Abstract
Dechloromonas strain RCB has been shown to be capable of anaerobic degradation of benzene coupled to nitrate reduction. As a continuation of these studies, the metabolic versatility and hydrocarbon biodegradative capability of this organism were investigated. The results of these revealed that in addition to nitrate, strain RCB could alternatively degrade benzene both aerobically and anaerobically with perchlorate or chlorate [(per)chlorate] as a suitable electron acceptor. Furthermore, with nitrate as the electron acceptor, strain RCB could also utilize toluene, ethylbenzene, and all three isomers of xylene (ortho-, meta-, and para-) as electron donors. While toluene and ethylbenzene were completely mineralized to CO2, strain RCB did not completely mineralize para-xylene but rather transformed it to some as-yet-unidentified metabolite. Interestingly, with nitrate as the electron acceptor, strain RCB degraded benzene and toluene concurrently when the hydrocarbons were added as a mixture and almost 92 μM total hydrocarbons were oxidized within 15 days. The results of these studies emphasize the unique metabolic versatility of this organism, highlighting its potential applicability to bioremediative technologies.
The monoaromatic hydrocarbons benzene, toluene, ethylbenzene, and xylene, collectively called BTEX, are commonly found in gasoline and are highly volatile substances (8, 10, 17). Due to their relatively high solubility and toxicity, they represent a significant health threat in contaminated environments. BTEX components are considered among the most prevalent groundwater pollutants (8, 17). Over the last 2 decades, there has been a major impetus to investigate the ability of microorganisms to biodegrade hydrocarbons in the absence of oxygen (8, 10). This was motivated by the fact that extensive anaerobic zones frequently develop when soils and sediments are contaminated with hydrocarbons, due to depletion of oxygen by the stimulated indigenous aerobic microbial population (3, 9, 11, 33). Studies of microbial degradation of monoaromatic hydrocarbons have resulted in the identification and isolation of a number of different anaerobic bacterial strains capable of degrading one or more monoaromatic hydrocarbons. Of these compounds, the anaerobic biodegradation of toluene is probably the most comprehensively understood. Toluene is biodegradable with nitrate, Mn(IV), Fe(III), humic substances, sulfate, and CO2 as terminal electron acceptors (7, 20-26, 32, 34, 36, 39, 45). More recently, it has been demonstrated that toluene can also be assimilated anaerobically as a carbon source by anoxygenic phototrophs (46).
Many organisms capable of anaerobic toluene degradation were alternatively capable of anaerobic degradation of other monoaromatic compounds such as ethylbenzene or xylenes (particularly the Azoarcus and Thauera species) (21, 25). Several organisms have been described that can completely mineralize meta- and ortho-xylene coupled to the reduction of nitrate (29, 40) or sulfate (28, 37, 38). Interestingly, although anaerobic para-xylene degradation has been reported by an undefined nitrate-reducing enrichment culture (27), to date there is no organism available in pure culture that can anaerobically mineralize this compound (8).
In contrast to anaerobic toluene degradation, only four organisms capable of anaerobic ethylbenzene degradation have been described (4, 30, 40). Three of these organisms, strains EbN1, PbN1 (40), and EB1 (4) were facultative anaerobic denitrifiers; the fourth, strain EbS7, was an obligate anaerobic marine sulfate reducer (30). None of these organisms oxidized hydrocarbons aerobically, and all were limited in their ability to oxidize alternative aromatic hydrocarbons anaerobically.
Previously, Dechloromonas strain RCB was described as being capable of anaerobic benzene oxidation (16). Here, we demonstrate the metabolic capability of strain RCB in degrading several other monoaromatic compounds including all of the BTEX components under a range of different electron-accepting conditions. In this regard, Dechloromonas strain RCB is the most metabolically versatile hydrocarbon-degrading organism described to date.
MATERIALS AND METHODS
Media and cultivation.
