Abstract
The distribution of 15 typical freshwater bacterial groups in 15 diverse lakes in northern Europe was investigated using reverse line blot hybridization. Statistical evaluation of the data in relation to the characteristics of the lakes showed that pH, temperature, and the theoretical hydrological retention time of the lakes were most strongly related to variations in the distribution of bacterial taxa. This suggests that pH and temperature are steering factors in the selection of taxa and supports the notion that communities in lakes with short water turnover times are influenced by the input of bacterial cells from the drainage areas. Within the beta subdivision of the Proteobacteria (Betaproteobacteria), as well as within the divisions Actinobacteria and Verrucomicrobia, different subgroups were associated differently with environmental variables.
While huge efforts to explore the diversity of the bacterial kingdom have been made, very little understanding of the factors that drive the actual composition of bacterial communities has been gained. A number of studies have sought associations between molecular community fingerprints, such as those obtained by denaturing gradient gel electrophoresis, and environmental factors. In studies of lakes, multivariate analyses showed that factors such as the biomass of other plankton groups (11, 15, 16, 22, 26), pH (24, 28), nutrient concentrations (24, 26, 29), temperature (11, 22, 24, 26, 28, 29), and water flow (11, 24) covary with bacterioplankton fingerprints. Such associations suggest that these environmental factors are important in determining the distribution of taxa. However, fingerprinting methods are not well suited for revealing the identities of the various taxa. An alternative method for characterizing bacterial communities is fluorescence in situ hybridization (FISH), in which taxon-specific probes are used, which improves identification. In addition, FISH provides information on cell number and shape. However, the amount of work involved in FISH soon prohibits analysis of a set of taxa in a set of habitats, a requirement for finding taxon-environment associations. Therefore, little information about which environmental variables determine the abundance of individual bacterial groups is available.
The aims of this study were to further explore possible determinative factors for bacterioplankton community composition in lakes and, in particular, to identify factors that influence the appearance of individual bacterial groups. We used reverse line blot hybridization with probes targeted at the 16S rRNA gene in order to analyze the distribution of 15 bacterial groups in relation to environmental gradients. The groups chosen were previously designated “putative typical freshwater bacterial clusters” since they were shown to occur in several freshwater bodies and the 16S rRNA gene sequences in the database for these groups contained more sequences from freshwater sources than from terrestrial or marine sources (30, 31).
MATERIALS AND METHODS
Study sites and sampling.
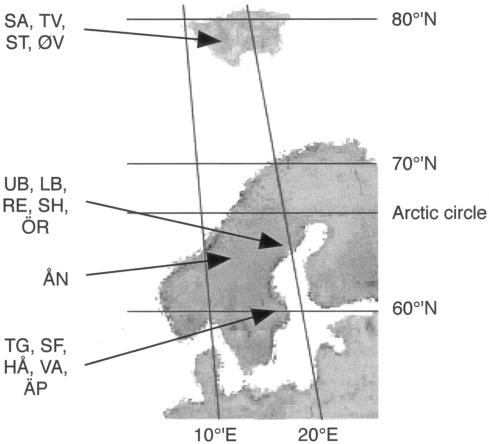
Fifteen lakes were included in the study (Table 1 and Fig. 1). Eleven of these lakes are located in three different regions in Sweden. The five Lappland lakes (northeast Sweden; 64°N, 19°E) are mesotrophic acidic and humic. Three of these lakes can even be designated polyhumic (16). All Lappland lakes have a relatively short (2) theoretical hydrological retention time (THRT), which expresses the average time that it takes for a parcel of water to pass through a lake. The lake in Jämtland (northwest Sweden; 63°N, 12°E) is an oligotrophic, slightly alkaline clearwater lake with a long THRT (calculated as described by Algesten et al [1]). The five lakes in Uppland (east central Sweden; 60°N, 17°E) have diverse characteristics and range from oligomesotrophic to hypereutrophic and from unstained to humic, and they are more or less alkaline (15). The THRT of the Uppland lakes range from approximately 2 months to more than 2 years (3, 4).
TABLE 1.
