Abstract
Electron microscopic analysis of contractile phage tail-like bacteriocins of three Pragia fontium strains and one Budvicia aquatica strain was performed. Fonticin and aquaticin are remarkably heat sensitive but trypsin resistant. Simultaneous production of contractile and flexible phage tail-like bacteriocins in the P. fontium 64613 strain is shown for the first time.
Phage tail-like (high-molecular-weight [HMW]) bacteriocins have been described in many species of gram-negative and gram-positive bacteria (4, 8, 11, 12, 15, 18, 21). Generally, phage tail-like bacteriocins belong to two different families: contractile phage tail-like (R-type) and noncontractile but flexible ones (F-type) (17). The former family is similar to tails of P2 or T-even phages of Escherichia coli (virus family Myoviridae), while the morphology of the latter family resembles the flexible tails of λ phage of E. coli (virus family Siphoviridae). Nakayama et al. (19) confirmed the relationship of R-type pyocin to P2 phage and F-type pyocin to coliphage λ. Simultaneous production of R-type and F-type bacteriocins was described in Pseudomonas aeruginosa strains (10, 16). The narrow specificity of phage tail-like bacteriocins and the absence of nucleic acid in their particles may play an important role in the construction of new therapeutic agents with directed action against bacterial strains (7, 23).
Šmarda (22) analyzed five strains of Pragia fontium and nine strains of Budvicia aquatica, the hydrogen sulfide-producing species of Enterobacteriaceae (1, 2, 5), for production of bacteriocin-like agents. All strains produced contractile phage tail-like particles, each killing two to six strains of the same set. Here, we confirm and extend these data and present a detailed comparative study of the morphology of these elements.
Culture conditions.
Strains of P. fontium 24613, 24647, and 25240 and B. aquatica 24522, kindly donated by E. Aldová (National Institute of Public Health, Prague, Czech Republic), were cultured in TY broth (8 g casein enzymatic hydrolysate type I, 5 g yeast extract powder [HI Media Laboratories, India], 5 g NaCl per 1,000 ml H2O). The maximum spontaneous production of phage tail-like particles was after 12 h at 30°C and heavy shaking. Their activity usually ranged from 103 to 106 arbitrary units. The production of phage tail-like particles could be raised by 1 to 2 orders of magnitude by UV light (Philips TUV 254-nm 15-W germicidal tube; 60 s from 50-cm distance) or mitomycin C (1 μg/ml) induction. Enhanced titers were used in all experiments. None of the phage tail-like bacteriocins we examined is plasmid encoded; no plasmid could be detected in cells of any of the strains.
Activity of isolated phage tail-like particles.
The phage tail-like bacteriocins of both Budvicia and Pragia killed sensitive strains of both species (Table 1), and the lethal effect was not transmissible in series. The standard agar double layer procedure was used in cross-experiments. In positive combinations, very narrow (0.7 to 2.5 mm wide) inhibition zones were formed: they were mostly clear, partly turbid, with isolated tiny colonies. No strain was sensitive to its own bacteriocin. All phage tail-like bacteriocins were temperature sensitive (Table 2) and lost their activity completely after 10 min at temperatures of 45°C to 60°C. On the other hand, they showed a high degree of resistance to trypsin (Table 3). Intergeneric activity was tested on seven Klebsiella spp. strains, five bacteriophage- and low-molecular-weight colicin-sensitive indicator strains of E. coli and one Shigella sonnei colicin indicator strain. In the 52 combinations tested, only 3 were positive: fonticin of the strain P. fontium 24342 inhibited one Klebsiella sp. strain plus the strain of E. coli ϕ, while that of the strain P. fontium 24613 inhibited the strain E. coli B. The other 49 combinations were negative. All bacterial strains under study originated from the collection at the Faculty of Medicine, Masaryk University, Brno, Czech Republic.
TABLE 1.
Strain-specific lethal action of HMW bacteriocins
| Sensitive strain | Lethal result with producer straina
|
||||
|---|---|---|---|---|---|
|
Pragia fontium
|
Budvicia aquatica 24522 | ||||
| 24342 | 24613 | 24647 | 25240 | ||
| Pragia fontium strains | |||||
| 24342 | + | + | |||
| 24613 | + | + | + | ||
| 24647 | + | + | + | ||
| 25240 | |||||
| Budvicia aquatica 24522 | |||||
Plates were incubated at room temperature, and the results were read after 24 hours.
TABLE 2.
