Abstract
The emerging concept of host specificity of Cryptosporidium spp. was exploited to characterize sources of fecal contamination in a watershed. A method of molecular forensic profiling of Cryptosporidium oocysts on microscope slides prepared from raw water samples processed by U.S. Environmental Protection Agency Method 1623 was developed. The method was based on a repetitive nested PCR-restriction fragment length polymorphism-DNA sequencing approach that permitted the resolution of multiple species/genotypes of Cryptosporidium in a single water sample.
Recent research on the molecular epidemiology of the genus Cryptosporidium has significantly changed our perceptions regarding the zoonotic potential of this waterborne parasite. There are currently 14 described species within the genus Cryptosporidium (3, 10) and more than 33 unique host-adapted genotypes, with only C. hominis, C. hominis monkey genotype, C. parvum, C. muris, C. felis, C. meleagridis, C. canis, C. suis, and Cryptosporidium cervine genotype demonstrated to cause infections in humans. Techniques commonly used for the identification of Cryptosporidium spp. in water, such as the U.S. Environmental Protection Agency (USEPA) Method 1623 (5), provide a quantitative assessment of the number of parasites present but do not discriminate those species or genotypes which pose a threat to public health.
Effective environmental risk assessment requires the development of methods for profiling the molecular composition of the Cryptosporidium species and/or genotypes that may be present in a water sample. In this paper we describe a method for profiling the molecular composition of Cryptosporidium parasites from raw water samples where mixed species or genotypes are present. The protocols are an extension of Method 1623, whereby DNA is extracted directly from fluorescently labeled oocysts on the microscope slide and profiled to determine the molecular composition of Cryptosporidium species and genotypes in the sample.
Raw water samples were collected from Old Man River in Lethbridge, Alberta, Canada. Raw river water samples (∼50 liters) were passed through Filta-max filters (IDEXX, Westbrook, ME) and transported to the lab for processing and enumeration according to USEPA Method 1623 (5).
Coverslips were removed from the enumerated microscope slides, and any remaining mounting medium was washed away with two rinses of warm (37°C) phosphate-buffered saline. The slide was allowed to air dry and stored at −20°C. Slides were placed on ice, and 50 μl of lysis buffer (2) was added to the well. Material was removed from the slide by scraping the entire well with a pipette tip and transferring the contents to a microcentrifuge tube. Further material was removed from the slide by placing 50 μl of lysis buffer on the well and scraping the surface with a tissue culture cell scraper. A final 100-μl rinse of lysis buffer was used to transfer any remaining material from the slide.
Some samples were collected and processed by Method 1623 through to the immunomagnetic separation procedure only. The resulting oocyst concentrates were combined with an equivalent volume of double-strength lysis buffer and frozen until DNA was extracted.
DNA was extracted from the samples with five freeze-thaw (liquid nitrogen/65°C) cycles and purified using the DNeasy tissue kit (QIAGEN, Mississauga, Ontario, Canada). To aid with DNA precipitation, 2 μg of carrier DNA (poly-deoxyadenosine; Roche Applied Sciences, Laval, Quebec, Canada) was added to each lysate sample prior to ethanol addition.
Molecular forensic profiling of Cryptosporidium spp. was carried out by nested PCR-restriction fragment length polymorphism (PCR-RFLP) analysis of the small ribosomal subunit RNA (18S rRNA) gene with primary and secondary primers and PCR conditions previously described by Xiao et al. (8, 11). A primary PCR was carried out using a 50-μl reaction mixture including 5 μl of template with 2 μl of product used for the 100-μl secondary reaction mixture. All reaction mixtures positive by nested PCR were digested with the restriction enzymes SspI, VspI, and DdeI (Promega Corp., Madison, WI). Restriction digests (20-μl volumes) included 8 μl of secondary PCR product and 8 U of enzyme and were digested according to the manufacturer's instructions for 3 h.
DNA sequencing was used to confirm Cryptosporidium species/genotypes obtained from secondary PCRs that contained only single species/genotypes as determined by RFLP. Bidirectional sequence analysis was performed using a Prism 3100-Avant genetic analyzer (Applied Biosystems) equipped with an 80-cm-long capillary array and POP-4 separation matrix.