Characteristics of the 15 lakes included in this study
| Lake | Abbreviation | Geographic region | Trophic status | pH | Temp (°C) | Lake area (km2) | THRT (days) |
|---|---|---|---|---|---|---|---|
| Sarsvatnet | SA | Svalbard | Ultraoligotrophic, unstained | 7.9 | 3.0 | 0.22 | 695a |
| Tvillingvatna | TV | Svalbard | Oligomesotrophic, unstained | 7.9 | 6.2 | 0.03 | 59a |
| Storvatnet | ST | Svalbard | Oligotrophic, unstained | 8.3 | 7.0 | 0.03 | 66a |
| Øvretjørna | ØV | Svalbard | Oligotrophic, unstained | 8.1 | 6.2 | 0.01 | 9a |
| Upper Bear | UB | Lappland | Mesotrophic, polyhumic | 6.3 | 14.3 | 0.05 | 62a |
| Lower Bear | LB | Lappland | Mesotrophic, polyhumic | 5.7 | 14.0 | 0.03 | 67 |
| Reindeer | RE | Lappland | Mesotrophic, polyhumic | 5.5 | 13.6 | 0.01 | 42a |
| Siholma | SH | Lappland | Oligomesotrophic, humic | 5.9 | 13.6 | 0.01 | 83a |
| Örträsket | ÖR | Lappland | Mesotrophic, humic | 6.3 | 11.6 | 7.30 | 100 |
| Ånnsjön | ÅN | Jämtland | Oligotrophic, unstained | 7.3 | 12.0 | 62.80 | 428 |
| Tvigölingen | TG | Uppland | Mesotrophic, humic | 7.5 | 18.5 | 0.07 | 147a |
| Siggeforasjön | SF | Uppland | Oligomesotrophic, humic | 7.3 | 17.9 | 0.76 | 180 |
| Hålsjön | HÅ | Uppland | Mesoeutrophic, weakly humic | 7.9 | 15.7 | 0.20 | 730 |
| Vallentunasjön | VA | Uppland | Eutrophic, unstained | 8.7 | 18.7 | 6.20 | 730 |
| Äs puss | ÄP | Uppland | Hypereutrophic, humic | 8.4 | 20.5 | 0.04 | 66a |
Headwater lake.
FIG. 1.
Map of Scandinavia showing the locations of the lakes sampled. The abbreviations for the lakes are defined in Table 1.
Four lakes are located close to the Ny-Ålesund research station on the Arctic Norwegian Svalbard islands (79°N, 15°E). These four lakes are all oligotrophic, slightly alkaline, clearwater lakes (20). The maximum depths of the lakes were estimated during sampling. Lake areas were obtained from maps available from the Norwegian Polar Institute (http://npweb.npolar.no/). By assuming a cone-shaped morphometry for all lakes except the most shallow lake (lake ST [see Table 1 for lake abbreviations]), which was assumed to be box shaped, approximate lake volumes could be calculated. The sizes of the drainage areas were estimated using the maps mentioned above. The average water flow (m3 year−1) into a lake was calculated using the average yearly precipitation in the area during the years from 1961 to 1990, 385 mm (http://met.no/observasjoner/svalbard/), multiplied by the size of the drainage area. The average air temperature at the sampling sites from 1961 to 1990 was −6.3°C. Since the vegetation at the sites is scarce, we assumed that the evapotranspiration was low and that the precipitation was a reasonable estimate of the water flow. THRT was calculated by dividing the lake volume by the average water flow rate. We estimated that three of the Svalbard lakes, which were small and shallow, had a short water retention time, which did not exceed 100 days. The fourth lake (lake SA), which was deeper and larger, had an estimated THRT of almost 2 years.
Most of the lakes are small, and only three of them, lake ÖR in Lappland, lake VA in Uppland, and lake ÅN in Jämtland, have a surface area that exceeds 1 km2; lake ÅN is the largest lake by far. There was a north-south gradient for temperatures of the lakes; i.e., the Svalbard lakes were the coldest lakes, with temperatures of 3 to 7°C, the temperatures of the Lappland and Jämtland lakes were 11 to 14°C, and the temperatures of the Uppland lakes were 15 to 20°C. Only two lakes are in the same catchment area; lake UB flows into lake LB.
All samples were collected in the summer; Lappland samples were collected on 25 to 28 June 1996, Jämtland samples were collected on 12 July 1996, Uppland samples were collected on 22 to 26 July 1996, and Svalbard samples were collected on 14 to 21 July 1997. In general, composite samples were collected from the whole water column (lakes in Uppland, Jämtland, and Svalbard) or the epilimnion (lakes in Lappland). Chemistry and temperature data for the lakes have been published previously (15, 16, 20).
Analysis of bacterioplankton communities.
