Abstract
Three species within a deeply branching cluster of the Chloroflexi are the only microorganisms currently known to anaerobically transform polychlorinated biphenyls (PCBs) by the mechanism of reductive dechlorination. A selective PCR primer set was designed that amplifies the 16S rRNA genes of a monophyletic group within the Chloroflexi including Dehalococcoides spp. and the o-17/DF-1 group. Assays for both qualitative and quantitative analyses by denaturing gradient gel electrophoresis and most probable number-PCR, respectively, were developed to assess sediment microcosm enrichments that reductively dechlorinated PCBs 101 (2,2′,4,5,5′-CB) and 132 (2,2′,3,3′,4,6′-CB). PCB 101 was reductively dechlorinated at the para-flanked meta position to PCB 49 (2,2′,4,5′-CB) by phylotype DEH10, which belongs to the Dehalococcoides group. This same species reductively dechlorinated the para- and ortho-flanked meta-chlorine of PCB 132 to PCB 91 (2,2′,3′,4,6′-CB). However, another phylotype designated SF1, which is more closely related to the o-17/DF-1 group, was responsible for the subsequent dechlorination of PCB 91 to PCB 51 (2,2′,4,6′-CB). Using the selective primer set, an increase in 16S rRNA gene copies was observed only with actively dechlorinating cultures, indicating that PCB-dechlorinating activities by both phylotype DEH10 and SF1 were linked to growth. The results suggest that individual species within the Chloroflexi exhibit a limited range of congener specificities and that a relatively diverse community of species within a deeply branching group of Chloroflexi with complementary congener specificities is likely required for the reductive dechlorination of different PCBs congeners in the environment.
Polychlorinated biphenyls (PCBs) have been an environmental concern for several decades due to their widespread use, chemical stability, and biological toxicity (31, 33). Historically, harbor regions have been heavily impacted by the accumulation of PCBs released during commercial activities. Commercial production of PCBs was banned in the United States in 1978, but reports of the distribution of PCBs in marine coastal harbor regions demonstrate the tenacity of PCB contamination (3, 18, 21, 34).
Although PCBs persist in the environment, some microbial processes are able to transform these chemically stable molecules. Aerobic degradation involves biphenyl ring cleavage. However, PCBs are hydrophobic and tend to adsorb to particles that settle and accumulate in the anaerobic zone of sediments, where microbial reductive dehalogenation results in the sequential removal of chlorine atoms from the biphenyl backbone (4, 6). Two anaerobic PCB-dechlorinating microorganisms, strains DF-1 and o-17, within the green non-sulfur Chloroflexi phylum have been shown to link their growth to the reductive dechlorination of PCBs (12, 29, 38, 40). Fennell and coworkers (16) reported that another species within the Chloroflexi, Dehalococcoides ethenogenes 195, dechlorinated the PCB 2,3,4,5,6-pentachlorobiphenyl and other aromatic organochlorines when grown with perchloroethene. This microorganism was the first species in the Dehalococcoides group to be isolated and described (26). Although other Dehalococcoides spp. including strains VS (10), FL2 (24), BAV1 (19), CBDB1 (1, 7), and KB-1/VC-H2 (13) use chlorinated ethenes and other chlorinated compounds as electron acceptors, no other species have been reported to reductively dechlorinate PCBs.
PCB-dechlorinating microorganisms are difficult to isolate and are generally a small portion of the total microbial community in natural sediments (29, 38). Little is therefore known about the catalytic diversity of PCB-dechlorinating bacteria and their distribution in nature. A recently developed PCR-based assay using primers specific for the 16S rRNA genes of PCB-dechlorinating microorganisms similar to o-17 and DF-1 revealed a diverse group of organisms within a deep branch of the Chloroflexi that are distinct from Dehalococcoides spp. (37). Sequence similarity among Dehalococcoides strains is very high (>98%), while the similarity between the o-17/DF-1 group and the Dehalococcoides strains is <90%. Nevertheless, all these microorganisms form a monophyletic clade within the Chloroflexi. Using PCR primers designed to detect both Dehalococcoides spp. and o-17/DF-1-like microorganisms by denaturing gradient gel electrophoresis (DGGE), we report that two phylotypes, one closely related to phylotype m-1 (37) within the o-17/DF-1 group and the second a Dehalococcoides sp., sequentially dechlorinate the double-flanked and single-flanked meta-chlorines of PCB 132 in Baltimore Harbor (BH) sediment microcosms. Enumeration by most-probable-number (MPN)-PCR with the specific primers shows that individual PCB congeners can be sequentially dechlorinated by a succession of two phylotypes that link their growth to reductive dehalogenation.
