 3′ end of arsR and the promoter for arsC further supports this hypothesis.
To determine if the putative arsenic resistance operon of G. sulfurreducens played a role in resistance to the metalloid in fumarate-containing medium, we quantified the aqueous species of arsenic remaining in supernatants after growth. After 20 days of incubation under anaerobic conditions, all of the arsenic in solution (498 μM) was in the pentavalent oxidation state and no As(III) was detected by IC-ICP-MS. As we could detect no As(III) in the growth medium under these conditions, we concluded that the putative arsenic resistance operon reported in G. sulfurreducens is unlikely to have played a role in mediating resistance to the metalloid in these experiments and is unlikely, therefore, to function as an arsenic resistance determinant in the subsurface in this organism. The mechanism of resistance to arsenate in this organism clearly warrants further investigation.
Interactions of As(V) with biogenic Fe(II).
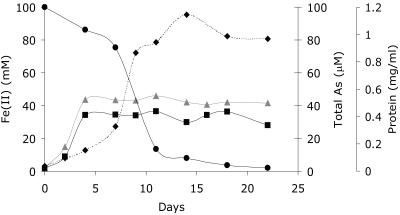
Having determined that Geobacter sulfurreducens did not reduce As(V) enzymatically under the conditions imposed in our experiments, we focused on the potential of Fe(II) formed by this organism to reduce As(V) abiotically (6). Initial experiments focused on actively growing cultures inoculated into medium containing 56 mM Fe(III)-citrate as the electron acceptor and supplemented with 100 μM As(V). In these experiments, growth was accompanied by the generation of Fe(II), the subsequent formation of a white precipitate in the cultures, and the removal of arsenic from the aqueous phase. At the end of the experiment (22 days), approximately 32% of the Fe(II) and 98% of the total arsenic were lost from the supernatant with the precipitate (Fig. 1). The remaining arsenic detected in the aqueous phase by IC-ICP-MS was As(V). Similar results were also noted at 500 μM As(V). Control experiments containing no bacterial cells showed negligible accumulation of Fe(II), no precipitate, and no significant reduction or removal of arsenic from the solution. In addition, we ran controls containing defined medium with no cells but with 50 mM Fe(II), 56 mM sodium citrate, and 100 μM As(V). After 8 days, a white precipitate also formed, which was able to remove 60% of the As(V) from the solution. The remaining arsenic in solution was exclusively As(V). These results suggest strongly that the arsenic was removed in Fig. 1 via sorption to the Fe(II)-bearing mineral phase precipitated by the Fe(III)-reducing bacterium.
FIG. 1.
Fe(II) (mM) before and after centrifugation and total soluble arsenic in cultures of G. sulfurreducens grown in defined medium using Fe(III) citrate as the electron acceptor and supplemented with 100 μM As(V). ▴, Fe(II) (mM) before centrifugation; ▪, Fe(II) (mM) after centrifugation; •, total As (μM); ⧫, protein concentration (mg/ml).
