Abstract
The deacetoxycephalosporin C synthase from Streptomyces clavuligerus was directly modified for enhancement of penicillin G expansion into phenylacetyl-7-aminodeacetoxycephalosporanic acid, an important intermediate in the industrial manufacture of cephalosporin antibiotics. Nine new mutants, mutants M73T, T91A, A106T, C155Y, Y184H, M188V, M188I, H244Q, and L277Q with 1.4- to 5.7-fold increases in the kcat/Km ratio, were obtained by screening 6,364 clones after error-prone PCR-based random mutagenesis. Subsequently, DNA shuffling was carried out to screen possible combinations of substitutions, including previous point mutations. One quaternary mutant, the C155Y/Y184H/V275I/C281Y mutant, which had a kcat/Km ratio that was 41-fold higher was found after 10,572 clones were assayed. The distinct mutants obtained using different mutagenesis methods demonstrated the complementarity of the techniques. Interestingly, most of the mutated residues that result in enhanced activities are located within or near the unique small barrel subdomain, suggesting that manipulation of this subdomain may be a constructive strategy for improvement of penicillin expansion. Several mutations had very distinct effects on expansion of penicillins N and G, perhaps due to different penicillin-interacting modes within the enzyme. Thus, the present study provided not only promising enzymes for cephalosporin biosynthesis but also a large number of mutants, which provided new insights into the structure-function relationship of the protein that should lead to further rational engineering.
Cephalosporins, a family of antimicrobial chemotherapeutic agents, have been efficacious for clinical treatment of many infectious diseases due to their broad activity spectra coupled with their low toxicity and resistance to β-lactamase (26). Many pharmaceutically important cephalosporins, such as cephradine, cephalexin, and cephadroxil, are derived from 7-aminodeacetoxycephalosporanic acid (7-ADCA) (3). The method currently used for industrial synthesis of 7-ADCA includes complicated chemical expansion of the five-member thiazolidine ring of penicillin G (PenG) into the six-member dihydrothiazine ring of phenylacetyl-7-aminodeacetoxycephalosporanic acid, followed by well-documented enzymatic removal of the phenylacetyl side chain. Since the chemical ring expansion is expensive and polluting, the development of alternative biosynthetic methods has been studied for decades (1, 4-6, 9-11, 30, 31).
Cephalosporins can be produced by several microorganisms, such as filamentous fungi, actinomycetes, and gram-negative bacteria, and the biosynthesis of cephalosporins by Streptomyces clavuligerus and Acremonium chrysogenum has been well studied (22, 30). The deacetoxycephalosporin C synthase (DAOCS) (expandase) catalyzes the ring expansion of penicillin N (PenN) into deacetoxycephalosporin C (DAOC) (13) (Fig. 1). Several DAOCSs from actinomycetes have been screened (7, 11-13), and the S. clavuligerus enzyme has been characterized extensively. These enzymes are Fe(II)- and α-ketoglutarate (αKG)-dependent oxygenases. They can catalyze the ring expansion of penicillins with various side chains into the corresponding cephalosporins and hence have become important possible compounds for 7-ADCA production. However, DAOCSs prefer their physiological substrate, PenN, rather than other penicillins (9).
FIG. 1.
Ring expansion of penicillins into cephalosporins catalyzed by DAOCS. G-7-ADCA, phenylacetyl-7-aminodeacetoxycephalosporanic acid.
The crystal structure of S. clavuligerus DAOCS has led to some rational engineering trials using site-directed mutagenesis (17, 21, 29). More than 20 residues have been replaced, including three cysteine residues (C100, C155, and C195) (18), various arginine residues (R74, R75, R160, R162, R266, R306, and R307) (4, 20), and other residues located in or near the active site cavity, including the ferrous ligands (H183, D185, and H243) (27), an αKG-selective residue (R258) (15, 16), and the C-terminal tail (G299, G300, N301, Y302, N304, R306, R307, and a range of deletions) (4, 5, 17, 19). However, only the N304L, R306L, ΔK310 (deletion of the last residue, A311), and +GQY (additional three residues at the C terminus) mutants have been shown to exhibit 1.5- to 2.0-fold increases in PenG expansion (4, 5, 20, 21). In a previous study, based on structural and mutational information, we changed M73, L158, R160, V303, N304, and I305 into alanine, lysine, aspartate, leucine, and methionine and identified three mutants, N304K, I305L, and I305M mutants, with 6- to 14-fold increases in the kcat/Km ratio (31). Due to the uncertainty of structure-based mutational analysis for improving the PenG expansion, we also developed an Escherichia coli ESS bioassay to select desirable mutants in random mutagenesis and family shuffling analyses (11, 31). We first employed simple random mutagenesis using hydroxylamine as a chemical mutagen, and only three mutants, G79E, V275I, and C281Y mutants, which had kcat/Km values that were two- to sixfold higher, were obtained. All 41 possible combined substitutions for six point mutations were subsequently carried out by site-directed mutagenesis, and eight multiple mutants with significantly enhanced activity were identified; in particular, the double mutant V275I/I305M, which exhibited a 32-fold increase in the kcat/Km value (31), was identified. In this study, we first utilized error-prone PCR-based random mutagenesis (epPCR), a diversity-generating method, to discover if new mutants with enhanced activities could be generated. Subsequently, the DNA shuffling method was used to screen mutants with all possible combinations of the available point mutations. Sixteen multiple mutants were obtained; in particular, a quaternary mutant, mutant C155Y/Y184H/V275I/C281Y, had a 41-fold-higher kcat/Km ratio. The large number of mutants may provide new insights into the structure-function relationship of this important enzyme and lead to further engineering experiments.