Heat sensitivity of HMW bacteriocins
| Strain | Activity (AUa) after 10 min at:
|
||||||
|---|---|---|---|---|---|---|---|
| 30°Cb | 40°C | 45°C | 50°C | 55°C | 60°C | 65°C | |
| Pragia fontium strains | |||||||
| 24342 | 25 | 25 | 0 | 0 | 0 | 0 | 0 |
| 24613 | 28 | 28 | 27 | 27 | 22 | 0 | 0 |
| 24647 | 26 | 26 | 22 | 0 | 0 | 0 | 0 |
| Budvicia aquatica 24522 | 25 | 25 | 25 | 24 | 0 | 0 | 0 |
AU, arbitrary units.
Control.
TABLE 3.
Trypsin sensitivity of HMW bacteriocinsa
| Strain | Activity (AU) after 30 min of incubation at trypsin concn (% wt/vol) of:
|
||
|---|---|---|---|
| 0b | 0.05 | 0.1 | |
| Pragia fontium strains | |||
| 24342 | 25 | 24 | 20 |
| 24613 | 28 | 28 | 24 |
| 24647 | 26 | 24 | 22 |
| Budvicia aquatica 24522 | 25 | 23 | 21 |
One-milliliter samples of phage tail-like bacteriocin suspensions in TY broth were mixed with stock trypsin solutions (in H2O) in prewarmed Eppendorf tubes and incubated at 37°C. Samples were serially diluted (2−1 to 2−8) with TY broth, and drops of all dilutions were placed on agar plates (1.5% [wt/vol] nutrient agar no. 2; Imuna, Slovakia) overlaid with a corresponding indicator strain in soft agar 0.7% (wt/vol). AU, arbitrary units.
Control.
Morphology of phage tail-like particles.
The negatively stained samples (see Fig. 1, 2, and 4 below) were prepared as follows: drops of phage tail-like bacteriocin suspension were applied onto glow discharge-activated (3) Formvar-carbon-coated grids. After adsorption (30 s), the grids were floated on 1-ml drops of 1% (wt/vol) ammonium molybdate, pH 7.1, for 10 min. Finally, the grids were negatively stained with 2% (wt/vol) uranyl acetate. The samples were analyzed in a Philips CM100 electron microscope.
FIG. 1.
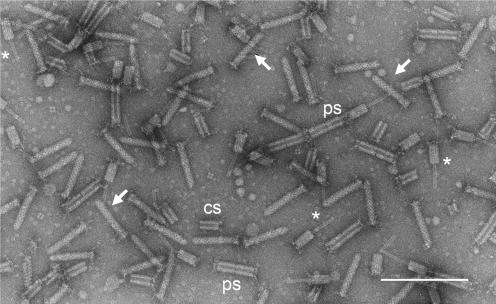
Contractile phage tail-like particles, aquaticin, produced by B. aquatica strain 24522: a mixture of native and contracted particles and their released components. Some bullet-like intact particles with base plate and attached tail fibers are marked with arrows. Intact bacteriocin particles show a typical cross-striation pattern. Contracted particles with a protruding core and shortened sheath with clearly visible tail fibers are marked with asterisks. Some of the polysheaths formed by at least two contracted sheaths are marked “ps,” and contracted sheath monomers are marked “cs.” The sample was prepared from cultivation media concentrated by ultracentrifugation. The image was recorded on Kodak 4489 EM film at a primary magnification of ×46,000. The negative was scanned with a Minolta Multi Pro film scanner in TEM mode at 2,400 pixels per inch. Bar, 200 nm.
FIG. 2.
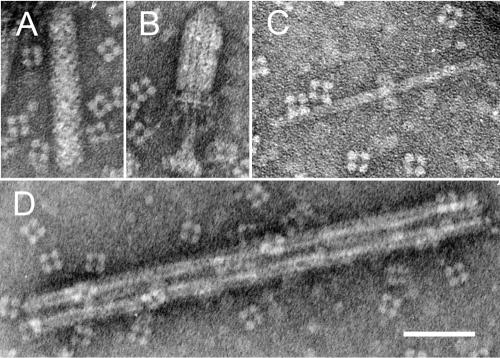
Set of typical structures found in cultivation media of P. fontium strain 24647. (A) Native form; (B) contracted form of R-type particle; (C) “polycore” composed of two units; (D) polysheath composed of five contracted sheaths (pentamer). In panel B, the remainders of the basal plate are clearly visible on the distal end of the contracted sheath. Bar, 50 nm.
FIG. 4.