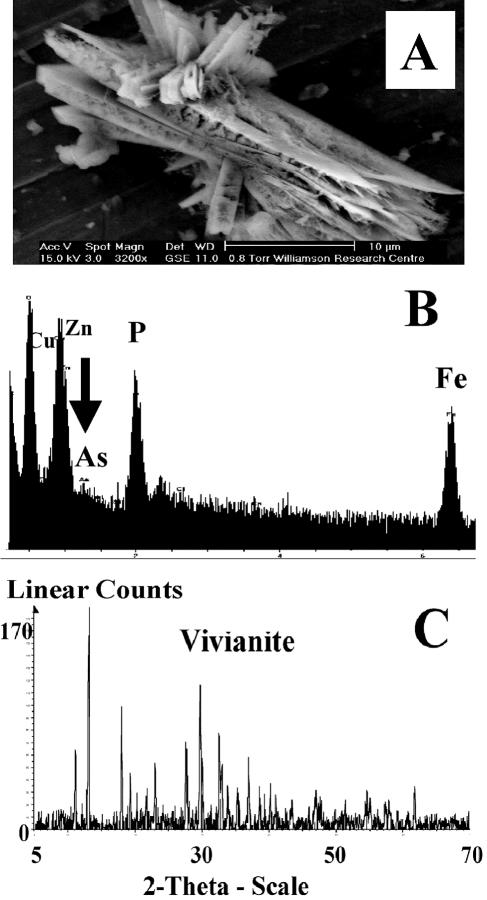
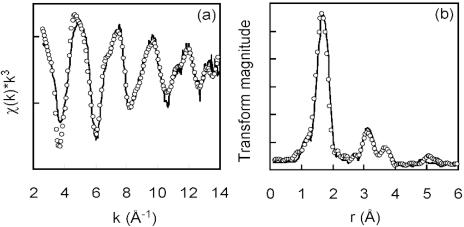
Analysis of the precipitate (after 13 days) by X-ray diffraction identified the mineral phase as vivianite [Fe3(PO4)2 · 8H2O] (Fig. 2C), with the presence of Fe, As, and P in biominerals obtained from the cultures confirmed using an ESEM with EDS (Fig. 2A and B). The formation of phosphate minerals has frequently been observed in sedimentary environments under high biological productivity (22), where organic matter serves as a source of phosphate to sediment pore water through bacterial degradation (5). In this experiment, the phosphate was present as a constituent of the growth medium, which reacted with biogenic ferrous iron, leading to the formation of a new mineral phase with the potential to sorb arsenic. XANES analysis of the vivianite indicated that the arsenic sorbed as As(V) (85%), with small amounts of As(III) (15%) also detected (Table 1). The EXAFS and associated Fourier transformed spectra are displayed in Fig. 3. The best fit of the EXAFS spectrum of arsenic sorbed to vivianite indicated that the metalloid was coordinated to four oxygen atoms at a distance of 1.70 Å, consistent with the geometry of tetrahedral arsenate. A second shell of two iron atoms was also resolved at 3.29 Å. A further shell of arsenic atoms was fitted at 3.40 Å. Inclusion of a contribution from multiple scattering within the arsenate tetrahedron made a small improvement to the EXAFS fit. These results suggest that G. sulfurreducens was incapable of efficient reduction of As(V) either enzymatically or via Fe(II) in solution or in the Fe(II)-bearing biomineral vivianite.
FIG. 2.
Removal of arsenic with biogenic vivianite formed by G. sulfurreducens. (A) ESEM image of biogenic vivianite; (B) EDS spectrum of vivianite; (C) identification of vivianite by XRD.
TABLE 1.
XANES analysis showing the oxidation state of arsenic associated with a range of Fe(II)-bearing biominerals formed by Fe(III)-reducing bacteria
| Fe biomineral | Composition (%)
|
|
|---|---|---|
| As(V) | As(III) | |
| Vivianite [Fe3(PO4)2 · 8H2O] | 85 | 15 |
| Siderite (FeCO3) | 50 | 50 |
| Magnetite (Fe3O4) | 100 | 0 |
FIG. 3.
EXAFS analysis of arsenic associated with biogenic vivianite formed by G. sulfurreducens. The EXAFS spectrum (a) with Fourier transformed data (b) is shown. Solid lines represent experimental data, and open circles represent the least-squares best fit using the parameters stated in the text.