MATERIALS AND METHODS
Sediment samples.
Sediments were sampled from the Northwest Branch of BH with a petite Ponar grab sampler at 39°16.8′N, 76°36.1′W as described by Berkaw et al. (5) and stored anaerobically under nitrogen prior to use.
Anaerobic enrichment cultures.
A defined low-sulfate (<0.3 mM) estuarine salts medium was prepared as described by Berkaw et al. (5) with the exclusion of Na2S · 9H2O, dispensed in 10-ml aliquots into 20-ml Balch anaerobe tubes, and sealed under N2-CO2 (80:20). The medium was autoclaved for 20 min and had a final pH of 6.8. All subsequent additions were performed aseptically in an anaerobic glove box under an N2-CO2-H2 (75:20:5) atmosphere. Prior to inoculation, a fatty acid mixture of sodium salts (acetate, propionate, and butyrate) was added to a final concentration of 2.5 mM each. Microcosms were initiated by the addition of 2 g of BH sediment slurry into 8 ml of medium. PCB congeners 91, 101, and 132 (AccuStandard, Inc., New Haven, CT) were solubilized in 10 μl acetone and separately added to triplicate microcosms to a final concentration of 50 ppm (in milligrams per liter). Sterile controls were prepared by autoclaving sediment-inoculated tubes containing medium and the fatty mixture twice, with a 48-h interval between treatments, followed by the addition of PCB. Active cultures were maintained by transference of 1 ml of homogenized slurry into freshly prepared medium containing 0.5 g dried, sterile BH sediment approximately every 8 months. One control microcosm containing 10 μl acetone without PCB was also transferred for each PCB congener set. Dried BH sediment was prepared by baking BH sediment at 115°C for 72 h, followed by autoclaving five times in a container sealed under N2 for 60 min. All cultures were incubated at 30°C in the dark.
Analytical techniques.
PCBs were analyzed by the extraction of 0.5 ml of culture with 3 ml of hexane for 12 h on a wrist shaker. The organic phase was passed through a copper/Florisil (1:4) column and analyzed using a Hewlett-Packard 5890 series II gas chromatograph (GC) with a DB-1 capillary column (30 m by 0.25 mm by 0.25 μm; JW Scientific, Folsom, CA) and a 63Ni electron capture detector as described by Berkaw et al. (5). Nine mixes containing 209 congeners in total (C-CSQ-SET; AccuStandard) were used to identify the PCB congeners by matching their retention times. Individual PCB congeners were quantified with a 10-point calibration curve using PCB 65 and PCB 204 as external and internal standards.
Bacterial community 16S rRNA gene analyses.
DNA from pooled samples (0.5 ml from each culture replicate) was extracted according to Pulliam Holoman et al. (29) with minor modifications. Briefly, samples were subjected to bead beating with a Fastprep Cell Disruptor (Qbiogene, Carlsbad, CA), and phenol chloroform extraction was followed by electrophoresis in a 1.3% (wt/vol) low-melt agarose gel containing 2% (wt/vol) polyvinylpyrrolidine. DNA was excised from the gel and recovered with the Promega Wizard PCR Prep kit (Promega, Madison, WI). Total DNA was probed for dechlorinating microorganisms within the o-17/DF-1 group with universal primer 14F (14) and specific primer Dehal1265R (36, 37). The same DNA samples were screened for the presence of Dehalococcoides spp. with forward primer DHC 1 and reverse primer DHC 1377 (20). Amplified rDNA restriction analyses (ARDRAs) were conducted as described by Pulliam Holoman et al. (29). The 16S rRNA gene clone library was generated with Dehalococcoides-specific primers DHC 1 and DHC 1377 (20) and fragments were ligated into pCR2.1 using the TA Cloning kit (Invitrogen, Carlsbad, CA). The library was screened using the primers DHC 1 and DHC 1377, followed by restriction fragment polymorphism analysis with restriction endonucleases HaeIII and HhaI. Digestion products were discriminated by gel electrophoresis on a 3% (wt/vol) Trevigel (Trevigen, Inc., Gaithersburg, MD) at 25 V for 3 h on ice. Five plasmids containing the 16S rRNA gene from strains DEH10, D. ethenogenes 195, strain o-17, DF-1, and Chloroflexus aurantiacus were constructed using the TA Cloning kit and used as controls.
A new group-specific primer set was developed by using Probe Design in the ARB software package (25). Forward primer Chl348F (5′-GAGGCAGCAGCAAGGAA-3′) is specific for Chloroflexi, and reverse primer Dehal884R (5′-GGCGGGACACTTAAAGCG-3′) is specific for putative dechlorinating microorganisms. The product size is approximately 470 bp. The primers were checked for compatibility and possible self annealing with Primer Express (Applied Biosystems, Foster City, CA).
