Abstract
Heterologous bacteriocin production in Propionibacterium freudenreichii is described. We developed an efficient system for DNA shuttling between Escherichia coli and P. freudenreichii using vector pAMT1. It is based on the P. freudenreichii rolling-circle replicating plasmid pLME108 and carries the cml(A)/cmx(A) chloramphenicol resistance marker. Introduction of the propionicin T1 structural gene (pctA) into pAMT1 under the control of the constitutive promoter (P4) yielded bacteriocin in amounts equal to those of the wild-type producer Propionibacterium thoenii 419. The P. freudenreichii clone showed propionicin T1 activity in coculture, killing 90% of sensitive bacteria within 48 h. The pamA gene from P. thoenii 419 encoding the protease-activated antimicrobial peptide (PAMP) was cloned and expressed in P. freudenreichii, resulting in secretion of the pro-PAMP protein. Like in the wild type, PAMP activation was dependent on externally added protease. Secretion of the antimicrobial peptide was obtained from a clone in which the pamA signal peptide and PAMP were fused in frame. The promoter region of pamA was identified by fusion of putative promoter fragments to the coding sequence of the pctA gene. The P4 and Ppamp promoters directed constitutive gene expression, and activity of both promoters was enhanced by elements upstream of the promoter core region.
Propionic acid bacteria (PAB) are economically important bacteria used in the production of Swiss-type cheese. The influence of PAB in the cheese-making process has been extensively studied by microbiological and biochemical methods (10, 23). During the last few years, much of the scientific focus has been directed towards studies of the antimicrobial potential of PAB. The dairy PAB species have achieved a “generally recognized as safe” status, which makes their antimicrobial substances attractive as food preservatives (1, 2). For instance, propionic acid is commonly used as a mold inhibitor (2). PAB also have a potential use as protective cultures for inhibition of pathogens and food spoilage organisms (30, 31). The antimicrobial capacity of PAB is only partly due to the production of organic acids, and it has become evident that PAB also produce other biologically active substances such as bacteriocins (4, 7, 9, 13, 26). Recently, Faye et al. (9) characterized the propionicin T1 bacteriocin from Propionibacterium thoenii. The propionicin T1 gene locus is organized in an operon structure with a putative ABC transporter (orf2) immediately downstream of the bacteriocin structural gene pctA. Propionicin T1 is an unmodified peptide that contains a signal sequence probably recognized by the general secretory (sec) pathway. The bacteriocin is inhibitory to all dairy PAB species except Propionibacterium freudenreichii. Faye et al. (7) purified a bacteriocin-like peptide, protease-activated antimicrobial peptide (PAMP), from protease-treated culture supernatants of Propionibacterium jensenii LMGT 3032. Biochemical and genetic analysis revealed that the PAMP-encoding gene, pamA, encodes a 225-amino-acid preproprotein with a 27-residue leader peptide. Mature PAMP is comprised of the 64 C-terminal residues of the secreted 198-residue proprotein. P. jensenii LMGT 3032 constitutively produces pro-PAMP during growth in sodium lactate broth. Besides pamA, no genes connected to pro-PAMP production have been identified. The function of the PAMP prodomain remains elusive, but an involvement in protection of the producer has been suggested (7).
Genetic investigations of dairy PAB have been limited, and characterization of gene function has previously only been possible through reverse genetics or expression in heterologous hosts such as Escherichia coli (11, 21, 24, 28). About 30 PAB genes have been characterized with an assigned function (33). However, the recent publication of the genome sequence of Propionibacterium acnes (5), a nondairy pathogenic species, provides an important source of information for the genetic study of dairy propionibacteria. Furthermore, the current improvements in tools for genetic manipulation of PAB will develop functional genetic characterization of dairy propionibacteria (16, 18, 19). Nevertheless, PAB transformation has proved to be difficult, especially with DNA prepared from E. coli (16, 18). This trait represents a major obstacle, since most cloning requires E. coli as an intermediate host. Compared to other bacterial transformation systems, the number of Propionibacterium shuttle vectors (including expression vectors) is limited, all of which originate from two theta-type replication plasmids (16, 18, 26). In this work, we have developed a new E. coli-Propionibacterium shuttle vector based on rolling-circle replication in propionibacteria and designed an efficient method for transformation of P. freudenreichii with plasmids constructed via E. coli. This protocol was used to study heterologous expression of the propionicin T1 and pro-PAMP-encoding genes in P. freudenreichii.
MATERIALS AND METHODS
Bacterial strains, vectors, and media.
The bacterial strains and vectors are shown in Table 1. E. coli was cultivated at 37°C in LB medium supplemented with 100 μg/ml of ampicillin or 50 μg/ml kanamycin where appropriate. Propionibacteria were grown anaerobically at 30°C in sodium lactate broth (SLB) (4). Lactobacilli were propagated anaerobically in MRS medium (Oxoid, Basingstoke, Hampshire, United Kingdom) at 30°C. Determination of the MIC of chloramphenicol was performed for propionibacteria on solidified SLB medium containing 1.6% agar with E-test strips (AB Biodisk, Sweden).
TABLE 1.
