Abstract
An industrially attractive l-specific amidase was purified to homogeneity from Ochrobactrum anthropi NCIMB 40321 wild-type cells. The purified amidase displayed maximum initial activity between pH 6 and 8.5 and was fully stable for at least 1 h up to 60°C. The purified enzyme was strongly inhibited by the metal-chelating compounds EDTA and 1,10-phenanthroline. The activity of the EDTA-treated enzyme could be restored by the addition of Zn2+ (to 80%), Mn2+ (to 400%), and Mg2+ (to 560%). Serine and cysteine protease inhibitors did not influence the purified amidase. This enzyme displayed activity toward a broad range of substrates consisting of α-hydrogen- and (bulky) α,α-disubstituted α-amino acid amides, α-hydroxy acid amides, and α-N-hydroxyamino acid amides. In all cases, only the l-enantiomer was hydrolyzed, resulting in E values of more than 150. Simple aliphatic amides, β-amino and β-hydroxy acid amides, and dipeptides were not converted. The gene encoding this l-amidase was cloned via reverse genetics. It encodes a polypeptide of 314 amino acids with a calculated molecular weight of 33,870. Since the native enzyme has a molecular mass of about 66 kDa, it most likely has a homodimeric structure. The deduced amino acid sequence showed homology to a few other stereoselective amidases and the acetamidase/formamidase family of proteins (Pfam FmdA_AmdA). Subcloning of the gene in expression vector pTrc99A enabled efficient heterologous expression in Escherichia coli. Altogether, this amidase has a unique set of properties for application in the fine-chemicals industry.
Enantiomerically pure α-amino acids, both natural and unnatural, form an important class of chiral building blocks for the pharmaceutical and agrochemical industries. Examples of important α-hydrogen-α-amino acids are d-phenylglycine (d-Phg) and d-p-hydroxyphenylglycine, which are produced as side chains for the manufacture of semisynthetic β-lactam antibiotics such as ampicillin, amoxicillin, and cephalexin (14, 53), and d-valine, an intermediate for the pyrethroid insecticide fluvalinate (28). Another frequently used α-hydrogen-α-amino acid is l-tert-leucine, which is used as a building block for a variety of antiviral (e.g., anti-human immunodeficiency virus), antiarthritis, and anticancer drugs under development and as a chiral auxiliary in chemical asymmetric synthesis (5, 10). Besides α-hydrogen-α-amino acids, α,α-disubstituted α-amino acids also constitute a group of compounds of increasing importance, as exemplified by the use of l-α-methyl-3,4-dihydroxyphenylalanine (l-α-methyldopa) as an antihypertensive drug (34, 42), α-methylvaline as an intermediate for the herbicide Arsenal and related herbicides (47, 49), and l-α-methylphenylglycine as a building block for the new fungicide fenamidone (27).
Enantiomerically pure α-amino acids can be produced on a commercial scale by a number of different processes, both chemical and enzymatic. Biocatalytic processes that have been commercialized include the acylase process operated by Degussa (8) and Tanabe Seiyake (16), the d-hydantoinase process operated by Kanegafuchi (58, 59), the aminotransferase process operated by NSC Technologies (1, 48), and the l-leucine dehydrogenase process operated by Degussa (10). At DSM, we have implemented several chemoenzymatic processes for the production of enantiopure α-hydrogen- and α,α-disubstituted α-amino acids, which are based on the stereoselective hydrolysis of racemic amino acid amides (46). These substrates are readily available on a large scale via Strecker synthesis on the corresponding aldehydes or ketones (6, 39). To further expand the scope of this technology, we isolated a new Ochrobactrum anthropi strain that combines high amidase activity toward α-hydrogen- and α,α-disubstituted α-amino acid amides, α-hydroxy acid amides, and α-N-hydroxyamino acid amides with strict l-selectivity (50). The industrial attractiveness of this strain is further increased by its broad pH optimum, enabling resolution reactions between pH 5 and 8.5, and its very good temperature stability. This strain has been deposited at the NCIMB culture collection as NCIMB 40321.
In this paper, we describe the purification, cloning, and heterologous expression in Escherichia coli of the l-amidase from O.anthropi NCIMB 40321. Furthermore, we report the main properties of this enzyme. This work showed that this amidase is solely responsible for the remarkably relaxed substrate specificity of this microorganism.
MATERIALS AND METHODS
Materials.
All standard chemicals used were of the highest grade obtainable. Protease inhibitors E-64 {N-[N-(l-3-trans-carboxirane-2-carbonyl)-l-leucyl]-agmatine} and Pefabloc SC [4-(2-aminoethyl)-benzenesulfonyl fluoride] were purchased from Roche Diagnostics (Mannheim, Germany); phenylmethylsulfonyl fluoride (PMSF) was from Sigma-Aldrich (St. Louis, MO).
Aliphatic amide substrates as well as glycinamide were obtained from commercial suppliers (acetamide from Merck [Darmstadt, Germany]; isobutyramide, propionamide, and glycinamide from Sigma-Aldrich). All other amides were prepared in our laboratory by a number of methods described previously. The α-hydrogen-α-amino acid amides were prepared by Strecker reactions on the aldehyde precursors, followed by selective hydrolysis to the amide (6, 7, 39) or by ammonolysis of the corresponding racemic amino acid methyl esters (23). The latter method was also applied for the preparation of the β-amino and β-hydroxy acid amides. The α,α-disubstituted α-amino acid amides were prepared either by Strecker reaction followed by acidic hydrolysis in concentrated H2SO4 (46) or by phase transfer-catalyzed alkylation of N-benzylidene α-hydrogen-α-amino acid amides (23). The chemical purity of these amides was more than 99% in all cases. The dipeptides H-l-Leu-l-Leu-OH, H-l-Leu-l-Phe-OH, H-l-Phe-l-Leu-OH, and H-l-Phe-l-Phe-OH were purchased from Bachem (Bubendorf, Switzerland).
All enzymes used in DNA manipulations were obtained from Invitrogen (Breda, The Netherlands) and used according to the supplier's instructions unless indicated differently. Oligonucleotides were ordered from Applied Biosystems (Warrington, United Kingdom) and Invitrogen.
Microorganisms, plasmids, and cultivation conditions.
O. anthropi NCIMB 40321 was routinely maintained on LB plates (tryptone, 10 g/liter; yeast extract, 5 g/liter; NaCl, 5 g/liter). For protein purification, this microorganism was precultured in two 1-liter Erlenmeyer flasks, each with 250 ml of a medium containing (per liter) 4 g of yeast carbon base (YCB; Difco, Detroit, MI), 2 g of dl-mandelic acid amide, and 50 mM potassium phosphate buffer (pH 7.0). After 24 h of incubation at 28°C with stirring (190 rpm), the 500-ml preculture was transferred to a 15-liter fermentor (MBR Bio Reactor AG, Wetzikon, Switzerland) containing 10 liters of fresh preculture medium with an increased concentration of YCB (20 g/liter). The fermentor was operated at 28°C and pH 7 with agitation (900 rpm); the dissolved O2 level was maintained at 80% by adjusting the aeration (about 3.5 liters/min). After 24 h, the cells were harvested by centrifugation at 14,000 × g for 15 min, washed once with standard buffer (20 mM Tris-HCl, pH 7.5, containing 1 mM dithiothreitol), and centrifuged again. The cell pellet (approximately 70 g [wet weight]) was resuspended in an equal amount of standard buffer and stored in aliquots at −80°C.
E. coli strains were cultivated in LB medium at 37°C. When needed, carbenicillin (Carb) was added to this medium at 100 mg/liter. When isopropyl-β-d-1-thiogalactopyranoside (IPTG) was required for induction, it was used at a final concentration of 0.1 mM. For blue/white selection, LB plates contained 0.1 mM of both IPTG and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). E. coli DH5α (Invitrogen) and XL1-Blue MRF′ (Stratagene, La Jolla, CA) were used for propagation of derivatives of pGEM-T (Promega, Madison, WI); the latter strain was also used for cloning in pKK233-2 and pTrc99A (both plasmids are available from The Netherlands Culture Collection of Bacteria [NCCB 3078 and 3260, respectively]).
Purification of the l-amidase.
The whole purification procedure was performed at 4°C on a standard fast protein liquid chromatography system (Amersham Biosciences, Freiburg, Germany) equipped with columns from the same supplier.
The cell suspension was thawed and diluted with standard buffer to 1 g of cells per 4 g of buffer. The cells were disrupted in aliquots of 30 ml by sonication (total sonication time, 80 min; 30 s on, 30 s off; ice-acetone cooling). Cell debris was removed by centrifugation for 30 min at 28,000 × g. The resulting supernatant was the cell extract (CFE).
Ammonium sulfate precipitation was performed by addition of solid ammonium sulfate to the cell extract. The desired amidase precipitated between 35 and 60% ammonium sulfate saturation and was collected by centrifugation at 28,000 × g for 20 min. The protein pellet obtained was dissolved in standard buffer (30 ml) and desalted by gel filtration in 2.5-ml aliquots on Sephadex PD-10 columns (Amersham Biosciences).
The desalted 35 to 60% ammonium sulfate fraction (2 × 17.5 ml) was loaded onto a Mono Q HR 10/10 anion-exchange column that had been equilibrated with standard buffer. The l-amidase was eluted from the column at a flow rate of 3 ml/min by applying a 200-ml linear gradient from 0 to 1 M NaCl. Fractions of 3 ml were collected. The l-amidase eluted between 100 and 220 mM NaCl. Active fractions from both runs (34 ml) were pooled and concentrated to 7.2 ml by ultrafiltration through a filter with a cutoff value of 10,000 Da (YM-10 filter; Millipore, Billerica, MA).