For ARDRA, clone libraries were generated by PCR with Chl348F and Dehal884R as described above, except that restriction enzymes RsaI and HinfI were used for restriction fragment analysis. For DGGE, a GC clamp (28) was added to primer Chl348F (5′-CGC CCG CCG CGC GCG GGA GGC AGC AGC AAG GAA-3′) (Genosys Biotechnologies) and designated Chl348FGC. PCRs (50 μl) with 10 ng DNA were performed using the GeneAmp PCR kit (PE Applied Biosystems, Foster City, CA) containing 1× PCR buffer, a mixture of deoxynucleoside triphosphates (200 nM each), 1.5 mM MgCl2, 160 nM each primer, 192 mM dimethyl sulfoxide, and 1 U of AmpliTaq DNA polymerase. Amplification was performed in a PTC200 thermal cycler (MJ Research, Watertown, MA) with the following cycle parameters: initial denaturing (1 min at 95°C), 26 cycles of denaturation (each cycle consisting of 45 s at 95°C), annealing (45 s at 60°C), elongation (45 s at 72°C), and a final extension (30 min at 72°C) (22). The sensitivity of the DGGE assay under the PCR conditions described above was determined by dilution of plasmids containing the 16S rRNA gene of o-17 (12). PCR products were checked for correct size and yield on a 0.8% (wt/vol) Tris-acetate-EDTA agarose gel (Fisher Biotech, New Jersey). DGGE was performed as described by Watts et al. (38) with the D-Code Universal Mutation Detection system (Bio-Rad, Hercules, CA). The 6% (wt/vol) polyacrylamide gels (Sigma, St. Louis, MO) contained a 39 to 48% denaturing gradient, and fragments were separated by electrophoresis for 18 h at 75 V. The gels were stained with SYBR-Green 1 DNA stain (Molecular Probes, Eugene, OR) and visualized with a Storm PhosphorImager (GE Healthcare, Piscataway, NJ). DGGE bands of interest were excised and eluted by incubation in 30 μl Tris-EDTA overnight at 4°C. PCR and DGGE were repeated twice to assure the purity of each eluted band; the last PCR used primers without the GC clamp before DNA sequencing as described below.
Sequencing and analysis.
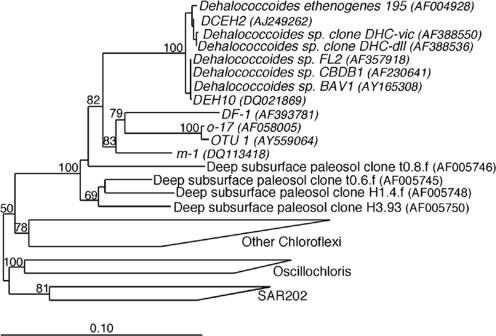
Plasmids from the two clone libraries were purified using the QIAGEN Plasmid Mini kit (QIAGEN, Chatsworth, CA) according to the manufacturer's protocol. Plasmids and PCR products were used as templates for dye terminator cycle sequencing with the Big Dye 3.1 kit (Applied Biosystems) and an ABI 3100 (Applied Biosystems). Sequences were examined for errors and assembled with the Pregap4 and Gap4 software of the Staden software package (http://sourceforge.net/projects/staden). Chimera formation was examined using Chimera Check (9). The sequences were aligned using the ARB software package (25), and a phylogenetic tree was generated based on published Chloroflexi sequences of >1,200 bp. A manual filter was developed to exclude hypervariable regions sequences (Escherichia coli positions 71 to 98, 452 to 483, 838 to 849, 1004 to 1037, 1126 to 1148, and 1163 to 1174). A second filter was created using the “filter by base frequency” tool in the ARB package that excluded positions in the alignment where gaps were more frequent than characters and positions with ambiguous characters. DNA distance matrices were generated with the ARB software package using the Kimura two-parameter evolutionary distance correction, and phylogenetic trees were generated with the neighbor-joining (32) algorithm. Bootstrap analyses (100 replicates) were performed using the PHYLIP package (15).
Quantitative assessment of PCB-dechlorinating populations.