Plasmids and strains used in this studya
| Plasmid or strain | Relevant characteristics | Source and/or reference |
|---|---|---|
| Plasmids | ||
| Topo 2.1AT vector | E. coli cloning vector with 5′-T overhang; Kmr Apr | Invitrogen |
| pLME108 | Cryptic plasmid in P. freudenreichii subsp. shermanii DF2; 2.051 kb; EMBL accession no. AJ006662 | This study |
| pAMT1 | E. coli-PAB shuttle vector Apr in E. coli and Cmr in PAB; derived from pUC18, pLME108, and cml/cmx PCR product; 6.25 kb | This study |
| pTD101 | pctA gene with native promoter (PpctS), cloned in pAMT1 | This study |
| pTD102 | pctA gene with extended native promoter (PpctE), cloned in pAMT1 | This study |
| pTD103 | pctA gene expressed from the P4 short promoter (P4S), cloned in pAMT1 | This study |
| pTD104 | pctA gene expressed from the P4 extended promoter (P4E), cloned in pAMT1 | This study |
| pTD105 | pctA-A gene expressed from the P4E promoter, cloned in pAMT1 | This study |
| pTD110 | Mutated pamA gene, containing a stop at codon 175, cloned in pAMT1 | This study |
| pTD112 | pamA gene expressed from the P4S promoter, cloned in pAMT1 | This study |
| pTD113 | pamA gene expressed from the P4E promoter, cloned in pAMT1 | This study |
| pTD114 | pamA gene with an introduced in-frame deletion (designated pamAΔpro) which results in a fusion of the leader peptide to PAMP, expressed from the P4S promoter and cloned into pAMT1 | This study |
| pTD115 | pctA gene expressed from the short Ppamp promoter (PpampS), cloned in pAMT1 | This study |
| pTD116 | pctA gene expressed from the extended Ppamp promoter (PpampE), cloned in pAMT1 | This study |
| Strains | ||
| E. coli Top Ten | Invitrogen | |
| E. coli JM109 | New England Biolabs | |
| E. coli JM110 | dam and dcm mutant conferring no methylation of DNA | Stratagene |
| P. thoenii 419b | Propionicin T1+, pro-PAMP+ | 9 |
| P. jensenii LMGT 3032 | pro-PAMP+ | 7 |
| P. jensenii LMGT 2942 | pctA-A allele, propionicin T1− | 8 |
| P. freudenreichii IFO12426 | High frequency of DNA transformation by electroporation | 18, IFO |
| P. freudenreichii subsp. shermanii DF2 | Carries plasmid pLME108 | 25 |
| P. freuenreichii subsp. freudenreichii ATCC 6207 | Type strain; recipient in DNA transformations | ATCC |
| P. acidipropionici ATCC 4965 | Highly sensitive to propionicin T1 | ATCC |
| P. acidipropionici ATCC 4965*Eryr | Highly sensitive to propionicin T1; spontaneous erythromycin-resistant mutant | This study |
| L. sakei NCDO 2714 | Highly sensitive to PAMP | NCDO |
Abbreviations: ATCC, American Type Culture Collection (Rockville, Md.); NCDO, National Collection of Food Bacteria (Reading, United Kingdom) LMGT, Laboratory of Microbial Gene Technology, Ås, Norway; cml/cmx, complete cml(A) and cmx(A) genes from Corynebacterium striatum plasmid pTP10 (32) conferring chloramphenicol resistance; Cmr, chloramphenicol selection; Apr, ampicillin selection; Kmr, kanamycin selection.
P. thoenii 419 originates from the Environmental Bacteriology Culture Collection, University of the Orange Free State, Bloemfontein, South Africa.
General methods.
General molecular biological techniques used in this study were performed as described previously by Sambrook et al. (29), unless otherwise stated. Transformation of E. coli was performed according to a method described previously by Inoue et al. (15). Plasmid DNA for cloning was purified with QIAprep spin columns, while plasmid DNA for transformation of P. freudenreichii was prepared by use of Midi Prep columns (QIAGEN, Hilden, Germany). Restriction enzymes and T4 DNA ligase were purchased from New England Biolabs, Inc. (Beverly, Mass.) or Fermentas (Vilnius, Lithuania). DNA amplified by PCR for cloning was done in 100-μl reaction mixtures using 2.5 units of Pfx polymerase (Invitrogen, Paisley, United Kingdom) and 100 picomoles of each primer. The PCR conditions included a polymerase activation/template denaturation step at 94°C (3 min) followed by 35 cycles of denaturing at 94°C (15 s), annealing at 57 to 60°C (30 s), and polymerization at 68°C. Taq polymerase (QIAGEN) was used to add single-nucleotide 3′-A overhangs to PCR products. DNA fragments from PCR amplification or restriction digests were analyzed by agarose gel electrophoresis and purified on QIAquick purification columns (QIAGEN). DNA sequencing was performed with the BigDye V.3.1 Terminator cycle sequencing ready reaction kit and an Applied Biosystems (Foster City, Calif.) model 3100 genetic analyzer. All products were used according to the manufacturers' instructions.
DNA transformation of propionibacteria.
Electrocompetent P. freudenreichii isolates were prepared from cells precultivated overnight in SLB. This preculture was diluted 1:50 in SLB and further incubated for 18 h (A620, ∼0.7), placed on ice for 30 min, and then harvested by centrifugation at 5,000 × g (4°C) for 4 min. The cells were washed twice in 1 volume of ice-cold distilled water and once in 1 volume of 10% glycerol. Finally, the cells were suspended in a 1/100 volume of 10% glycerol. The cells were dispensed in 70-μl aliquots and stored at −80°C. Electroporation was performed with a Gene Pulser apparatus (Bio-Rad, Hercules, Calif.) using 35 μl of the cell suspension mixed with DNA in a cooled 1-mm electroporation cuvette. An electric pulse was delivered at 200-Ω resistance and 25-μF capacitance at 20 kV/cm. Immediately after the pulse, 950 μl of SLB medium was added to the cell suspension. The cells were further incubated at 30°C for 3 h before appropriate volumes were plated onto SLB agar supplemented with 3.4 μg/ml and 10 μg/ml chloramphenicol for P. freudenreichii IFO12426 and P. freudenreichii ATCC 6207, respectively. The plates were incubated at 30°C under anaerobic conditions, and transformants could be detected after 5 to 10 days.
DNA preparation from Propionibacterium freudenreichii cells.
Plasmid minipreparations from P. freudenreichii were performed using cells from a 5-ml overnight culture. The cells were washed in 1 volume of STE buffer (100 mM NaCl, 1 mM EDTA, and 10 mM Tris-HCl at pH 8.0) before they were suspended in 0.25 ml GTE buffer (50 mM glucose, 10 mM EDTA, and 25 mM Tris-HCl at pH 8.0) containing 100 μg/ml RNase (Sigma, St. Louis, Mo.) and 10 mg/ml lysozyme (Sigma). The cell suspension was incubated at 37°C for 15 min prior to the addition of 0.25 ml of alkaline lysis solution P2 (1% sodium dodecyl sulfate, 0.2 N NaOH) and further incubated at room temperature for 5 min. Next, 0.35 ml of neutralization buffer P3 (3 M potassium acetate, 2 M acetic acid, pH 5.4) was added before the cell debris was removed by centrifugation (13,000 rpm, 10 min). The resulting supernatant was applied onto a QIAprep spin column (QIAGEN). Subsequent steps in the procedure were performed according to the plasmid Mini Prep protocol of QIAGEN. Large-scale plasmid preparations from 200-ml PAB cultures were performed with the Nucleobond AX 500 kit (Macherey-Nagel) including an additional lysis step by incubating the cells with 10 mg/ml lysozyme and 30 U/ml mutanolysin at 37°C for 30 min. Isolation of total DNA from P. freudenreichii was done from 5-ml overnight cultures (A600, ∼0.5) using Advamax beads according to manufacturer's recommendations (Advanced Genetic Technologies Corp., Gaithersburg, Md.).