The concentrated fractions from the anion-exchange column (2 × 3 ml) were applied to a HiLoad 26/60 Superdex 200 preparative gel filtration column that had been equilibrated with standard buffer containing 150 mM NaCl and were eluted at 2 ml/min. The fractions (3 ml) of both runs containing the highest amidase activity were pooled. To these pooled fractions an equal volume of 2.6 M ammonium sulfate was added. The resulting protein solution (2 × 30 ml) was loaded onto an Alkyl Superose HR 5/5 hydrophobic interaction chromatography column, which had been equilibrated with standard buffer containing 1.3 M ammonium sulfate. The l-amidase was eluted in 1-ml fractions by using a gradient from 1.3 to 0.65 M ammonium sulfate in 12.5 ml. The desired protein eluted as a free symmetrical peak between 1.2 and 1.0 M ammonium sulfate. The pooled fractions (total volume, 8 ml) were desalted by gel filtration through PD-10 columns and used for characterization studies.
Amidase activity assay.
The activity of this l-amidase was assayed in glass vials closed with screw caps and containing a reaction mixture (total volume, 10 ml) composed of 100 mM HEPES-NaOH buffer (pH 8.0), 1 mM MnCl2, 0.1 mg/ml bovine serum albumin (BSA), 50 mM substrate l-Val-NH2, and different amounts (1 to 10 μg) of protein. The reaction was initially performed at 40°C, which was changed to 55°C after the temperature stability of this enzyme had been determined. After regular time intervals (e.g., after 5, 10, 15, 20, 30, 60, and 120 min), 1-ml samples were withdrawn and the reaction was stopped by the addition of 1 ml of 1 M phosphoric acid. Routinely, the samples were quantitatively analyzed by measuring the amount of ammonia formed, which is equivalent to the amino acid production, using an ion-selective ammonia electrode system (Orion model 95-12; Thermo Electron Corporation, Milford, MA). However, in the experiments to establish the optimum pH of the enzyme, high-performance liquid chromatography (HPLC) analysis as described previously (19) was used to directly quantify the amino acid production. A chemical blank proved that both l-Val-NH2 and l-Val are stable for at least 6 h under standard conditions (55°C, pH 8.0). One unit of enzyme activity was defined as the amount of amidase that catalyzes the formation of 1 μmol of amino acid per min.
Identification of amidase-containing fractions in enzyme purification studies was performed using 175 μl of a reaction mixture composed of 50 mM l-Val-NH2 in 100 mM of HEPES-NaOH buffer, pH 8.0, and 25 μl of each fraction. After 1 h of incubation at 37°C, 2 μl of the reaction mixture was applied to precoated thin-layer chromatography (TLC) plates (silica gel 60 F254; Merck), dried, and developed in a chloroform-methanol-ammonia (60:45:20, vol/vol/vol) solvent system. After drying, both Val-NH2 and Val were stained by spraying the plates with ninhydrin reagent and incubating for 10 min at 120°C.
Protein determination.
Protein concentrations were determined by the method of Bradford (11) using BSA (SERVA Electrophoresis GmbH, Heidelberg, Germany) as the standard.
PAGE.
Both analytical sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and native PAGE were performed in PhastGel gradient (8 to 25%) gels using the PhastSystem according to the supplier's protocols (Amersham Biosciences). Gels were calibrated with the low (SDS-PAGE)- and high (native PAGE)-molecular-weight markers from the same supplier. The isoelectric point of the purified l-amidase was determined using isoelectric focusing with PhastGel IEF 3-9 gels that were calibrated with the Broad pI marker (also from Amersham Biosciences). In all cases, protein bands were visualized by the silver-staining method as described in the instruction manual of the PhastGel silver kit (Amersham Biosciences).
Preparative SDS-PAGE was performed as described by Laemmli (36) using a 12.5% separation gel. The protein bands were stained for 20 min in a solution containing 0.2% Coomassie brilliant blue G250, 20% methanol, and 0.5% acetic acid, followed by destaining for 1 h in 30% methanol.
Determination of the NH2-terminal and internal peptide amino acid sequences.
Approximately 500 pmol of purified amidase was dialyzed against Milli-Q grade water in a Spectra/Por CE (Spectrum Laboratories, Inc., Rancho Dominguez, CA) membrane with a cutoff value of 15,000 Da for 16 h. The dialyzed material was then dried to completion in a Savant SpeedVac (Thermo Electron Corporation). Approximately 350 pmol of this material was further pretreated on a preparative SDS-PAGE gel as described above. The l-amidase-containing slice was excised from the gel and used for determination of the amino acid sequences of internal peptides. The remaining 150 pmol of dialyzed material was used for determination of the NH2-terminal sequence. For both sequence analyses, Eurosequence BV (Groningen, The Netherlands) carried out further sample preparation (e.g., in situ tryptic digestion, followed by isolation and purification of the generated peptides) and automated Edman degradation on an Applied Biosystems 477A pulse-liquid sequenator connected online to a reversed-phase HPLC unit for identification of the phenylthiohydantoin-amino acids that were released stepwise.
Effect of pH on amidase activity.
Quantitative analysis of the amidase activity as a function of pH values between 4.0 and 10.5 was carried out by using substrate solutions (10 ml) containing 50 mM l-Val-NH2, 1 mM MnCl2, and 0.1 mg/ml BSA in the Britton-Robinson universal buffer consisting of 40 mM of each of the components H3PO4, CH3COOH, and H3BO3. The pHs of the different substrate solutions were adjusted by the addition of NaOH at 55°C (reaction temperature). Per reaction, 1.6 μg of purified amidase was used.
Effect of temperature on amidase stability.
The temperature stability of the enzyme was analyzed by preincubation of 4.4 μg of purified amidase for 1 h at temperatures between 30 and 80°C in 110 μl of standard buffer. Subsequently, 100 μl of the pretreated enzyme solution was used to determine the remaining activities according to the standard activity assay at 55°C.
Effects of inhibitors on amidase activity.
To determine the effects of protease inhibitors on the l-amidase, 2.4 μg of the purified enzyme in 200 mM HEPES-NaOH buffer, pH 8.0, was pretreated for 30 min at 0°C with EDTA (4 and 20 mM), 1,10-phenanthroline (2 and 20 mM), dithiothreitol (2 and 20 mM), iodoacetamide (IAA; 0.2 and 2 mM), PMSF (0.2 and 2 mM), E-64 (0.2 and 2 μM), or Pefabloc SC (0.2 and 2 mM) in a total volume of 2.25 ml. After subsequent addition of 250 μl of 40 mM MnCl2 and a further preincubation for 20 min at 0°C, the residual activity was analyzed.
Reactivation of EDTA-inhibited amidase was investigated by pretreating 8 μg of purified enzyme with 2 mM EDTA at 0°C for 30 min in 200 mM HEPES-NaOH buffer (pH 8.0; total volume, 4.875 ml). Then different divalent metal ions were added (125 μl of 0.16 M solutions), followed by another incubation at 0°C for 20 min. Residual activities were determined at 55°C by the addition of 5 ml of 100 mM l-Val-NH2, pH 8.0.
Determination of substrate specificity.
The activities of the purified l-amidase toward different types of amides were determined by the standard amidase activity assay. Per reaction, 1.6 to 3.4 μg of enzyme was incubated with 33 to 100 mM of substrate. For the amide substrate threo-3-[4-(methylsulfanyl)phenyl]serine amide (threo-amide) (structure given in Tables 3 and 5) (32), 100 mM 2-morpholinoethanesulfonic acid (MES)-NaOH buffer, pH 6.0 (instead of the standard HEPES buffer of pH 8), was used to increase the solubility. Conversion of N-hydroxy-phenylalanine amide was done under anaerobic conditions by N2 flushing. All reactions were performed at 55°C and stopped after a maximum of 120 min.
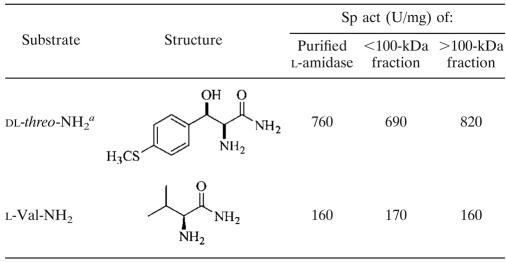
TABLE 3.
Specific activities toward dl-threo-NH2 and l-Val-NH2 of the purified l-amidase preparation, as well as of the retentate and the filtrate after 100-kDa ultrafiltration
Enzyme activity was determined at pH 6.
TABLE 5.
Substrate specificity of the purified l-amidase from O. anthropi NCIMB 40321 Substrate model 
Activities are given relative to the activity toward dl-valine amide (100%; corresponds to 300 U/mg). ND, not detected (corresponding to <1% conversion).
Reaction is performed under anaerobic conditions by N2 flushing.
Activities for the dipeptides H-l-Leu-l-Leu-OH, H-l-Leu-l-Phe-OH, H-l-Phe-l-Leu-OH, and H-l-Phe-l-Phe-OH were investigated by incubating 160 ng of purified amidase with 2 mM of each of the dipeptides under standard reaction conditions. Reaction products were analyzed by TLC as described above.
Determination of enantioselectivity.
The enantioselectivity of this enzyme for α-hydrogen-α-amino acid amides was qualitatively assayed by chiral TLC. Reaction mixtures from the experiment to determine the substrate specificity were diluted 1:1 with methanol-ammonia (3:1, vol/vol), and 5 μl was applied to chiral TLC plates (CHIRAL-PLATE, a reversed-phase silica gel plate coated with Cu2+ and chiral reagent; Macherey-Nagel, Düren, Germany). After drying, plates were developed in a solvent system consisting of methanol-water-acetonitrile (50:50:200, vol/vol/vol). After drying, both the amino acid amides and the amino acids were visualized by spraying the plates with ninhydrin reagent (Merck) and incubating them for 10 min at 120°C.