MATERIALS AND METHODS
Generation of mutant libraries by epPCR.
The S. clavuligerus DAOCS gene was cloned from pYB4 (31) and inserted into the BamHI-HindIII site of pET30a (Novagen) to produce pYB202. Plasmid pYB4 was used as a template for the epPCR. A ratio of Mg2+ ions to Mn2+ ions of 12:1 was used to induce random mutation in the epPCR. The mutated DAOCS genes were treated with BamHI and HindIII, cleaned up using a PCR clean up-M kit (Viogene), and then ligated into the BamHI-HindIII site of pET30a. The resultant ligation mixture was used to transform BL21(DE3) cells (Novagen).
Generation of mutant libraries by DNA shuffling.
DNA shuffling was carried out as reported previously (8, 28), with minor modifications. The DNase digestion conditions were changed as follows: 30 μg of DNA substrates from the selected DAOCS mutated genes was digested with 1.5 U of DNase I (Roche) in a 600-μl (total volume) mixture for 20 min at room temperature. The fragments, which were 100 to 300 bp long, were purified for reassembly. The shuffled fragments were treated with NcoI and HindIII and ligated into the NcoI-HindIII site of pET30a, and this was followed by transformation and selection.
Screening, purification, and activity assay for DAOCS mutants.
Transformants with increased activities were selected by a two-step screening process as described previously (31), with some modifications. The cells bearing pYB202 were used as a positive control in the E. coli ESS bioassay, and the final concentration of PenG in the thin-layer chromatography assay was 2.5 mM. The selected DAOCS variants were subjected to sequencing for identification of the mutation sites. To eliminate any possible interference from the vector linkers, such as a His tag at the N terminus of the enzyme, all mutants selected were further reconstructed as the 311-residue native form without any extra residues at the N and C termini. The epPCR-selected genes and the combined mutant genes were cloned into the NdeI-HindIII site of pET30a and the NcoI-HindIII site of pET24d, respectively, and then transformed into cells of E. coli Tuner(DE3) (Novagen), a lacYZ deletion mutant of E. coli BL21(DE3) that allows more tightly controlled expression by isopropyl-β-d-thiogalactopyranoside (IPTG). The resultant mutants were confirmed by DNA sequencing.
The recombinant proteins were isolated by anion-exchange and gel filtration chromatography as described previously (31). Each of the purified DAOCSs was >90% pure as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. The relative expansion activity of the purified protein was then determined using 1 mM and 10 mM PenG with a high-performance liquid chromatography system (31). Only the mutants with enhanced activities toward PenG and no substrate inhibition were used for further measurements, and their kinetic parameters with 0.1 to 10 mM PenG or 15 to 100 μM PenN were determined (31). To study the possible effects of the point mutations, the residue substitutions were modeled with energy minimization using the Insight II package (Accelrys). Figures 2 and 3A were generated using MOLSCRIPT (14) and Raster 3D (23), and Fig. 3B was generated by using GRASP (24).
FIG. 2.
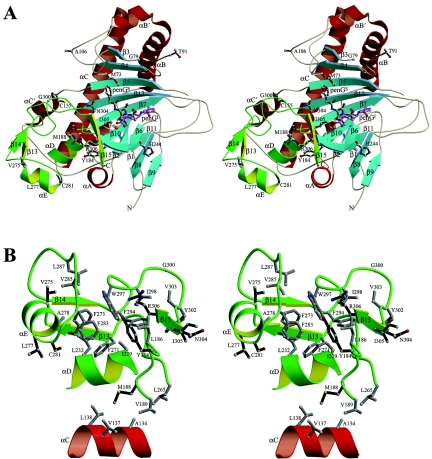
Possible effects of point mutations on enhancement of PenG expansion. (A) Stereo view of the spatial positions of the substituted residues. The ferrous ion is indicated by an orange sphere, the mutated residues and PenGS are black, and PenGI is magenta. The unique small barrel subdomain is green. The long loops between the β3 and β4 strands, between the β5 and β6 strands, between the β7 and β8 strands, and between the β11 and β12 strands are quite flexible, and their conformations are different upon binding of various penicillins. Interestingly, 9 of the 14 mutated residues are located within or near the small subdomain. (B) Hydrophobic core of the small subdomain. Several layers of hydrophobic packing are formed from the C-terminal tail to the αD and αC helices and the β-barrel. R306, V303, I298, W297, L287, V285, and V275 are responsible for the first layer; F294, F283, C281, V278, F273, F271, L232, I229, and L186 are responsible for the second layer; and M188, V189, and L232 are responsible for extension to A134, V137, L138, F152, C155, and L265. Y302 stacks on R266, which forms a hydrogen bond with N304.
FIG. 3.