Construction of Propionibacterium-E. coli shuttle vector pAMT1.
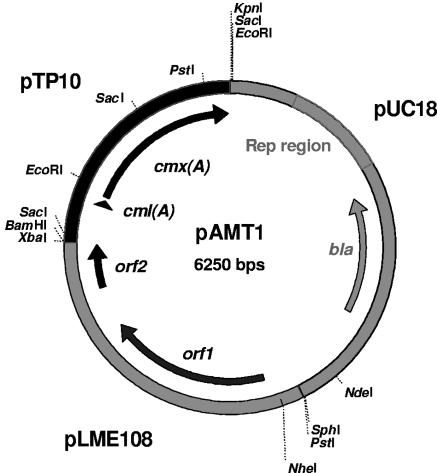
A 1.5-kb PCR fragment containing the cml(A) and cmx(A) genes was amplified from the Corynebacterium striatum pTP10 plasmid (32) using primer pair cmx1-cmx2 (Table 2). This fragment was cloned into SmaI-digested pUC18 DNA. The resulting plasmid was cut at the SalI site and ligated with XhoI-digested pLME108, resulting in the pAMT1 vector (Table 1 and Fig. 1).
TABLE 2.
List of primers used in this study
| Primer | Sequence (5′-3′) | Relevant characteristicsa |
|---|---|---|
| PAMP1 | GG CGC TGG CAG ATG GTA GGA | pamA sec, Rev |
| PAMP3 | CAT CGG GGC TTG GCC TCC TC | Ppamp promoter, Rev |
| PAMP5 | GA GGA GGC CAA GCC CCG ATG AAG AAG ACC CTC CTG CGA AGT | pctA-Ppamp fusion, Fwd |
| PAMP2 | TCC TAC CAT CTG CCA GCG CC AGA GCG AGG GCG CCT CAC AAG | sec-PAMP fusion, Fwd |
| PAMP6 | GAA CTC TGT CAG TAC TTG CTC CG | pamA, Rev |
| PAMP7 | CCG CAC GAT ATG GTT TCG GGT ATG AGA ATT CCA GTC ACG ATC AC | pamA-P41 promoter fusion, Fwd |
| PAMP8 | TCT AGA CCT TCA ACC CTA CAC TCC TCG | PpampS promoter, Fwd |
| PAMP4 | GATC TCT AGA CCA TGC GTT TAT TCC AGA TC | PpampE promoter, Fwd |
| PCTA1 | CCG CAC GAT ATG GTT TCG GGT ATG AAG AAG ACC CTC CTG CGA AGT | pctA-P4 promoter fusion, Fwd |
| P4A | ACC CGA AAC CAT ATC GTC CG | P4 promoter, Rev |
| P4B | CGA ACG TCT CGG AAA ATG C | P4S promoter, Fwd |
| P4C | TCG AGT TGC AGG GCG AGG | P4E promoter, Fwd |
| 419PC | GTC TCA TGG GGT TCC CTT TTT | pctA, Rev |
| 419PG | ACC TTC CAC CAA GAT CGA ACC | PpctS promoter, Fwd |
| 419P5 | ACG CAC TGA TGG CGA ATC G | PpctE promoter, Fwd |
| cmx1 | GAT GGG TCA TCA ATT GGC CTC | Complete cml(A)/cmx(A), Fwd |
| cmx2 | CGT CAC ACC CGA ACA TGT CG | Complete cml(A)/cmx(A), Rev |
Rev, reverse primer; Fwd, forward primer.
FIG. 1.
Restriction map of Propionibacterium-E. coli shuttle vector pAMT1. Parts originating from pTP10 (thick black line), pUC18 (thick gray line), and pLME108 (empty double lines) are indicated. Genes and open reading frames are designated by arrows, and the positions of restriction sites are designated by dotted lines. The “Rep region” contains the origin of pUC18 replication, whereas genes assigned to the open reading frames in pLME108 are further described in GenBank under accession number AJ006662.
Construction of propionicin T1 and PAMP expression plasmids.
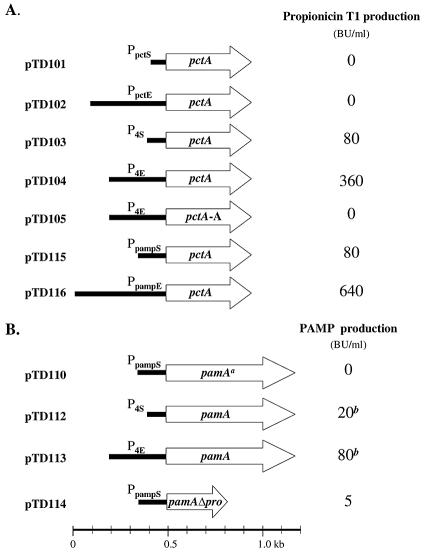
A number of propionicin T1 and PAMP expression plasmids were devised and introduced into P. freudenreichii IFO12426. First, the desired promoter and bacteriocin gene fragments were generated by PCR, cloned into the pCR2.1 Topo AT vector (Invitrogen), and subsequently cloned as XbaI-SpeI or XbaI-BamHI fragments in the E. coli-PAB shuttle vector pAMT1 (Table 1 and Fig. 2).
FIG. 2.
Schematic representation of PCR-derived inserts in pAMT1. Plasmid designations are indicated on the left. Maximum bacteriocin production conferred by the plasmids introduced into P. freudenreichii IFO12426 is indicated on the right. (A) Propionicin T1 inserts cloned as XbaI-SpeI fragments in the XbaI site of pAMT1. Propionicin T1 activity was measured using P. acidipropionicii 4965 as an indicator. (B) PAMP and pro-PAMP expression constructs cloned as XbaI-BamHI fragments in pAMT1. PAMP activity was measured using L. sakeii NCDO 2714 as an indicator. a, the pamA gene in the pTD110 plasmid contains a frameshift mutation that results in the expression of a 174-amino-acid pro-PAMP protein without the C-terminal PAMP domain; b, PAMP activity was only obtained after proteinase K treatment.