All media and solutions were prepared using strict anaerobic techniques as previously described (5, 16). All culturing was performed at 30°C in the dark in sterile sealed serum bottles or pressure tubes amended with 10 ml of bicarbonate-buffered (pH 7.2) freshwater basal medium under a headspace of N2-CO2 (80-20[vol/vol]) (5). For all experiments, a 10% cell culture inoculum was used to inoculate the experimental samples. Sterile anoxic aqueous stock solutions of sodium chlorate (1 M), sodium perchlorate (1 M), and sodium nitrate (1 M) were prepared and stored in the dark until required. Amendments to the respective experimental samples were made using sterile N2-flushed syringes as required. In aerobic cultures, air (5 ml) was added to the headspace of the previously prepared 30-ml medium tubes containing 10 ml of anoxic culture medium. Benzene, toluene, ethylbenzene, and all three isomers of xylene (meta-, para-, and ortho-xylene) were added from sterile anoxic aqueous 3 mM stock solutions as previously described (13). When necessary, universally labeled [14C]benzene, [14C]toluene, and [14C]p-xylene were purchased from Sigma Chemicals Corp., St. Louis, MO, with a specific activity of 40 to 60 mCi (1.5 × 103 to 2.2 × 103 MBq) mmol−1. Anaerobic working aqueous stock solutions were prepared to give a final radioactivity of 4 μCi (0.15 MBq) ml−1. For experiments using radioactive compounds, 1 μCi (0.037 MBq) of the respective 14C-labeled hydrocarbon was added to 10 ml of the anoxic basal medium.
Analytical techniques.
14CO2 production in the headspace of cultures amended with 14C-labeled hydrocarbon was determined by gas chromatography with gas proportional counting detection as previously described (16). Gaseous-phase unlabeled hydrocarbons in 0.1-ml headspace samples were analyzed with a Shimadzu GC-14A gas chromatograph equipped with a flame ionization detector. Chromatography was performed isothermally at 70°C using a 30-m (5% diphenyl) dimethylpolysiloxane column (Quadrex Corp.) with nitrogen at a flow rate of 1 ml/min as the carrier gas. The injector and the detector temperatures were 180°C and 200°C, respectively.
Cell numbers were determined by direct microscopic counting under an oil immersion lens.
RESULTS AND DISCUSSION
Oxidation and growth on benzene by strain RCB with different electron acceptors.
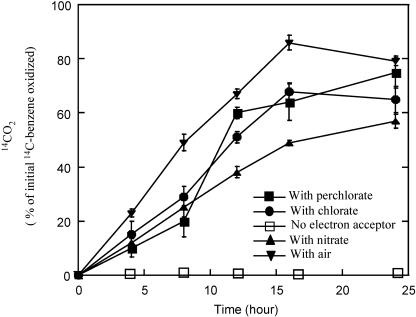
Dechloromonas strain RCB was isolated from aquatic sediments using 4-chlorobenzoate as the sole electron donor (16). In addition to 4-chlorobenzoate, strain RCB also oxidized benzene, which was completely degraded anaerobically to CO2 with nitrate as the electron acceptor (16); growth was coupled to this metabolism (16). Members of the Dechloromonas genus are generally recognized for their ability to grow by dissimilatory perchlorate or chlorate [(per)chlorate] reduction, and they represent the dominant (per)chlorate-reducing bacteria in most environments (12, 19). In support of this, Dechloromonas strain RCB, could also anaerobically metabolize [14C]benzene to 14CO2 with (per)chlorate as an alternative electron acceptor (Fig. 1). Benzene degradation rates were similar with these alternative electron acceptors and slightly faster than that observed with nitrate (Fig. 1). No [14C]benzene degradation occurred in the absence of the electron acceptor (Fig. 1). With chlorate as the sole electron acceptor, the degradation of 66 μM benzene by strain RCB resulted in the reduction of 343 μM chlorate. This represented 104% of the theoretical ratio for benzene degradation coupled to chlorate reduction according to the following equation: C6H6 + 5ClO3− → 6CO2 + 5Cl− + 3H2O.
FIG. 1.
Anaerobic oxidation of [14C]benzene to 14CO2 by strain RCB in the presence and absence of various alternative electron acceptors. The results depicted are the averages of triplicate determinations.
