Abstract
Ulcer-associated dyspepsia is caused by infection with Helicobacter pylori. H. pylori is linked to a majority of peptic ulcers. Antibiotic treatment does not always inhibit or kill H. pylori with potential for antibiotic resistance. The objective of this study was to determine the potential for using phenolic phytochemical extracts to inhibit H. pylori in a laboratory medium. Our approach involved the development of a specific phenolic profile with optimization of different ratios of extract mixtures from oregano and cranberry. Subsequently, antimicrobial activity and antimicrobial-linked urease inhibition ability were evaluated. The results indicated that the antimicrobial activity was greater in extract mixtures than in individual extracts of each species. The results also indicate that the synergistic contribution of oregano and cranberry phenolics may be more important for inhibition than any species-specific phenolic concentration. Further, based on plate assay, the likely mode of action may be through urease inhibition and disruption of energy production by inhibition of proline dehydrogenase at the plasma membrane.
Dyspepsia and related problems with peptic ulcers linked to Helicobacter pylori are major problems in many parts of the world (37). H. pylori is a gram-negative, spiral-shaped bacterium that lives in the stomach and duodenum. By releasing an enzyme called “urease,” H. pylori is able to survive in the stomach. Urease converts urea into ammonia, which then counters the stomach acid. This creates a neutralizing environment for protecting H. pylori from the acid in the stomach. Gastric infection with H. pylori may lead to the onset of various gastric-related diseases (13). Most patients specifically with duodenal ulcer can be cured by killing H. pylori with antibiotics and proton pump inhibitors (22, 29). In recent studies, H. pylori infection was also suspected to be associated with coronary artery and ischemic heart disease (4, 9, 21, 23). Many antibiotic-linked treatments have been recommended for eradication of H. pylori, but the emergence of antibiotic resistance makes the treatments more complicated, and the infection is sustained at higher levels when the drug treatment is stopped (12, 29). Two common antibiotics used for treatment of H. pylori infection are metronidazole and clarithromycin. Several triple- or quadruple-antibiotic therapies with proton pump inhibitors have been shown to be effective in eradication of H. pylori (14), but no single treatment regimen is considered the final treatment of choice.
Research has indicated that urease of H. pylori is located in the cytoplasm in freshly prepared cultures and in the outer membrane in older cultures (15). In addition to pathogenicity from H. pylori, evidence indicates that ammonia generated by urease can cause injury to the gastroduodenal mucosa (33, 42). Specific inhibition of urease activity has been proposed as a possible strategy to inhibit this microorganism (25). It has been demonstrated that a urease-negative mutant does not cause gastritis in nude mice due to difficulty in colonization (40). These results suggest the important role of urease in bacterial colonization.
Many naturally occurring compounds found in dietary and medicinal plants, herbs, and fruit extracts have been shown to possess antimicrobial activities (7, 18, 19, 41). Recent research has indicated that some key phenolic phytochemicals in plant extracts have antimicrobial properties that inhibit the bacteria that cause common types of food poisoning, such as the food-borne pathogens Listeria monocytogenes (20, 30) and Staphylococcus aureus (1). These results also indicated the potential of using plant extracts as antimicrobial ingredients in food to inhibit H. pylori (10, 16, 24, 34, 38). Previous research also indicated that host antioxidant stimulation is related to enhanced H. pylori inhibition (2). Therefore, we have proposed to develop a specific phenolic antioxidant profile to inhibit H. pylori. Our strategy couples the benefits of antioxidant activity with specific phenolic profiles to inhibit H. pylori. Further, we have previously investigated whether botanical phytochemical mixtures contribute to antioxidant functionality and antimicrobial effects through synergy (41). In the present study, we also made initial investigations into the likely mode of action by using simple plate assays to evaluate inhibition of urease and proline dehydrogenase at the plasma membrane level.
MATERIALS AND METHODS
Preparation of plant extracts.