A fragment containing the propionicin T1-encoding gene (pctA) and 75 bp of the putative promoter (PpctS) was amplified from P. thoenii 419 using primers 419PG and 419PC and cloned into pAMT1, resulting in the pTD101 plasmid. Next, a fragment encompassing 400 bp of the putative propionicin promoter (PpctE) and the pctA gene was amplified from P. thoenii 419 with primers 419P5 and 419PC and used to construct the pTD102 plasmid. The PAMP structural gene (pamA) with its putative short Ppamp promoter, PpampS, was amplified from P. thoenii 419 using primers PAMP8 and PAMP6 and cloned into pAMT1, resulting in pTD110. Hybrid promoter-gene fusions were constructed by use of a two-step PCR strategy as described previously by Higuchi (12). Using this approach, the P4 (20) and Ppamp promoter elements were spliced to the coding sequences of either the pctA or the pamA genes by extension overlaps at the ATG initiation codon. A short promoter fragment (P4S) and an extended promoter fragment (P4E) containing 100 and 300 bp of P4 were amplified using primer P4A in combination with primer P4B or P4C, respectively. Generation of P4 promoter-compatible extensions on the pctA and pamA genes was obtained by PCR amplification using primer combinations PCTA1 and 419PC and PAMP7 and PAMP6, respectively. Subsequently, chimeric promoter-gene fusions were generated by a second round of PCR where either the P4S or P4E promoter fragments in combination with the pctA or the pamA gene fragments served as templates. The generated promoter-gene fusion fragments were cloned into pAMT1, resulting in expression plasmids pTD103, pTD104, pTD112, and pTD113 (Table 1). The pTD105 plasmid contains the P4E promoter fused to the pctA-A allele amplified from P. jensenii LMGT 2942 using primers PCTA1 and 419PC.
A fragment containing the P4S promoter and the pamA sec leader from pTD112 was amplified with primers PAMP1 and P4B. Next, the PAMP-encoding part of pamA was amplified with primers PAMP2 and PAMP6, which produces a 20-bp add-on complementary to the pamA leader peptide. These two fragments were mixed and served as a template in the second round of PCR with primers P4B and PAMP6, which generated a new prebacteriocin gene where the pamA sec leader is fused to the N terminus of the mature PAMP peptide. Thus, in the pTD114 plasmid, the P4S promoter directs expression of pamA with an in-frame deletion of the prodomain-encoding part of the gene.
The putative promoter region of the pamA gene was analyzed for its ability to direct expression of the pctA gene. The PpampS fragment, which covers 150 bp upstream from the pamA initiation codon, was amplified using primers PAMP8 and PAMP3. The elongated Ppamp (PpampE) fragment, which encompasses 480 bp upstream from the pamA initiation codon, was amplified using primer PAMP3 in combination with PAMP4. Subsequently, these fragments were fused to the pctA gene amplified with primers PAMP5 and 419PC. The resulting promoter-gene fusions were used to construct plasmids pTD115 and pTD116, respectively.
All constructs were electroporated into P. freudenreichii IFO12426 where correct transformants were confirmed by restriction fragment analysis and DNA sequencing and subsequently screened for bacteriocin production.
Propionicin T1 and PAMP bioassays.
P. freudenreichii IFO12426 carrying different bacteriocin expression constructs was grown on SLB plates without antibiotic for 120 or 240 h. A lawn of 5 ml SLB soft agar containing 500 μl of an overnight culture of the indicator organism was then poured over the plates. For propionicin T1 expression, the standard indicator was Propionibacterium acidipropionici ATCC 4965. After incubation for 24 to 48 h at 30°C, the plates were examined for zones of growth inhibition. PAMP production was measured by spotting 1 μl of proteinase K (20 μg/μl) near the colonies before an additional incubation of 1 h at room temperature. MRS soft agar (0.7%) with a 1% inoculum of a Lactobacillus sakei NCDO 2714 culture was added, and the plates were incubated at 30°C overnight before they were inspected for growth inhibition zones. Quantification of bacteriocin production in liquid culture was determined by a microtiter plate assay (14). The culture supernatants were precipitated with ammonium sulfate in order to remove the antibiotic from the sample prior to testing. PAMP samples were also tested with a proteinase K (20 μg/ml) addition. Each well of the microtiter plate contained 50 μl of twofold serial dilutions in SLB or MRS of the bacteriocin samples and 150 μl of a 50-fold-diluted overnight culture of the indicator strains P. acidipropionici ATCC 4965 and L. sakei NCDO 2714 for propionicin T1 and PAMP, respectively. The plates were incubated at 30°C for 24 h, and growth inhibition of the indicator organisms was measured spectrophotometrically (A620) using a microtiter plate reader (Multiscan Ascent; Labsystems, Finland). One bacteriocin unit (BU) was defined as the amount of bacteriocin that produced 50% growth inhibition of the indicator bacterium compared to a culture without added bacteriocin.
Plasmid stability.
In order to assess the ability of P. freudenreichii IFO12426 to stably maintain plasmids and propionicin T1 production, stationary-phase cultures of these strains were diluted 1/50 in SLB without chloramphenicol and cultivated for 7 days. Samples of the cultures were taken at time intervals and plated onto SLB agar without antibiotics and cultivated for 5 days. At each time point, 96 colonies were evaluated by replica plating onto SLB agar with chloramphenicol selection and tested for propionicin T1 production in agar overlay assays as described above.
RESULTS AND DISCUSSION
Development of vector and transformation procedure for efficient E. coli-Propionibacterium gene shuttling.