Distinct penicillin-binding modes. (A) Stereo view of superposition of the enzyme in a complex with αKG (green), PenGS (black), PenGI (magenta), ampicillin (Amp) (gray), and DAOC (cyan). The penicillin-binding sites overlap each other and clash with the αKG site, suggesting that there is a sequential reaction with a trapped oxidizing intermediate (29). Distinct interaction networks of DAOCS with DAOC, PenGS, and PenGI lead to different conformations, particularly with some substrate-contacting residues, such as R160, R162, F164, M180, and R258. R160 forms a hydrogen bond with PenN; however, it becomes disordered upon PenGS binding due to a steric clash. The guanidino group of R162 forms hydrogen bonds with both PenN and PenGS but stacks on the phenyl group of PenGI with a distance of 5 Å. Y97, R179, and R258 are disordered upon DAOC binding, whereas Y97 hydrogen bonds with R179 during PenGI binding. (B) Molecular surfaces of the DAOCS-PenGI complex (Protein Data Bank code 1UOF) colored for electrostatic potential from −25 kBT (red) to 25 kBT (blue). The ferrous ion is embedded in the deepest part, whereas the polar aminoadipoyl side chain of DAOC is more solvent exposed. The phenyl groups of PenGS and ampicillin are covered by R160. This structural comparison suggests that the hydrophobic contact for the phenyl group of PenGI is a good candidate for selective damage of the inhibitory sites but not the substrate sites.
RESULTS AND DISCUSSION
epPCR-selected mutants.
After assays of 6,364 clones, 21 mutants with increased expansion activity were selected. Fifteen variants had one residue substitution, and seven replacements were selected twice. Thus, eight kinds of point mutants (M73T, T91A, M188V, M188I, H244Q, C281Y, G300V, and I305L mutants) were found. The C281Y mutant was also obtained in our previous study using the hydroxylamine mutagenesis procedure, and the I305L mutant was constructed by site-direct mutagenesis previously (31). In addition, six double mutants, D53H/C281Y, M73T/P145L, A106T/C155Y, A106T/M188V, D107G/L277Q, and Y184H/C281R mutants, were identified. The D53H/C281Y and M73T/P145L double mutants exhibited relative activities similar to those of the C281Y and M73T single mutants, respectively, suggesting that neither the D53H substitution nor the P145L substitution enhanced the activity significantly. In order to identify the effect of each substitution independently, individual point mutants, including the A106T, D107G, C155Y, Y184H, L277Q, and C281R mutants, were constructed from the double mutants. Compared with the three mutants obtained using hydroxylamine mutagenesis (31), many more mutants with increased kcat/Km ratios were found using epPCR random mutagenesis, demonstrating that this mutagenesis method is a more powerful tool. However, different mutants were obtained by the epPCR and hydroxylamine mutagenesis methods, suggesting that there is complementarity between the two methods.
Kinetic analyses of mutants with point mutations.
Substitutions at 16 residues were observed in the present epPCR study. The C281Y and I305L mutants were characterized in our previous work. D53H and P145L replacements had little effect on the enzyme activity. The D107G and C281R variants exhibited catalytic properties similar to those of the wild-type enzyme, and G300V exhibited substrate inhibition. Thus, the kinetic parameters of the remaining nine mutants with point mutations were determined, and they are shown in Table 1. Among these mutants, the L277Q mutant had the highest catalytic efficiency for PenG (a 2.3-fold increase in kcat and a 2.5-fold reduction in Km). However, this substitution had little effect on PenN expansion, and this was also true for all other mutations except M73T, Y184H, and M188V. Both the T91A and H244Q mutants exhibited similarly increased affinity for PenG (1.9-fold reductions in Km) and increased oxidation rates (1.2- to 1.5-fold increases in kcat). In contrast, the A106T and C155Y mutants showed 1.5-fold reductions in Km for PenG, but there were only minor effects on the reaction rate. The M73T mutation enhanced the binding affinities for both penicillins N and G, with 3.5-fold and 2.3-fold reductions in Km, respectively. Interestingly, replacement of M188 with leucine had little effect on expansion of penicillins N and G; however, replacement with valine enhanced PenG binding, with a 3.4-fold reduction in Km, but there were nearly 70% decreases in PenN binding and the reaction rate. More surprisingly, mutation of Y184 to a histidine improved the catalytic efficiency with PenG, resulting in a 4.7-fold increase in the kcat/Km value, but almost completely abolished PenN conversion (>99% decrease in the kcat/Km ratio).
TABLE 1.