Bacterioplankton cells were collected on 0.2-μm Durapore filters after prefiltration through a 1-μm glass fiber filter, and DNA was extracted as described previously by cell lysis in sodium dodecyl sulfate and subsequent extraction with phenol and chloroform (14). This protocol has previously been shown to efficiently extract DNA from lake bacterioplankton (14), although underrepresentation of larger cells and colonies (e.g., cyanobacteria) is expected for the most eutrophic lakes (lakes ÄP, HÅ, and VA) due to the prefiltration step (15).
Reverse line blot hybridization with oligonucleotide probes targeted at 16S rRNA gene sequences was performed as described previously (31). This is a fairly simple method which allows rapid screening of multiple samples with multiple probes (12, 31). In short, the extracted DNA to be tested was first subjected to PCR using universal bacterial primers targeting positions 27 to 518 in the 16S rRNA gene. The amplified DNA of all samples was then tested simultaneously with 15 different oligonucleotide probes by hybridization and stringent washing. Bound products were subsequently visualized through a chemiluminescence reaction. The signals were quantified by adding all pixel values within the signal dot boundaries after subtraction of the background value. The reactivity values used were the averages of the values for two reactions.
The probes used (Table 2) were designed to specifically bind bacterial 16S rRNA genes from previously described putative freshwater clusters (30, 31). Tests with PCR products that had either perfect matches or one or more mismatches showed that the discrimination was at approximately the single-mismatch level (31). The probes and conditions were optimized such that signal levels with equal amounts of perfectly matching PCR products were comparable (31).
TABLE 2.
Results from reverse line blot hybridization with 15 probes specific for freshwater bacterial groups
| Probea | Bacterial cluster targetedb | Phylogenetic affiliation | Similarity within cluster (%) | No. of positive lakes | Positive lakes in regionsc
|
|||
|---|---|---|---|---|---|---|---|---|
| Svalbard | Lappland | Jämtland | Uppland | |||||
| LD12-143 | LD12 cluster | Alphaproteobacteria | 99 | 11 | SA | All | ÅN | TG, SF, HÅ, VA |
| Rho-BAL47-396 | Rhodoferax sp. strain BAL 47 cluster | Betaproteobacteria | 95 | 15 | All | All | ÅN | All |
| Rpicketti-490 | Ralstonia pickettii cluster | Betaproteobacteria | 95 | 12 | All | All | ÅN | HÅ, ÄP |
| CR-FL23-464 | PnecA1 cluster | Betaproteobacteria | 98 | 8 | None | All | ÅN | TG, SF |
| LD28-451 | LD28 cluster | Betaproteobacteria | 97 | 12 | SA, TV | All | ÅN | TG, SF, HÅ, VA |
| GKS98-442 | GKS98 cluster | Betaproteobacteria | 98 | 12 | SA, TV, ØV | All | ÅN | TG, SF, HÅ |
| Mpsychro-211 | Methylobacter psychrophilus cluster | Gammaproteobacteria | 99 | 1 | None | None | None | HÅ |
| ACK-M1-193 | ACK-M1 cluster | Actinobacteria | 91 | 15 | All | All | ÅN | All |
| Sta230-187 | Sta2-30 cluster | Actinobacteria | 99 | 13 | SA, TV, ØV | All | ÅN | TG, SF, HÅ, VA |
| Urk014-126 | Urk-014 cluster | Actinobacteria | 98 | 2 | TV | None | None | TG |
| CL0-14-464 | CL0-14 cluster | Verrucomicrobia | 98 | 14 | SA, TV, ØV | All | ÅN | All |
| FukuN18-221 | FukuN18 cluster | Verrucomicrobia | 93 | 3 | None | UB | None | HÅ, VA |
| Syn/Pro-415 | Synechococcus cluster 6b | Cyanobacteria | 95 | 2 | None | None | ÅN | VA |
| Oagardhii-425 | Planktothrix agardhii cluster | Cyanobacteria | 99 | 0 | None | None | None | None |
| Aflosaquae-199 | A. flos-aquae cluster | Cyanobacteria | 96 | 0 | None | None | None | None |
Targeted bacterial groups.