In a previous work, we did an extensive search for plasmids in propionibacteria (25). Plasmid pLME108 (2,051 bp) was isolated from Propionibacterium freudenreichii subsp. shermanii, and its replicon was identified by comparative DNA analysis. It contained a putative replicase gene (rep) showing an identity of 42% to the rep gene of the Arcanobacterium pyogenes plasmid pAP1, which uses the rolling-circle mechanism for replication (3). The replicon of pLME108 was fused with the E. coli replicon from pUC18 and the cml(A)/cmx(A) chloramphenicol resistance marker genes (Table 1) from Corynebacterium striatum (32). The resulting construct (pAMT1) (Fig. 1) was successfully transformed into E. coli JM110 and P. freudenreichii subsp. freudenreichii ATCC 6207 using ampicillin and chloramphenicol selection, respectively. The MIC of chloramphenicol could be augmented by about 100-fold from 0.05 to 4 to 6 μg/ml considering the MIC of nontransformed recipient strains of Propionbacterium. Electroporation of P. freudenreichii ATCC 6207 with pAMT1 from E. coli only gave 10 to 20 transformants/μg DNA, while a high efficiency of 108 transformants/μg DNA was obtained when the vector was prepared from P. freudenreichii. The difference is probably due to the presence of restriction-modification systems in PAB (16, 18). The low number of transformants achieved with DNA prepared from E. coli represented a major obstacle for studying gene function in PAB. In a previous study, Kiatpapan et al. (18) described transformation of P. freudenreichii using the E. coli/PAB shuttle vector pPK705, which contains a hygromycin B selection marker. According to the authors, this vector could be propagated in E. coli and then transformed into a P. freudenreichii subsp. shermanii strain with an efficiency of 103 transformants/μg vector DNA. However, we experienced a high background of nontransformed colonies using hygromycin B selection, which hampered the use of the pPK705 vector. In contrast, the cml(A)/cmx(A) chloramphenicol resistance marker of pAMT1 provided efficient selection without any background. The data did, however, indicate that the efficiency of the restriction barrier to foreign plasmid DNA could be strain dependent. Based on these premises, we devised an optimized protocol for transformation of P. freudenreichii with vector DNA from E. coli. Electroporation of competent P. freudenreichii subsp. shermanii IFO12426 cells produced 104 and >107 transformants/μg DNA with vector prepared from E. coli and P. freudenreichii, respectively. With DNA from E. coli, this is an improvement in transformation efficiency by 3 orders magnitude compared to the transformation of P. freudenreichii ATCC 6207. Accordingly, all constructions made in subsequent cloning experiments were based on pAMT1, and P. freudenreichii subsp. shermanii IFO12426 served as the recipient for the bacteriocin expression plasmids.
Heterologous expression of propionicin T1 in P. freudenreichii.
As part of a continued effort to study and exploit the antimicrobial potential of PAB, we used P. freudenreichii as a host for heterologous expression of a P. thoenii bacteriocin, propionicin T1 (9). The propionicin T1-encoding gene pctA was cloned in pAMT1 with either 75 or 400 bp of the upstream promoter region, resulting in expression plasmids pTD101 and pTD102, respectively. As shown in Table 3, the resulting P. freudenreichii clones did not produce any detectable amounts of propionicin T1. This could indicate that expression from the native propionicin T1 promoter is dependent on regulatory factors that are not present in P. freudenreichii. We therefore investigated if expression from the constitutive P. freudenreichii P4 promoter (20) improved bacteriocin production. The pctA gene was cloned behind either a short (P4S) or extended (P4E) version of the P4 promoter. As shown in Table 3, the resulting plasmids were able to facilitate propionicin T1 production in P. freudenreichii. Thus, the pctA structural gene encodes the information required for production and secretion of propionicin T1 in P. freudenreichii. The propionicin T1 locus contains an ABC transporter (orf2) directly downstream of the pctA gene (9). The presence of a sec leader in prepropionicin T1 and the fact that transport in P. freudenreichii occurred independently of orf2 indicate that the orf2 ABC transporter does not function as part of the propionicin T1 secretion apparatus. On agar plates, P. freudenreichii harboring the pTD104 plasmid produced large inhibition zones, while zones of inhibition with pTD103 were minute (Fig. 3). The difference in bacteriocin production between these clones was quantified in SLB cultures, where P. freudenreichii IFO12426 transformed with pTD103 and pTD104 reached a maximum propionicin T1 activity of 80 and 320 BU/ml, respectively (Table 3). Thus, the P4E promoter directed bacteriocin activity that was approximately five times higher than that of P4S. Since the P4S fragment contains the predicted ribosome binding site and the −10 and −35 promoter elements, it appears that the P4E fragment contains unidentified elements upstream of the core promoter that contribute to activity.
TABLE 3.
Bacteriocin activity in SLB cultures of P. freudenreichii IFO 12426 carrying propionicin T1 or PAMP expression plasmidsa
| Time (days) | Bacteriocin production (Bu/ml) in P. freudenreichii IFO12426 carrying:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Propionicin T1 plasmids (promoter::gene)
|
PAMP plasmids (promoter::gene)
|
||||||||||
| pTD101 (PpctS::pctA) | pTD102 (PpctE::pctA) | pTD103 (P4S::pctA) | pTD104 (P4E::pctA) | pTD105 (P4E::pctA-A) | pTD115 (PpampS::pctA) | pTD116 (PpampE::pctA) | pTD110 (PpampE::pamA)c | pTD112b (P4S::pamA) | pTD113b (P4E::pamA) | pTD114 (P4S::pamA)d | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 40 | 160 | 0 | 20 | 320 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 80 | 320 | 0 | 80 | 640 | 0 | 0 | 10 | 0 |
| 4 | 0 | 0 | 80 | 320 | 0 | 80 | 640 | 0 | 10 | 40 | 5 |
| 5 | 0 | 0 | 80 | 320 | 0 | 80 | 640 | 0 | 20 | 80 | 5 |
P. acidipropionici ATCC 4965 was used as an indicator for propionicin T1, and L. sakei NCDO 2714 was used as an indicator for PAMP.
Bacteriocin activity was only obtained after proteinase K treatment.
The pamA gene in the pTD110 plasmid contains a frameshift mutation that results in expression of a 174-amino-acid pro-PAMP protein without the C-terminal PAMP domain.
The pTD114 plasmid carries the pamAΔpro gene which encodes the leader peptide fused directly to PAMP.
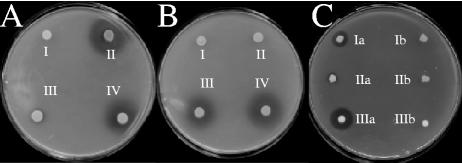
FIG. 3.