Putative dehalogenating Chloroflexi members were enumerated by MPN-PCR using primers Chl348F and Dehal884R. DNA samples (each, 10 μg/ml) were serially diluted 10 fold and amplified as described above with 40 PCR cycles. 16S rRNA gene copies per microliter of DNA sample were determined with a standard MPN table (8). Dilutions of plasmids with the 16S rRNA genes of the PCB-dechlorinating strains o-17 (12), DF-1 (40), and phylotype DEH10 were used as controls and to determine the sensitivity of the assay. To test whether nonhomologous DNA would interfere with the MPN assay, 10 ng of DNA from a Chloroflexus aurantiacus isolate was added to the dilution series, and the MPN numbers were calculated as described above.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences for phylotype DEH10 and phylotype SF1 have been submitted to GenBank under accession numbers DQ21869 and DQ21870, respectively.
RESULTS
PCB dechlorination in initial enrichment cultures.
Cultures containing BH sediment were amended with PCBs 101 (2,2′,4,5,5′-CB) and 132 (2,2′,3,3′,4,6′-CB), which are predominant congeners (about 4 and 3 mol%, respectively) in Aroclor 1260 (17). Complete reductive dehalogenation of congeners in only the meta positions was detected within 3 to 6 months by the pathways shown in Fig. 1. These cultures were transferred with their respective PCB congener and then screened for dechlorinating organisms with primers specific for the 16S rRNA genes of strains DF-1 and o-17 (36, 37) and Dehalococcoides spp. (20). Unexpectedly, cultures dechlorinating PCB 101 were positive only for Dehalococcoides spp., while the cultures dechlorinating PCB 132 were positive for both groups.
FIG. 1.
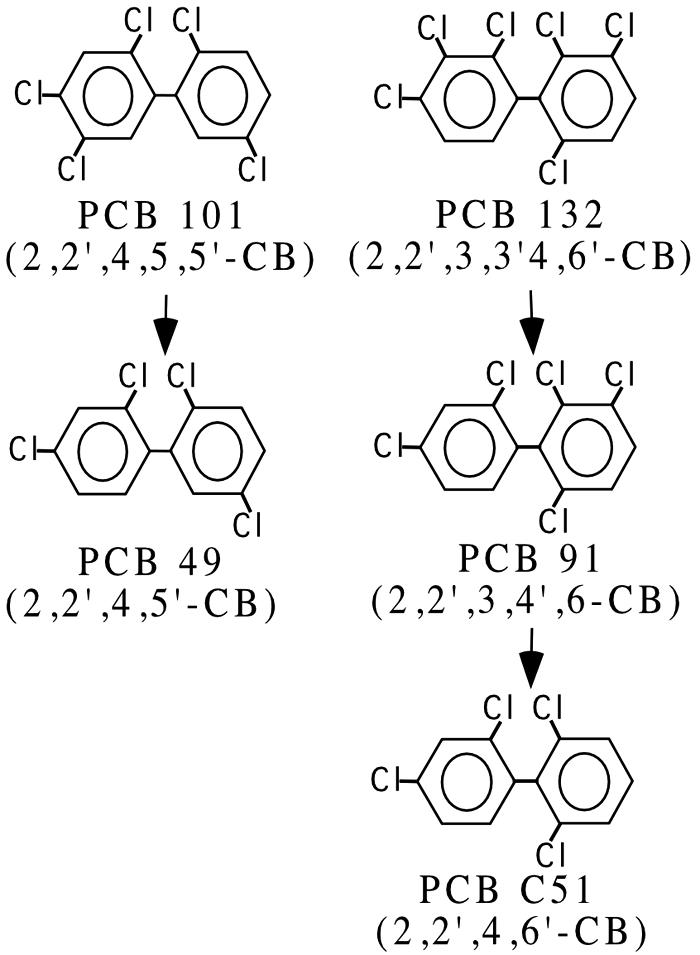
Dechlorination patterns and products observed from Baltimore Harbor sediment microcosms containing PCBs 91, 101, and 132.
To investigate if one specific Dehalococcoides phylotype was enriched in the PCB101 microcosms, we performed an ARDRA with PCR products generated with primers targeting Dehalococcoides spp. (20) from cultures dechlorinating PCB 101 and from the no-PCB control after 6 months of incubation. There was a clear enrichment of a single phylotype (17 out of 18 clones), which we designated DEH10, with no apparent enrichment of an individual ARDRA pattern in the no-PCB control (8 different patterns out of 9 clones). The restriction fragment length polymorphism pattern representing DEH10 was not found in the clone library from the no-PCB control.
Development of PCR primers for detection of PCB-dechlorinating species.