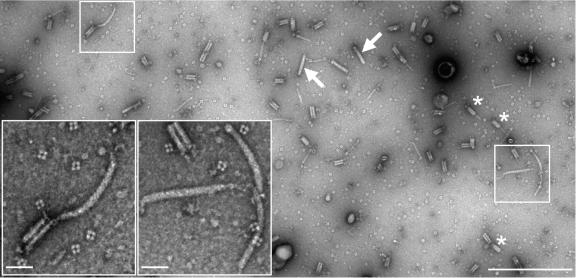
Simultaneous production of contractile phage tail-like particles (R-type) and flexible F-type particles by P. fontium strain 24613. (Inserts) Higher magnifications of marked areas with intact F-type particles. The image was recorded using a MegaView II digital slow scan camera. Nine full-frame images (12 bits; 1,280 by 1,024 pixels; 3-by-3 matrix), which covered about 15 μm2 of the negatively stained sample on a support film, were recorded and stitched together using the MIA module functions of the Analysis 3.2 software. Only part of the recorded area is shown. The F-type particles were found at low frequencies (less than 5%) compared with the occurrence of intact contractile R-type particles (arrows) and contracted sheaths (asterisks). The sample was prepared from cultivation medium concentrated by an Ultrafree-15 centrifugal device (Millipore). The images were recorded at a primary magnification of ×46,000, which gives a pixel size of 1.37 nm, resulting in an image resolution of 2.7 nm, according to the Nyqiust criterion. Bars: main image, 500 nm; inserts, 50 nm.
The cultivation media of all tested bacterial strains (P. fontium 24613, 24647, and 25240 and B. aquatica 24522) contain phage tail-like particles resembling isolated tails of T-even bacteriophages of E. coli. A narrow, hollow inner core encompassed by a wider outer sheath, which is contractile but not flexible, forms their typical structure. A perpendicular basal plate endowed with spikes and several tail fibers complements this structure. Two superimposed streams of spiral coiling of the contractile protein, including an angle of some 45°, forming the sheath, are recognizable by a general regular superficial cross-striation pattern, oblique to the length (Fig. 1).
Contracted tail-like forms show two structural components: a native narrow full-length core protrudes from the contracted sheath through the central aperture of the base plate or on both ends. The spiral coiling of the sheath disappears with its contraction. Two- or severalfold longitudinal polymers of contracted sheaths show “polysheath” formations. Interestingly enough, native sheaths never show analogous polysheath formations. Apparently, they first become contracted and eject their cores; only after that do they bind with other ones, forming polysheaths of varied numbers of monomers (Fig. 1 and 2D). Inner cores frequently slip out of the contracted sheaths completely; also, freed native cores form polymers, called polycores (Fig. 2C).
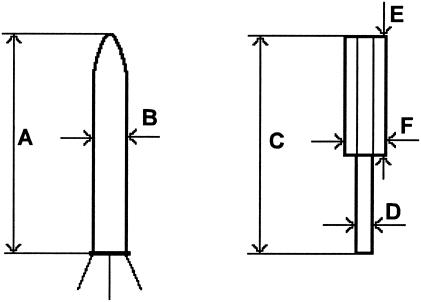
The native and contracted forms of four types of contractile phage tail-like particles were measured in detail (Table 4). We did not find any significant morphological difference between them. For P. fontium fonticin, the calculated length (Fig. 3, dimension A) of a native particle was 115 nm, and the width (dimension B) was 20 nm. B. aquatica aquaticin length was 107 nm, and the width was 18 nm. The difference between the lengths of native forms of fonticin and aquaticin was statistically significant on the 95% level. Table 5 compares all the dimensions of phage tail-like bacteriocins published for gram-negative bacteria with those found here for P. fontium and B. aquatica.
TABLE 4.
Dimensions of HMW bacteriocin particles produced by strains of P. fontium and B. aquatica
| Particle type and measurement |
Pragia fontium
|
Budvicia aquatica 24522 | ||
|---|---|---|---|---|
| 24613 | 24647 | 25240 | ||
| Extended particles | ||||
| No.a | 49 | 8 | 18 | 86 |
| Dimension Ab | 116 ± 4 | 119 ± 5 | 112 ± 5 | 107 ± 7 |
| Dimension B | 20 ± 1 | 20 ± 1 | 21 ± 2 | 18 ± 2 |
| Contracted particles | ||||
| No.a | 19 | 15 | 8 | |
| Dimension C | 116 ± 7 | 113 ± 8 | 111 ± 6 | |
| Dimension D | 10 | 10 ± 1 | 9 ± 1 | |
| Dimension E | 63 ± 5 | 64 ± 13 | 47 ± 2 | |
| Dimension F | 26 ± 1 | 26 ± 1 | 24 ± 1 | |
Number of measured particles.