Kinetic parameters for PenN or PenG expansiona
| Amino acid substitution(s) | PenG
|
PenN
|
||||||
|---|---|---|---|---|---|---|---|---|
| Km (mM) | kcat (s−1) | kcat/Km (M−1 s−1) | Relative activityb (%) with:
|
Km (mM) | kcat (s−1) | kcat/Km (M−1 s−1) | ||
| 1 mM PenG | 10 mM PenG | |||||||
| Wild type | 2.58 ± 0.220 | 0.0453 ± 0.0010 | 18 | 100 | 160 | 0.014 ± 0.0060 | 0.307 ± 0.038 | 22,000 |
| M73T | 0.74 ± 0.160 | 0.0627 ± 0.0021 | 85 | 180 | 250 | 0.006 ± 0.0030 | 0.239 ± 0.009 | 40,000 |
| G79Ec | 0.75 ± 0.020 | 0.0315 ± 0.0003 | 42 | 90 | 130 | 0.009 ± 0.0030 | 0.178 ± 0.019 | 20,000 |
| T91A | 1.36 ± 0.090 | 0.0522 ± 0.0004 | 38 | 110 | 200 | 0.009 ± 0.0005 | 0.247 ± 0.006 | 27,000 |
| A106T | 1.77 ± 0.150 | 0.0453 ± 0.0009 | 26 | 80 | 170 | 0.013 ± 0.0020 | 0.277 ± 0.014 | 21,000 |
| C155Y | 1.76 ± 0.050 | 0.0476 ± 0.0012 | 27 | 90 | 180 | 0.016 ± 0.0005 | 0.235 ± 0.008 | 15,000 |
| Y184H | 0.95 ± 0.050 | 0.0798 ± 0.0012 | 84 | 200 | 330 | 0.295 ± 0.0300 | 0.048 ± 0.005 | 163 |
| M188I | 1.96 ± 0.090 | 0.0507 ± 0.0006 | 26 | 90 | 190 | 0.011 ± 0.0010 | 0.277 ± 0.004 | 25,000 |
| M188V | 0.76 ± 0.170 | 0.0456 ± 0.0016 | 60 | 150 | 190 | 0.047 ± 0.0005 | 0.084 ± 0.002 | 2,000 |
| H244Q | 1.39 ± 0.090 | 0.0698 ± 0.0012 | 50 | 140 | 270 | 0.009 ± 0.0015 | 0.260 ± 0.006 | 29,000 |
| V275Ic | 1.68 ± 0.200 | 0.0502 ± 0.0012 | 30 | 100 | 190 | 0.012 ± 0.0030 | 0.252 ± 0.020 | 20,000 |
| L277Q | 1.02 ± 0.020 | 0.1036 ± 0.0012 | 102 | 270 | 410 | 0.011 ± 0.0015 | 0.297 ± 0.020 | 27,000 |
| C281Yc | 0.68 ± 0.340 | 0.0744 ± 0.0033 | 73 | 200 | 300 | 0.006 ± 0.0010 | 0.273 ± 0.014 | 47,000 |
| N304Kc | 0.22 ± 0.030 | 0.0564 ± 0.0003 | 256 | 220 | 240 | 0.004 ± 0.0010 | 0.366 ± 0.023 | 92,000 |
| I305Lc | 0.66 ± 0.070 | 0.0759 ± 0.0015 | 115 | 230 | 310 | 0.006 ± 0.0020 | 0.284 ± 0.030 | 44,000 |
| I305Mc | 0.75 ± 0.040 | 0.1452 ± 0.0020 | 194 | 380 | 580 | 0.012 ± 0.0010 | 0.310 ± 0.014 | 26,000 |
| V275I/I305Mc | 0.25 ± 0.05 | 0.1458 ± 0.0014 | 583 | 500 | 610 | 0.013 ± 0.002 | 0.316 ± 0.016 | 24,000 |
| C155Y/Y184H/V275I/C281Y | 0.19 ± 0.002 | 0.1398 ± 0.0015 | 736 | 580 | 670 | 0.092 ± 0.0005 | 0.048 ± 0.001 | 521 |
The values for kinetic parameters are means ± standard errors for three independent experiments.
The relative activity was determined using 1 and 10 mM PenG, and the activity of the wild-type enzyme with 1 mM PenG was defined as 100%.
DAOCS mutant obtained in previous studies (31).
The relative specific activity in the presence of 1 mM and 10 mM PenG is mainly correlated with the reaction rate, kcat (Table 1). The I305M and L277Q mutants had the highest kcat values and hence the highest relative activities. The G300V mutant should have a high kcat value. The higher substrate-binding affinities of some mutants, such as N304K, also enhanced the relative activity with 1 mM PenG, but the activity was not improved further when the substrate concentration was increased to 10 mM. Therefore, the relative activity, and hence the kcat value, should be the target when workers engineer improvements in DAOCS for PenG expansion. It should be possible to combine the point mutations that result in high kcat values, such as Y184H, H244Q, L277Q, C281Y, G300V, I305L, and I305M, to generate more active enzymes. Indeed, C281Y, I305L, and I305M were quite often observed in our previously selected combined mutants with high relative activities, such as the V275I/C281Y/I305M and G79E/V275I/I305M mutants (31) (Table 2).
TABLE 2.