Water-soluble oregano (Origanum vulgare) and cranberry (Vaccinium macrocarpon) extract powders were obtained from Barrington Chemicals, NY, and Decas Cranberry Products, Wareham, MA, respectively. Ten grams of different ratios of oregano/cranberry powder mixture (Table 1) were added to 90 ml water to make concentrated stock extracts and stored at 4°C. Different ratios of concentrated extracts were then diluted on a total phenolic basis and filter sterilized before use. The total phenolic contents in oregano and cranberry powders were a minimum of 16 mg/g (wt/wt) (dry weight basis) and 5 mg/g (wt/wt), respectively. The major phenolic found in oregano extracts was rosmarinic acid at a minimum of 3% of total phenolics. Cranberry extracts had a range of phenolic compounds, such as ellagic acid, p-hydroxybenzoic acid, cinnamic acid, hydroxycinnamic acid, caffeic acid, chlorogenic acid, ferulic acid, sinapic acid, and p-coumaric acids (43), with each being less than 0.5% of total phenolics.
TABLE 1.
Total phenolic contents of oregano and cranberry in various mixtures
| Mixture | Amt of phenolics (mg/g, dry wt) |
|---|---|
| 100% oregano | 18.2 |
| 75% oregano, 25% cranberry | 15.1 |
| 50% oregano, 50% cranberry | 12.2 |
| 25% oregano, 75% cranberry | 10.0 |
| 100% cranberry | 7.4 |
Total phenolic assay.
The total phenolic content was determined spectrophotometrically by the Folin-Ciocalteu assay. This method was originally developed by Chandler and Dodds (5) and later modified for oregano phenolics (31). Approximately 50 mg of different ratios of phytochemical powder mixtures were added into 150- by 15-mm glass tubes. Distilled water (2.5 ml) was added to the tubes. The samples were homogenized using a Tissue-Tearor (Biospec Products, Bartleville, OK) and centrifuged at 13,000 × g for 10 min (25°C). One milliliter of the supernatant fluid was transferred to a test tube, to which 6 ml of distilled water and 0.5 ml of Folin-Ciocalteu phenol reagent (Sigma Chemical Co., St. Louis, MO) were added. After incubation for 5 min (25°C), 1 ml of 5% Na2CO3 was added. The tubes were mixed well and kept in the dark (25°C) for an hour. The samples were vortexed, and absorbance at 725 nm was measured (Spectronic Genesys 5; MiltonRoy Company, Rochester, NY). The phenolic content was then calculated from a gallic acid standard curve.
Agar diffusion assay.
H. pylori was cultured using the method of Stevenson et al. (35). The isolate of H. pylori (strain ATCC 43579, which originated from human gastric samples) was obtained from the American Type Culture Collection (Manassas, VA). Standard plating medium were prepared by using 10 g of special peptone (Oxoid Ltd., Basingstoke, England), 15 g of granulated agar (Difco Laboratories, Detroit, MI), 5 g of sodium chloride (EM Science, Gibbstown, NJ), 5 g of yeast extract (Difco), and 5 g of beef extract (Becton Dickinson and Co., St. Louis, MO) per liter of water. Activity against H. pylori was tested by the standard agar diffusion method. Broth media were prepared in the same way without agar. One hundred microliters of stock H. pylori was added to test tubes containing 10 ml of broth medium. The tubes were incubated at 37°C for 48 h before being used for spread plate assays.
Agar diffusion assay was done aseptically using sterile 1/4-in. (0.635-cm)-diameter paper disks (Schleicher & Schuell, Inc., Keene, NH). Individual phytochemical extracts and mixtures of extracts were added to paper disks (100 μl, containing a total phenolic content of 0.1 mg) by using a micropipette. Phytochemical-saturated disks were placed on surfaces of seeded agar plates. Plates were incubated at 37°C for 48 h in GasPak jars (BBL Microbiology Systems, Cockeysville, MD) with BBL CampyPacks (BBL Microbiology Systems, Cockeysville, MD). The diameter of the inhibition zone surrounding each disk was measured, and the zone of inhibition was determined. Various combinations of oregano and cranberry (Table 1) were evaluated for antimicrobial efficacy. Controls consisted of disks with distilled water only.
A2C/proline assay.
The antimicrobial effect of phenolic phytochemicals was compared to that of azetidine-2-carboxylate (A2C) based on the rationale that small phenolics in phytochemical profiles could behave like proline analogs and likely inhibit proline dehydrogenase (32). Further the effects of phenolic phytochemicals or A2C could be overcome by proline if the site of action is proline dehydrogenase.