(A and B) Bacteriocin activity conferred by different propionicin T1 expression plasmids in P. freudenreichii IFO12426 compared to wild-type producer strain P. thoenii 419. (A) I, P. freudenreichii IFO12426(pAMT1); II, P. thoenii 419; III, P. freudenreichii IFO12426(pTD103); IV, P. freudenreichii IFO12426(pTD104). (B) I, P. freudenreichii IFO12426(pTD102); II, P. freudenreichii IFO12426(pTD105); III, P. freudenreichii IFO12426(pTD115); IV, P. thoenii 419. (C) Protease-dependent bacteriocin activity conferred by pro-PAMP expression plasmids in P. freudenreichii IFO12426 compared to that of wild-type producer strain P. jensenii LMGT 3032. I, P. freudenreichii IFO12426(pTD112); II, P. freudenreichii IFO12426(pTD113); III, P. jensenii LMGT 3032. a indicates bacteriocin activity with proteinase K treatment; b indicates bacteriocin activity without proteinase K treatment.
The naturally occurring pctA-A allele encodes an inactive propionicin T1 variant.
A recent survey on the prevalence of the pctA gene revealed that 13 of 24 P. jensenii strains contained this gene. However, only five strains produced antimicrobial activity corresponding to propionicin T1 (8). Intriguingly, six of the propionicin T1-negative P. jensenii strains contained a G→A transition mutation in the pctA gene, resulting in the amino acid substitution G55D in the mature bacteriocin (8). In order to investigate the biological activity of this propionicin T1 variant, the mutated gene (pctA-A) was cloned under the control of the P4E promoter in the pAMT1 vector. Transfer of the resulting plasmid, pTD105 (Fig. 2A), into P. freudenreichii IFO12426 was confirmed by restriction analysis and DNA sequencing. This clone showed no antimicrobial activity in agar overlay assays (Fig. 3B) or in liquid cultures (Table 3). Thus, the point mutation in the pctA-A allele results in drastic changes of the antimicrobial properties of the encoded peptide. In vitro mutagenesis studies of the bacteriocin pediocin Ac-H have demonstrated that most amino acid substitutions that change either structural or physicochemical properties of the peptide greatly influence its antimicrobial properties (27). Similarly, amino acid substitutions that reduce the net positive charge of sakacin P result in a less potent bacteriocin (17). The G55D substitution only slightly reduces the net positive charge of propionicin T1, but it is possible that introduction of the negatively charged aspartate residue causes a structural change that diminishes the antimicrobial activity of the peptide.
Antagonistic activity of a propionicin T1-producing P. freudenreichii in cocultures.
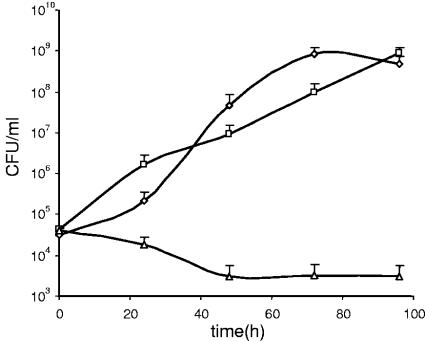
Purified propionicin T1 has been demonstrated to kill sensitive bacteria (9). We investigated whether it was possible to achieve the same antagonistic effect in situ from a propionicin T1-producing P. freudenreichii towards sensitive bacteria. A vector stability experiment was conducted on the P. freudenreichii IFO12426(pTD104) clone where all tested colonies were chloramphenicol resistant and produced propionicin T1 (data not shown). Encouraged by the fact that the expression plasmid and bacteriocin production were stably maintained in the culture, we designed a cocultivation competition assay. SLB broth was inoculated with ∼5 × 104 CFU/ml of a spontaneous erythromycin-resistant mutant of P. acidipropionici ATCC 4965 and ∼5 × 106 CFU/ml of either P. freudenreichii IFO12426(pAMT1) or P. freudenreichii IFO12426(pTD104). The 100:1 ratio of P. freudenreichii and P. acidipropionici was chosen to mimic the situation of an industrial process where starter bacterium inoculum sizes are in the order of 106 CFU/ml and contamination levels higher than 104 CFU/ml are rarely seen. The growth of the P. freudenreichii IFO12426 clones was unaffected by the presence of P. acidipropionici ATCC 4965*Eryr (data not shown). Furthermore, bacteriocin production of P. freudenreichii IFO12426(pTD104) was also unaltered by the presence of P. acidipropionici ATCC 4965*Eryr (data not shown). The growth of propionicin T1-sensitive P. acidipropionici ATCC 4965*Eryr was monitored for 96 h by plate counting on SLB agar with erythromycin. As shown in Fig. 4, P. freudenreichii IFO12426(pAMT1) did not prevent growth of P. acidipropionici. In contrast, the P. freudenreichii IFO12426(pTD104) clone efficiently prevented growth of P. acidipropionici. This effect appeared to be immediate and resulted in a 90% reduction in P. acidipropionici cell counts after 48 h. The effect was sustained throughout the test period and led to a 5-log10 reduction in P. acidipropionici viable counts compared to those of the P. acidipropionici-P. freudenreichii IFO12426(pAMT1) control culture. The fact that the propionicin T1 expression plasmid was stably maintained without selection and rendered high levels of bacteriocin production demonstrates the potential of propionicin T1-producing P. freudenreichii for practical applications. Growth of nonstarter pigmented and psycrophilic PAB in Swiss-type cheeses may cause brown spots and “anomalous blowing,” resulting in devaluated products and economic losses (6, 22). The use of a propionicin T1-producing P. freudenreichii as a secondary starter would be a convenient method to prevent growth of nonstarter PAB without affecting the lactic acid bacterial culture and facilitate a more controlled ripening of the cheese.
FIG. 4.
Effect of propionicin T1 production by P. freudenreichii IFO12426 on growth of P. acidipropionici ATCC 4965*Eryr in coculture. Approximately 5 × 104 CFU/ml of an erythromycin-resistant mutant strain of P. acidipropionici ATCC 4965 was inoculated with 5 × 106 CFU/ml of P. freudenreichii IFO12426 in SLB medium. ⋄, P. acidipropionici ATCC 4965*Eryr only; □, P. acidipropionici ATCC 4965*Eryr cocultured with P. freudenreichii IFO12426(pAMT1); ▵, P. acidipropionici ATCC 4965*Eryr cocultured with P. freudenreichii IFO12426(pTD104). Appropriate dilutions of the cultures were plated out on SLB plates containing 10 μg/ml of erythromycin and incubated for 5 days before cell numbers of P. acidipropionici ATCC 4965*Eryr were determined. The results represent the averages of three independent experiments, and standard deviations are indicated.