Dimensions are given in nanometers (mean ± standard deviation). The individual measured values headed by capital letters correspond to the diagrams shown in Fig. 3.
FIG. 3.
Dimensions measured in extended and contracted forms of phage tail-like bacteriocins. For an explanation of the symbols, see Tables 4 and 5.
TABLE 5.
Dimensions of HMW bacteriocins of gram-negative producers
| Bacteriocin type | Dimension (nm)a
|
Reference | ||||
|---|---|---|---|---|---|---|
| A | B | D | E | F | ||
| Budvicia aquatica aquaticinb | 107 ± 7 | 18 ± 2 | 9 ± 1 | 47 ± 2 | 24 ± 1 | This paper |
| Erwinia carotovora | 184 ± 4 | 22 | 13 | 69 ± 2 | 25 | 20 |
| Pragia fontium fonticinb,c | 115 ± 5 | 20 ± 1 | 10 ± 1 | 63 ± 10 | 26 ± 1 | This paper |
| Proteus vulgaris | 128 | 18 | 7 | 6 | ||
| Pseudomonas aeruginosa (R-type pyocin) | 130 | 15 | 13 | |||
| Serratia plymithicum | 133 | 16 | 50 | 20 | 14 | |
| Xenorhabdus nematophilus | 170 | 24 | ||||
| Yersinia enterocolitica | 80 ± 5 | 15 ± 1 | 5 ± 1 | 35 ± 2 | 20 ± 2 | 23 |
Besides the contractile-type bacteriocins, the samples of cultivation media of P. fontium strain 24613 contained typical F-type bacteriocin (Fig. 4) at low frequencies. The presence of F-type bacteriocin particles in P. fontium strain 24613 was a surprise. It has never been observed before in this strain nor in the other examined strains of P. fontium or B. aquatica.
Acknowledgments
This research was supported by grants 310/01/0013 and 310/03/1091 from the Grant Agency of the Czech Republic and by Institutional Research Concept no. AV0Z50200510.
REFERENCES
- 1.Aldová, E., O. Hausner, D. J. Brenner, Z. Kocmoud, J. Schindler, B. Potužníková, and P. Petráš. 1988. Pragia fontium gen. nov., sp. nov. of the family Enterobacteriaceae, isolated from water. Int. J. Syst. Bacteriol. 38:183-189. [Google Scholar]
- 2.Aldová, E., O. Hausner, M. Gabrhelová, J. Schindler, P. Petráš, and H. Braná. 1983. A hydrogen sulphide producing gram-negative rod from water. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 254:95-108. [PubMed] [Google Scholar]
- 3.Benada, O., and V. Pokorný. 1990. Modification of the Polaron sputter-coater unit for glow-discharge activation of carbon support films J. Electron Microsc. Tech. 16:235-239. [DOI] [PubMed] [Google Scholar]
- 4.Bondi, M., P. Messi, C. Sabia, M. B. Contri, and G. Manicardi. 1999. Antimicrobial properties and morphological characteristics of two Photorhabdus luminescens strains. Microbiologica 22:117-127. [PubMed] [Google Scholar]
- 5.Bouvet, O. M. M., P. A. D. Grimont, C. Richard, E. Aldová, O. Hausner, and M. Gabrhelová. 1985. Budvicia aquatica gen. nov., sp. nov.: a hydrogen sulfide-producing member of the Enterobacteriaceae. Int. J. Syst. Bacteriol. 35:60-64. [Google Scholar]
- 6.Coetzee, H. L., H. C. de Klerk, and J. N. Coetzee. 1968. Bacteriophage tail-like particles associated with intra-species killing of Proteus vulgaris. J. Gen. Virol. 2:29-36. [DOI] [PubMed] [Google Scholar]
- 7.Damasko, C., A. Konietzny, H. Kaspar, B. Appel, P. Dersch, and E. Strauch. 2005. Studies of the efficacy of enterocoliticin, a phage-tail like bacteriocin, as antimicrobial agent against Yersinia enterocolitica serotype O3 in a cell culture system and in mice. J. Vet. Med. B 52:171-179. [DOI] [PubMed] [Google Scholar]
- 8.Daw, M. A., and F. R. Falkiner. 1996. Bacteriocins: nature, function and structure. Micron 26:467-479. [DOI] [PubMed] [Google Scholar]
- 9.Dykes, G. A. 1995. Bacteriocins: ecological and evolutionary significance. Trends Ecol. Evol. 10:186-189. [DOI] [PubMed] [Google Scholar]