To detect both Dehalococcoides spp. and o-17/DF-1-like PCB-dechlorinating species, a group-specific primer set was developed to target the 16S rRNA genes of this group of Chloroflexi (Fig. 2). Chl348F and Dehal884R amplify the 16S rRNA genes from o-17, DF-1, phylotype DEH10, and Dehalococcoides ethenogenes 195 but not Chloroflexus aurantiacus (Fig. 3). PCR product was not detected with species outside of the green non-sulfur bacteria, including those from several Bacteria and Archaea phyla (data not shown). Furthermore, sequences retrieved from a clone library generated using Chl348F and Dehal884R with Baltimore Harbor sediments included only sequences within the Dehalococcoides/o-17/DF-1 Chloroflexi group. The detection limit of these primers was ≥105 copies per 50 μl of PCR mixture with 26 PCR cycles and 8 μl loaded in an agarose gel. The detection limit in 8 μl with 40 PCR cycles ranged between 10 and 65 gene copies per 50-μl PCR mixture for strains o-17, DF-1, and phylotype DEH10. The addition of up to 10 μg of Chloroflexus aurantiacus DNA had no effect.
FIG. 2.
Phylogenetic analysis (neighbor joining) of Chloroflexi 16S rRNA genes. Tree reconstruction was based on 998 positions between E. coli, positions 44 and 1232, from published sequences. The tree is rooted with Bacillus subtilis (AB016721). Bootstrap analysis was performed using the PHYLIP software package (15), and values of >50 are indicated at the branch points. The scale bar indicates 10 substitutions per 100 nucleotide positions. Microorganisms that are confirmed dechlorinators are italicized.
FIG. 3.
Primer specificity. Alignment of 16S rRNA genes of o-17, DF-1, DEH10, Dehalococcoides ethenogenes 195 (DHE), and Chloroflexus aurantiacus (Chl.). Numbering is based on the E. coli 16S rDNA positions. The panel on the right shows an agarose gel of PCR products using primers Chl348F and Dehal884R and plasmids with cloned 16S rRNA gene templates from the organisms indicated. The top lane shows the DNA size marker (φXDNA-HaeIII digest).
Analysis of PCB-dechlorinating activity and microbial community profiles.
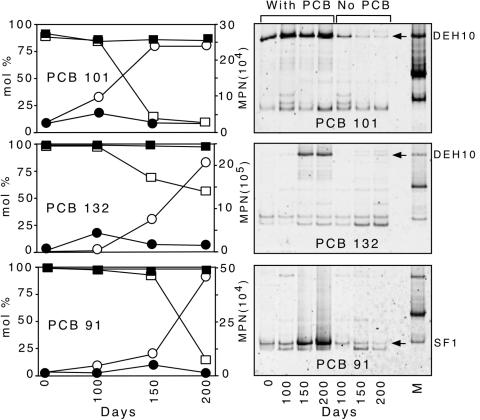
Group-specific primer set Chl348F and Dehal884R were used to identify putative PCB-dechlorinating bacteria in sediment microcosms actively dechlorinating PCBs 101, 132, and 91. The progressive dechlorination of the congeners 132, 101, and 91 at intervals of 0, 100, 150, and 200 days is shown in Fig. 4. PCB101 was dechlorinated in a flanked meta position to PCB 49 (2,2′,4,5′-CB), and no further dechlorination was observed after 250 days. PCB 132 was dechlorinated sequentially in two meta positions to PCB 91 and then to PCB 51 (2,2′,4,6′-CB), which was the terminal product after incubation for 300 days. Inoculum from PCB 132 microcosms was also used to initiate PCB 91 microcosms. Negligible dechlorinating activity was detected in sterile controls (<6 mol% over 200 days).
FIG. 4.
Quantitative and qualitative analyses of 16S rRNA genes during active dechlorination of PCBs 132, 101, and 91. (Left) Dechlorination of PCB congeners 101, 91, and 132, showing moles percent of the parent compound in active culture (□) and sterile control (▪) and MPN-PCR analyses of 16S rDNA copies per μl of DNA in active culture (○) and sterile controls (•). (Right) DGGE results from dechlorinating cultures and the no-PCB controls. Cultures were sequentially transferred four times on the respective PCB congener prior to analysis. All bands were excised and sequenced. Bands in the far right lane are products from (reading from top to bottom) DEH10, DF-1, and o-17.
DGGE profiles in Fig. 4 show enrichment of a single phylotype in each of the dechlorinating cultures over the course of 200 days. The DGGE band representing phylotype DEH10 was enriched in cultures dechlorinating PCB 101 and PCB 132. Another phylotype, designated SF1, was enriched in PCB 91-dechlorinating cultures. Although other PCR amplified 16S rRNA genes appear in both the actively dechlorinating cultures and the no-PCB controls, there was no apparent enrichment of these bands during the incubation period. The bottommost bands in the DGGE gels (Fig. 4) were chimeras.
Quantitative assessment of PCB-dechlorinating populations.