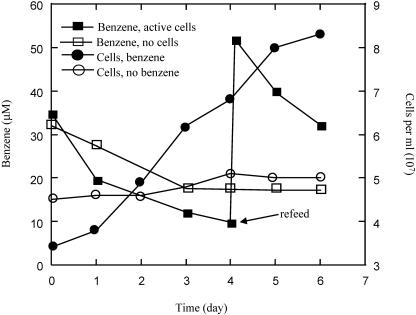
In an active benzene-degrading culture of strain RCB with chlorate as the electron acceptor, cell number increase was concomitant with benzene disappearance (Fig. 2). Minimal growth occurred in the absence of benzene (Fig. 2) and was probably the result of carryover from the inoculum. The small amount of benzene lost in the heat-killed controls was due to absorption into the butyl rubber stoppers. Previous studies have demonstrated similar losses of benzene into butyl rubber stoppers due to absorption (14, 16, 26). When strain RCB was grown anaerobically with chlorate and [U-14C]benzene, 2.6% of the [U-14C] label was associated with the retentate after the culture was filtered through a 0.2-μm-pore-size filter. A similar filtration of a heat-killed control retained 0.8% of the [U-14C] label, suggesting that 1.8% of the [U-14C] label was incorporated into biomass. In contrast, 3.0% of the [U-14C] label was incorporated into the biomass in an aerobically grown culture (16). These low values of incorporation of [U-14C] label into biomass are similar to values reported previously for strain RCB grown on benzene and nitrate (16) and for a nitrate-reducing benzene-oxidizing enrichment culture (5 to 8%) (6) and are supportive of the low cell yield for growth on benzene observed for strain RCB shown in Fig. 2.
FIG. 2.
Anaerobic growth and benzene oxidation by strain RCB anaerobically with 2 mM chlorate as the electron acceptor. The arrows represent the readdition of benzene. The results depicted are the averages of triplicate determinations. The replicate samples did not deviate from each other by >6%.
In addition to anaerobic oxidation of benzene with nitrate or (per)chlorate, strain RCB also oxidized benzene completely to CO2 aerobically. As expected, aerobic benzene oxidation was faster than anaerobic benzene oxidation, probably due to the more favorable thermodynamics of the reaction. Aerobic cultures of strain RCB oxidized almost 111.43 μM benzene completely to CO2 within 6 days (data not shown). As with anaerobic cultures, cell growth was concomitant with benzene removal, and no significant amount of benzene was removed in the heat-killed controls.
These results demonstrate that strain RCB is capable of both aerobic and anaerobic benzene degradation with several alternative terminal electron acceptors. Whether or not this organism plays an important role in the natural attenuation of environments contaminated with benzene is currently unknown. However, Dechloromonas species are widely distributed in a broad diversity of environments, both pristine and contaminated (12, 19), and a recent study demonstrated that the dominant microbial species in a nitrate-dependent benzene-degrading enrichment culture (70% of the cloned 16S rRNA gene sequences) was 93% identical to Dechloromonas strain JJ (43).
Oxidation and growth on toluene by strain RCB.
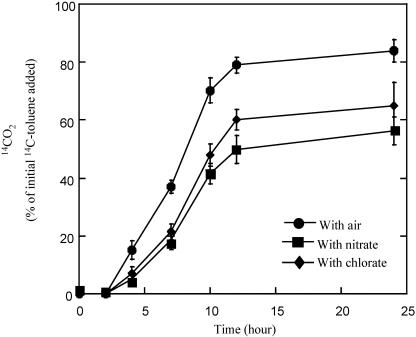
Similarly to benzene, strain RCB also completely mineralized [14C]toluene to 14CO2, either aerobically or anaerobically with (per)chlorate or nitrate as an alternative electron acceptor (Fig. 3). No production of 14CO2 was observed in the absence of an electron acceptor (data not shown). Both the rate and extent of toluene oxidation were greatest under aerobic conditions, and similar oxidation rates were observed with either chlorate or nitrate as alternative electron acceptors (Fig. 3).
FIG. 3.
Aerobic and anaerobic oxidation of 14C-toluene to 14CO2 by strain RCB in the presence and absence of various alternative electron acceptors. The results depicted are the averages of triplicate determinations.
Growth concomitant with aerobic and anaerobic toluene oxidation was not observed in the absence of toluene (data not shown). Almost 256 μM toluene was aerobically degraded relative to the heat-killed control over a period of 7 days. In contrast, only 71 μM toluene was consumed by active cells of strain RCB with nitrate as the sole electron acceptor in a similar time frame (data not shown). This cumulative removal of toluene under nitrate-reducing conditions by strain RCB was significantly lower than those observed for previous studies for nitrate-reducing, toluene-degrading isolates belonging to the Azoarcus genus, which metabolized >216 μM toluene in 14 days on average (25). However, these differences may potentially be due to differences in the biomass content used in the various studies.