H. pylori was cultured using the method of Stevenson et al. (35). Plating medium were prepared by using standard plating medium as described for the agar diffusion assay, with some modifications. Proline (Sigma, St. Louis, MO) was added to the medium to give a final concentration of 1.0 mM. The antimicrobial assay was done in the same way as the agar diffusion assay. Individual phytochemical extracts and mixtures were added to paper disks (100 μl, containing 0.1 mg of total phenolics) using a micropipette. A2C was prepared at a concentration of 0.1 mM and added at 100 μl to paper disk. Saturated disks were placed on the surfaces of seeded agar plates. Plates were incubated at 37°C for 48 h in GasPak jars (BBL Microbiology Systems, Cockeysville, MD) with BBL CampyPacks (BBL Microbiology Systems, Cockeysville, MD). The diameter of the inhibition zone surrounding each disk was measured, and the zone of inhibition was determined. Each experiment consisted of three replicates with various phytochemical concentrations. Each experiment was repeated three times. Controls consisted of disks with distilled water only.
Assay of urease activity in disks.
We developed the urease plate assay based on the rationale that if urease is located in the cytoplasmic membrane or excreted by the bacteria under low-pH conditions, it will convert urea to ammonia and counter the low pH. When this happens, bromocresol purple will be converted to purple due to the pH increase. If urease activity was inhibited, a yellow zone would be observed due to low pH. Plating medium was modified from the standard plating medium described for the agar diffusion assay. Urea (Schwarz/Mann Biotech, Cleveland, OH) was added to the medium to a final concentration of 10 mM. Bromocresol purple was added to the medium at 0.01 g per liter. The final pH of the medium was adjusted to 6.0. Individual phytochemical extracts and mixtures (pH 7.0) were adding to paper disks (50 μl, containing 0.05 mg of total phenolics) by using a micropipette. Saturated disks were placed on the surfaces of seeded agar plates. Plates were incubated at 37°C for 48 h in GasPak jars (BBL Microbiology Systems, Cockeysville, MD) with BBL CampyPacks (BBL Microbiology Systems, Cockeysville, MD). The diameter of the yellow zone surrounding each disk was measured. Each experiment consisted of three replicates with various phytochemical concentrations. Each experiment was repeated three times. Controls consisted of disks with distilled water only.
Assay of urease activity in broth (26).
Urease activity of H. pylori was also determined by measuring the release of ammonia by a modification of the Berthelot reaction (8). Special peptone broth was made from the plating medium described for the agar diffusion assay but without agar. The pH of the broth was adjusted to 6.0 prior to use. Cells were grown in the special peptone broth for 48 h at 37°C (A560 of 1.0) and then incubated with oregano, cranberry, and an extract mixture (25%/75%) at a concentration of 0.05 mg phenolic/ml for 10 min at 28°C. The incubated cell cultures were centrifuged at 4°C (4,000 × g, 5 min) and resuspended in 0.5 volume of ice-cold 0.1 M sodium phosphate buffer (pH 7.3) containing 10 mM EDTA. Cells were disrupted by sonication, and the supernatant obtained after centrifugation at 4°C (12,000 × g, 5 min) was used for the urease assay. The reaction mixture contained 50 mM urea, 100 mM sodium phosphate buffer (pH 7.3), and an aliquot of the supernatant in a total volume of 1.0 ml. After incubation for 10 min at 37°C, the reaction was terminated by addition of 2 ml of 0.5% phenol and 0.0025% sodium nitroprusside solution, after which 2 ml of 0.25% sodium hydroxide and 0.21% sodium hypochlorite solution were added, the mixture was incubated for 6 min at 55°C for color development, and the absorbance at 625 nm was determined. Blanks were cells treated similarly but without phytochemical mixtures. The amount of ammonia produced was equivalent to the hydrolysis of urea. A high absorption value indicated high urease activity in the supernatant.
Total protein assay.
Cell cultures with phytochemical extracts prepared in the previous urease assay were then used for total protein assay. The samples were centrifuged at 4°C (4,000 × g, 5 min) and resuspended in 0.5 volume of ice-cold 0.1 M sodium phosphate buffer (pH 7.3) containing 10 mM EDTA. Cells were disrupted by sonication, and the supernatant was obtained after centrifugation at 4°C (12,000 × g, 5 min). The supernatant was used for the estimation of total protein.
Protein content was measured by the method of Bradford (3). The dye reagent concentrate (Bio-Rad protein assay kit II; Bio-Rad Laboratory, Hercules, CA) was diluted 1:4 with distilled water. Five milliliters of diluted dye reagent was added to 100 μl of the supernatant. After vortexing and incubating for 5 min, the absorbance was measured at 595 nm against a 5-ml reagent blank and 100 μl buffer by using a UV-visible Genesys spectrophotometer (Milton Roy, Inc., Rochester, NY). The urease activity was expressed as the amount of ammonia produced per unit protein compared to the control without phytochemical extract treatment.