We have optimized the reverse line blot assay with 15 16S rRNA gene oligonucleotide probes that target a selection of the “putative freshwater bacterial clusters” (30, 31). The targeted groups, to which the probes exhibit perfect matches, have within-group levels of similarity for known 16S rRNA gene sequences of 91 to 99% (Table 2). Actinobacterial cluster ACK-M1 and verrucomicrobial cluster FukuN18 have 91 and 93% within-group similarity, respectively; these levels are around genus-level similarity. At the other end of the spectrum is cluster LD12, in which there is more than 99% similarity among all of the 16S rRNA gene sequences collected (from at least 12 freshwater systems worldwide); this is probably species-level similarity.
The CR-FL23-464 probe covers a small subgroup (designated PnecA1, with 98% within-group similarity) of the Polynucleobacter necessarius cluster (which has 93% within-group similarity). With one sequence from Toolik Lake (Alaska) and three sequences from the Columbia River (31), this PnecA1 subgroup is in fact a monophyletic branch within the PnecA subcluster of the P. necessarius cluster (9).
The Oagardhii-425 probe targets the Planktothrix agardhii cluster, which contains Planktothrix agardhii, Planktothrix rubescens, Planktothrix mougeotii, and Planktothrix pseudagardhii. The Aflosaquae-199 probe targets both Aphanizomenon strains and Anabaena strains. More information on the phylogeny of targeted groups and probe design has been published previously (30, 31).
Statistics.
In order to reveal patterns among lakes regarding the distribution of bacterial groups, detrended correspondence analysis (DCA) (10) was performed using CANOCO 4.53 (Biometris-Plant Research International). DCA was chosen instead of correspondence analysis to avoid the arch effect, which is a distortion in the ordination diagram. In DCA the correspondence analysis axes are divided into segments, and the sample scores of the second axis are reassigned to be centered around the centroid to remove distortion. The hybridization signals of the different probes were first converted to proportions of the signal of the universal probe, UNI518 (31), for the same sample (expressed as a percentage) and transformed as log(1 + x). The two bacterial groups that did not show a positive signal for any lake were excluded from the analyses.
In order to reveal relationships between the appearance of bacterial groups and environmental variables from the lakes, a redundancy analysis (RDA) was used with the software CANOCO. This method requires a linear species-environment relationship (25), which can be assumed in cases in which the length of the first DCA axis is <2, which was the case in our data set (Fig. 2). The environmental factors that best described the most influential gradients were identified by forward selection. This procedure gives information about the importance of individual variables (25). Explanatory variables were added until addition of further variables failed to improve significantly (P < 0.05) the model's explanatory power. This was assessed in permutation tests with 499 unrestricted Monte Carlo permutations.
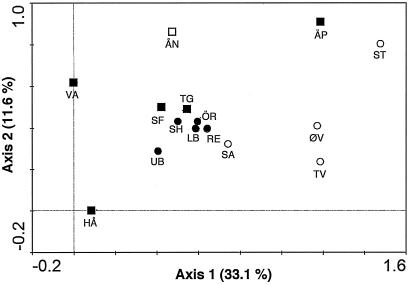
FIG. 2.
DCA of the hybridization data from the 15 lakes. Open circles, lakes in Svalbard; solid circles, lakes in Lappland; open square, lake in Jämtland; solid squares, lakes in Uppland. The abbreviations for the lakes are defined in Table 1.
The environmental variables tested were pH, water color (measured by measuring the absorbance of the water at 420 nm), total phosphorus concentration, total nitrogen concentration, concentration of dissolved organic carbon, temperature, and THRT. All of the environmental data except pH data were also transformed as log(1 + x). In a separate analysis a qualitative variable “region” was included in addition to the set of environmental variables. This was done by including three binary dummy variables (Svalbard, Lappland, and Uppland).
RESULTS
Distribution of bacterial groups among lakes and in relation to environmental variables.
In each lake 3 to 10 of the bacterial groups were detected; the average was 7.9 bacterial groups per lake (Table 2). There was no apparent connection between the number of groups detected per lake and geographic location.
The DCA showed that the samples from the lakes in Lappland had similar reactivities to the probe panel, since the samples from these lakes clustered together (Fig. 2). Otherwise, there was no apparent clustering consistent with the geographic locations of the lakes.