For quantitative analysis of enantioselectivity, the purified l-amidase (1.6 to 4.0 μg) was incubated with dl-Val-NH2, dl-Met-NH2, dl-Phg-NH2, racemic threo-NH2 (50 mM for each of these four substrates), and dl-α-(CH3)-Phe-NH2 (16.7 mM) under standard reaction conditions. However, in the case of threo-NH2, 100 mM MES-NaOH buffer, pH 6.0, was used. After a maximum of 6 h of incubation at 55°C, the reaction was stopped by the addition of an equal volume of 1 M phosphoric acid. Finally, the amino acid and amino acid amide enantiomers were converted into diastereomers by derivatization with o-phthaldehyde and d-3-mercapto-2-methylpropionic acid, and the diastereomers were analyzed by reversed-phase HPLC (20).
Cloning of part of the O. anthropi l-amidase gene (lamA).
Genomic DNA (gDNA) from O. anthropi NCIMB 40321 was isolated by the SDS-proteinase K-cetyltrimethylammonium bromide method essentially as described previously (54). However, use of cultures in the early-logarithmic-growth phase (optical density at 620 nm, 0.4 to 0.5) instead of the stationary phase appeared to be essential for isolation of sufficient amounts of good-quality gDNA. Another adaptation of this procedure was incubation of the resuspended cells with lysozyme (5 mg/g, wet weight) for 2 h at 37°C to weaken the cell wall before the lysis step with SDS and proteinase K. Furthermore, an incubation with RNAse A (20 μg/ml) for 30 min at 37°C, followed by a standard phenol-chloroform-isoamyl alcohol extraction, replaced the CsCl gradient centrifugation step in the original procedure. The gDNA (1 μg per reaction) was subsequently used as a template for PCR to amplify part of the lamA gene. Two combinations of degenerate oligonucleotides (AOA-1-AOA-6 and AOA-2-AOA-5; sequences given in Table 1) were used as primer pairs (1,000 nM per primer). The sequences of these oligonucleotides were based on the amino acid sequences of the two internal peptides (Table 1). After initial denaturation of the gDNA for 12 min at 94°C, amplification was performed using AmpliTaq DNA polymerase (Invitrogen) and 2 mM MgCl2 in 30 cycles of 2 min at 94°C, 1 min at 55°C, and 2 min at 72°C, with an additional 3 s per cycle. The 640-nucleotide (nt) fragment obtained with primers AOA-1 and AOA-6 was purified from the agarose gel with the QIAEXII kit (QIAGEN, Hilden, Germany) before being ligated into vector pGEM-T. The ligation mixture was used to transform E. coli DH5α, which was plated on LB-plus-Carb medium containing IPTG and X-Gal. The insert in a correct plasmid (as established by restriction enzyme analysis) from one of the white colonies obtained, pGEMDA-14, was bidirectionally sequenced.
TABLE 1.
Sequences of the NH2 terminus, tryptic peptides, and deduced oligonucleotides
| Peptide
|
Deduced oligonucleotide
|
||
|---|---|---|---|
| Name | Amino acid sequencea | Name | nt sequenceb |
| NH2 terminus | (C?)NNC(H/W)YTIX(G)(R?) | ||
| Peptide 1 | ITI(H)QFEPSGFG(W)TANIPGFGLLAD | AOA-1 | 5′-ACNATHCAYCARTTYGARCC-3′ |
| AOA-2 | 5′-GGYTCRAAYTGRTGDATNGT-3′ | ||
| Peptide 2 | ISEIVDQPNWVVSFYFPR | AOA-5 | 5′-GTNGAYCARCCNAAYTGGGT-3′ |
| AOA-6 | 5′-ACCCARTTNGGYTGRTCNAC-3′ | ||
Parentheses indicate an amino acid with about 70% certainty; parentheses with a question mark indicate an amino acid with <70% certainty. Segments used for oligonucleotide design are underlined.
N stands for A, C, T, or G; D stands for G, A, or T; Y stands for C or T; R stands for A or G; H stands for A, C, or T.
Preparation of a lamA-specific probe and Southern blotting.
A lamA-specific probe was prepared by digestion of plasmid pGEMDA-14 with PstI and NcoI. The isolated insert fragment was labeled with fluorescein-11-dUTP using the ECL Random-Prime Labeling and Detection system (Amersham Biosciences). This labeled probe was used in a Southern blotting experiment with O. anthropi gDNA digested to completion with BamHI, EcoRI, HindIII, PstI, SalI, or XbaI. These restriction enzymes were chosen because they are compatible with the LA PCR in vitro cloning kit (see below). After electrophoresis, the DNA was transferred to a Photogene nylon membrane (Invitrogen) via overnight capillary blotting and then baked to the filter for 2 h at 80°C. Hybridization and detection were performed according to the protocol of the ECL Random-Prime Labeling and Detection system, with overnight hybridization at 60°C and a probe concentration of 10 ng/ml. Washing steps (two washes with 1× SSC [0.15 M NaCl plus 0.015 M sodium citrate]-0.1% SDS solution followed by two with 0.5× SSC-0.1% SDS solution) were also performed at 60°C.
Identification of the complete lamA gene.
Starting from the part of the lamA gene cloned into vector pGEMDA-14, the nucleotide sequence of the complete amidase gene, including its flanking regions, was obtained using the LA PCR in vitro cloning kit (TaKaRa Bio Inc., Otsu, Japan). Primers AOA-7 (5′-ATATTCCGGGCTTCGGTCTTCTCGCCGACG-3′) (outer primer) and AOA-8 (5′-TCTCCATTGGTGATACCCATGCGGCACAGG-3′) (inner primer) were used at 200 nM each to amplify the 5′ end of lamA. The 3′ end of lamA was amplified using primers AOA-9 (5′-CGATTTCGCTGATACGCAGATCACCGCAG-3′) (outer primer) and AOA-10 (5′-GGGTTGAGGTTCTGCGTCTGGCAAAGAAGG-3′) (inner primer). As a template, O. anthropi gDNA digested with BamHI and ligated to the corresponding cassettes of the LA PCR in vitro cloning kit was used (0.5 μg per PCR). All amplification reactions were started with an initial denaturation for 1 min at 94°C, followed by 30 cycles of 20 s at 98°C and 3 min at 68°C, with an additional 15 s per cycle in the last 16 cycles. Both amplification products obtained were purified via gel filtration over ChromaSpin + TE-200 spin columns (BD Biosciences Clontech, Franklin Lakes, NJ) and cloned into vector pGEM-T. After transformation of E. coli XL1-Blue MRF′ and plating onto LB-plus-Carb plates containing IPTG and X-Gal, a correct clone from each of the two transformation mixtures, pGEMDA(−) and pGEMDA(+), was bidirectionally sequenced.
Heterologous expression of lamA in E. coli.
For expression of the l-amidase in E. coli, the lamA gene was cloned between the NcoI and HindIII sites of expression vectors pKK233-2 and pTrc99A, leading to constructs pKKLAM and pTrcLAM, respectively. The largest part of lamA (nt 831 to 1742; numbering as in Fig. 3) was amplified by PCR using Expand High Fidelity polymerase (Roche Diagnostics), 300 nM forward primer 5′-GATTACGGCCGGCATCATCATTTCGGCTGGGACA-3′ (XmaIII site underlined, nucleotide introducing the silent mutation double underlined), 300 nM reverse primer 5′-TCTGCAAGCTTATTCGAAAACGGAACGCGGGAAG-3′ (HindIII site underlined), and O. anthropi gDNA (100 ng) as a template. PCR was started with an initial denaturation for 4 min at 94°C, followed by 30 cycles of 1 min at 94°C, 30 s at 55°C, and 1.5 min at 72°C, with an additional 15 s per cycle. The amplification product (930 nt) was digested with HindIII and XmaIII, after which the desired fragment was purified using Chromaspin TE-100 spin columns. The connection between the NcoI site of the vector and the XmaIII site of the insert was established using a linker which was prepared by mixing 2.6 nmol of each of the oligonucleotides 5′-CATGTGCAATAATTGCCATTACACCATTCAC-3′ (NcoI overhang underlined) and 5′-GGCCGTGAATGGTGTAATGGCAATTATTGCA-3′ (XmaIII overhang underlined), heating for 5 min at 70°C, and slowly cooling down to room temperature to allow duplex formation. The vector, insert, and linker (molar ratio, 1:3:50) were then ligated (16 h, 15°C) using T4 DNA ligase. After removal of the excess linker molecules using a Chromaspin TE-400 spin column, the eluent was heated at 75°C for 5 min, followed by slow cooling to room temperature and a second spin column step to secure the presence of only one linker molecule per recombinant vector. The two ligation mixtures were used to transform E. coli XL-1 Blue MRF′ cells. The correctness of one of the pTrcLAM constructs was confirmed by nucleotide sequence analysis.
FIG. 3.
Positions of the lamA gene and flanking ORFs on the 2,973-bp BamHI fragment from O. anthropi NCIMB 40321, including their N- and C-terminal parts, and encoding nucleotide sequences. The deduced amino acid sequences are given below the nucleotide sequences. Stop codons are marked by number signs (#). Putative ribosome binding sites are boldfaced. The experimentally determined amino acid sequences of the N terminus and the two internal peptides from LamA are underlined. ORFs 1 and 2 are described in the text.
Nucleotide sequence accession number.
The nucleotide sequence of the l-amidase-encoding gDNA fragment from O. anthropi NCIMB 40321, including the 5′- and 3′-flanking regions, has been submitted to GenBank. The accession number is AY841125.
RESULTS
Enzyme purification.
The amidase was purified from O. anthropi NCIMB 40321 cells as described in Materials and Methods. Disintegration of the whole cells appeared to be difficult but could be effected by prolonged sonication (40 min). This procedure led to the release of more than 60% of the total amidase activity into the CFE. Starting from this extract, the amidase was purified to homogeneity by ammonium sulfate precipitation (35 to 60% saturation) and three chromatographic techniques, anion-exchange chromatography, gel filtration, and hydrophobic interaction chromatography (Table 2). By use of l-Val-NH2 as a substrate, the amidase activity consistently eluted as a single peak in all three chromatographic steps. By this procedure, the amidase could be purified approximately 100-fold with a 35% yield, which yielded a homogeneous preparation, as concluded from a single band on SDS-PAGE (Fig. 1) and a single NH2-terminal amino acid sequence. The specific activity of the purified enzyme preparation toward l-Val-NH2 (50 mM) was 200 U/mg of protein at pH 8 and 40°C.