Relative activities of the remaining selected mutants
| Amino acid substitution(s) | Relative activitya (%) with:
|
|
|---|---|---|
| 1 mM PenG | 10 mM PenG | |
| Wild type | 100 | 160 |
| G300V | 410 | 370 |
| M73T/I277Q | 350 | 350 |
| M73T/C281Y | 610 | 530 |
| V275I/C281Y | 260 | 310 |
| V275I/I305L | 310 | 330 |
| L277Q/I305M | 610 | 520 |
| M73T/G300V/I305L | 640 | 380 |
| G79E/V275I/C281Yb | 430 | 200 |
| G79E/V275I/I305Lb | 470 | 240 |
| G79E/V275I/I305Mb | 1,110 | 540 |
| M188V/V2751/G300V | 440 | 310 |
| V275I/C281Y/G300V | 620 | 380 |
| V275I/C281Y/I305Mb | 1,290 | 690 |
| V275I/N304K/I305Lb | 300 | 130 |
| C281Y/N304K/I305Mb | 650 | 230 |
| A11V/T91A/C281Y/I305L | 220 | 200 |
| G79E/V275I/C281Y/I305Lb | 250 | 90 |
| Y184H/M188I/C281Y/I305L | 610 | 460 |
| Y184H/H244Q/T259I/L277Q | 320 | 310 |
| M73T/I118V/A140V/H244Q/C281Y | 450 | 350 |
| A106T/E169K/V275I/C281Y/I305L | 450 | 380 |
| R135Q/C155Y/R179Q/M188V/I305M | 360 | 290 |
| E144K/M188I/A198T/V275I/C281Y | 160 | 200 |
The relative activity was determined using 1 and 10 mM PenG, and the activity of the wild-type enzyme with 1 mM PenG was defined as 100%.
DAOCS mutant obtained in previous studies (31).
Possible effects of mutations.
In order to identify the possible effects of the mutations of point mutants with enhanced activities for PenG expansion, the substituted residues were mapped on the enzyme structures (Fig. 2) (29). Residues M73, N304, and I305 are located within the active site cavity. T73 and K304 interact with the penicillin side chains, whereas L305 contacts the penicillin core. Our structural modeling suggests that T73 rather than M73, K304 or L304 rather than N304, and L305 or M305 rather than I305 make better contact with both penicillins N and G and hence decrease the Km values. For instance, the distance between the β-lactam ring of PenG and the I305 side chain is shortened from 4.8 Å to 3.2 Å after a leucine substitution. K304 may hydrogen bond with the aminoadipoyl group of PenN and interact with the phenylacetyl group of PenG. Among the point mutants with measured kinetic parameters, the N304K mutant exhibited the lowest Km values for both penicillins N and G (Table 1). Interestingly, the I305M mutant exhibited the highest kcat value with PenG, but there was little effect on PenN. Structural modeling suggests that the sulfur atom of M305 has close contact with Fe(II) (3.1 Å), and this may affect the penicillin binding and the reaction distance between the β-methyl group of the thiazolidine ring and the ferryl oxygen during catalysis.
In contrast, some mutations may be involved in structural integrity, through hydrophobic contacts or polar interactions. DAOCS and some other Fe(II)-dependent oxygenases, such as isopenicillin N synthase and anthocyanidin synthase, have a common “double-stranded beta-helix” fold (2, 25, 32). However, DAOCS has a unique small barrel subdomain, which is made up of residues 186 to 189, residues 228 to 235, and residues 266 to 311 (Fig. 2). Almost all the hydrophobic residues in these regions are involved in the hydrophobic core of the small subdomain. The hydrophobic contacts involve several layers of packing from the C-terminal tail to helices αD and αC and the β-barrel. Interestingly, the mutations of most of the available point and combined mutants with enhanced PenG expansion are located within or near this small subdomain. Replacement of M188 with isoleucine does not affect the expansion of penicillins N and G; however, replacement with valine improves PenG binding but decreases PenN binding and the reaction rate. M188, near the ferrous ligands H183 and D185, is buried in a hydrophobic pocket within the small subdomain. Thus, the M188I and M188V mutants demonstrate that even a subtle change in the hydrophobic packing can affect enzyme activity significantly. Similarly, the C155Y, V275I, C281Y, and R306L mutations may make closer contact, resulting in increased enzymatic efficiency. Y155 may have closer interactions with L265 and Y127; I275 may have closer interactions with F273, A278, V285, L287, and A292; Y281 may have closer interactions with T231, L232, A278, and F283; and L306 may have closer interactions with L186, W297, I298, and V303. In addition, the hydroxyl group of Y281 may form a hydrogen bond with the backbone oxygen of V238. Replacement of G300 with valine may affect the γ-turn made up of G299, G300, and N301 and hence change the orientation of the C-terminal tail. Therefore, it should be possible to improve penicillin expansion further by modification of this small subdomain, as well as the surrounding residues.
On the other hand, Y184 is more solvent accessible, and its hydroxyl group forms hydrogen bonds with two ordered water molecules. Modeling of the Y184H mutant suggested that one imidazole nitrogen atom forms a hydrogen bond with the backbone oxygen of D185 (3.0 Å), and hence H184 may affect ferrous ligation. Similarly, replacement of H244 with glutamine may affect the ferrous ligation of H243 and thus change enzyme activity. L277 is on the protein surface and is not surrounded by any hydrophobic residues. Replacement with glutamine may lead to formation of hydrogen bonds with E280 and water molecules. However, residues G79, T91, and A106 are also on the protein surface and seem to be very flexible in the structure. Therefore, at this point, we cannot predict their function in terms of enzyme activity.
Combined mutants.