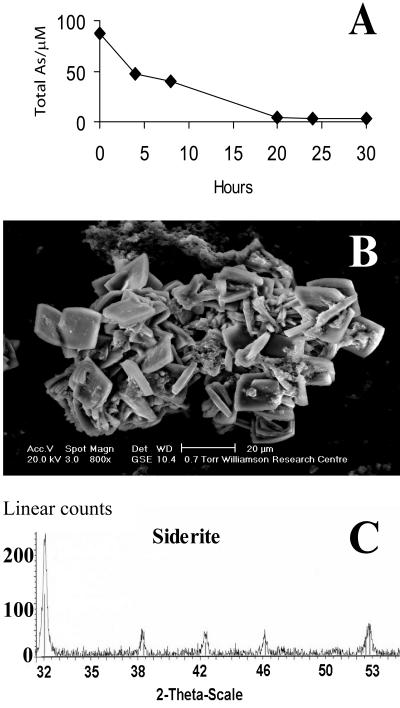
In addition to vivianite, a range of alternative Fe(II) biominerals is produced by Fe(III)-reducing bacteria depending on geochemical and mineralogical constraints (15, 37, 45). For example, siderite (FeCO3) is frequently observed as a diagenic precipitate in aquatic sediments (31), where its formation is generally associated with the bacterial respiration of organic matter or hydrogen coupled to dissimilatory Fe(III) reduction in a reducing environment, with CO2 production and high alkalinity (15)—conditions encountered in sediments in the Ganges Delta (38). Given the results obtained with vivianite, we also studied the interactions of biogenic siderite with As(V), and attempts were made to generate siderite using washed cell suspensions of G. sulfurreducens suspended in 30 mM carbonate buffer (pH 7.0) supplemented with 56 mM Fe(III)-citrate and 20 mM acetate as the electron donor. Although efficient Fe(III) reduction was noted, siderite did not form in these experiments, despite an excess of carbonate ions. For this reason, biogenic siderite was obtained from a stable Fe(III)-reducing consortium enriched from brackish waters where siderite was implicated as a dominant iron biomineral (2). Samples of siderite (78 mg/ml) from these cultures (Fig. 4A) were incubated in carbonate buffer in the presence of 100 μM As(V), and approximately 96% of the metalloid was removed from solution over a 20-h incubation period (Fig. 4A). A similar level of removal was also noted with 500 μM As (data not shown). The low concentrations of arsenic remaining in solution were analyzed by IC-ICP-MS and were approximately 3% As(V) and 98% As(III) at both starting concentrations. XAS analysis showed that the As associated with the biomineral phase was a mix of 50% As(V) and 50% As(III), suggesting abiotic reduction of the pentavalent arsenic by Fe(II) and retention of the reduced As(III) by the siderite (Table 1). These results demonstrate that the Fe(II)-bearing mineral siderite is also able to sorb As(V) and As(III) effectively and is implicated in the abiotic reduction of As(V). Hence, siderite, like vivianite, could be a potential sink for arsenic in the subsurface. Indeed, field evidence supports a role for siderite in arsenic sorption in sediments from the Bengal Delta (38).
FIG. 4.
Removal of arsenic (as arsenate) by biogenic siderite. (A) Arsenic removal by preprepared siderite; (B) ESEM image of biogenic siderite; (C) identification of siderite by XRD.
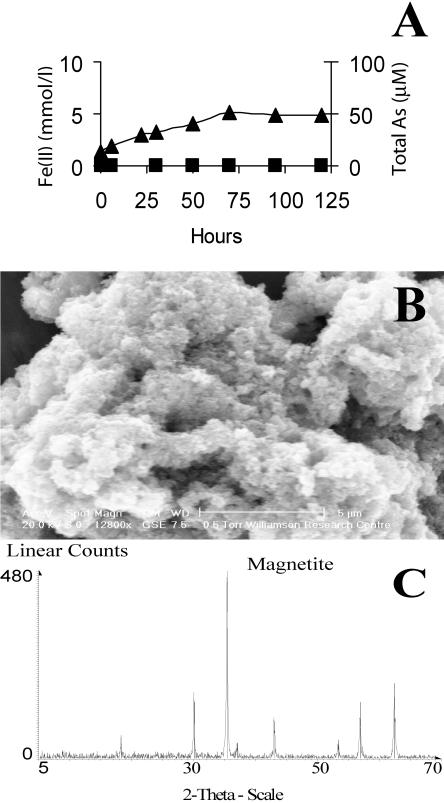
An additional set of experiments using G. sulfurreducens addressed the fate of As(V) sorbed to an insoluble poorly crystalline Fe(III) oxide phase undergoing reduction by washed cell suspensions. These experiments were important, as insoluble poorly crystalline Fe(III) oxides and oxy-hydroxides such as ferrihydrite are widespread in aquifer sediments and exhibit high adsorptive capacities for arsenic (36). Furthermore, the reductive dissolution of arsenic-bearing Fe(III) phases by Fe(III)-reducing bacteria has been proposed as a mechanism for arsenic release into groundwater in aquifers (39). In these experiments, the reduction of poorly crystalline Fe(III) oxides resulted in the formation of a black magnetic mineral, the Fe(II)-containing mixed oxide magnetite (Fe3O4) (Fig. 5A to C). Approximately 99.9% of the As remained sorbed to the mineral phase throughout these incubations, and the trace amounts of arsenic in solution were detected as As(V) by IC-ICP-MS (Fig. 5A). XAS analysis of the arsenic-bearing biomagnetite suggested that As(III) was not present in the samples; the position of the absorption edge and the near-edge structure showed that arsenic was present entirely as As(V) in the magnetite precipitates (Table 1). These results do not support the hypothesis that the reduction of As(V)-bearing Fe(III) oxides results in the efficient mobilization of arsenic, but they do add weight to the view that biogenic Fe(II)-bearing minerals can act as a sink for the metalloid.