A MPN-PCR-based assay with primers Chl348F and Dehal884R was used to determine whether the apparent enrichment of selected 16S rRNA genes showed by DGGE analyses of actively dechlorinating microcosms was the result of growth by specific phylotypes. PCB 101 microcosms exhibited a 10-fold increase in 16S rRNA gene copies during active dechlorination (Fig. 4). The controls initiated without added PCB showed a steady decrease of dehalogenating Chloroflexi 16S rRNA gene copies during the same incubation period. During active dechlorination of PCB 132 and PCB 91, the cultures exhibited a 20-fold and 50-fold increase in dehalogenating Chloroflexi 16S rRNA gene copies, respectively.
DISCUSSION
Patterns of dechlorination in sediment microcosms.
All microcosms incubated with PCB 132, 101, and 91 exhibited reductive dechlorination in the meta position. Dechlorination of ortho-, para-, and unflanked meta-chlorines was not detected, indicating that these enrichment microcosms selectively dechlorinated double- and single-flanked meta-chlorines. Dechlorination of double-flanked chlorines on a biphenyl backbone has been reported previously for bacterium DF-1 (39, 40) and D. ethenogenes 195 (16). This study provided compelling evidence that phylotype DEH10 was responsible for both double-flanked meta-dechlorination of PCB132 and single-flanked meta-dechlorination of PCB 101. As D. ethenogenes 195 was only tested with PCB 116 (2,3,4,5,6-CB) (17), which contains two double-flanked meta-chlorines, the ability to reductive dechlorinate a PCB congener such as PCB 101 with a single-flanked meta-chlorine cannot be discounted.
These results are consistent with the reductive dechlorination of Aroclor 1260 in microcosms with Baltimore Harbor sediments (41), which showed significant decreases in PCB 101 and PCB 132 and significant accumulation of PCB 51 and PCB 49 after 181 days of incubation. This pattern of dechlorination is most similar to “Process N” described in enrichment microcosms from Silver Lake and Woods Ponds (4), which exhibited extensive dechlorination of flanked meta-chlorines. Other studies have also reported this to be a frequent dechlorination pattern (2, 6, 23, 27, 30).
Identification of dechlorinating microorganisms.
One of the goals of this study was to develop a rapid and comprehensive assay for monitoring the microorganisms responsible for the different dechlorination patterns observed in sediment microcosms. Although primers for both the Dehalococcoides group (20) and the o-17/DF-1 group (36, 37) are available, the group-specific primer set developed in this study selectively amplified both groups of putative PCB-dechlorinating bacteria within this Chloroflexi clade in a single PCR. Figure 2 shows that the bootstrap values separating this clade from the rest of the Chloroflexi are high, suggesting that this group is monophyletic.
Enrichment of phylotype DEH10 in the PCB 101 and PCB 132 cultures compared to the no-PCB control is apparent in the DGGE gel (Fig. 4). Phylotype DEH10 16S rRNA gene sequence has the “Pinellas group” signature of Dehalococcoides spp. in variable regions 2 and 6 (20), and a single base pair difference over 1,378 bp compared to Dehalococcoides sp. strain FL2 (24). However, due to the small size of the PCR products, the DGGE assay described here will not distinguish between different members of the Pinellas group.
Phylotype SF1 was clearly enriched in the microcosm dechlorinating PCB 91 compared to the no-PCB control. Phylotype SF1 is most similar to phylotype m-1 (only 1-bp difference from the 16S rRNA gene sequence over 466 bp), which was detected in cultures dechlorinating 3,5-dichlorobiphenyl (37). Previously, DGGE using universal primers (28) was performed on these same cultures, and no changes were observed in the microbial community. Although the DNA concentrations were normalized among samples and PCR cycles were kept at a minimum to minimize PCR biases, DGGE is only a semiquantitative method. The use of MPN-PCR confirmed the DGGE assay results (Fig. 4). MPN-PCR was used instead of real-time quantitative PCR because fluorescence quenching and autofluorescence associated with sediment samples can adversely affect enumeration accuracy by the latter assay (35).
Isolation of PCB-dechlorinating microorganisms has proven difficult (5, 11, 12, 29, 38-40). Several isolates in the Dehalococcoides group have been reported (1, 10, 13, 19, 24), but a direct link between growth and PCB dechlorination activity has not been shown for any of these isolates. The development of primers targeting a broader range of putative dehalogenating phylotypes within Chloroflexi, including uncultured microorganisms, is an important advance in the study of this diverse group of bacteria in laboratory microcosms, as well as in situ. Increases in 16S rRNA gene copies were only observed in cultures actively dechlorinating PCBs. This is the strongest evidence reported thus far to indicate that PCB-dechlorinating activity is linked to growth of dehalogenating bacteria, in this case, DEH10 and bacterium SF1, possibly by the proposed mechanism of dehalorespiration. The predominance of these meta-dechlorinating pathways in the reductive dechlorination of Aroclor 1260 in BH sediments further suggests that phylotypes DEH10 and SF1 may have a significant and complementary role in this process.