Degradation of ethylbenzene.
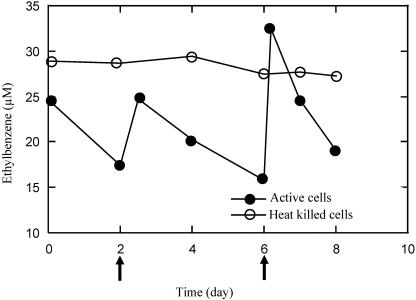
In addition to benzene and toluene, strain RCB also readily degraded ethylbenzene anaerobically with nitrate as the electron acceptor (Fig. 4). Ethylbenzene was completely mineralized to CO2 (data not shown), and no ethylbenzene removal was observed in heat-killed controls (Fig. 4). To date, only three other organisms have been described that are capable of anaerobic nitrate-dependent ethylbenzene degradation (4, 40). These organisms, strains EbN1, PbN1 (40), and EB1 (4), were facultative anaerobes and coupled ethylbenzene oxidation to the reduction of nitrate to N2. The three isolates were closely related to each other and to the previously described toluene-oxidizing Thauera species in the β subclass of the Proteobacteria. Strains EbN1 and PbN1 were isolated with ethylbenzene and propylbenzene, respectively, from enrichments prepared with homogenized mixtures of river and ditch mud samples (40), while strain EB1 was isolated from ethylbenzene-degrading enrichments prepared with sediment from an oil refinery treatment pond (4). Similarly to strain RCB, ethylbenzene was completely mineralized to CO2 by these isolates (4, 40). In contrast to strain RCB, however, these strains did not oxidize any hydrocarbons aerobically and were very limited in their ability to anaerobically oxidize alternative aromatic hydrocarbons other than ethylbenzene. In addition to ethylbenzene, strain EbN1 could only utilize toluene, while strain PbN1 could alternatively utilize propylbenzene (40). In contrast, strain EB1 did not utilize any other hydrocarbons other than ethylbenzene (4).
FIG. 4.
Anaerobic ethylbenzene degradation by Dechloromonas strain RCB with nitrate as the sole electron acceptor. The arrows represent refeeds of ethylbenzene. The results depicted are the averages of triplicate determinations. The replicate samples did not deviate from each other by >5%.
Degradation of xylene isomers.
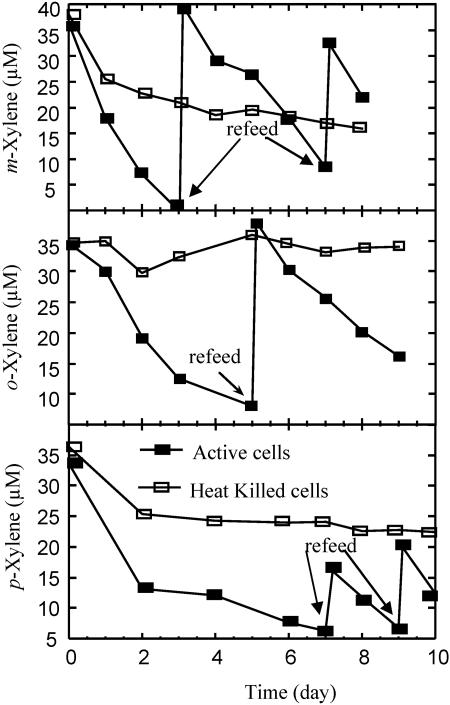
Anaerobic biodegradation of the three structural isomers of dimethylbenzene (meta-, ortho-, and para-xylene) has been predominantly studied under nitrate- and sulfate-reducing conditions. Several xylene-degrading organisms have now been isolated (29, 40). Many of these are closely related to each other and to the previously identified toluene-degrading denitrifiers belonging to the β subclass of the Proteobacteria. Analysis of active cultures of strain RCB supplemented with each of the isomers of xylene revealed that concentrations of all three isomers rapidly decreased with nitrate as the sole electron acceptor (Fig. 5). As before with the other BTEX components, no significant xylene degradation was observed in the respective heat-killed controls, demonstrating that the observed xylene removal was enzymatic. In general, m-xylene was more rapidly removed than either o- or p-xylene. Similar preferential use of m-xylene has been previously observed in studies performed with a novel sulfate-reducing Desulfotomaculum species strain OX39 (38). However, the rate of anaerobic nitrate-dependent degradation of meta-xylene by strain RCB (ca. 11 μM · day−1) was almost twice that reported for sulfate-reducing strain OX39 (5 μM · day−1) (38), although this may again be a function of differences in the biomass used in the respective studies.