FIG. 5.
Reduction of As(V)-bearing, insoluble, poorly crystalline Fe(III) oxide by G. sulfurreducens. (A) Formation of Fe(II) (mmol/liter) ▴ and negligible release of As (μM) ▪; (B) ESEM image of magnetite formed by the reduction of insoluble poorly crystalline Fe(III) oxide; (C) identification of magnetite by XRD.
Geobacter-arsenic interactions in sediments.
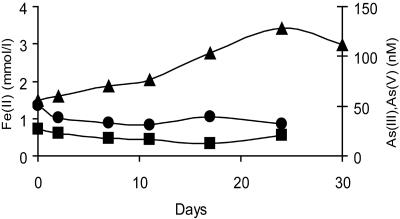
Several studies have shown that metal reduction activities in defined laboratory media can vary significantly in comparison to those results obtained from more-complex aquifer sediments (for an example, see reference 32). To determine if Geobacter sulfurreducens could play a role in the release of arsenic from West Bengal sediments, the organism was inoculated into heat-sterilized sediments collected from an aquifer from the Nadia district, West Bengal, with elevated arsenic concentrations (21) and mixed with a synthetic artificial groundwater (described in reference 21). However, the axenic culture of G. sulfurreducens was not able to reduce or mobilize arsenic from the sediment, even though the indigenous microorganisms were able to mobilize As(III) under these conditions (21). There was a negligible increase in As(V) or As(III) concentrations detected in the pore waters, even though there was a significant increase in Fe(II) concentrations in the sediments, reaching a 3-mmol/kg sediment slurry after 24 days of incubation (Fig. 6). This confirms that metabolically active cells of G. sulfurreducens were not able to reduce and mobilize As(V) in sediments and suggests that the reduction of As(V)-bearing Fe(III) oxides alone is not sufficient to mobilize arsenic in these sediments by this microorganism.
FIG. 6.
Reduction of Fe(III) and impact on the speciation of arsenic in microcosms containing heat-sterilized Bengal sediments inoculated with pure culture of G. sulfurreducens. ▴, Fe(II) (mmol/liter); •, As(III) (nM); ▪, As(V) (nM).
Conclusion.
These results show that although the model Geobacter species G. sulfurreducens has been reported to have the genetic potential to reduce As(V) (30), it did not reduce As(V) in culture or in As(V)-bearing sediments. These experiments illustrate the importance of verifying inferences from genome-sequencing initiatives with physiological experiments. The mechanism of tolerance to high (0.5 mM) concentrations of As(V) remains to be identified in G. sulfurreducens, and it remains to be determined if Geobacter species play a direct role in As reduction and mobilization in aquifers, as implicated in previous studies (21; H. A. L. Rowland, R. L. Pederick, D. A. Polya, R. D. Pancost, A. G. Gault, J. M. Charnock, D. J. Vaughan, and J. R. Lloyd, unpublished observations). Although there may be specialist Geobacter species with the ability to respire using As(V), it is also possible that other metal-reducing prokaryotes play a role in mobilizing arsenic in such environments.