Heterologous expression of pro-PAMP and PAMP in P. freudenreichii.
Faye et al. (7) reported that P. jensenii LMG 3032 secretes large amounts of the 20-kDa pro-PAMP protein. Processing of pro-PAMP by proteinase K produces the bacteriocin-like peptide PAMP. The production of pro-PAMP is prevalent among strains of P. jensenii and P. thoenii (8). It has been suggested that the secretion of an inactive probacteriocin, whose activation relies on proteases in the environment, might represent a novel strategy for production of antimicrobial peptides and producer self-protection (7). We investigated the PAMP system by cloning pamA under the control of the P4S or P4E promoter fragment in plasmids pTD112 and pTD113, respectively. In P. freudenreichii IFO12426, both plasmids conferred protease-dependent inhibition of L. sakei NCDO 2714 (Fig. 2B and 3C). The same pattern of antimicrobial activity was observed in liquid culture, but the amounts produced were less than 10% of that produced by the wild-type producer P. jensenii LMG 3032. The pTD110 plasmid contains the pamA gene with a frameshift mutation that results in the C-terminal deletion of 54 amino acids corresponding to the PAMP-specific part of pro-PAMP. This clone did not produce any antimicrobial activity (Fig. 2B and Table 3). Next, we designed another deletion variant of the pamA gene that encodes the pamA leader peptide fused directly to the N terminus of mature PAMP. The resulting gene (pamAΔpro) expressed from the P4S promoter was cloned in pTD114. In contrast to the pTD112 and pTD113 clones, protease activation was not necessary. The pTD114 clone displayed reduced growth capacity in broth, and only low levels of bacteriocin activity were produced. Since P. freudenreichii IFO12426 is sensitive to PAMP, it is possible that the growth reduction was caused by suicide expression. In terms of specific activity (BU · ml−1 · A620−1), pro-PAMP expression by pTD113 was approximately 20 times higher than PAMP production by pTD114 (data not shown). These results indicate that the presence of the prodomain protected P. freudenreichii IFO12426 from the antimicrobial activity of PAMP.
Identification of the pamA promoter region.
The promoter region of pamA was analyzed using the pctA gene as a reporter. To achieve this, different segments of the putative PAMP promoter region were fused to the pctA gene and ligated into the pAMT1 vector (Fig. 2A). The fact that P4 promoter activity depended on elements only present in the extended version of the P4 promoter encouraged us to investigate if the putative pamA promoter inherited similar features. The short promoter fragment PpampS was designed to encompass the ribosome binding sites and −10 and −35 promoter sequences predicted previously by Faye et al. (7), while the PpampE fragment includes 480 bp upstream of the pamA initiation codon. The P. freudenreichii clone carrying the extended promoter fragment PpampE produced the most bacteriocin. In liquid culture, PpampE directed bacteriocin production that was eight times higher than that of PpampS (Table 3). Hence, like P4, the PAMP promoter appears to contain upstream sequence elements that contribute to increased transcriptional activity. The nature of these signals remains elusive, but a detailed investigation of such is beyond the scope of this study. Nevertheless, the identification of the PAMP promoter demonstrates the potential of the pctA gene as an in vivo reporter for quantitative assessment of promoter activity in P. freudenreichii. Hopefully, more detailed knowledge on transcriptional regulation and promoter structure in PAB will be available in the near future.
Concluding remarks.
This work describes the first successful cloning and heterologous expression of bacteriocins in P. freudenreichii. The results demonstrate the utility of the described genetic manipulation system for the study of gene function in P. freudenreichii and a potential for generation of strains with improved genetic features.
Acknowledgments
We thank Y. Murooka for providing us with P. freudenreichii IFO12426 and A. Tauch for providing plasmid pTP10. We also thank M. Teuber for support and O. Johnsborg for critical reading of the manuscript.
D.A.B. was funded by a grant from the Norwegian Research Council, and T.F. was funded by a postdoctoral fellowship from the Norwegian Research Council.
REFERENCES
- 1.Al-Zoreky, N., J. W. Ayres, and W. E. Sandine. 1991. Antimicrobial activity of Microgard against food spoilage and pathogenic microorganisms. J. Dairy Sci. 74:758-763. [DOI] [PubMed] [Google Scholar]
- 2.Barefoot, S. F., and C. G. Nettles. 1993. Antibiosis revisited: bacteriocins produced by dairy starter cultures. J. Dairy Sci. 76:2366-2379. [DOI] [PubMed] [Google Scholar]
- 3.Billington, S. J., B. H. Jost, and J. G. Songer. 1998. The Arcanobacterium (Actinomyces) pyogenes plasmid pAP1 is a member of the pIJ101/pJV1 family of rolling circle replication plasmids. J. Bacteriol. 180:3233-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brede, D. A., T. Faye, O. Johnsborg, I. Odegard, I. F. Nes, and H. Holo. 2004. Molecular and genetic characterization of propionicin F, a bacteriocin from Propionibacterium freudenreichii. Appl. Environ. Microbiol. 70:7303-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruggemann, H., A. Henne, F. Hoster, H. Liesegang, A. Wiezer, A. Strittmatter, S. Hujer, P. Durre, and G. Gottschalk. 2004. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 305:671-673. [DOI] [PubMed] [Google Scholar]
- 6.Carcano, M. R., R. Todesco, R. Lodi, and M. Brasca. 1995. Propionibacteria in Italian hard cheeses. Lait 75:415-425. [Google Scholar]
- 7.Faye, T., D. A. Brede, T. Langsrud, I. F. Nes, and H. Holo. 2002. An antimicrobial peptide is produced by extracellular processing of a protein from Propionibacterium jensenii. J. Bacteriol. 184:3649-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faye, T., D. A. Brede, T. Langsrud, I. F. Nes, and H. Holo. 2004. Prevalence of the genes encoding propionicin T1 and protease-activated antimicrobial peptide and their expression in classical propionibacteria. Appl. Environ. Microbiol. 70:2240-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faye, T., T. Langsrud, I. F. Nes, and H. Holo. 2000. Biochemical and genetic characterization of propionicin T1, a new bacteriocin from Propionibacterium thoenii. Appl. Environ. Microbiol. 66:4230-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grappin, R., E. Beuvier, Y. Bouton, and S. Pochet. 1999. Advances in the biochemistry and microbiology of Swiss-type cheeses. Lait 79:3-22. [Google Scholar]
- 11.Hashimoto, Y., M. Yamashita, and Y. Murooka. 1997. The Propionibacterium freudenreichii hemYHBXRL gene cluster, which encodes enzymes and a regulator involved in the biosynthetic pathway from glutamate to protoheme. Appl. Microbiol. Biotechnol. 47:385-392. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi, R. 1990. Recombinant PCR, p. 177-183. In M. A. Innis (ed.), PCR protocols. A guide to methods and applications. Academic Press, San Diego, Calif.