Acknowledgments
This work was supported by the Office of Naval Research, U.S. Department of Defense, grant N000014-03-1-0035 to K.R.S. and J.E.M.W. and grant N000014-03-1-0034 to H.D.M.
We thank Robert Blankenship for kindly providing us with Chloroflexus aurantiacus genomic DNA. We also thank Marcelino T. Suzuki and Birthe Kjellerup for useful comments and for reviewing the manuscript.
REFERENCES
- 1.Adrian, L., U. Szewzyk, J. Wecke, and J. Görisch. 2000. Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580-583. [DOI] [PubMed] [Google Scholar]
- 2.Alder, A. C., M. M. Häggblom, S. R. Oppenheimer, and L. Y. Young. 1993. Reductive dechlorination of polychlorinated biphenyls in anaerobic sediments. Environ. Sci. Technol. 27:530-538. [Google Scholar]
- 3.Ashley, J. T. F., and J. E. Baker. 1999. Hydrophobic organic contaminants in surficial sediments of Baltimore Harbor: inventories and sources. Environ. Toxicol. Chem. 18:838-849. [Google Scholar]
- 4.Bedard, D. L., and J. F. Quensen. 1995. Microbial reductive dechlorination of polychlorinated biphenyls, p. 127-216. In L. Y. Young and C. E. Cerniglia (ed.), Microbial transformation and degradation of toxic organic chemicals. John Wiley, New York, N.Y.
- 5.Berkaw, M., K. R. Sowers, and H. D. May. 1996. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl. Environ. Microbiol. 62:2534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, J. J. F., R. E. Wagner, H. Feng, D. L. Bedard, M. J. Brennan, J. C. Carnahan, and R. J. May. 1987. Environmental dechlorination of PCBs. Environ. Toxicol. Chem. 6:579-593. [Google Scholar]
- 7.Bunge, M., L. Adrian, A. Kraus, M. Opel, W. G. Lorenz, J. R. Andreesen, H. Görisch, and U. Lechner. 2003. Reductive dehalogenation of chlorinated dioxins by an anaerobic bacterium. Nature 421:357-360. [DOI] [PubMed] [Google Scholar]
- 8.Cochran, W. G. 1950. Estimation of bacterial densities by means of the “most probable number”. Biometrics 6:105-116. [PubMed] [Google Scholar]
- 9.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cupples, A. M., A. M. Spormann, and P. L. McCarty. 2003. Growth of Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl. Environ. Microbiol. 69:953-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutter, L., K. R. Sowers, and H. D. May. 1998. Microbial dechlorination of 2,3,5,6-tetrachlorobiphenyl under anaerobic conditions in the absence of soil or sediment. Appl. Environ. Microbiol. 64:2966-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutter, L. A., J. E. M. Watts, K. R. Sowers, and H. D. May. 2001. Identification of a microorganism that links its growth to the reductive dechlorination of 2,3,5,6-chlorobiphenyl. Environ. Microbiol. 3:699-709. [DOI] [PubMed] [Google Scholar]
- 13.Duhamel, M., K. Mo, and E. A. Edwards. 2004. Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl. Environ. Microbiol. 70:5538-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards, U., T. Rogall, H. Blocker, M. Emde, and E. C. Bottger. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felsenstein, J. 1989. PHYLIP—Phylogeny Inference Package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 16.Fennell, D. E., I. Nijenhuis, S. F. Wilson, S. H. Zinder, and M. M. Häggblom. 2004. Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ. Sci. Technol. 38:2075-2081. [DOI] [PubMed] [Google Scholar]
- 17.Frame, G. M., J. W. Cochran, and S. S. Bøwadt. 1996. Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J. High Resolut. Chromatogr. 19:657-668. [Google Scholar]
- 18.Garton, L. S., J. S. Bonner, A. N. Ernest, and R. L. Autenrieth. 1996. Fate and transport of PCBs at the New Bedford Harbor superfund site. Environ. Toxicol. Chem. 15:736-745. [Google Scholar]
- 19.He, J., K. M. Ritalahti, K. L. Yang, S. S. Koenigsberg, and F. E. Löffler. 2003. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62-65. [DOI] [PubMed] [Google Scholar]
- 20.Hendrickson, E. R., J. A. Payne, R. M. Young, M. G. Starr, M. P. Perry, S. Fahnestock, D. E. Ellis, and R. C. Ebersole. 2002. Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Appl. Environ. Microbiol. 68:485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannuzzi, T. J., S. L. Huntley, N. L. Bonnevie, B. L. Finley, and R. J. Wenning. 1995. Distribution and possible sources of polychlorinated biphenyls in dated sediments from the Newark Bay estuary, New Jersey. Arch. Environ. Contam. Toxicol. 28:108-117. [DOI] [PubMed] [Google Scholar]