These results also demonstrate that the reduction of As(V)-bearing Fe(III) oxides may not be sufficient to mobilize As(V) in aquifer sediments as previously hypothesized (43) but instead could lead to the formation of new Fe(II)-bearing mineral assemblages that have the capacity to sorb As(V). Our results also show that a range of biogenic Fe(II) minerals (e.g., vivianite and magnetite) are unable to abiotically reduce As(V) efficiently [no reduction with magnetite and only 15% supplied As(V) reduced by the vivianite] in sharp contrast to other high-valence metals such as Cr(VI) (14) and Tc(VII) (25). More efficient reduction of As(V) was noted with siderite but with the subsequent sorption of most of the As(III) by the Fe(II)-bearing mineral. Instead, other mechanisms are implicated in arsenic release, including the involvement of other specialist As(V)-reducing prokaryotes, possibly sustained initially through respiration using the more abundant bioavailable Fe(III) oxides in the sediments (21). This model of As(III) mobilization is supported by several recent microcosm-based studies that have shown that Fe(III) reduction and As(V) reduction are sequential and not necessarily linked directly (21, 43). The identification of these organisms remains a crucial step in understanding the biogeochemical basis of As(V) reduction and mobilization of As(III) in “at risk” aquifers. There is also a clear need for further work on the detailed mechanism of As mobilization through the biological reduction of As(V), as our studies suggest that As(III) formed through reduction by biogenic Fe(II) in siderite is able to sorb effectively to the Fe(II)-bearing mineral. Thus, other factors associated with the local chemistry of the metal-microbe interface may potentially play a crucial role in maintaining solubility of the bioreduced As(III).
Acknowledgments
This work was supported by EPSRC (GR/S30207/01), University of Manchester; CVCP (ORS award); and CCLRC (Daresbury SRS Beamtime awards 42/217 and 40/172).
We thank C. Boothman for preliminary microbial community analysis and R. L. Bilsborrow for his support as a station 16.5 scientist at the Daresbury SRS. Peter Morris is thanked for XRD analysis.
REFERENCES
- 1.Acharyya, S. K., P. Chakraborty, S. Lahiri, B. C. Raymahashay, S. Guha, and A. Bhowmik. 1999. Arsenic poisoning in the Ganges delta. Nature 401:545. [DOI] [PubMed] [Google Scholar]
- 2.Adams, L. K. 2004. Iron reduction in sediments associated with shell beds: refining models of bacterial Fe(III)-reduction in marginal marine sedimentary systems. Ph.D. thesis. University of Manchester, Manchester, United Kingdom.
- 3.Akai, J., K. Izumi, H. Fukuhara, H. Masuda, S. Nakano, T. Yoshimura, H. Ohfuji, A. H. Md, and K. Akai. 2004. Mineralogical and geomicrobiological investigations on groundwater arsenic enrichment in Bangladesh. Appl. Geochem. 19:215-230. [Google Scholar]
- 4.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. H. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beveridge, T. J., J. D. Meloche, W. S. Fyfe, and R. J. E. Murray. 1983. Diagenesis of metals chemically complexed to bacteria: laboratory formation of metal phosphates, sulfides and organic condensates in artificial sediments. Appl. Environ. Microbiol. 45:1094-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bose, P., and A. Sharma. 2002. Role of iron in controlling speciation and mobilization of arsenic in subsurface environment. Water Res. 36:4916-4926. [DOI] [PubMed] [Google Scholar]
- 7.Caccavo, F., Jr., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury, T. R., G. K. Basu, B. K. Mandal, B. K. Biswas, G. Samanta, U. K. Chowdhury, C. R. Chanda, D. Lodh, S. L. Roy, K. C. Saha, S. Roy, S. Kabir, Q. Quamruzzaman, and D. Chakraborti. 1999. Arsenic poisoning in the Ganges delta. Nature 401:545-546. [DOI] [PubMed] [Google Scholar]