FIG. 5.
Anaerobic biotransformation of meta-, ortho-, and para-xylene by Dechloromonas strain RCB. The arrows represent refeeds of the respective xylene isomers. The results depicted are the averages of triplicate determinations. The replicate samples did not deviate from each other by >5%.
Although strain RCB quantitatively produced 14CO2 when amended with either 14C-labeled toluene or benzene, 14CO2 was not produced during the catabolism of [U-14C] labeled p-xylene (data not shown), suggesting that this substrate was biotransformed into some as-yet-unidentified intermediate. This was not entirely unexpected, as no pure culture exists to date that can mineralize p-xylene completely to CO2, and p-xylene mineralization in the absence of oxygen has only been observed in studies based on sediments or enrichment cultures (27, 31). Furthermore, these studies have indicated the accumulation of nondegradable dead-end products of anaerobic p-xylene metabolism. Similarly, it has been previously demonstrated that one of the intermediates arising from the transformation of p-xylene under aerobic conditions also leads to the formation of 3,6-dimethyl catechol, which represents an inhibitory dead-end product (42). Whether or not strain RCB produces similar type dead-end products is currently unknown.
Cometabolism of monoaromatic substrates by strain RCB.
Although several organisms have now been identified that can oxidize the individual components of BTEX, in general, these organisms are not very versatile in their ability to utilize more than one hydrocarbon. Furthermore, of those that have been identified that can utilize more than one component, only the m-xylene-degrading strain mXyN1 and the ethylbenzene-degrading strain EbN1 (40) have been demonstrated to oxidize these compounds in a mixture (41). These prior studies were performed using crude oil as the hydrocarbon mixture, and the results indicated that the utilization of alkylbenzenes was in agreement with the substrate spectra of the individual organisms determined with the pure compounds (41). In this instance, strains mXyN1 and EbN1 utilized m-xylene and ethylbenzene, respectively, in addition to toluene from the crude oil (41). However, it was unclear from these studies whether or not these substrates were used concomitantly or sequentially by the respective strains.
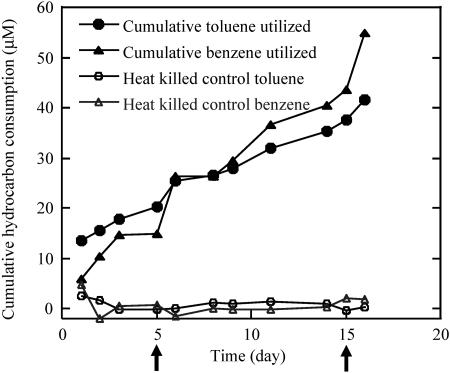
When an active anaerobic culture of strain RCB was transferred from acetate-nitrate (each, 10 mM) medium to anaerobic nitrate (10 mM) medium supplemented with an equal mixture of toluene and benzene (each, 30 μM) as the combined electron donors, rapid and simultaneous degradation of both hydrocarbons was observed (Fig. 6). Almost 49 μM toluene and 43 μM benzene were oxidized in the mixture within 16 days with 10 mM nitrate as the electron acceptor. The rates of oxidation of the benzene and toluene when added as cosubstrates to the medium were almost identical to the rate of oxidation of the individual components (data not shown). Interestingly, although benzene is considered more recalcitrant, its cumulative degradation was almost always identical to the cumulative degradation of toluene by strain RCB (Fig. 6). This result was in contrast to previous observations made with column studies bioaugmented with BTEX-degrading anaerobic methanogenic enrichments, where the presence of toluene was shown to inhibit the rate of benzene removal (35).
FIG. 6.
Cumulative consumption of toluene and benzene by active cells of strain RCB under anaerobic nitrate-reducing conditions when inoculated into medium containing equal amounts of both benzene and toluene. The arrows represent points at which the cultures were refed with the hydrocarbon mixture. The results depicted are the averages of triplicate determinations.
Conclusions.