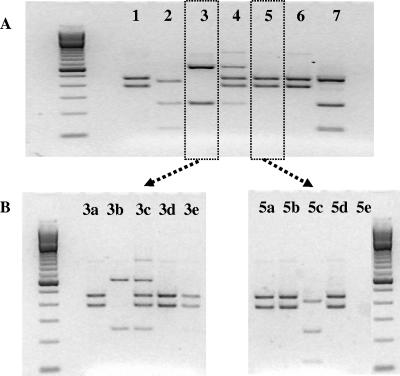
Initial attempts to identify Cryptosporidium species or genotypes in water samples were carried out using a single nested PCR-RFLP reaction from immunomagnetic separation purified oocyst concentrates (Fig. 1A). Restriction enzyme digests (SspI) yielded four different banding patterns among seven water samples analyzed, with one pattern representing a mixed profile (Fig. 1A, lane 4).
FIG. 1.
Composite image demonstrating the value of using repetitive nested PCR-RFLP to characterize mixed species/genotypes of Cryptosporidium in a water sample. (A) SspI RFLP profiles from a single nested PCR for water samples collected on 25 August 2003 (lane 1, C. andersoni), 2 September 2003 (lane 2, Cryptosporidium skunk genotype), 9 September 2003 (lane 3, C. baileyi), 29 September 2003 (lane 4, mixed C. andersoni and C. baileyi), 17 February 2004 (lane 5, C. andersoni), and 1 March 2004 (lane 6, C. andersoni). Lane 7 represents SspI RFLP patterns for a laboratory control strain of C. parvum. (B) Five replicates of nested PCR-RFLP reactions carried out from DNA extracts from 9 September and 17 February samples (boxed areas). Unlike the single PCR-RFLP analysis, repetitive nested PCR-RFLP revealed that the water sample from 22 September contained a mixture of C. andersoni (lanes 3a, 3c, 3d, and 3e) and C. baileyi (lanes 3b and 3c). Repetitive analysis also detected multiple genotypes/species of Cryptosporidium in the 17 February sample (C. andersoni in lanes 5a, 5b, and 5d and Cryptosporidium skunk genotype in lane 5c).
In order to resolve and assess mixed Cryptosporidium compositions within a single water sample, five replicates of the nested PCR were conducted on each of the water samples shown in Fig. 1A. Replicate reactions demonstrated that multiple species/genotypes were present in a water sample where previously only one was recovered from the single PCR-RFLP reaction (Fig. 1, compare panels A and B). These data indicate that incomplete and/or biased data may be obtained when only a single nested PCR-RFLP reaction is used to determine Cryptosporidium species/genotypes present in a water sample.
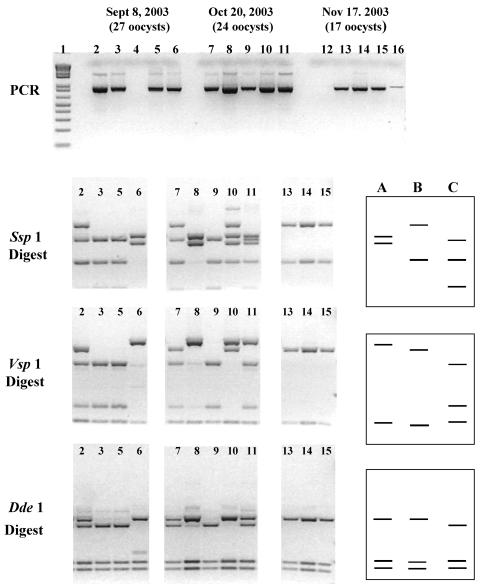
Repetitive nested PCR-RFLP was subsequently adapted to DNA extracted from stained microscope slides prepared from water samples processed by USEPA method 1623 in order to provide a composite picture of the species/genotypes found in the water sample. Figure 2 demonstrates the analysis from three microscope slides processed from water samples collected at the Lethbridge Drinking Water Utility on 8 September 2003, 20 October 2003, and 17 November 2003. Not all replicates of nested PCR analysis resulted in positive bands (Fig. 2), 8 September and 17 November, which is likely due to a low number of DNA template copies. Furthermore, replicating the PCR analysis greatly reduced the occurrence of the false negatives that occurred with single PCRs (data not shown).
FIG. 2.