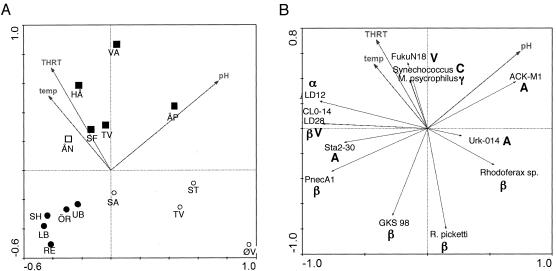
In the RDA model pH, THRT, and temperature were found to be the environmental variables that statistically best explained the variations in distribution of bacterial groups among lakes (Fig. 3 and Table 3). Including region with the variables tested did not significantly improve the explanatory power of the model, and none of the regions was among the best environmental variables. Thus, geographic region in itself did not “explain” more of the variation in the hybridization data set than the environmental variables included. The RDA model (i.e., pH, THRT, and temperature) statistically explained 64% of the variation of the hybridization data (Table 3). The first axis explained 44% of the variation (Table 3). The first axis also had a considerably better eigenvalue than the second and third axes, and, thus, the first axis must be considered the most important. The variable that correlated most strongly with this axis was pH, while temperature and THRT were more strongly correlated with axis 2 or 3; i.e., pH was the environmental variable that was most strongly related to patterns in the distribution of bacterial groups (Table 3).
FIG. 3.
RDA biplots. (A) Different samples in relation to the strongest environmental variables. Open circles, lakes in Svalbard; solid circles, lakes in Lappland; open square, lake in Jämtland; solid squares, lakes in Uppland. (B) Different bacterial groups in relation to the strongest environmental variables. α, Alphaproteobacteria; β, Betaproteobacteria; γ, Gammaproteobacteria; A, Actinobacteria; C, Cyanobacteria; V, Verrucomicrobia. The abbreviations for the lakes are defined in Table 1.
TABLE 3.
Results from RDAa
| Parameter | Value | Results
|
||
|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 3 | ||
| Sum of canonical eigenvalues | 0.642 | |||
| Sum of variance of “species” data explained (%) | 64.2 | |||
| Eigenvalue | 0.437 | 0.156 | 0.049 | |
| Variance of “species” data explained (%) | 43.7 | 15.6 | 4.9 | |
| Correlation with pH | 0.6745 | 0.5630 | 0.2218 | |
| Correlation with temperature | −0.3887 | 0.4712 | −0.6258 | |
| Correlation with THRT | −0.3713 | 0.6474 | 0.4853 | |
The variables selected by forward selection (P < 0.05) were pH, THRT, and temperature.
Appearance of individual bacterial groups.
Nine bacterial groups were detected in more than eight lakes (i.e., had a rather ubiquitous distribution) (Table 2). All of these groups except PnecA1, which was not found in Svalbard, were detected in lakes in all of the geographic regions sampled. Two probes, targeting the Rhodoferax sp. strain BAL 47 and ACK-M1 groups or clusters, gave positive results for all lakes.
The remaining six bacterial groups were detected in less than three lakes (Table 2), and two of these groups were not detected in any lake.
All five groups in the beta subdivision of the Proteobacteria (Betaproteobacteria) were detected in more than eight lakes (Table 2). All these groups were clearly associated very differently with the environmental variables included (Fig. 3b). For instance, PnecA1 was negatively correlated with pH, and Ralstonia pickettii was negatively correlated with temperature and THRT.
The three Actinobacteria groups were detected in 2, 13, and 15 lakes. These three groups were correlated completely differently with environmental variables. ACK-M1 was associated with high pH, Sta2-30 was associated with low pH, and the third group, Urk-014, appeared to be not correlated with pH.
Verrucomicrobia group CL0-14 was detected in 14 lakes and was associated with low pH and high THRT and temperature. The other Verrucomicrobia group screened for (FukuN18) was detected in only three lakes.
The only Alphaproteobacteria group screened for, LD12, was detected in 12 lakes, and it was associated with high temperature and long THRT.
Thus, the different bacterial groups, even within major bacterial groups, showed different patterns in terms of abundance and relationship to environmental variables.
The only Gammaproteobacteria group investigated was rare and was detected in only one lake. No signals were obtained for two of the cyanobacterial groups. The third cyanobacterial group, Synechococcus cluster 6b, was detected in only two lakes. The rarity of cyanobacterial signals is consistent with microscopic observations since cyanobacteria were observed in only four lakes (15, 16).
DISCUSSION
The 15 bacterial groups that were targeted in this study are putative typical freshwater bacterial clusters that were described previously on the basis of 16S rRNA gene sequence analysis (30). The probe set is not comprehensive for all freshwater clusters that have been described, and probably many common freshwater bacteria have not been described yet. Still, a number of very common groups were included, and the set allowed testing for environmental associations of subgroups within important bacterial divisions.