The results of these studies demonstrate that Dechloromonas strain RCB was capable of metabolizing benzene coupled to the reduction of a broad range of alternative electron acceptors including oxygen, perchlorate, chlorate, or nitrate. Furthermore, Dechloromonas strain RCB utilized all of the BTEX components under a range of alternative electron-accepting conditions either individually or as mixtures. In most cases, the hydrocarbons were completely mineralized to CO2.
Whether or not strain RCB or other members of the Dechloromonas genus play an important role in environmental hydrocarbon degradation is still unknown. Members of this genus are generally recognized for their ability to grow by dissimilatory perchlorate reduction (1, 2, 5, 12, 15); perchlorate is another common groundwater contaminant associated with the activity of the munitions industry (44). In support of this, Dechloromonas strain RCB did couple growth and carbon assimilation to the reduction of perchlorate. The Dechloromonas species, together with the closely related Azospira (formerly Dechlorosoma) species, are considered to represent the predominant perchlorate-reducing bacteria in the environment and have been found to be ubiquitous, regardless of whether or not there has been previous exposure of the environment to perchlorate (18, 19). Because perchlorate-reducing bacteria are found in several pristine environments, the ubiquity of these organisms is unlikely to be related to their ability to grow by dissimilatory perchlorate reduction (19). Previous studies have demonstrated that these organisms are, in general, metabolically versatile and can use a broad range of alternative electron donors (18). As such, the selective pressures for Dechloromonas species in the environment may be based on the diversity of their metabolic capabilities rather than any individual metabolism.
Since Dechloromonas strain RCB possesses the capability of degrading such a broad range of monoaromatic hydrocarbons, the results hold great promise in developing strategies for the bioremediation of a myriad of hydrocarbon-contaminated environments. In addition, this organism also offers great potential for the bioremediation of environments contaminated with both BTEX and perchlorate in a single treatment strategy.
Acknowledgments
Studies of Dechloromonas aromatica strain RCB were supported by independent grants to J.D.C. from the U.S. Department of Energy NABIR program (DE-FG02-98-ER-62689) and the U.S. Department of Defense SERDP program (DACA72-00-C-0016).
REFERENCES
- 1.Achenbach, L. A., U. Michaelidou, R. A. Bruce, J. Fryman, and J. D. Coates. 2001. Dechloromonas agitata gen. nov., sp. nov. and Dechlorosoma suillum gen. nov., sp. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. Evol. Microbiol. 51:527-533. [DOI] [PubMed] [Google Scholar]
- 2.Achenbach, L. A., and J. D. Coates. 2000. Disparity between bacterial phylogeny and physiology. ASM News 66:714-716. [Google Scholar]
- 3.Anderson, R. T., and D. R. Lovley. 1997. Ecology and biogeochemistry of in situ groundwater bioremediation. Adv. Microb. Ecol. 15:289-350. [Google Scholar]
- 4.Ball, H. A., H. A. Johnson, M. Reinhard, and A. M. Spormann. 1996. Initial reactions in anaerobic ethylbenzene oxidation by a denitrifying bacterium, strain EB1. J. Bacteriol. 178:5755-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce, R. A., L. A. Achenbach, and J. D. Coates. 1999. Reduction of (per)chlorate by a novel organism isolated from paper mill waste. Environ. Microbiol. 1:319-329. [DOI] [PubMed] [Google Scholar]
- 6.Burland, S. M., and E. A. Edwards. 1999. Anaerobic benzene biodegradation linked to nitrate reduction. Appl. Environ. Microbiol. 65:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervantes, F. J., W. Dijksma, T. Duong-Dac, A. Ivanova, G. Lettinga, and J. A. Field. 2001. Anaerobic mineralization of toluene by enriched sediments with quinones and humus as terminal electron acceptors. Appl. Environ. Microbiol. 67:4471-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty, R., and J. D. Coates. 2004. Anaerobic degradation of monoaromatic hydrocarbons. Appl. Microbiol. Biotechnol. 64:437-446. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, T., P. Kjeldsen, H. Albrechtsen, and G. Heron. 1994. Attenuation of pollutants in landfill leachate polluted aquifers. Crit. Rev. Environ. Sci. Technol. 24:119-202. [Google Scholar]
- 10.Coates, J. D. 2003. Anaerobic biodegradation of hydrocarbons, p. 58-81. In A. Singh and O. Ward (ed.), Bioremediation, phytoremediation, and natural attenuation. Springer-Verlag, Heidelberg, Germany.