Molecular forensic profiling of Cryptosporidium parasites by repetitive nested PCR-RFLP analysis using microscope slides containing three water samples processed by USEPA method 1623. (Top) Results of five repetitive nested PCRs for three water samples in which 27 oocysts (lanes 2 to 6, 8 September 2004), 24 oocysts (lanes 7 to 11, 20 October 2004), and 17 oocysts (lanes 12 to 16, 17 November 2004) were enumerated from the microscope slides. Results of RFLP analysis with restriction enzymes SspI, VspI, and DdeI of positive PCRs are shown in the lower panels. Single-genotype patterns were verified through DNA sequencing as Cryptosporidium skunk genotype (lane 3), C. andersoni (lane 8), and C. baileyi (lane 13). The RFLP banding patterns for these species/genotypes have been illustrated on the right (lane A, C. andersoni; lane B, C. baileyi; lane C, Cryptosporidium skunk genotype) and can be used to explain all the observed banding patterns. The 8 September water sample was composed of C. andersoni (lane 6), C. baileyi (lane 2 [contains both C. baileyi and Cryptosporidium skunk genotype]), and Cryptosporidium skunk genotype (lanes 2, 3, and 5). The 20 October water sample was composed of the same Cryptosporidium species/genotypes. C. andersoni was detected in lanes 8, 10 (mixed with C. baileyi), and 11 (mixed with Cryptosporidium skunk genotype). C. baileyi was detected in lanes 7 (mixed with Cryptosporidium skunk genotype) and 10 (mixed with C. andersoni). Cryptosporidium skunk genotype was detected in lanes 9 and 11 (mixed with C. andersoni). In the 17 November sample only C. baileyi (lanes 13, 14, and 15) was detected.
Water samples collected from the Oldman River were shown to contain three predominant species/genotypes of Cryptosporidium: C. andersoni, C. baileyi, and Cryptosporidium skunk genotype. All single species/genotype RFLP patterns were subjected to DNA sequence analysis to verify the presence of the three species/genotypes of Cryptosporidium in the river. All C. andersoni sequences had 100% identity match to GenBank accession number L19069. C. baileyi sequences had 100% identity match to GenBank accession number L19068, and all Cryptosporidium skunk genotype sequences were virtually identical to GenBank accession number AY120903, except for a consistent single threonine deletion at position 259.
There has been little focus on addressing the challenges of resolving mixed populations of Cryptosporidium oocysts in environmental water samples. In one study, ambiguous sequencing results indicative of a mixed population of Cryptosporidium species/genotypes in the sample were excluded from further analysis (7). This approach focuses on apparently dominant populations of Cryptosporidium oocysts in a water sample but results in loss of detectable resolution of Cryptosporidium species and/or genotype populations occurring at low frequency even though these organisms (i.e., C. hominis) may represent the more significant threat to human health. Other approaches to assess population heterogeneity have focused on generating multiple PCR products by using different volumes of sample template in the PCR (12). However, this method may not be suitable for samples having relatively few oocysts since limiting the template increases the likelihood of losing low frequency species/genotypes. Attempts have also been made to clone PCR products prior to DNA sequencing when mixed genotypes were present; however, this has been shown to result in artifacts during sequencing and is extremely labor intensive (15).
The repetitive nested PCR approach carried out in this study identified the composite diversity of species/genotypes in a water sample and also successfully resolved single species/genotypes into a PCR product that subsequently allows for accurate DNA sequence analysis. The adaptation and extension of this method to microscope slides obtained from water processed by USEPA method 1623 alleviates the need for additional sampling and processing to acquire molecular forensic profiling of Cryptosporidium from environmental samples. Quantifying and genotyping oocysts from a single sample not only reduces overall costs but also eliminates the disparity between duplicate samples caused by the random distribution of small numbers of oocysts in water (1).
Molecular analysis of water samples collected at the Lethbridge Water Utility suggests that three predominant host sources of Cryptosporidium parasites contaminate the river. These include cattle (C. andersoni), skunks or raccoons (Cryptosporidium skunk genotype), and birds (C. baileyi) (9, 13, 14). Although the data are limited, they suggest a low risk to public health since these three species/genotypes of Cryptosporidium are not known to infect humans. More-extensive watershed studies using the described method have been used in our laboratory to identify unique temporal and spatial patterns of Cryptosporidium contamination in the environment (data not shown). Such data are imperative for assessing the public health risk associated with waterborne Cryptosporidium oocyst contamination occurring in the environment and the host sources that pose those risks.
Cryptosporidium represents a model organism for tracking sources of fecal contamination in a watershed, backed by a strong and extensive scientific database correlating parasite host specificity to DNA sequence analysis at several genetic loci (4, 13). The molecular epidemiology of this organism can be exploited to provide a detailed assessment of the impact that wildlife or domestic agricultural animals have on contamination of water resources and the subsequent risks to human or animal health that are associated with these contamination events. Future policy changes requiring utilities to routinely monitor for Cryptosporidium in raw water (i.e., the Long Term 2 Enhanced Surface Water Treatment Rule [6]) may provide an ideal opportunity to exploit the described method to track sources of fecal contamination in a watershed and assess the public health impact of detected Cryptosporidium oocysts.