- 13.Holo, H., T. Faye, D. A. Brede, T. Nilsen, I. Ødegård, T. Langsrud, J. Brendehaug, and I. F. Nes. 2002. Bacteriocins of propionic acid bacteria—review. Lait 82:59-68. [Google Scholar]
- 14.Holo, H., O. Nilssen, and I. F. Nes. 1991. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J. Bacteriol. 173:3879-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 16.Jore, J. P., N. van Luijk, R. G. Luiten, M. J. van der Werf, and P. H. Pouwels. 2001. Efficient transformation system for Propionibacterium freudenreichii based on a novel vector. Appl. Environ. Microbiol. 67:499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazazic, M., J. Nissen-Meyer, and G. Fimland. 2002. Mutational analysis of the role of charged residues in target-cell binding, potency and specificity of the pediocin-like bacteriocin sakacin P. Microbiology (Reading) 148:2019-2027. [DOI] [PubMed] [Google Scholar]
- 18.Kiatpapan, P., Y. Hashimoto, H. Nakamura, Y.-Z. Piao, H. Ono, M. Yamashita, and Y. Murooka. 2000. Characterization of pRGO1, a plasmid from Propionibacterium acidipropionici, and its use for development of a host-vector system in propionibacteria. Appl. Environ. Microbiol. 66:4688-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiatpapan, P., and Y. Murooka. 2001. Construction of an expression vector for propionibacteria and its use in production of 5-aminolevulinic acid by Propionibacterium freudenreichii. Appl. Microbiol. Biotechnol. 56:144-149. [DOI] [PubMed] [Google Scholar]
- 20.Kiatpapan, P., M. Yamashita, N. Kawaraichi, T. Yasuda, and Y. Murooka. 2001. Heterologous expression of a gene encoding cholesterol oxidase in probiotic strains of Lactobacillus plantarum and Propionibacterium freudenreichii under the control of native promoters. J. Biosci. Bioeng. 92:459-465. [DOI] [PubMed] [Google Scholar]
- 21.Ladror, U. S., L. Gollapudi, R. L. Tripathi, S. P. Latshaw, and R. G. Kemp. 1991. Cloning, sequencing, and expression of pyrophosphate-dependent phosphofructokinase from Propionibacterium freudenreichii. J. Biol. Chem. 266:16550-16555. [PubMed] [Google Scholar]
- 22.Langsrud, T., and G. W. Reinbold. 1973. Flavor development and microbiology of Swiss cheese. III. Ripening and flavor production. J. Milk Food Technol. 36:593-609. [Google Scholar]
- 23.Langsrud, T., and G. W. Reinbold. 1973. Flavor development and microbiology of Swiss cheese. II. Starters, manufacturing processes and procedures. J. Milk Food Technol. 36:531-542. [Google Scholar]
- 24.Marsh, E. N., N. McKie, N. K. Davis, and P. F. Leadlay. 1989. Cloning and structural characterization of the genes coding for adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. Biochem. J. 260:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meile, L., G. Dasen, S. Miescher, M. Stierli, and M. Teuber. 1999. Classification of propionic acid bacteria and approaches to applied genetics. Lait 79:71-78. [Google Scholar]
- 26.Miescher, S., M. P. Stierli, M. Teuber, and L. Meile. 2000. Propionicin SM1, a bacteriocin from Propionibacterium jensenii DF1: isolation and characterization of the protein and its gene. Syst. Appl. Microbiol. 23:174-184. [DOI] [PubMed] [Google Scholar]
- 27.Miller, K. W., R. Schamber, O. Osmanagaoglu, and B. Ray. 1998. Isolation and characterization of pediocin AcH chimeric protein mutants with altered bactericidal activity. Appl. Environ. Microbiol. 64:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roessner, C. A., K. X. Huang, M. J. Warren, E. Raux, and A. I. Scott. 2002. Isolation and characterization of 14 additional genes specifying the anaerobic biosynthesis of cobalamin (vitamin B12) in Propionibacterium freudenreichii (P. shermanii). Microbiology (Reading) 148:1845-1853. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., T. Maniatis, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Schwenninger, S. M., and L. Meile. 2004. A mixed culture of Propionibacterium jensenii and Lactobacillus paracasei subsp. paracasei inhibits food spoilage yeasts. Syst. Appl. Microbiol. 27:229-237. [DOI] [PubMed] [Google Scholar]
- 31.Soumalainen, T. H., and A. M. Mäyrä-Mäkinen. 1999. Propionic acid bacteria as protective cultures in fermented milks and breads. Lait 79:165-174. [Google Scholar]
- 32.Tauch, A., S. Krieft, J. Kalinowski, and A. Puehler. 2000. The 51409-bp R-plasmid pTP10 from the multiresistant clinical isolate Corynebacterium striatum M82B is composed of DNA segments initially identified in soil bacteria and in plant, animal, and human pathogens. Mol. Gen. Genet. 263:1-11. [DOI] [PubMed] [Google Scholar]
- 33.Van Luijk, N., M. P. Stierli, S. M. Schwenninger, C. Herve, G. Dasen, J. P. M. Jore, P. H. Pouwels, M. J. van der Werf, M. Teuber, and L. Meile. 2002. Genetics and molecular biology of propionibacteria. Lait 82:45-57. [Google Scholar]