- 11.Coates, J. D., and L. A. Achenbach. 2001. The biogeochemistry of aquifer systems, p. 719-727. In C. J. Hurst, G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. W. Walter (ed.), Manual of environmental microbiology, 2nd ed. ASM Press, Washington, D.C.
- 12.Coates, J. D., and L. A. Achenbach. 2004. Microbial perchlorate reduction: rocket fuelled metabolism. Nat. Rev. Microbiol. 2:569-580. [DOI] [PubMed] [Google Scholar]
- 13.Coates, J. D., R. T. Anderson, and D. R. Lovley. 1996. Anaerobic hydrocarbon degradation in petroleum-contaminated harbor sediments under sulfate-reducing and artificially imposed iron-reducing conditions. Environ. Sci. Technol. 30:2784-2789. [Google Scholar]
- 14.Coates, J. D., R. T. Anderson, and D. R. Lovley. 1996. Anaerobic oxidation of polycyclic aromatic hydrocarbons under sulfate-reducing conditions. Appl. Environ. Microbiol. 62:1099-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coates, J. D., and R. Chakraborty. 2003. Anaerobic bioremediation—an emerging resource for environmental cleanup, p. 227-257. In I. Singleton, M. G. Milner, and I. M. Head (ed.), Bioremediation: a critical review. Horizon Press, Norfolk, United Kingdom.
- 16.Coates, J. D., R. Chakraborty, J. G. Lack, S. M. O'Connor, K. A. Cole, K. S. Bender, and L. A. Achenbach. 2001. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 411:1039-1043. [DOI] [PubMed] [Google Scholar]
- 17.Coates, J. D., R. Chakraborty, and M. J. McInerney. 2002. Anaerobic benzene biodegradation—a new era. Res. Microbiol. 153:621-628. [DOI] [PubMed] [Google Scholar]
- 18.Coates, J. D., and D. R. Lovley. 2005. Genus Geobacter, p. 1017-1020. In D. Brenner, N. Krieg, J. Staley, and G. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 2. Springer-Verlag, New York, N.Y. [Google Scholar]
- 19.Coates, J. D., U. Michaelidou, R. A. Bruce, S. M. O'Connor, J. N. Crespi, and L. A. Achenbach. 1999. The ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coates, J. D., E. J. P. Phillips, D. J. Lonergan, H. Jenter, and D. R. Lovley. 1996. Isolation of Geobacter species from a variety of sedimentary environments. Appl. Environ. Microbiol. 62:1531-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolfing, J., J. Zeyer, P. Binder-Eicher, and R. P. Schwarzenbach. 1990. Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch. Microbiol. 134:336-341. [DOI] [PubMed] [Google Scholar]
- 22.Edwards, E. A., L. E. Wills, M. Reinhard, and D. Grbic-Galic. 1992. Anaerobic degradation of toluene and xylene by aquifer microorganisms under sulfate-reducing conditions. Appl. Environ. Microbiol. 58:794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans, P. J., D. T. Mang, K. S. Kim, and L. Y. Young. 1991. Anaerobic degradation of toluene by a denitrifying bacterium. Appl. Environ. Microbiol. 57:1139-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans, P. J., D. T. Mang, and L. Y. Young. 1991. Degradation of toluene and m-xylene and transformation of o-xylene by denitrifying enrichment cultures. Appl. Environ. Microbiol. 57:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fries, M. R., J. Zhou, J. Chee-Sanford, and J. M. Tiedje. 1994. Isolation, characterization, and distribution of denitrifying toluene degraders from a variety of habitats. Appl. Environ. Microbiol. 60:2802-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grbic-Galic, D., and T. Vogel. 1987. Transformation of toluene and benzene by mixed methanogenic cultures. Appl. Environ. Microbiol. 53:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haner, A., P. Hohener, and J. Zeyer. 1995. Degradation of p-xylene by a denitrifying enrichment culture. Appl. Environ. Microbiol. 61:3185-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harms, G., R. Rabus, and F. Widdel. 1999. Anaerobic oxidation of the aromatic plant hydrocarbon p-cymene by newly isolated denitrifying bacteria. Arch. Microbiol. 172:303-312. [DOI] [PubMed] [Google Scholar]