- 9.Coppi, M. V., C. Leang, S. J. Sandler, and D. R. Lovley. 2001. Development of genetic system for Geobacter sulfurreducens. Appl. Environ. Microbiol. 67:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings, L., L. Riley, L. Black, A. Souvorov, S. Resenchuk, I. Dondoshansky, and T. Tatusova. 2002. Genomic BLAST: custom-defined virtual databases for complete and unfinished genomes. FEMS Microbiol. Lett. 216:133-138. [DOI] [PubMed] [Google Scholar]
- 11.Das, D., A. Chatterjee, B. K. Mandal, G. Samanta, D. Chakraborti, and B. Chanda. 1995. Arsenic in ground water in six districts of West Bengal, India: the biggest arsenic calamity in the world. Part 2. Arsenic concentration in drinking water, hair, nail, urine, skin-scale and liver tissue (biopsy) of the affected people. Analyst 120:917-924. [DOI] [PubMed] [Google Scholar]
- 12.Das, D., G. Samanta, B. K. Mandal, T. R. Chowdhury, C. R. Chanda, P. P. Chowdhury, G. K. Basu, and D. Chakraborti. 1996. Arsenic in groundwater in six districts of West Bengal, India. Environ. Geochem. Health 18:5-15. [DOI] [PubMed] [Google Scholar]
- 13.Delcher, A. L., D. Harmon, S. Kasif, O. White, and S. L. Salzberg. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fendorf, S., B. W. Wielinga, and C. M. Hansel. 2000. Chromium transformations in natural environments: the role of biological and abiological processes in chromium(VI) reduction. Int. Geol. Rev. 42:691-701. [Google Scholar]
- 15.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, H. Dong, T. C. Onstott, N. W. Hinman, and S. M. Li. 1998. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 62:3239-3257. [Google Scholar]
- 16.Gault, A. G., J. Jana, S. Chakraborty, P. Mukherjee, M. Sarkar, B. Nath, D. A. Polya, and D. Chatterjee. 2005. Preservation strategies for inorganic arsenic species in high iron, low-Eh groundwater from West Bengal, India. Anal. Bioanal. Chem. 381:347-353. [DOI] [PubMed] [Google Scholar]
- 17.Gordon, L., A. Y. Chervonenkis, A. J. Gammerman, I. A. Shahmuradov, and V. V. Solovyev. 2003. Sequence alignment kernel for recognition of promoter regions. Bioinformatics 19:1964-1971. [DOI] [PubMed] [Google Scholar]
- 18.Harvey, C. F., C. H. Swartz, A. B. M. Badruzzaman, N. Keon-Blute, W. Yu, M. Ashraf Ali, J. Jay, R. Beckie, V. Niedan, D. Brabander, P. M. Oates, K. N. Ashfaque, S. Islam, H. F. Hemond, and M. F. Ahmed. 2002. Arsenic mobility and groundwater extraction in Bangladesh. Science 298:1602-1606. [DOI] [PubMed] [Google Scholar]
- 19.Holmes, D. E., K. T. Finneran, and D. R. Lovley. 2002. Enrichment of Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horneman, A., A. van Geen, D. V. Kent, P. E. Mathe, Y. Zheng, R. K. Dhar, S. O'Connell, M. A. Hoque, Z. Aziz, M. Shamsudduha, A. A. Seddique, and K. M. Ahmed. 2004. Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions. Part I: Evidence from sediment profiles. Geochim. Cosmochim. Acta 68:3459-3473. [Google Scholar]
- 21.Islam, F. S., A. G. Gault, C. Boothman, D. A. Polya, J. M. Charnock, D. Chatterjee, and J. R. Lloyd. 2004. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68-71. [DOI] [PubMed] [Google Scholar]
- 22.Konhauser, K. O. 1997. Bacterial iron biomineralisation in nature. FEMS Microbiol. Rev. 20:315-326. [Google Scholar]
- 23.Lloyd, J. R. 2003. Microbial reduction of metals and radionuclides. FEMS Microbiol. Rev. 27:411-425. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd, J. R., C. Leang, A. L. Hodges Myerson, S. Ciufo, S. J. Sandler, B. Methe, and D. R. Lovley. 2003. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem. J. 369:153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd, J. R., V. A. Sole, C. V. Van Praagh, and D. R. Lovley. 2000. Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl. Environ. Microbiol. 66:3743-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovley, D. R. 2003. Cleaning up with genomics: applying molecular biology to bioremediation. Nat. Rev. Microbiol. 1:36-44. [DOI] [PubMed] [Google Scholar]