Hybridization of PCR products to solid-phase-bound probes does not allow absolute quantification. We assumed that biases in DNA extraction, PCR efficiency, and probe binding efficiency are constant in the sense that preferences for one group over another are equal for different ecosystems. In this manner we used the relative proportions of hybridization signals as quantitative measures to monitor bacterial groups along environmental gradients.
The results indicated that pH, retention time, and temperature were strong explanatory variables and together explained 64% of the variation in probe signals among lakes.
The relatively strong relationship to pH is not an unexpected finding. In a study in which the bacterial communities in 30 lakes in Wisconsin were investigated by a fingerprinting method, automated ribosomal intergenic spacer analysis, pH was one of the factors that were most strongly related (28). In experimental studies in which lake bacteria were inoculated into media having a neutral or alkaline pH, it was shown that growth of bacteria originating from acidic lakes was inhibited (13), suggesting that different taxa could be selected in acidic and alkaline environments.
Especially obvious from our hybridization results was the greater occurrence of PnecA1 in environments with low pH (Fig. 3b). This result was supported to some extent by a previous combined field and experimental study in which bacteria belonging to the P. necessarius cluster were favored by humic and acidic conditions (5), although our probe covered only a relatively narrow subcluster of the P. necessarius group (9, 31); thus, comparisons should be made with caution.
Temperature was another environmental variable that covaried with the appearance of bacterial groups. The betaproteobacterial taxon R. pickettii was negatively associated with temperature. There was a clear north-south gradient for temperature, and R. picketii was generally more abundant in the northern lakes. Several field investigations have shown that temperature covaries with bacterioplankton community composition (24, 28, 29). Since mechanistic relationships cannot be revealed by statistical relationships alone, it cannot be proven that a temperature difference of about 15°C, such as that between the warmest and coldest lakes in this study (Table 1), could select different taxa. Whether individual bacteria from such lakes have different temperature optima remains to be shown experimentally.
It was also not unexpected that THRT was an important explanatory variable. The development of a “complete” community adapted to lake conditions takes time. Previous field studies have shown that bacterioplankton community composition can be related to water flow and the import of bacterial cells from the drainage area (6, 11, 17-19, 24). Therefore, in a system with a short retention time, the community can be expected to be shaped by the import of river and/or terrestrial bacteria, while a community in a system with a long retention time is shaped by processes in the lake (19). The two types of lakes could, therefore, harbor different bacteria to some extent.
The type of bacteria being imported from the drainage area should depend on the position of the lake in the landscape; i.e., headwater lakes should receive a larger share of terrestrial bacteria, while lakes further down in the drainage area should receive aquatic bacteria from lakes upstream. Therefore, it is interesting that the betaproteobacterial group R. pickettii, which was associated with a short THRT, was especially abundant in the Svalbard lakes, which are all headwater lakes. Thus, these bacteria may originate to a greater extent from soils in the drainage areas. The fact that this cluster contains several terrestrial sequences (30) and the fact that members of this group are often particle associated (31) and thus can be supposed to be easily transported in rivers support this idea to some extent, but it needs further verification.
In contrast to R. picketti, alphaproteobacterial group LD12 was associated with long THRT and high temperature; i.e., the probe gave a stronger signal in lakes with longer THRT and high temperatures (Fig. 3b). It was not detected at all in four of the lakes with the shortest retention times (lakes ÄP, ST, TV, and ØV, all of which are headwater lakes). In a study of a delta system, bacteria belonging to the LD12 cluster were found to be associated with low water flow (24). These combined observations further support designation of bacteria in the LD12 cluster as typical pelagic freshwater bacteria. The previously proposed relationship between the appearance of this bacterium and high pH (alkalinity), based on denaturing gradient gel electrophoresis data from the lakes (20), was, not supported by the results of this study (Fig. 3b).
Thus, in summary, we conclude however, that both local and regional factors appear to have influenced the distribution of bacterial groups among the lakes. This result is in agreement with the results from a study of 30 Wisconsin lakes (28), as well as experimental evidence (13), introducing stochastic component into the shaping of lake bacterioplankton communities (7).
One other important finding of this study is that subgroups within major groups are distributed differently among the lakes and along environmental gradients. Sometimes the different subgroups even show opposite patterns; one example of this is actinobacterial groups ACK-M1 and Sta2-30 (Fig. 3 and Table 2). The different subgroups within the Betaproteobacteria and Verrucomicrobia were also differently correlated with the environment. Thus, in order to investigate determinative factors for bacterioplankton community composition, general probes designed to cover bacterial groups at the division or subdivision level have too little resolution. We expect that in order to understand more about the distribution of individual bacterial groups in nature, the use of more specific probes (for instance, in reverse line blot hybridization) will be of interest in the future.