- 10.Govan, J. R. 1974. Studies on the pyocins of Pseudomonas aeruginosa: morphology and mode of action of contractile pyocins. J. Gen. Microbiol. 80:1-15. [DOI] [PubMed] [Google Scholar]
- 11.Gratia, J.-P. 1987. Macrocin G: a phage tail related to, but distinct from the temperate coliphage phi-gamma and produced by Serratia marcescens SMG 38. Arch. Int. Physiol. Biochim. 95:B30. [Google Scholar]
- 12.Hernández-Romero, D., P. Lucas-Elio, D. Lopez-Serrano, F. Solano, and A. Sanchez-Amat. 2003. Marinomonas mediterranea is a lysogenic bacterium that synthesizes R-bodies. Microbiology 149:2679.-2686. [DOI] [PubMed] [Google Scholar]
- 13.Ito, S., and M. Kageyama. 1970. Relationship between pyocins and a bacteriophage in Pseudomonas aeruginosa. J. Gen. Appl. Microbiol. 16:231.-240. [Google Scholar]
- 14.Jabrane, A., A. Sabri, P. Compère, P. Jacques, I. Vandenberghe, J. Van Beeumen, and P. Thonart. 2002. Characterization of serracin P, a phage-tail-like bacteriocin, and its activity against Erwinia amylovora, the fire blight pathogen. Appl. Environ. Microbiol. 68:5704-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krügel, H., N. J. Solovjeva, G. Fiedler, I. W. Avraleva, and G. Schumann. 1987. Occurrence of phage-like particles and growth inhibition in Streptomyces chrysomallus. J. Basic Microbiol. 27:99-105. [Google Scholar]
- 16.Kuroda, K., and M. Kageyama. 1979. Biochemical properties of a new flexuous bacteriocin, pyocin F1, produced by Pseudomonas aeruginosa. J. Biochem. (Tokyo) 85:7-19. [DOI] [PubMed] [Google Scholar]
- 17.Michel-Briand, Y., and C. Baysse. 2002. The pyocins of Pseudomonas aeruginosa. Biochimie 84:449-510. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz, J., A. L. Extremera, J. M. Arias, and E. Montoya. 1987. Induction, purification and some properties of phage tail-like particles from Myxococcus coralloides D. Microbios 49:161-169. [PubMed] [Google Scholar]
- 19.Nakayama, K., K. Takashima, H. Ishihara, T. Shinomyia, M. Kageyama, S. Kanaya, M. Ohnishi, T. Murata, H. Mori, and T. Hayashi. 2000. The R-type pyocin of Pseudomonas aeruginosa is related to P2 phage, and the F-type is related to lambda phage. Mol. Microbiol. 38:213-231. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen, A. H., T. Tomita, M. Hirota, T. Sato, and Y. Kamio. 1999. A simple purification method and morphology and component analyses for carotovoricin Er, a phage-tail-like bacteriocin from the plant pathogen Erwinia carotovora Er. Biosci. Biotechnol. Biochem. 63:1360-1369. [DOI] [PubMed] [Google Scholar]
- 21.Nieves, B. M., F. Gil, and F. J. Castillo. 1981. Growth inhibition activity and bacteriophage and bacteriocin-like particles associated with different species of Clostridium. Can. J. Microbiol. 27:216-225. [DOI] [PubMed] [Google Scholar]
- 22.Šmarda, J. 1987. Production of bacteriocin-like agents of Budvicia aquatica and “Pragia fontium.” Zentralbl. Bakteriol. Hyg. A 265:74-81. [PubMed] [Google Scholar]
- 23.Strauch, E., H. Kaspar, Ch. Schaudinn, P. Dersch, K. Madela, Ch. Gewinner, S. Hertwig, J. Wecke, and B. Appel. 2001. Characterization of enterocoliticin, a phage tail-like bacteriocin, and its effect on pathogenic Yersinia enterocolitica strains. Appl. Environ. Microbiol. 67:5634-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thaler, J.-O., S. Baghdiguian, and N. Boemare. 1995. Purification and characterization of xenorhabdicin, a phage tail-like bacteriocin, from the lysogenic strain F1 of Xenorhabdus nematophilus. Appl. Environ. Microbiol. 61:2049-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]