- 27.Lovley, D. R., and E. J. P. Phillips. 1986. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl. Environ. Microbiol. 52:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malasarn, D., C. W. Saltikov, K. M. Campbell, J. M. Santini, J. G. A. Hering, and D. K. Newman. 2004. arrA is a reliable marker for As(V) respiration. Science 306:455. [DOI] [PubMed] [Google Scholar]
- 29.Messens, J., J. C. Martins, K. Van Belle, E. Brosens, A. Desmyter, M. De Gieter, J. M. Wieruszeski, R. Willem, L. Wyns, and I. Zegers. 2002. All intermediates of the arsenate reductase mechanism, including an intramolecular dynamic disulfide cascade. Proc. Natl. Acad. Sci. USA 99:8506-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Methe, B. A., K. E. Nelson, J. A. Eisen, I. T. Paulsen, W. Nelson, J. F. Heidelberg, D. Wu, M. Wu, N. Ward, M. J. Beanan, R. J. Dodson, R. Madupu, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, M. Gwinn, J. F. Kolonay, S. A. Sullivan, D. H. Haft, J. Selengut, T. M. Davidsen, N. Zafar, O. White, B. Tran, C. Romero, H. A. Forberger, J. Weidman, H. Khouri, T. V. Feldblyum, T. R. Utterback, S. E. Van Aken, D. R. Lovley, and C. M. Fraser. 2003. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302:1967-1969. [DOI] [PubMed] [Google Scholar]
- 31.Mortimer, R. J. G., and M. L. Coleman. 1997. Microbial influence on the oxygen isotopic composition of diagenetic siderite. Geochim. Cosmochim. Acta 61:1705-1711. [Google Scholar]
- 32.Nevin, K. P., and D. R. Lovley. 2000. Potential for nonenzymatic reduction of Fe(III) during microbial oxidation of organic matter coupled to Fe(III) reduction. Environ. Sci. Technol. 34:2472-2478. [Google Scholar]
- 33.Nickson, R., J. McArthur, W. Burgess, K. M. Ahmed, P. Ravenscroft, and M. Rahman. 1998. Arsenic poisoning of Bangladesh groundwater. Nature 395:338. [DOI] [PubMed] [Google Scholar]
- 34.Nickson, R. T., J. M. McArthur, P. Ravenscroft, W. G. Burgess, and K. M. Ahmed. 2000. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl. Geochem. 15:403-413. [Google Scholar]
- 35.Oremland, R. S., and J. F. Stolz. 2003. The ecology of arsenic. Science 300:939-944. [DOI] [PubMed] [Google Scholar]
- 36.Pierce, M. L., and C. B. Moore. 1982. Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Res. 16:1247-1253. [Google Scholar]
- 37.Postma, D. 1981. Formation of siderite and vivianite and the porewater composition of a recent bog sediment in Denmark. Chem. Geol. 31:225-244. [Google Scholar]
- 38.Shanker, R., T. Pal, P. K. Mukherjee, S. Shome, and S. Sengupta. 2001. Association of microbes with arsenic-bearing siderite concretions from shallow aquifer sediments of Bengal delta and its implication. J. Geol. Soc. India 58:269-271. [Google Scholar]
- 39.Smedley, P. L., and D. G. Kinniburgh. 2002. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 17:517-568. [Google Scholar]
- 40.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. R. Fujimoto, N. M. Goeke, B. J. Olsen, and D. C. Kleck. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 41.Snoeyenbos-West, O., K. P. Nevin, R. T. Anderson, and D. R. Lovley. 2000. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microbial Ecol. 39:153-167. [DOI] [PubMed] [Google Scholar]
- 42.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmoughin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Geen, A., J. Rose, S. Thoral, J. M. Garnier, Y. Zheng, and J. Y. Bottero. 2004. Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions. Part II: evidence from sediment incubations. Geochim. Cosmochim. Acta 68:3475-3486. [Google Scholar]
- 44.Wheeler, D. L., D. M. Church, S. Federhen, A. E. Lash, T. L. Madden, J. U. Pontius, G. D. Schuler, L. M. Schriml, E. Sequeira, T. A. Tatusova, and L. Wagner. 2003. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 31:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, C., S. Liu, T. J. Phelps, D. R. Cole, J. Horita, S. M. Fortier, M. Elless, and J. W. Valley. 1997. Physiochemical, mineralogical, and isotopic characterization of magnetite rich iron oxides formed by thermophilic bacteria. Geochim. Cosmochim. Acta 61:4621-4632. [Google Scholar]