TABLE 2.
Purification of the l-amidase from O. anthropi NCIMB 40321
| Purification step | Total activity (U) | Total protein (mg) | Sp act (U/mg) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| CFE | 2,000 | 980 | 2.0 | 100 | 1.0 |
| (NH4)2SO4 | 1,800 | 580 | 3.1 | 93 | 1.6 |
| Mono Q | 1,500 | 96 | 16 | 76 | 7.8 |
| Superdex 200 | 1,100 | 31 | 35 | 56 | 18 |
| Alkyl Superose | 680 | 3.5 | 200 | 35 | 98 |
FIG. 1.
SDS-PAGE of enzyme fractions obtained during purification of the l-amidase from O. anthropi NCIMB 40321. Lane 1, low-molecular-weight marker; lane 2, CFE (120 ng); lane 3, protein after (NH4)2SO4 fractionation (110 ng); lane 4, protein after Mono Q chromatography (170 ng); lane 5, protein after HiLoad Superdex 200 gel filtration (85 ng); lanes 6 and 7, protein after Alkyl Superose chromatography (64 ng and 32 ng, respectively).
Molecular mass, subunit composition, and isoelectric point.
By using SDS-PAGE, a single protein band corresponding to a subunit molecular mass of 36 kDa (Fig. 1) was obtained. The molecular mass of the native amidase was determined by native gradient gel electrophoresis. Contrary to expectations, the purified amidase preparation gave a number of consecutive protein bands corresponding to molecular masses of approximately 65, 125, and 180 kDa (data not shown). Most likely, the band corresponding to the protein with the lowest molecular mass belonged to the native amidase, meaning that the native enzyme is a dimer of identical subunits. The other two bands observed were most likely caused by aggregates of this native enzyme. To establish if the formation of these aggregates influences the catalytic properties of this amidase, the aggregates were separated from the native enzyme by ultrafiltration with a 100-kDa filter, followed by direct determination of the specific activities of the retentate (>100-kDa fraction) and the filtrate (<100-kDa fraction) toward l-Val-NH2 and racemic threo-NH2. The specific activities of both fractions toward these two structurally different α-H-α-amino acid amides were nearly the same as those of the enzyme preparation obtained after purification, indicating that aggregate formation did not significantly influence catalytic performance (Table 3). Therefore, the enzyme preparation obtained after the purification procedure was used for the biochemical characterization of this enzyme.
The isoelectric point of the purified amidase was estimated to be 5.4 by isoelectric focusing.
Determination of the NH2-terminal and internal peptide amino acid sequences.
The NH2-terminal amino acid sequence of the purified amidase was determined by automated Edman degradation (Table 1). The purity of the enzyme preparation was high (over 95%), leading to a single sequence. Rapid buildup of a high background signal due to nonselective peptide bond cleavage led to a very unfavorable signal-to-noise ratio, which complicated correct assignment for the first 11 amino acid residues and even prevented it from position 12 on.
The large number of tentative assignments in the NH2-terminal sequence complicated its use for the design of gene-specific oligonucleotides. Therefore, two internal peptides obtained after in situ tryptic digestion of the purified l-amidase were sequenced. The sequences obtained are given in Table 1.
Effect of pH on enzyme activity.
Enzyme activity was determined between pH 4.0 and 10.5 with a universal Britton-Robinson buffer, because use of different buffers to cover this pH range led to strong buffer-to-buffer effects (data not shown). The purified amidase was active toward l-Val-NH2 at pH values between 4.0 and 9.5 (Fig. 2). Maximum activity was displayed over a broad pH range of 6.0 to 8.5, but even at pH 4.5 the amidase still had about 65% of its maximum activity. At pH values above 8.5, the activity of the amidase dropped sharply.
FIG. 2.
Hydrolysis of l-Val-NH2 by the purified l-amidase from O. anthropi NCIMB 40321 as a function of pH at 55°C. The experiment was performed as described in Materials and Methods. Activity is given relative to the activity at pH 8 (corresponding to 280 U/mg).
Effect of temperature on enzyme stability.
The thermal stability of the l-amidase was determined by measuring the remaining activity after preincubation of the purified enzyme for 60 min at temperatures between 20 and 80°C. The amidase appeared to be fully stable at temperatures up to 60°C (data not shown). At higher temperatures, however, considerable thermal deactivation occurred within 1 h.
Effects of inhibitors.
The effects of different types of inhibitors were determined by measuring the remaining activity of the purified amidase from O. anthropi NCIMB 40321 toward l-Val-NH2 after it had been preincubated with the inhibitors for 30 min. For each inhibitor, two concentrations, chosen on the basis of literature data, were tested. Strong inhibition of the amidase occurred only with the metal-chelating reagents EDTA (93% inhibition at 2 mM) and 1,10-phenanthroline (80% inhibition at 10 mM) (Table 4). The other inhibitors tested, including serine (Pefabloc SC) and cysteine (IAA and E-64) protease inhibitors, had no clear inhibitory effect. An exception was the serine protease inhibitor PMSF, which moderately inhibited the enzyme at the highest concentration tested (28% inhibition at 1 mM).
TABLE 4.
Effects of potential inhibitors on purified amidase from O. anthropi NCIMB 40321
| Compound added | Concn (mM) | % Remaining activitya |
|---|---|---|
| None | 100 | |
| Dithiothreitol | 1 | 83 |
| 10 | 90 | |
| Iodoacetamide | 0.1 | 102 |
| 1 | 120 | |
| PMSF | 0.1 | 94 |
| 1 | 72 | |
| E-64 | 0.0001 | 109 |
| 0.001 | 109 | |
| Pefabloc SC | 0.1 | 108 |
| 1 | 92 | |
| EDTA | 2 | 7 |
| 10 | 4 | |
| 1,10-Phenanthroline | 1 | 64 |
| 10 | 20 |
Remaining activities are given relative to the activity of the untreated enzyme (100% corresponds to 280 U/mg).
To test if the inhibition by metal-chelating compounds is reversible, the apoenzyme obtained by pretreatment with EDTA was preincubated with different divalent metal ions for 20 min before the activity toward l-Val-NH2 was determined. Treatment of the apoenzyme (15% activity relative to that of untreated amidase [holoamidase]) with Ca2+ or Cu2+ resulted in a further decrease of the activity to 5 or 0%, respectively. On the other hand, reactivation occurred with Co2+ (to 40%), Fe2+ (to 30%), Zn2+ (to 80%), Mn2+ (to 400%), and Mg2+ (to 560%). Thus, Mn2+ and Mg2+ had especially marked effects, leading to activities four- to sixfold higher than that of the holoenzyme. In a similar experiment, it was demonstrated that activation of the amidase by preincubation with metal ions did not require the apoenzyme as an intermediate (e.g., Mn2+ increased the activity of the holoenzyme to 325%).
Substrate specificity.
The substrate specificity of the purified amidase was investigated by testing its activity toward a broad range of amide substrates. The results are given in Table 5. This enzyme showed excellent activity toward the majority of the aliphatic and aromatic α-hydrogen-α-amino acid amides tested. The highest activity within this substrate category was observed toward dl-Phe-NH2 and the cyclic amide dl-Pro-NH2. However, the activity for dl-tLeu-NH2, with the very bulky tert-butyl side chain, and for dl-Glu-NH2, the only α-hydrogen-α-amino acid amide containing a charged side chain tested, was low. This enzyme also displayed good activity toward α,α-disubstituted α-amino acid amides. For this type of substrate, the second α-alkyl substituent could range from a methyl group to an n-butyl group. Furthermore, the enzyme was active with the α-hydroxy acid amide dl-mandelic acid amide and the α-N-hydroxyamino acid amide dl-N-hydroxy-phenylalanine amide. No activity was observed with amide substrates without an —NH2, —OH, or —NHOH group at the Cα position: the aliphatic amides acetamide, propionamide, and isobutyramide, the β-amino acid amides dl-β-aminobutyric acid amide and dl-β-phenyl-β-aminopropionamide, and the β-hydroxy acid amide dl-β-hydroxybutyric acid amide. Moreover, none of the dipeptide substrates H-l-Leu-l-Leu-OH, H-l-Leu-l-Phe-OH, H-l-Phe-l-Leu-OH, and H-l-Phe-l-Phe-OH were converted by the purified amidase.
Enantioselectivity.
The dl-Val-NH2, dl-Leu-NH2, dl-Phg-NH2, dl-Phe-NH2, dl-Met-NH2, and dl-Pro-NH2 reaction mixtures from the substrate specificity test were analyzed by chiral TLC. This showed that in all cases only the l-amino acid was formed (data not shown). The high enantioselectivity of the purified amidase for α-hydrogen- and α,α-disubstituted α-amino acid amides was quantitatively confirmed using chiral HPLC (Table 6). The enantiomeric excess of the l-acids formed was more than 99% for Val, Phg, and α-(CH3)-Phe at conversions of at least 40%, which corresponded to a calculated E value of more than 200. Also, racemic threo-amide was converted completely enantioselectively, yielding the (2S,3R)-3-[4-(methylsulfanyl)phenyl]serine in an enantiomeric excess of 99.5% at 49% conversion (E > 200). For dl-Met-NH2, however, the enantiomeric excess of the l-amino acid formed was somewhat lower (96.5% at 47% conversion).
TABLE 6.