Clearly, the relative proportions of the hybridization signals used here are hampered by the methodological biases mentioned above. However, some of the results obtained here by reverse line blot hybridization are in agreement with results of FISH and the analyses of clone libraries, such as the generally wide occurrence of bacteria belonging to the LD12, LD28, Rhodoferax sp. strain BAL47, ACK-M1, and STA2-30 clusters (27, 30) and the fact that Betaproteobacteria are generally common in freshwater (8, 21). Also, the finding that cyanobacteria were uncommon in our lakes was in agreement with microscopic observations. Thus, we expect that reverse line blot hybridization should give a relatively good picture of the situation in the lakes, especially when a larger number of probes can be used, although exact quantification of bacterial populations is not possible.
Acknowledgments
We thank Dag Hessen for information about the Norwegian lakes.
This work was supported by grants from the Olsson-Borgh and YMER-80 foundations and from the Training and Mobility of Researchers program of the Commission of the European Communities.
REFERENCES
- 1.Algesten, G., S. Sobek, A.-K. Bergström, A. Ågren, L. J. Tranvik, and M. Jansson. 2004. Role of lakes for organic carbon cycling in the boreal zone. Global Change Biol. 10:141-147. [Google Scholar]
- 2.Bergström, A.-K. 2000. The impact of allochthonous organic carbon on bacterial production and pelagic food web interactions in humic lakes in northern Sweden. Ph.D. thesis. Umeå University, Umeå, Sweden.
- 3.Brunberg, A.-K., and P. Blomqvist. 1998. Vatten i Uppsala län 1997.8/1998. Upplandsstiftelsen, Uppsala, Sweden.
- 4.Brunberg, A.-K., and B. Boström. 1992. Coupling between benthic biomass of Microcystis and phosphorus release from the sediments of a highly eutrophic lake. Hydrobiologia 235/236:375-385. [Google Scholar]
- 5.Burkert, U., F. Warnecke, D. Babenzien, E. Zwirnmann, and J. Pernthaler. 2003. Members of a readily enriched β-proteobacterial clade are common in surface waters of a humic lake. Appl. Environ. Microbiol. 69:6550-6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump, B. C., C. S. Hopkinson, M. L. Sogin, and J. E. Hobbie. 2004. Microbial biogeography along an estuarine salinity gradient: combined influences of bacterial growth and residence time. Appl. Environ. Microbiol. 70:1494-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis, T. P., and W. T. Sloan. 2004. Prokaryotic diversity and its limits: microbial community structure in nature and implications for microbial ecology. Curr. Opin. Microbiol. 7:221-226. [DOI] [PubMed] [Google Scholar]
- 8.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton composition of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn, M. W. 2003. Isolation of strains belonging to the cosmopolitan Polynucleobacter necessarius cluster from freshwater habitats located in three climatic zones. Appl. Environ. Microbiol. 69:5248-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill, M. O., and H. G. J. Gauch. 1980. Detrended correspondence analysis: an improved ordination technique. Vegetatio 42:47-58. [Google Scholar]
- 11.Jardillier, L., M. Basset, I. Domaizon, A. Belan, C. Amblard, M. Richardot, and D. Debroas. 2004. Bottom-up and top-down control of bacterial community composition in the euphotic zone of a reservoir. Aquat. Microb. Ecol. 35:259-273. [Google Scholar]
- 12.Kaufhold, A., A. Podbielski, G. Baumgarten, M. Blokpoel, J. Top, and L. Schouls. 1994. Rapid typing of group-a streptococci by the use of DNA amplification and nonradioactive allele-specific oligonucleotide probes. FEMS Microbiol. Lett. 119:19-25. [DOI] [PubMed] [Google Scholar]
- 13.Langenheder, S., E. S. Lindström, and L. J. Tranvik. 2005. Weak coupling between community composition and functioning of aquatic bacteria. Limnol. Oceanogr. 50:957-967. [Google Scholar]
- 14.Lindström, E. S. 1998. Bacterioplankton community composition in a boreal forest lake. FEMS Microbiol. Ecol. 27:163-174. [Google Scholar]
- 15.Lindström, E. S. 2000. Bacterioplankton community composition in five lakes differing in trophic status and humic content. Microb. Ecol. 40:104-113. [DOI] [PubMed] [Google Scholar]
- 16.Lindström, E. S. 2001. Investigating influential factors on bacterioplankton community composition—results from a field study of five mesotrophic lakes. Microb. Ecol. 42:598-605. [DOI] [PubMed] [Google Scholar]
- 17.Lindström, E. S., and A.-K. Bergström. 2005. Community composition of bacterioplankton and cell transport in lakes in two different drainage areas. Aquat. Sci. 67:210-219. [Google Scholar]
- 18.Lindström, E. S., and A.-K. Bergström. 2004. Influence of inlet bacteria on bacterioplankton assemblage composition in lakes of different hydraulic retention time. Limnol. Oceanogr. 49:125-136. [Google Scholar]
- 19.Lindström, E. S., M. Forslund, G. Algesten, and A.-K. Bergström. External control of bacterial community structure in lakes. Limnol. Oceanogr., in press.