Together with our previously described point mutants, including the G79E, V275I, C281Y, N304K, I305L, and I305M mutants (31), the mutants mentioned above were used as inputs for DNA shuffling to generate random combinations. After 10,572 clones were screened by comparison to the best of the parents, 16 mutants with increased activities were selected (Tables 1 and 2). Eleven mutants showed substrate inhibition because the activity decreased when the PenG concentration was increased from 1 mM to 10 mM. Among the remaining five mutants, only the quaternary mutant with significantly enhanced activity, the C155Y/Y184H/V275I/C281Y mutant, was subjected to measurement of the kinetic parameters. This mutant showed marked 13.6-fold and 3.1-fold increases in binding and the rate of conversion of PenG, whereas with PenN both abilities were almost eliminated (>97% decrease in the kcat/Km ratio), perhaps due to the Y184H mutation. As a result, the quaternary mutant exhibited a 41-fold increase in the kcat/Km ratio for PenG expansion. Compared to the 1.5-, 4.7-, 1.7-, and 6.1-fold increases for the C155Y, Y184H, V275I, and C281Y point mutants, this mutant exhibited a significantly additive effect for kcat/Km values.
PenG inhibition.
The mutations of most of our combined mutants had negative effects on PenG expansion, and only a small portion had additive, even synergistic effects. Substrate inhibition might have been a major contributor to the negative effects. However, there were still a few mutants that produced more active enzymes with no substrate inhibition. This implies that there are two PenG-binding sites; one site is responsible for the expansion activity (the substrate-binding site [S site]; PenGS indicates the PenG binding at the S site), whereas the other has an attenuation effect (the inhibitory site [I site]; PenGI indicates the PenG binding at the I site). Most of the combined mutants also exhibited enhanced PenGI binding and thus attenuated activity. Interestingly, the absence of substrate inhibition in the V275I/C281Y, C155Y/Y184H/V275I/C281Y, and E144K/M188I/A198T/V275I/C281Y mutants suggests that both the V275I and C281Y substitutions may not enhance PenGI. Already, these two mutations apparently are the most frequent replacements in selected clones during direct evolution (Tables 1 and 2). The V275I mutation was observed in seven of the eight mutants in previous combinations (31) and in nine selected mutants resulting from the present DNA shuffling. On the other hand, the N304K mutation was not observed in any selected mutant resulting from the present DNA shuffling.
Substrate inhibition is a major disadvantage for industrial cephalosporin production. In order to destroy the structural integrity of the I site but not the S site or the αKG site, the available DAOCS structures were analyzed and compared (Fig. 3) (29). The similar moieties of PenGS, ampicillin, and the product DAOC make similar contact with DAOCS, whereas the distinct side chains display different interaction networks. Thus, the β-lactam and thiazolidine rings and the aminoadipoyl group of PenN were proposed to occupy positions similar to those of PenG and DAOC, respectively. In the absence of the cosubstrate αKG, PenG prefers the I site. The phenylacetyl ring has close contact with R162, F164, R179, and V245, and the β-lactam and thiazolidine rings have close contact with M180, I192, L204, and V262. In addition, there are several hydrogen bonds of the acetyl oxygen with R258 and of the NH group and the β-lactam oxygen with S260. The carboxylate group of the thiazolidine ring ligates the ferrous ion. However, αKG occupies most of the PenGI site through Fe(II) ligation, hydrogen bonding with R258 and S260, and hydrophobic contact with M180, I192, L204, V245, and V262 and hence changes the preference of PenG toward the S site. The phenylacetyl ring of PenGS makes close contact with M73, L158, and F264, and the β-lactam and thiazolidine rings make close contact with M180, T190, I192, L204, V245, V262, and I305. Several hydrogen bonds are formed between the acetyl and β-lactam oxygen and R162 and between the carboxylate group of the thiazolidine ring and S260. Fe(II) ligates the thiazolidine sulfur of either PenGS or PenN. Ampicillin has a side chain similar to that of PenG with an extra NH group, and hence the modes of binding to DAOCS of these compounds are virtually identical. In contrast to the hydrophobic side chain of PenG, the polar aminoadipoyl group of PenN is more solvent accessible and forms hydrogen bonds with several ordered water molecules, R160, and the backbone NH of N304 and I305. In addition, PenN makes close contact with R160, R162, I192, F225, V262, F264, N304, and I305.
The structural comparison provided some new insights. First, the distinct interaction networks of DAOCS with PenN, PenGS, and PenGI result in different structural conformations, particularly for some substrate-contacting residues and the long loops between the β3 and β4 strands, between the β5 and β6 strands, between the β7 and β8 strands, and between the β11 and β12 strands. These differences may explain why many substitutions have very distinct effects on the ring expansion of penicillins N and G (Table 1). Second, there are more interactions of DAOCS with PenN than with PenG; thus, PenN has a Km that is approximately 200-fold lower than that of PenGS. The kcat of PenN that is sevenfold higher than that of PenG may be due to subtle differences in Fe(II) ligation. Finally, the structural comparison suggests that disruption of the hydrophobic contact with the phenyl group of PenGI is a good candidate for selective site damage (Fig. 3A). This could be carried out by replacement of the surrounding apolar residues, such as F164, with charged residues.
Future rational engineering.
Selective disruption of the I site will be carried out first in the V275I/C281Y/I305M and G79E/V275I/I305M mutants, which have the highest relative activities for PenG expansion known to date but exhibit severe substrate inhibition (Table 2). After the substrate inhibition problem is solved, the available mutants with high kcat values can then be combined. Since the binding modes of PenG and ampicillin are virtually identical, our engineered DAOCS mutants should enhance the ring expansion of not only PenG but also other related penicillins, such as ampicillin, phenethicillin, and penicillin V, and this should provide important possibilities for the manufacture of new and better oral cephalosporins.