Enantioselectivity of the l-amidase from O. anthropi NCIMB 40321a
| Substrate | eed-amideb | eel-acidc | cd | Ee |
|---|---|---|---|---|
| dl-Val-NH2 | 0.70 | >0.995 | 0.41 | >200 |
| dl-Met-NH2 | 0.85 | 0.965 | 0.47 | >150 |
| dl-Phg-NH2 | 0.64 | >0.995 | 0.39 | >200 |
| dl-threo-NH2 | 0.94 | 0.995 | 0.49 | >200 |
| dl-(α-CH3)Phe-NH2 | 0.67 | >0.995 | 0.40 | >200 |
ee, enantiomeric excess; c, conversion.
eed-amide = (damide − lamide)/(damide + lamide).
eel-acid = (lacid − dacid)/(lacid + dacid).
c = (eed-amide)/(eed-amide + eel-acid).
E = {ln[1 − c(1 + eel-acid)]}/{ln[1 − c(1 − eel-acid)]}.
Cloning and sequence analysis of the gene encoding the l-amidase.
Based on the amino acid sequences of the two internal peptide fragments, degenerate oligonucleotides were designed (Table 1) for use in PCR experiments. Since the relative order of the two peptides in the amidase was unknown, two oligonucleotides with opposite directions were designed per peptide. The two possible primer combinations AOA-1-AOA-6 and AOA-2-AOA-5 were tested in PCR experiments using genomic DNA from O. anthropi NCIMB 40321 as a template. As expected, only one of the two primer combinations, AOA-1-AOA-6, led to amplification of a clear PCR product. Cloning of this fragment, which had a size of about 640 nt, into vector pGEM-T resulted in plasmid pGEMDA-14. Nucleotide sequence determination proved that part of the desired l-amidase gene had been cloned, since the DNA downstream of primer AOA-1 encoded the remaining 17 amino acids identified in peptide 1. Similarly, the DNA upstream of the AOA-6 primer sequence encoded the four N-terminal amino acids of peptide 2.
The complete l-amidase gene sequence, including its flanking regions, was then obtained with the LA PCR in vitro cloning kit in combination with gene-specific primers AOA-7 to AOA-10. These primers were designed in such a way that two PCR products with a 271-nt overlap would be obtained, one covering the 5′ end of the l-amidase gene and the other covering the 3′ end. Because Southern blotting with the insert of pGEMDA-14 as an l-amidase gene-specific probe had shown that this gene was localized on a 3,250-nt-BamHI fragment (data not shown), it was decided to use this restriction enzyme to fragment the O. anthropi gDNA. The procedure applied resulted in two clear PCR fragments of about 1,550 and 1,540 nt, which were cloned into vector pGEM-T, resulting in the pGEMDA(−) and pGEMDA(+) constructs, respectively. Sequence analysis of both constructs showed that together they contained a 2,973-bp BamHI region of the gDNA from O. anthropi NCIMB 40321. Three tentative open reading frames (ORFs) were identified in this DNA region (Fig. 3). The middle ORF clearly encoded the l-amidase, since the amino acid sequences of the N terminus and both internal peptides that were derived from the purified protein could be traced in the deduced amino acid sequence of this ORF. This gene, which was designated lamA, encoded a 314-amino-acid protein with a calculated molecular weight of 33,870. Because N-terminal sequencing of the amidase purified from O. anthropi wild-type cells showed that Cys-2 is the N-terminal residue, the calculated molecular weight of the mature amidase is 33,739, which sufficiently agrees with the experimentally determined subunit molecular mass of 36 kDa (SDS-PAGE). The theoretical isoelectric point of 5.2 corresponds very well with the value determined by isoelectric focusing (5.4). At 11 bp upstream of the ATG start codon, the nucleotide sequence ACGAGGT was found; this sequence most likely acts as a ribosome binding site (45), because it is highly complementary to the O. anthropi 16S rRNA 3′ end (OHUUCCUCCACT; accession no. AB120120).
BLASTP searches of the nr, pat, and env_nr protein sequence databases at the server of the National Center for Biotechnology Information (NCBI) (2) and the Derwent patentprot database with the deduced amino acid sequence of LamA as a query showed that this amidase has homology with a large number of (putative) proteins, most of which have been annotated as acetamidase and/or formamidase. This result was supported by a conserved domain search (38) at NCBI using RPS-BLAST, which produced a significant alignment (E value, 2e-42) of LamA with the acetamidase/formamidase family of proteins as defined by the Pfam database (FmdA_AmdA; accession no. PF03069). Since the majority of these homologous proteins were derived from genome-sequencing projects, either from isolated microorganisms or from an environmental sample (51), experimental evidence for the annotated activity of these proteins is lacking. The highest degree of sequence identity of LamA (80%) was obtained to such a hypothetical protein from Sinorhizobium meliloti (accession no. CAC46529). The l-amidase from Enterobacter cloacae N-7901 is the most closely related protein (67% identity) for which an activity has been described (40) (Table 7). This 315-amino-acid protein catalyzes the l-selective hydrolysis of racemic tert-leucine amide, resulting in the formation of the enantiomerically pure l-amino acid. The biochemical features of this amidase have not been described yet. The two least related proteins with proven biochemical function are the formamidase from Methylophilus methylotrophus (56) and the well-characterized acetamidase from Mycobacterium smegmatis (37). These two enzymes, which both showed 25% overall sequence identity to LamA, enable the bacteria to grow on formamide as a source of nitrogen (18, 57).
TABLE 7.
Amino acid sequence similarities of the O. anthropi NCIMB 40321 l-amidase
| Entry | Accession no.a | Enzyme source | Strain | Function (reference) | Amino acid residues | Mol wt | Identityb (%) | E value |
|---|---|---|---|---|---|---|---|---|
| 1 | AAV87210 | Ochrobactrum anthropi | NCIMB 40321 | l-Amidase (this study) | 314 | 33,870 | 100 | 0.0 |
| 2 | AAR56843 | Enterobacter cloacae | N-7901 | l-Amidase (40) | 315 | 33,755 | 67 | e-127 |
| 3 | ABR57499c | Thermus sp. | 0-3-1 | l-Amidase (33) | 309 | 33,091 | 53 | 1e-90 |
| 4 | CAB42722 | Klebsiella oxytoca | PRS1 | (R)-Amidase (12, 43) | 328 | 36,344 | 28 | 6e-33 |
| 5 | AAG60585 | Aspergillus nidulans | FGSC A4 | Formamidase (24) | 411 | 44,907 | 26 | 7e-24 |
| 6 | CAA67953 | Methylophilus methylotrophus | NCIMB 10515 | Formamidase (56) | 407 | 44,466 | 25 | 2e-22 |
| 7 | CAA40462 | Mycobacterium smegmatis | NCTC 8159 | Acetamidase (37) | 406 | 43,964 | 25 | 3e-21 |
Accession numbers in the protein sequence database of the NCBI. Analyses of the nr, pat, and env_nr protein sequence databases were performed in April 2005.
Determined by the Compare Two sequences option in the Align Plus 4 software program using the Blosum 62 scoring matrix (Scientific and Educational Software).
Accession number of the Derwent patentprot database. Database analysis was performed in April 2005.
As already indicated above, the sequenced BamHI region of the O. anthropi gDNA contained two more putative ORFs surrounding the amidase gene, which are transcribed in the same direction as lamA. Sequence analysis showed that the last 11 nucleotides of the upstream ORF (ORF-1) overlapped with the 5′ end of lamA (Fig. 3). This incomplete ORF encodes an N-terminally truncated protein which has the highest degree of sequence identity (78%) to the C-terminal 268 amino acids of a putative peptide-binding periplasmic ABC transporter protein from S. meliloti (accession no. CAC46530). When the comparison was limited to proteins with proven activity, the highest identity (32%) was observed to the C terminus of the heme-binding protein A precursor from Haemophilus influenzae (accession no. P33950). The ORF downstream of lamA (ORF-2) is also incomplete, but at the 3′ end. A Prosite search revealed that ORF-2 encodes a protein with two ATP/GTP-binding site A motifs (P-loop; PS00017) and one ABC transporter family signature sequence (PS00211). The highest sequence identity (67%) of this protein was observed with the N-terminal 388 amino acids from a putative peptide transport ATP-binding ABC transporter protein from S. meliloti (accession no. CAC46528).
Expression of the l-amidase gene in E. coli.
Production of the l-amidase via fermentation of wild-type O. anthropi cells was rather inefficient, since the amount of amidase formed amounted to, at most, 1% of the total soluble protein. Furthermore, commercial-scale implementation was hampered by the fact that O. anthropi is a risk class 2 microorganism. To enable more-efficient production of the l-amidase, the lamA gene was amplified from the O. anthropi gDNA by PCR and cloned into the NcoI and HindIII restriction sites of two E. coli expression vectors, pKK233-2 and pTrc99A. This yielded plasmids pKKLAM and pTrcLAM, respectively. Since use of NcoI to cut the PCR fragment would have led to a mutation of the amidase's second amino acid (Cys2Gly), the desired translational fusion of vectors and the amidase gene was created with an NcoI-XmaIII linker. This linker contained the first 33 nucleotides of the lamA gene up to an XmaIII site that was engineered into this gene via the forward PCR primer by a silent point mutation at position 33. Introduction of pKKLAM into E. coli XL-1 Blue MRF′ and plating onto LB plates containing carbenicillin resulted in extremely heterogeneous colony sizes. Investigation of the plasmids isolated showed that only the very small colonies contained the correct expression vector, whereas five of the six larger colonies investigated contained plasmids with deviating restriction patterns. Transformation of E. coli XL-1 Blue MRF′ cells with pTrcLAM, on the other hand, resulted in colonies of uniform size. Since no plasmid variation was observed, E. coli XL-1 Blue MRF′(pTrcLAM) was chosen for lab-scale protein production. Expression of the l-amidase from the strong trc promoter with 0.1 mM of IPTG as an inducer resulted in a CFE with a specific activity of 140 U/mg of protein (substrate, l-phenylglycine amide; 55°C), compared with only 8.1 U/mg of protein for the O. anthropi CFE, corresponding to 17-fold overexpression.