- 20.Lindström, E. S., and E. Leskinen. 2002. Do neighbouring lakes share common taxa of bacterioplankton? Comparison of 16S rDNA fingerprints and sequences from three geographic regions. Microb. Ecol. 44:1-9. [DOI] [PubMed] [Google Scholar]
- 21.Methé, B. A., W. D. Hiorns, and J. P. Zehr. 1998. Contrasts between marine and freshwater bacterial community composition: analyses of communities in Lake George and six other Adirondack lakes. Limnol. Oceanogr. 43:368-374. [Google Scholar]
- 22.Muylaert, K., K. Van der Gucht, N. Vloemans, L. De Meester, M. Gillis, and W. Vyverman. 2002. Relationship between bacterial community composition and bottom-up versus top-down variables in four eutrophic shallow lakes. Appl. Environ. Microbiol. 68:4740-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson, B. R., N. Tezuka, and M. M. Watanabe. 2001. Phylogenetic analyses of Synechococcus strains (cyanobacteria) using sequences of 16S rDNA and part of the phycocyanin operon reveal multiple evolutionary lines and reflect phycobilin content. Int J. Syst. Evol. Microbiol. 51:861-871. [DOI] [PubMed] [Google Scholar]
- 24.Stepanauskas, R., M. A. Moran, B. A. Bergamaschi, and J. T. Hollibaugh. 2003. Covariance of bacterioplankton composition and environmental variables in a temperate delta system. Aquat. Microb. Ecol. 31:85-98. [Google Scholar]
- 25.ter Braak, C. J. F., and P. F. M. Verdonschot. 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 57:255-289. [Google Scholar]
- 26.Van der Gucht, K., K. Sabbe, L. De Meester, N. Vloemans, G. Zwart, M. Gillis, and W. Vyverman. 2001. Contrasting bacterioplankton community composition and seasonal dynamics in two neighbouring hypertrophic freshwater lakes. Environ. Microbiol. 3:680-690. [DOI] [PubMed] [Google Scholar]
- 27.Van der Gucht, K., T. Vandekerckhove, N. Vloemans, S. Cousin, K. Muylaert, K. Sabbe, M. Gillis, S. Declerck, L. De Meester, and W. Vyverman. 2005. Characterization of bacterial communities in four freshwater lakes differing in nutrient load and food web structure. FEMS Microbiol. Ecol. 53:205-220. [DOI] [PubMed] [Google Scholar]
- 28.Yannarell, A. C., and E. W. Triplett. 2005. Geographic and environmental sources of variation in lake bacterial community composition. Appl. Environ. Microbiol. 71:227-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yannarell, A. C., and E. W. Triplett. 2004. Within- and between-lake variability in the composition of bacterioplankton communities: investigations using multiple spatial scales. Appl. Environ. Microbiol. 70:214-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwart, G., B. C. Crump, M. P. Kamst-van Agterveld, F. Hagen, and S.-K. Han. 2002. Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat. Microb. Ecol. 28:141-155. [Google Scholar]
- 31.Zwart, G., E. J. van Hannen, M. P. Kamst-van Agterveld, K. van der Gucht, E. S. Lindström, J. Van Wichelen, T. Lauridsen, B. C. Crump, S.-K. Han, and S. Declerck. 2003. Rapid screening for freshwater bacterial groups by using reverse line blot hybridization. Appl. Environ. Microbiol. 69:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]