- 22.Janse, I., J. Bok, and G. Zwart. 2004. A simple remedy against artificial double bands in denaturing gradient gel electrophoresis. J. Microbiol. Methods 57:279-281. [DOI] [PubMed] [Google Scholar]
- 23.Lake, J. L., R. J. Pruell, and F. A. Osterman. 1992. An examination of dechlorination processes and pathways in New Bedford Harbor sediments. Mar. Environ. Res. 33:31-47. [Google Scholar]
- 24.Löffler, F. E., Q. Sun, J. Li, and J. M. Tiedje. 2002. 16S rRNA gene-based detection of tetrachloroethene-dechlorinating Desulfuromonas and Dehalcoccoides species. Appl. Environ. Microbiol. 66:1369-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüβmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maymo-Gatell, X., Y.-T. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 27.Morris, P. J., W. W. Mohn, J. F. Quensen III, J. M. Tiedje, and S. A. Boyd. 1992. Establishment of polychlorinated biphenyl-degrading enrichment culture with predominantly meta dechlorination. Appl. Environ. Microbiol. 58:3088-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of PCR amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulliam Holoman, T. R., M. A. Elberson, L. A. Cutter, H. D. May, and K. R. Sowers. 1998. Characterization of a defined 2,3,5,6-tetrachlorobiphenyl-ortho-dechlorinating microbial community by comparative sequence analysis of genes coding for 16S rRNA. Appl. Environ. Microbiol. 64:3359-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quensen, J. F., III, S. A. Boyd, and J. M. Tiedje. 1990. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl. Environ. Microbiol. 56:2360-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safe, S. 1992. Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environ. Health Perspect. 100:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 33.Stone, R. 1992. Swimming against the PCB tide. Science 255:798-799. [DOI] [PubMed] [Google Scholar]
- 34.Stull, J. K., D. J. P. Swift, and A. W. Niedoroda. 1996. Contaminant dispersal on the Palos Verdes continental margin. 1. Sediments and biota near a major California wastewater discharge. Sci. Total Environ. 179:73-90. [Google Scholar]
- 35.Stults, J. R., O. Snoeyenbos-West, B. Methe, D. R. Lovley, and D. P. Chandler. 2001. Application of the 5′ fluorogenic exonuclease assay (TaqMan) for quantitative ribosomal DNA and rRNA analysis in sediments. Appl. Environ. Microbiol. 67:2781-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts, J. E. M., S. K. Fagervold, G. S. Miller, C. E. Milliken, H. D. May, and K. R. Sowers. 2004. Microbial reductive dechlorination of organohalide pollutants in marine environment. Mar. Biotechnol. 6:S378-S383. [Google Scholar]
- 37.Watts, J. E. M., S. K. Fagervold, K. R. Sowers, and H. D. May. 2005. A PCR based specific assay reveals a population of bacteria within the Chloroflexi associated with the reductive dehalogenation of polychlorinated biphenyls. Microbiology 151:2039-2046. [DOI] [PubMed] [Google Scholar]
- 38.Watts, J. E. M., Q. Wu, S. B. Schreier, H. D. May, and K. R. Sowers. 2001. Comparative analyses of PCB dechlorinating communities in enrichment cultures using three different molecular screening techniques. Environ. Microbiol. 2:710-719. [DOI] [PubMed] [Google Scholar]
- 39.Wu, Q., K. R. Sowers, and H. D. May. 2000. Establishment of a polychlorinated biphenyl-dechlorinating microbial consortium, specific for doubly flanked chlorines in a defined, sediment-free medium. Appl. Environ. Microbiol. 66:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, Q., J. E. M. Watts, K. R. Sowers, and H. D. May. 2002. Identification of a bacterium that specifically catalyzes the reductive dechlorination of polychlorinated biphenyls with doubly flanked chlorines. Appl. Environ. Microbiol. 68:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, Q. Z., K. R. Sowers, and H. D. May. 1998. Microbial reductive dechlorination of Aroclor 1260 in anaerobic slurries of estuarine sediments. Appl. Environ. Microbiol. 64:1052-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]