DISCUSSION
Stereoselective amidases and aminopeptidases are widely used in industry and academia for the synthesis of enantiomerically pure carboxylic acids. Examples include the production of both enantiomers of the α,α-disubstituted α-hydroxy acid 3,3,3-trifluoro-2-hydroxy-2-methylpropionic acid using an amidase from Klebsiella oxytoca PRS1 (12, 43), the synthesis of the cyclic α-hydrogen-α-amino acid (S)-piperazine-2-carboxylic acid by an amidase from Klebsiella terrigena DSMZ 9174 (21) and by the leucine aminopeptidase from porcine kidney (13), the use of a d-amidase from Variovorax paradoxus DSMZ 14468 for the synthesis of aliphatic α-hydrogen-α-amino acids such as d-leucine and d-tert-leucine (35, 52), and the lab-scale synthesis of both enantiomeric forms of a set of unsaturated α-hydrogen-α-amino acids using the leucine aminopeptidase from Pseudomonas putida ATCC 12633 (55).
In this paper we describe the purification and characterization of a unique l-amidase from O. anthropi NCIMB 40321 as well as the cloning of the encoding gene and its expression in E. coli. This enzyme is one of the available biocatalysts for use in DSM's chemoenzymatic resolution process for the commercial production of enantiomerically pure d- and l-α-hydrogen- and α,α-disubstituted α-amino acids (9, 46).
The amidase could be purified from the cell extract by a combination of ammonium sulfate fractionation, anion-exchange chromatography, gel filtration, and hydrophobic interaction chromatography. This purification procedure yielded a homogeneous preparation of the l-amidase enzyme with a subunit molecular mass of 36 kDa and a native molecular mass of 66 kDa. These results suggest that this enzyme has a homodimeric structure.
The purified l-amidase can convert an extremely broad range of substrates (Table 5). It readily converted α-hydrogen- as well as α,α-disubstituted α-amino acid amides, but the presence of the second alkyl side chain on the α-carbon atom in the latter class of substrates without exception led to at least 10-fold-lower activities. Besides α-amino acid amides, the α-hydroxy acid amide (dl-mandelic acid amide) and α-N-hydroxy-amino acid amide (dl-N-hydroxy-phenylalanine amide) investigated were also hydrolyzed by this enzyme. Furthermore, we recently reported on the resolution of two racemic α,α-disubstituted α-azido acid amides, 2-azido-2,4-dimethylpentanamide and 2-azido-2-methyl-3-phenylpropanamide, using a cell extract containing the O. anthropi amidase (30). Therefore, the α-amino group is not essential for activity. On theother hand, all substrates without an —NH2, —OH, or—NHOH group tested in this study, as in simple aliphatic amides, or with these groups at a C atom different from the Cα, as in β-amino and β-hydroxy acid amides, were not converted by the purified enzyme. Because this enzyme did not hydrolyze dipeptides, either, we classify this enzyme as an amidase. With the exception of activity for dipeptides, the substrate spectra of the purified amidase and dry cells of O. anthropi NCIMB 40321 (50) correspond rather well. Therefore, we conclude that the purified amidase reported in this paper is solely responsible for the broad-spectrum amidase activity of O. anthropi cells. This conclusion is supported by the fact that the relative activity for five structurally different substrates did not change significantly throughout the enzyme purification process (data not shown). The very broad substrate specificity of the purified amidase does not come at the expense of its enantioselectivity. Of all racemic substrates tested, only the l-enantiomer was hydrolyzed, resulting in E values of more than 150 (Table 6).
To circumvent fermentation of a risk class 2 microorganism, the gene encoding this l-amidase was cloned and expressed in E. coli using plasmid pTrc99A. This resulted in 17-fold overexpression compared to production in wild type O. anthropi cells, assuming that the specific activities of the l-amidases purified from the recombinant E. coli strain and the wild-type cells are identical. Uncoupling of growth and enzyme production phase, as is possible with vector pTrc99A due to the presence of the lacIq gene, appeared to be essential for successful heterologous expression. Overexpression of lamA throughout the cultivation severely hampered the growth of the recombinant E. coli production strain, as exemplified by the results with vector pKK233-2, which lacks a lacIq gene, leading to almost constitutive expression of the cloned lamA gene. Obviously, large amounts of the O. anthropi l-amidase are toxic for E. coli for reasons that are still unclear.
Database searches with the deduced amino acid sequence of the l-amidase revealed clear homology with more than 100 proteins that in many cases have been annotated as putative acetamidases or formamidases. About 45 of these homologous proteins have been grouped into the acetamidase/formamidase family as defined in the Pfam database (accession no. PF03069). Members of this protein family are widespread in nature; they are found in archaea, prokaryotes, and eukaryotes (e.g., in plants and fungi). Including the O. anthropi l-amidase, actual enzymatic activity has been experimentally proven for only seven of these proteins to date (Table 7). Based on the nature of this activity, these seven enzymes can be divided into two groups. The first group consists of the stereoselective amidases from E. cloacae (40), a Thermus sp. (33), and Klebsiella oxytoca (43). The Enterobacter and Thermus amidase genes have been isolated by Mitsubishi researchers. They encode two amidases of 315 and 309 amino acids, respectively, which are 67 and 53% identical to the O. anthropi l-amidase (Table 7). Both amidases are highly l-selective toward dl-tert-leucine amide and are also active toward lactate amide, implying that these amidases can also convert α-hydroxy acid amides. Unfortunately, no further biochemical properties have been described in the patents or open literature. More information is available on the amidase from K. oxytoca; this amidase, which is 328 amino acids long and has 28% identity with LamA, has been developed by Lonza for the (R)-selective hydrolysis of racemic 3,3,3-trifluoro-2-hydroxy-2-methylpropanamide (12). Recently, the results of a limited study on the substrate specificity of this amidase have been reported. It appeared that the specific activity of this amidase for the α-amino compound, 3,3,3-trifluoro-2-amino-2-methylpropanamide, was about 28 times higher than that for the corresponding α-hydroxy compound (44). A similar effect was observed with the l-amidase from O. anthropi; the specific activity for racemic phenylglycine amide was 37-fold higher than that for dl-mandelic acid amide. Clearly, both amidases prefer an amino group to a hydroxy group at the α-carbon atom. The two amidases also have similarly good heat stabilities. The effects of pH on the activities of the two enzymes, on the other hand, are quite different. The K. oxytoca amidase prefers alkaline conditions and has an optimum pH between 9 and 10 (43), whereas LamA prefers neutral to slightly acidic pH conditions, with a broad pH optimum at 6.0 to 8.5, and retains more than 50% of its maximum activity at pH 4.5. This high activity at a slightly acidic pH is of particular industrial relevance, because it enables the hydrolysis of amino acid amides, which are only very poorly soluble under alkaline conditions.
The second group consists of the formamidase of M. methylotrophus (56, 57), the acetamidase of M. smegmatis (18, 37), and the formamidase of Aspergillus nidulans (24). These three enzymes, which allow these microorganisms to grow on formamide and other short-chain amides as the sole nitrogen source, are significantly larger, with 406 to 411 amino acid residues, than the amidases in the first group and the O. anthropi amidase. The overall sequence identity of these enzymes with LamA is 25 to 26%. The enzymes from M. methylotrophus and M. smegmatis have been (partially) purified and biochemically characterized and appear to be closely related, as reflected by their 57% sequence identity and the cross-reactivity of antisera prepared against the two enzymes (56). They exhibit an extremely narrow substrate specificity, with the highest activity toward formamide and much lower activities toward short-chain aliphatic amides such as propionamide, butyramide, and acetamide (18, 57), exactly the substrates that the O. anthropi l-amidase cannot convert. Activities toward other types of substrates as well as investigations of potential enantioselectivity have not been reported. In contrast to the homodimeric structure first reported (57), it is now generally accepted that the active M. methylotrophus formamidase is a homotrimer in solution (56), which also distinguishes this enzyme from O. anthropi LamA. Although crystal data and a preliminary X-ray analysis of the formamidase of M. methylotrophus have been published (41), neither the detailed 3-dimensional structure of this protein nor that of any other member of the acetamidase/formamidase family has been solved yet. However, O'Hara et al. have found by optimal sequence threading that the formamidase of M. methylotrophus has a fold very similar to that of the Salmonella enterica serovar Typhimurium sialidase (41), which consists almost completely of antiparallel β-sheets arranged like the blades of a propeller around an axis passing through the active site (17). Interestingly, secondary-structure prediction of the O. anthropi l-amidase using the Pedant-Pro Sequence Analysis Suite (25, 26) classified this enzyme as an all-β protein.
Notwithstanding their clearly different substrate ranges and sizes, alignment of these seven biochemically proven amidases showed significant conservation across the entire proteins, except for the N and C termini and a large insertion of 48 amino acid residues in the central parts (between residues 148 to 149 [LamA numbering]) of the enzymes from M. smegmatis, M. methylotrophus, and A. nidulans (Fig. 4). Furthermore, these three enzymes contain a 22- to 24-amino-acid extension at their C termini. Alignment of other enzymes annotated as acetamidases or formamidases showed that this central insertion is without exception associated with a C-terminal extension, thereby suggesting a clear subdivision within this acetamidase/formamidase family of proteins (data not shown). To clarify the influence of both insertions on, e.g., the differences in substrate specificity, elucidation of the structure of a representative of each group is essential. Solving these amidase 3-dimensional structures will also reveal the amino acid residues that are important for catalysis as well as their catalytic mechanism. Since nothing has been published to date about the catalytic mechanism of (any member of) this family of proteins, we tried to classify O. anthropi LamA as a serine, cysteine, or metallodependent enzyme (31) based on inhibition by a standard set of inhibitors. IAA and E-64, typical inhibitors of cysteine proteases, had no effect on the purified amidase. Of the serine protease inhibitors tested, only the highest concentration of PMSF (1 mM) showed moderate inhibition. However, because Pefabloc SC, the second inhibitor of serine proteases tested, did not cause significant inhibition, it was concluded that serine and cysteine residues do not play a prominent role in the catalytic mechanism of this enzyme. This observation is in line with the fact that none of the l-amidase's cysteine or serine residues is conserved in the other six biochemically proven amidases (Fig. 4). Treatment of the l-amidase with the metalloprotease inhibitors EDTA and 1,10-phenanthroline led to very strong inhibition. Therefore, this enzyme can be classified as a metalloenzyme. The activity of the EDTA-inhibited enzyme could be (partially) restored by addition of the divalent metal ion Co2+, Fe2+, or Zn2+, whereas addition of Mn2+ or Mg2+ led to an amidase preparation with a four- to sixfold-higher specific activity than that obtained after purification. Similarly drastic effects of metal ion exchange on kinetic properties have been observed for other metalloenzymes, e.g., for the leucine aminopeptidase family of enzymes. The l-aminopeptidase from P. putida ATCC 12633 is a typical member of this enzyme family (46). This enzyme is activated no less than 12-fold by Mn2+ even without prior treatment with a metal-chelating agent (29).