Acknowledgments
This research was supported by funds from the University of Calgary (N.N.), the Canadian Water Research Network (J.I.-R., C.O., and N.N.), and the Natural Science and Engineering Research Council of Canada (Strategic Research Grant to N.N.).
We gratefully acknowledge the logistical support for water sampling provided by Alberta Environment (Wendell Koning and Ray Walker) and the City of Lethbridge (Doug Kaupp and Dave Kubik). Thanks to Marnie Andersen for DNA sequencing and to Provincial Lab staff (Joanna Cumming and Ben Villeneuve) for the technical assistance with method 1623.
REFERENCES
- 1.Jellison, K. L., H. F. Hemond, and D. B. Schauer. 2002. Sources and species of Cryptosporidium oocysts in the Wachusett Reservoir watershed. Appl. Environ. Microbiol. 68:569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim, K., L. Gooze, C. Petersen, J. Gut, and R. G. Nelson. 1992. Isolation, sequence and molecular karyotype analysis of the actin gene of Cryptosporidium parvum. Mol. Biochem. Parasitol. 50:105-113. [DOI] [PubMed] [Google Scholar]
- 3.Ryan, U. M., P. Monis, H. L. Enemark, I. Sulaiman, B. Samarasinghe, C. Read, R. Buddle, I. Robertson, L. Zhou, R. C. Thompson, and L. Xiao. 2004. Cryptosporidium suis n. sp. (Apicomplexa: Cryptosporidiidae) in pigs (Sus scrofa). J. Parasitol. 90:769-773. [DOI] [PubMed] [Google Scholar]
- 4.Sulaiman, I. M., L. Xiao, and A. A. Lal. 1999. Evaluation of Cryptosporidium parvum genotyping techniques. Appl. Environ. Microbiol. 65:4431-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Environmental Protection Agency. 2003. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. Publication no. 815-R-03-XXX. Office of Ground Water and Drinking Water Technical Support Center, U.S. Environmental Protection Agency, Washington, D.C.
- 6.U.S. Environmental Protection Agency. 2003. National primary drinking water regulations: long term 2 enhanced surface water treatment rule. Fed. Regist. 68:47639-47795. [Google Scholar]
- 7.Ward, P. I., P. Deplazes, W. Regli, H. Rinder, and A. Mathis. 2002. Detection of eight Cryptosporidium genotypes in surface and waste waters in Europe. Parasitology 124:359-368. [DOI] [PubMed] [Google Scholar]
- 8.Xiao, L., K. Alderisio, J. Limor, M. Royer, and A. A. Lal. 2000. Identification of species and sources of Cryptosporidium oocysts in storm waters with a small-subunit rRNA-based diagnostic and genotyping tool. Appl. Environ. Microbiol. 66:5492-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao, L., L. Escalante, C. Yang, I. Sulaiman, A. A. Escalante, R. J. Montali, R. Fayer, and A. A. Lal. 1999. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 65:1578-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao, L., R. Fayer, U. Ryan, and S. J. Upton. 2004. Cryptosporidium taxonomy: recent advances and implications for public health. Clin. Microbiol. Rev. 17:72-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao, L., U. M. Morgan, J. Limor, A. Escalante, M. Arrowood, W. Shulaw, R. C. Thompson, R. Fayer, and A. A. Lal. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao, L., A. Singh, J. Limor, T. K. Graczyk, S. Gradus, and A. Lal. 2001. Molecular characterization of Cryptosporidium oocysts in samples of raw surface water and wastewater. Appl. Environ. Microbiol. 67:1097-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao, L., I. M. Sulaiman, U. M. Ryan, L. Zhou, E. R. Atwill, M. L. Tischler, X. Zhang, R. Fayer, and A. A. Lal. 2002. Host adaptation and host-parasite co-evolution in Cryptosporidium: implications for taxonomy and public health. Int. J. Parasitol. 32:1773-1785. [DOI] [PubMed] [Google Scholar]
- 14.Zhou, L., R. Fayer, J. M. Trout, U. M. Ryan, F. W. Schaefer III, and L. Xiao. 2004. Genotypes of Cryptosporidium species infecting fur-bearing mammals differ from those of species infecting humans. Appl. Environ. Microbiol. 70:7574-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou, L., C. Yang, and L. Xiao. 2003. PCR-mediated recombination between Cryptosporidium spp. of lizards and snakes. J. Eukaryot. Microbiol. 50(Suppl.):563-565. [DOI] [PubMed] [Google Scholar]