Acknowledgments
We thank Arnold L. Demain for kindly providing E. coli strain ESS used in the screening procedure.
This work was supported by grants from the Ministry of Economic Affairs of the Republic of China (grant 90J027), by an intramural fund from the National Yang-Ming University of the Republic of China, and by Synmax Biochemical Co. Ltd., Taiwan.
REFERENCES
- 1.Adrio, J. L., J. Velasco, G. Soler, M. Rodriguez-Saiz, J. L. Barredo, and M. A. Moreno. 2001. Extracellular production of biologically active deacetoxycephalosporin C synthase from Streptomyces clavuligerus in Pichia pastoris. Biotechnol. Bioeng. 75:485-491. [DOI] [PubMed] [Google Scholar]
- 2.Andreeva, A., D. Howorth, S. E. Brenner, T. J. P. Hubbard, C. Chothia, and A. G. Murzin. 2004. SCOP database in 2004: refinements integrate structure and sequence family data. Nucleic Acids Res. 32:D226-D229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, B. J., J. E. Dotzlaf, and W. K. Yeh. 1991. Deacetoxycephalosporin C hydroxylase of Streptomyces clavuligerus. Purification, characterization, bifunctionality, and evolutionary implication. J. Biol. Chem. 266:5087-5093. [PubMed] [Google Scholar]
- 4.Chin, H. S., J. Sim, and T. S. Sim. 2001. Mutation of N304 to leucine in Streptomyces clavuligerus deacetoxycephalosporin C synthase creates an enzyme with increased penicillin analogue conversion. Biochem. Biophys. Res. Commun. 287:507-513. [DOI] [PubMed] [Google Scholar]
- 5.Chin, H. S., and T. S. Sim. 2002. C-terminus modification of Streptomyces clavuligerus deacetoxycephalosporin C synthase improves catalysis with an expanded substrate specificity. Biochem. Biophys. Res. Commun. 295:55-61. [DOI] [PubMed] [Google Scholar]
- 6.Cho, H., J. L. Adrio, J. M. Luengo, S. Wolfe, S. Ocran, G. Hintermann, J. M. Piret, and A. L. Demain. 1998. Elucidation of conditions allowing conversion of penicillin G and other penicillins to deacetoxycephalosporins by resting cells and extracts of Streptomyces clavuligerus NP1. Proc. Natl. Acad. Sci. USA 95:11544-11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coque, J. J., J. F. Martin, and P. Liras. 1993. Characterization and expression in Streptomyces lividans of cefD and cefE genes from Nocardia lactamdurans: the organization of the cephamycin gene cluster differs from that in Streptomyces clavuligerus. Mol. Gen. Genet. 236:453-458. [DOI] [PubMed] [Google Scholar]
- 8.Crameri, A., S. A. Raillard, E. Bermudez, and W. P. Stemmer. 1998. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature 391:288-291. [DOI] [PubMed] [Google Scholar]
- 9.Crawford, L., A. M. Stepan, P. C. McAda, J. A. Rambosek, M. J. Conder, V. A. Vinci, and C. D. Reeves. 1995. Production of cephalosporin intermediates by feeding adipic acid to recombinant Penicillium chrysogenum strains expressing ring expansion activity. Bio/Technology 13:58-62. [DOI] [PubMed] [Google Scholar]
- 10.Demain, A. L., and M. A. Baez-Vasquez. 2000. Immobilized Streptomyces clavuligerus NP1 cells for biotransformation of penicillin G into deacetoxycephalosporin G. Appl. Biochem. Biotechnol. 87:135-140. [DOI] [PubMed] [Google Scholar]
- 11.Hsu, J.-S., Y.-B. Yang, C.-H. Deng, C.-L. Wei, S.-H. Liaw, and Y.-C. Tsai. 2004. Family shuffling of expandase genes to enhance substrate specificity for penicillin G. Appl. Environ. Microbiol. 70:6257-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura, H., H. Miyashita, and Y. Sumino. 1996. Organization and expression in Pseudomonas putida of the gene cluster involved in cephalosporin biosynthesis from Lysobacter lactamgenus YK90. Appl. Microbiol. Biotechnol. 45:490-501. [DOI] [PubMed] [Google Scholar]
- 13.Kovacevic, S., B. J. Weigel, M. B. Tobin, T. D. Ingolia, and J. R. Miller. 1989. Cloning, characterization, and expression in Escherichia coli of the Streptomyces clavuligerus gene encoding deacetoxycephalosporin C synthetase. J. Bacteriol. 171:754-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraulis, P. J. 1991. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24:946-950. [Google Scholar]
- 15.Lee, H.-J., Y.-F. Dai, C.-Y. Shiau, C. J. Schofield, and M. D. Lloyd. 2003. The kinetic properties of various R258 mutants of deacetoxycephalosporin C synthase. Eur. J. Biochem. 270:1301-1307. [DOI] [PubMed] [Google Scholar]