FIG. 4.
Amino acid sequence alignment of the l-amidase (LamA) from O. anthropi with six other biochemically proven amidases. The alignment was generated with ClustalW 1.82 (15) and viewed with Boxshade 3.21. Residues that are identical or similar in at least four of the amidases are indicated by dark shading or light shading, respectively. Amidase numbers refer to the entry numbers in Table 7.
Sequence analysis of the regions flanking the O. anthropi lamA gene showed that it is surrounded by genes encoding different units of an ABC transporter. Upstream of lamA, a gene encoding a putative peptide-binding periplasmic ABC transporter protein was localized, whereas the gene downstream of lamA coded for a putative peptide transport ATP-binding ABC transporter protein. Since these two flanking genes are expressed in the same direction as lamA, it is very likely that lamA is part of an operon. An identical genetic organization is present in the genome of Sinorhizobium meliloti 1021 (region 2108013-2112414). Further upstream of the lamA homologue in this sequenced microorganism, two more genes are localized that encode further units (i.e., permeases) of a peptide ABC transporter. Based on the high sequence identity between the O. anthropi and S. meliloti DNA fragments (73%), it can be expected that homologues of these two additional ABC transporter genes are also present in O. anthropi. Since the genetic organization of the region surrounding a gene can give indications about the physiological role of the protein it encodes, it is tempting to conclude that lamA plays a role in intracellular breakdown of small peptides that are transported into the cell by the coexpressed peptide transporter. This hypothesis, however, conflicts with the fact that no activity was observed toward the four dipeptides tested whereas the corresponding amino acid amides were converted smoothly. Therefore, the actual biological function of this amidase remains unclear.
In recent decades, O. anthropi has become a source of interesting biocatalysts. Among these are a number of amide bond-hydrolyzing enzymes, i.e., the d-aminopeptidase from O. anthropi SCRC C1-38 (4), which is identical to DmpB from Ochrobactrum anthropi LMG7991 (22), the l-aminopeptidase (DmpA) from strain LMG7991 (22), and the d-amino acid amidase from O. anthropi SV3 (3). Comparison of the nucleotide sequences of these biocatalysts with the lamA gene sequence clearly shows that the l-amidase from strain NCIMB 40321, described in this paper, is not related to any of these amide hydrolases described before.
In conclusion, the l-amidase from O. anthropi NCIMB 40321 combines a very broad substrate specificity with a high enantioselectivity. These characteristics, together with the enzyme's good stability and broad pH tolerance, constitute a set of unique properties for application in the fine-chemicals industry. The industrial potential of this enzyme is increased even further by its ability to be overexpressed in E. coli, which is one of DSM's enzyme production hosts.
Acknowledgments
This work was financed by DSM's corporate research program.
We thank B. Dassen for the preparation of the amino acids and derivatives, L. Duchateau and M. Boesten for analytical support, and H. J. Bak and W. J. Weijer (Eurosequence B.V., Groningen, The Netherlands) for performing the amino acid sequence analyses. R. Wever (University of Amsterdam, Amsterdam, The Netherlands) is kindly acknowledged for critical comments on the manuscript.
REFERENCES
- 1.Ager, D. J., I. G. Fotheringham, T. Li, D. P. Pantaleone, and R. F. Senkpeil. 2000. The large scale synthesis of “unnatural” amino acids. Enantiomer 5:235-243. [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano, Y., T. Mori, S. Hanamoto, Y. Kato, and A. Nakazawa. 1989. A new d-stereospecific amino acid amidase from Ochrobactrum anthropi. Biochem. Biophys. Res. Commun. 162:470-474. [DOI] [PubMed] [Google Scholar]
- 4.Asano, Y., A. Nakazawa, Y. Kato, and K. Kondo. 1989. Properties of a novel d-stereospecific aminopeptidase from Ochrobactrum anthropi. J. Biol. Chem. 264:14233-14239. [PubMed] [Google Scholar]
- 5.Baxter, A. D., R. Bhogal, J. Bird, J. F. Keily, D. T. Manallack, J. G. Montana, D. A. Owen, W. R. Pitt, R. J. Watson, and R. E. Wills. 2001. Arylsulphonyl hydroxamic acids: potent and selective matrix metalloproteinase inhibitors. Bioorg. Med. Chem. Lett. 11:1465-1468. [DOI] [PubMed] [Google Scholar]
- 6.Boesten, W. H. J. July. 1979. Process for preparing α-amino-acid amides. GB patent 1,548,032 to DSM/Stamicarbon B.V.
- 7.Boesten, W. H. J., and J. Kamphuis. November. 1990. Process for the preparation of α-amino-α-methylcarboxylic acid amides and α-amino-α-cycloalkylcarboxylic acid amides. EP patent 0,231,546 to DSM/Stamicarbon B.V.
- 8.Bommarius, A. S., K. Drauz, and U. Groeger. 1992. Membrane reactors for the production of enantiomerically pure α-amino acids, p. 371-397. In A. N. Collins, G. N. Sheldrake, and J. Crosby (ed.), Chirality in industry. John Wiley & Sons, Chichester, United Kingdom.
- 9.Bommarius, A. S., M. Schwarm, and K. Drauz. 2001. Comparison of different chemoenzymatic process routes to enantiomerically pure amino acids. Chimia 55:50-59. [Google Scholar]
- 10.Bommarius, A. S., M. Schwarm, K. Stingl, M. Kottenhahn, K. Huthmacher, and K. Drauz. 1995. Synthesis and use of enantiomerically pure tert-leucine. Tetrahedron Asymmetry 6:2851-2888. [Google Scholar]
- 11.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 12.Brieden, W., A. Naughton, K. Robins, N. M. Shaw, A. Tinschert, and T. Zimmermann. August. 2004. Method of preparing (S)- or (R)-3,3,3-trifluoro-2-hydroxy-2-methylpropionic acid. U.S. patent 6,773,910 to Lonza AG.
- 13.Bruce, M. A., D. R. St. Laurent, G. S. Poindexter, I. Monkovic, S. Huang, and N. Balasubramanian. 1995. Kinetic resolution of piperazine-2-carboxamide by leucine aminopeptidase. An application in the synthesis of the nucleoside transport blocker (−)-draflazine. Synth. Commun. 25:2673-2684. [Google Scholar]
- 14.Bruggink, A., and P. D. Roy. 2001. Industrial synthesis of semisynthetic antibiotics, p. 12-54. In A. Bruggink (ed.), Synthesis of β-lactam antibiotics: chemistry, biocatalysis and process integration. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 15.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chibata, I., T. Tosa, and T. Shibatani. 1992. The industrial production of optically active compounds by immobilized biocatalysts, p. 351-370. In A. N. Collins, G. N. Sheldrake, and J. Crosby (ed.), Chirality in industry. John Wiley & Sons, Chichester, United Kingdom.
- 17.Crennell, S. J., E. F. Garman, W. G. Laver, E. R. Vimr, and G. L. Taylor. 1993. Crystal structure of a bacterial sialidase (from Salmonella typhimurium LT2) shows the same fold as an influenza virus neuraminidase. Proc. Natl. Acad. Sci. USA 90:9852-9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draper, P. 1967. The aliphatic acylamide amidohydrolase of Mycobacterium smegmatis: its inducible nature and relation to acyl-transfer to hydroxylamine. J. Gen. Microbiol. 46:111-123. [DOI] [PubMed] [Google Scholar]
- 19.Duchateau, A. L. L., and M. Crombach. 1987. Determination of α-aminonitriles, α-amino acid amides and α-amino acids by means of HPLC, post-column reaction and fluorescence detection. Chromatographia 24:339-344. [Google Scholar]
- 20.Duchateau, A. L. L., H. Knuts, J. M. M. Boesten, and J. J. Guns. 1992. Enantioseparation of amino compounds by derivatization with o-phthalaldehyde and d-3-mercapto-2-methylpropionic acid. J. Chromatogr. A 623:237-245. [Google Scholar]
- 21.Eichhorn, E., J.-P. Roduit, N. M. Shaw, K. Heinzmann, and A. Kiener. 1997. Preparation of (S)-piperazine-2-carboxylic acid, (R)-piperazine-2-carboxylic acid, and (S)-pipecolic acid by kinetic resolution of the corresponding racemic carboxamides with stereoselective amidase in whole bacterial cells. Tetrahedron Asymmetry 8:2533-2536. [Google Scholar]
- 22.Fanuel, L., I. Thamm, V. Kostanjevecki, B. Samyn, B. Joris, C. Goffin, J. Brannigan, J. Van Beeumen, and J. M. Frere. 1999. Two new aminopeptidases from Ochrobactrum anthropi active on d-alanyl-p-nitroanilide. Cell. Mol. Life Sci. 55:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feenstra, R. W., E. H. M. Stokkingreef, A. M. Reichwein, W. B. H. Lousberg, H. C. J. Ottenheijm, J. Kamphuis, W. H. J. Boesten, and H. E. Schoemaker. 1990. Oxidative preparation of optically active N-hydroxy-α-amino acid amides. Tetrahedron 46:1745-1756. [Google Scholar]
- 24.Fraser, J. A., M. A. Davis, and M. J. Hynes. 2001. The formamidase gene of Aspergillus nidulans: regulation by nitrogen metabolite repression and transcriptional interference by an overlapping upstream gene. Genetics 157:119-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frishman, D., K. Albermann, J. Hani, K. Heumann, A. Metanomski, A. Zollner, and H. W. Mewes. 2001. Functional and structural genomics using PEDANT. Bioinformatics 17:44-57. [DOI] [PubMed] [Google Scholar]
- 26.Frishman, D., M. Mokrejs, D. Kosykh, G. Kastenmuller, G. Kolesov, I. Zubrzycki, C. Gruber, B. Geier, A. Kaps, K. Albermann, A. Volz, C. Wagner, M. Fellenberg, K. Heumann, and H. W. Mewes. 2003. The PEDANT genome database. Nucleic Acids Res. 31:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genix, P., J.-L. Guesnet, and G. Lacroix. 2003. Chemistry and stereo-chemistry of fenamidone. Pflanzenschutz-Nachrichten Bayer 56:421-434. [Google Scholar]
- 28.Henrick, C. A., and B. A. Garcia. April. 1981. Esters and thiolesters of amino acids, processes for their production, and compositions including them. GB patent 1,588,111 to Zoecon Corporation.