- 29.Hess, A., B. Zarda, D. Hahn, A. Haner, D. Stax, P. Hohener, and J. Zeyer. 1997. In situ analysis of denitrifying toluene- and m-xylene-degrading bacteria in a diesel fuel-contaminated laboratory aquifer column. Appl. Environ. Microbiol. 63:2136-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kniemeyer, O., T. Fischer, H. Wilkes, F. Glockner, and F. Widdel. 2003. Anaerobic degradation of ethylbenzene by a new type of marine sulfate-reducing bacterium. Appl. Environ. Microbiol. 69:760-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn, E. P., J. Zeyer, P. Eicher, and R. P. Schwarzenbach. 1988. Anaerobic degradation of alkylated benzenes in denitrifying laboratory aquifer columns. Appl. Environ. Microbiol. 54:490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langenhoff, A. A. M., D. L. Brouwers-Ceiler, J. H. L. Engelberting, J. J. Quist, J. G. P. N. Wolkenfelt, A. J. B. Zehnder, and G. Schraa. 1997. Microbial reduction of manganese coupled to toluene oxidation. FEMS Microbiol. Ecol. 22:119-127. [Google Scholar]
- 33.Lovley, D. R. 1997. Potential for anaerobic bioremediation of BTEX in petroleum-contaminated aquifers. J. Ind. Microbiol. 18:75-81. [Google Scholar]
- 34.Lovley, D. R., M. J. Baedecker, D. J. Lonergan, I. M. Cozzarelli, E. J. P. Phillips, and D. I. Siegel. 1989. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 339:297-299. [Google Scholar]
- 35.Marcio, L., B. Da Silva, and P. J. J. Alvarez. 2004. Enhanced anaerobic biodegradation of benzene-toluene-ethylbenzene-xylene-ethanol mixtures in bioaugmented aquifer columns. Appl. Environ. Microbiol. 70:4720-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meckenstock, R. U. 1999. Fermentative toluene degradation in anaerobic defined syntrophic cocultures. FEMS Microbiol. Lett. 177:67-73. [DOI] [PubMed] [Google Scholar]
- 37.Meckenstock, R. U., M. Safinowski, and C. Griebler. 2004. Anaerobic degradation of polycyclic aromatic hydrocarbons. FEMS Microbiol. Ecol. 49:27-36. [DOI] [PubMed] [Google Scholar]
- 38.Morasch, B. S., C. C. Tebbe, and R. U. Meckenstock. 2004. Degradation of o-xylene and m-xylene by a novel sulfate-reducer belonging to the genus Desulfotomaculum. Arch. Microbiol. 181:407-417. [DOI] [PubMed] [Google Scholar]
- 39.Rabus, R., R. Nordhaus, W. Ludwig, and F. Widdel. 1993. Complete oxidation of toluene under strictly anoxic conditions by a new sulfate-reducing bacterium. Appl. Environ. Microbiol. 59:1444-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabus, R., and F. Widdel. 1995. Anaerobic degradation of ethylbenzene and other aromatic hydrocarbons by new denitrifying bacteria. Arch. Microbiol. 163:96-103. [DOI] [PubMed] [Google Scholar]
- 41.Rabus, R., and F. Widdel. 1996. Utilization of alkylbenzenes during anaerobic growth of pure cultures of denitrifying bacteria on crude oil. Appl. Environ. Microbiol. 62:1238-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsao, C. W., H. G. Song, and R. Bartha. 1998. Metabolism of benzene, toluene, and xylene hydrocarbons in soil. Appl. Environ. Microbiol. 64:4924-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulrich, A. C., and E. A. Edwards. 2003. Physiological and molecular characterization of anaerobic benzene-degrading mixed cultures. Environ. Microbiol. 5:92-102. [DOI] [PubMed] [Google Scholar]
- 44.Urbansky, E. T. 1998. Perchlorate chemistry: implications for analysis and remediation. Bioremed. J. 2:81-95. [Google Scholar]
- 45.Vogel, T. M., and D. Grbic-Galic. 1986. Incorporation of oxygen from water into toluene and benzene during anaerobic fermentative transformation. Appl. Environ. Microbiol. 52:200-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zengler, K., J. Heider, R. Rosselló-Mora, and F. Widdel. 1999. Phototrophic utilization of toluene under anoxic conditions by a new strain of Blastochloris sulfoviridis. Arch. Microbiol. 172:204-212. [DOI] [PubMed] [Google Scholar]