- 16.Lee, H. J., M. D. Lloyd, I. J. Clifton, K. Harlos, A. Dubus, J. E. Baldwin, J. M. Frere, and C. J. Schofield. 2001. Alteration of the co-substrate selectivity of deacetoxycephalosporin C synthase. The role of arginine 258. J. Biol. Chem. 276:18290-18295. [DOI] [PubMed] [Google Scholar]
- 17.Lee, H. J., M. D. Lloyd, K. Harlos, I. J. Clifton, J. E. Baldwin, and C. J. Schofield. 2001. Kinetic and crystallographic studies on deacetoxycephalosporin C synthase (DAOCS). J. Mol. Biol. 308:937-948. [DOI] [PubMed] [Google Scholar]
- 18.Lee, H. J., M. D. Lloyd, K. Harlos, and C. J. Schofield. 2000. The effect of cysteine mutations on recombinant deacetoxycephalosporin C synthase from S. clavuligerus. Biochem. Biophys. Res. Commun. 267:445-448. [DOI] [PubMed] [Google Scholar]
- 19.Lee, H. J., C. J. Schofield, and M. D. Lloyd. 2002. Active site mutations of recombinant deacetoxycephalosporin C synthase. Biochem. Biophys. Res. Commun. 292:66-70. [DOI] [PubMed] [Google Scholar]
- 20.Lipscomb, S. J., H. J. Lee, M. Mukherji, J. E. Baldwin, C. J. Schofield, and M. D. Lloyd. 2002. The role of arginine residues in substrate binding and catalysis by deacetoxycephalosporin C synthase. Eur. J. Biochem. 269:2735-2739. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd, M. D., H. J. Lee, K. Harlos, Z. H. Zhang, J. E. Baldwin, C. J. Schofield, J. M. Charnock, C. D. Garner, T. Hara, A. C. Terwisscha van Scheltinga, K. Valegard, J. A. Viklund, J. Hajdu, I. Andersson, A. Danielsson, and R. Bhikhabhai. 1999. Studies on the active site of deacetoxycephalosporin C synthase. J. Mol. Biol. 287:943-960. [DOI] [PubMed] [Google Scholar]
- 22.Martin, J. F. 1998. New aspects of genes and enzymes for beta-lactam antibiotic biosynthesis. Appl. Microbiol. Biotechnol. 50:1-15. [DOI] [PubMed] [Google Scholar]
- 23.Merritt, E. A., and D. J. Bacon. 1997. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277:505-524. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls, A., K. A. Sharp, and B. Honig. 1991. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11:281-296. [DOI] [PubMed] [Google Scholar]
- 25.Roach, P. L., I. J. Clifton, V. Fulop, K. Horlos, G. J. Barton, J. Hajdu, I. Andersson, C. J. Schofield, and J. E. Baldwin. 1995. Crystal structure of isopenicillin N synthase is the first from a new structural family of enzymes. Nature 375:700-704. [DOI] [PubMed] [Google Scholar]
- 26.Scholar, E. M., and W. B. Pratt. 2000. The inhibitors of cell wall synthesis. II. Pharmacology and adverse effects of the penicillins, cephalosporins, carbapenems, monobactams, vanomycin, and bacitracin, p. 81-126. In E. M. Scholar and W. B. Pratt (ed.), Antimicrobial drugs, 2nd ed. Oxford University Press, New York, N.Y.
- 27.Sim, J., and T. S. Sim. 2000. Mutational evidence supporting the involvement of tripartite residues His183, Asp185, and His243 in Streptomyces clavuligerus deacetoxycephalosporin C synthase for catalysis. Biosci. Biotechnol. Biochem. 64:828-832. [DOI] [PubMed] [Google Scholar]
- 28.Stemmer, W. P. C. 1994. DNA shuffling by random fragmentation and reassembly: in vitro recombination for molecular evolution. Proc. Natl. Acad. Sci. USA 91:10747-10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valegard, K., A. C. Terwisscha van Scheltinga, A. Dubus, G. Ranghino, L. M. Oster, J. Hajdu, and I. Andersson. 2004. The structural basis of cephalosporin formation in a mononuclear ferrous enzyme. Nat. Struct. Mol. Biol. 11:95-101. [DOI] [PubMed] [Google Scholar]
- 30.Velasco, J., J. Luis Adrio, M. A. Moreno, B. Diez, G. Soler, and J. L. Barredo. 2000. Environmentally safe production of 7-aminodeacetoxycephalosporanic acid (7-ADCA) using recombinant strains of Acremonium chrysogenum. Nat. Biotechnol. 18:857-861. [DOI] [PubMed] [Google Scholar]
- 31.Wei, C.-L., Y.-B. Yang, W.-C. Wang, W.-C. Liu, J.-S. Hsu, and Y.-C. Tsai. 2003. Engineering Streptomyces clavuligerus deacetoxycephalosporin C synthase for optimal ring expansion activity toward penicillin G. Appl. Environ. Microbiol. 69:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilmouth, R. C., J. J. Turnbull, R. W. Welford, I. J. Clifton, A. G. Prescott, and C. J. Schofield. 2002. Structure and mechanism of anthocyanidin synthase from Arabidopsis thaliana. Structure 10:93-103. [DOI] [PubMed] [Google Scholar]