- 29.Hermes, H. F. M., T. Sonke, P. J. H. Peters, J. A. M. Van Balken, J. Kamphuis, L. Dijkhuizen, and E. M. Meijer. 1993. Purification and characterization of an l-aminopeptidase from Pseudomonas putida ATCC 12633. Appl. Environ. Microbiol. 59:4330-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jost, M., T. Sonke, B. Kaptein, Q. B. Broxterman, and N. Sewald. 2005. Synthesis and enzymatic resolution of Cα-dialkylated α-azido carboxamides: new enantiopure α-azido acids as building blocks in peptide synthesis. Synthesis 2005:272-278. [Google Scholar]
- 31.Kalisz, H. M. 1988. Microbial proteinases. Adv. Biochem. Eng. Biotechnol. 36:1-65. [DOI] [PubMed] [Google Scholar]
- 32.Kaptein, B., T. J. G. M. van Dooren, W. H. J. Boesten, T. Sonke, A. L. L. Duchateau, Q. B. Broxterman, and J. Kamphuis. 1998. Synthesis of 4-sulfur-substituted (2S,3R)-3-phenylserines by enzymatic resolution. Enantiopure precursors for thiamphenicol and florfenicol. Org. Process Res. Dev. 2:10-17. [Google Scholar]
- 33.Katoh, O., T. Akiyama, and T. Nakamura. June. 2004. Novel amide hydrolase gene. EP patent application 1,428,876 to Mitsubishi Rayon Co., Ltd.
- 34.Kleemann, A., J. Engel, D. Reichert, and B. Kutschesr. 1999. Pharmaceutical substances: syntheses, patents, applications. Thieme, Stuttgart, Germany.
- 35.Krieg, L., M. B. Ansorge-Schumacher, and M.-R. Kula. 2002. Screening for amidases: isolation and characterization of a novel d-amidase from Variovorax paradoxus. Adv. Synth. Catal. 344:965-973. [Google Scholar]
- 36.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 37.Mahenthiralingam, E., P. Draper, E. O. Davis, and M. J. Colston. 1993. Cloning and sequencing of the gene which encodes the highly inducible acetamidase of Mycobacterium smegmatis. J. Gen. Microbiol. 139:575-583. [DOI] [PubMed] [Google Scholar]
- 38.Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meijer, E. M., W. H. J. Boesten, H. E. Schoemaker, and J. A. M. Van Balken. 1985. Use of biocatalysis in the industrial production of specialty chemicals, p. 135-156. In J. Tramper, H. C. van der Plas, and P. Linko (ed.), Biocatalysts in organic syntheses. Elsevier Science Publishers B.V., Amsterdam, The Netherlands.
- 40.Nakamura, T., and F. Yu. September. 2003. Amidase gene. U.S. patent 6,617,139 to Mitsubishi Rayon Co., Ltd.
- 41.O'Hara, B. P., S. A. Wilson, N. R. Wyborn, C. W. Jones, and L. H. Pearl. 1994. Crystallisation, preliminary X-ray analysis and secondary structure determination of formamidase from Methylophilus methylotrophus. Protein Pept. Lett. 1:202-205. [Google Scholar]
- 42.Reinhold, D. F., R. A. Firestone, W. A. Gaines, J. M. Chemerda, and M. Sletzinger. 1968. Synthesis of l-α-methyldopa from asymmetric intermediates. J. Org. Chem. 33:1209-1213. [DOI] [PubMed] [Google Scholar]
- 43.Shaw, N. M., A. Naughton, K. Robins, A. Tinschert, E. Schmid, M.-L. Hischier, V. Venetz, J. Werlen, T. Zimmermann, W. Brieden, P. de Riedmatten, J.-P. Roduit, B. Zimmermann, and R. Neumüller. 2002. Selection, purification, characterisation, and cloning of a novel heat-stable stereospecific amidase from Klebsiella oxytoca, and its application in the synthesis of enantiomerically pure (R)- and (S)-3,3,3-trifluoro-2-hydroxy-2-methylpropionic acids and (S)-3,3,3-trifluoro-2-hydroxy-2-methylpropionamide. Org. Process Res. Dev. 6:497-504. [Google Scholar]
- 44.Shaw, N. M., and A. B. Naughton. 2004. The substrate specificity of the heat-stable stereospecific amidase from Klebsiella oxytoca. Tetrahedron 60:747-752. [Google Scholar]
- 45.Shine, J., and L. Dalgarno. 1975. Determinant of cistron specificity in bacterial ribosomes. Nature 254:34-38. [DOI] [PubMed] [Google Scholar]
- 46.Sonke, T., B. Kaptein, W. H. J. Boesten, Q. B. Broxterman, J. Kamphuis, F. Formaggio, C. Toniolo, F. P. J. T. Rutjes, and H. E. Schoemaker. 2000. Amino amidase catalyzed preparation and further transformations of enantiopure α-hydrogen- and α,α-disubstituted α-amino acids, p. 23-58. In R. N. Patel (ed.), Stereoselective biocatalysis. Marcel Dekker, Inc., New York, N.Y.
- 47.Stepek, W. J., and M. M. Nigro. October. 1988. Novel process for the preparation of aminonitriles useful for the preparation of herbicides. EP patent 0,123,830 to American Cyanamid Company.
- 48.Taylor, P. P., D. P. Pantaleone, R. F. Senkpeil, and I. G. Fotheringham. 1998. Novel biosynthetic approaches to the production of unnatural amino acids using transaminases. Trends Biotechnol. 16:412-418. [DOI] [PubMed] [Google Scholar]
- 49.Tomlin, C. D. S. 2003. The pesticide manual. British Crop Protection Council (BCPC), Alton, Hampshire, United Kingdom.
- 50.van den Tweel, W. J. J., T. J. G. M. van Dooren, P. H. de Jonge, B. Kaptein, A. L. L. Duchateau, and J. Kamphuis. 1993. Ochrobactrum anthropi NCIMB 40321: a new biocatalyst with broad-spectrum l-specific amidase activity. Appl. Microbiol. Biotechnol. 39:296-300. [Google Scholar]
- 51.Venter, J. C., K. Remington, J. F. Heidelberg, A. L. Halpern, D. Rusch, J. A. Eisen, D. Wu, I. Paulsen, K. E. Nelson, W. Nelson, D. E. Fouts, S. Levy, A. H. Knap, M. W. Lomas, K. Nealson, O. White, J. Peterson, J. Hoffman, R. Parsons, H. Baden-Tillson, C. Pfannkoch, Y.-H. Rogers, and H. O. Smith. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66-74. [DOI] [PubMed] [Google Scholar]
- 52.Verseck, S., K. Drauz, A. Bommarius, M.-R. Kula, L. Krieg, H. Slusarczyk, and M. Ansorge. June. 2003. d-Amidase aus Variovorax. EP patent application 1,318,193 to Degussa AG.
- 53.Wegman, M. A., M. H. A. Janssen, F. van Rantwijk, and R. A. Sheldon. 2001. Towards biocatalytic synthesis of β-lactam antibiotics. Adv. Synth. Catal. 343:559-576. [Google Scholar]
- 54.Wilson, K. 1997. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4. 5. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 55.Wolf, L. B., T. Sonke, K. C. M. F. Tjen, B. Kaptein, Q. B. Broxterman, H. E. Schoemaker, and F. P. J. T. Rutjes. 2001. A biocatalytic route to enantiomerically pure unsaturated α-H-α-amino acids. Adv. Synth. Catal. 343:662-674. [Google Scholar]
- 56.Wyborn, N. R., J. Mills, S. G. Williams, and C. W. Jones. 1996. Molecular characterisation of formamidase from Methylophilus methylotrophus. Eur. J. Biochem. 240:314-322. [DOI] [PubMed] [Google Scholar]
- 57.Wyborn, N. R., D. J. Scherr, and C. W. Jones. 1994. Purification, properties and heterologous expression of formamidase from Methylophilus methylotrophus. Microbiology 140:191-195. [Google Scholar]
- 58.Yamada, H., S. Shimizu, H. Shimada, Y. Tani, S. Takahashi, and T. Ohashi. 1980. Production of d-phenylglycine-related amino acids by immobilized microbial cells. Biochimie 62:395-399. [DOI] [PubMed] [Google Scholar]
- 59.Yamada, H., S. Takahashi, and K. Yoneda. December. 1980. Process for preparing d-N-carbamoyl-α-amino acids. U.S. patent 4,237,227 to Kanegafuchi Kagaku Kogyo K.K.