RESULTS AND DISCUSSION
Agar diffusion assay.
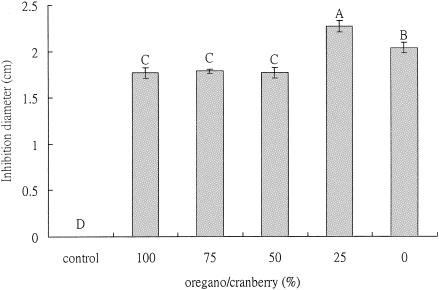
A level of 0.1 mg phenolic/disk was the concentration determined from preliminary investigations to be the effective dose. The results indicated that the best antimicrobial activity against H. pylori was observed when the mixture contained 25% (wt/wt) oregano with 75% (wt/wt) cranberry (Fig. 1). At pH 7.0, disks containing this extract mixture (0.1 mg total phenolic per disk) had larger inhibition zones than disks with extracts mixed in other ratios (Fig. 1).
FIG. 1.
Antimicrobial activities of oregano and cranberry extract mixtures against H. pylori at pH 7.0 and 37°C after 48 h of incubation (100 μl extract mixture per disk with 0.1 mg equivalent phenolic content). Each experiment consisted of three replicates with various phytochemical concentrations. Each experiment was repeated three times. The error bars represent ±1 standard deviation from the mean. Bars with the same letters are not significantly different. Statistical analysis was done by analysis of variance; P < 0.05. Controls consisted of disks with distilled water only.
A2C/proline assay.
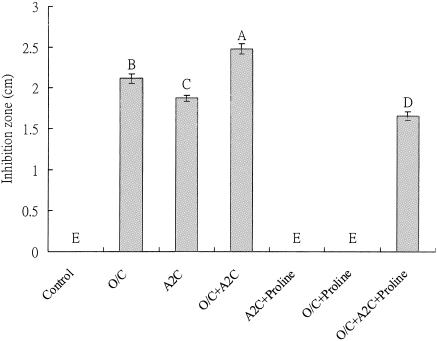
Previous studies in our laboratory have provided indications that in animal cell and yeast model systems, phenolics could modulate the cellular redox response through the proline-linked pentose phosphate pathway (32). Therefore, the rationale for the A2C/proline assay was to evaluate if small phenolics in the phytochemical mixtures behave as proline analogs and, if so, could inhibit proline dehydrogenase at the plasma membrane level in a prokaryotic cell (which is associated with energy production) and inhibit the bacterium. If this is the case, then only addition of proline could overcome the inhibition of A2C or A2C-linked phenolics with aromatic ring structures. In this study, A2C, phenolics, and combinations of A2C and phenolics enhanced the antimicrobial activity, and addition of proline helped to overcome the inhibition by A2C and/or phenolics, therefore indicating that site of action of phenolics could be proline dehydrogenase.
Specifically, the antimicrobial effect of phytochemical extracts was further enhanced by adding 100 μl of 0.1 mM A2C to paper disks, and a larger inhibition zone was observed (Fig. 2). Disks containing phytochemical extracts (at 0.1 mg phenolics per disk) showed no inhibition when the plates contained 1.0 mM proline, indicating that the antimicrobial effect from phytochemicals was overcome by proline. When both A2C and the phytochemical extract mixture were applied on the plates containing 1.0 mM proline, a smaller inhibition zone was observed than without proline, indicating that the antimicrobial effect was reduced in the presence of proline. Since phytochemicals enhance the effect of A2C and respond to proline similarly to A2C, these results provided clues that the likely site of action of phenolic phytochemicals was proline dehydrogenase. Future studies will further evaluate this enzyme response in detail.
FIG. 2.
Synergistic effects of oregano and cranberry extracts on inhibition of H. pylori in the presence of A2C/proline. Proline (Sigma, St. Louis, MO) was added into the medium to a final concentration of 1.0 mM. A2C was prepared at a concentration of 0.1 mM and added at 100 μl to paper disks. The total phenolic concentration was 0.1 mg/disk, and the inhibition diameter was monitored after incubation at 37°C for 48 h. Controls were at the same conditions with the same volume of water. O/C, oregano and cranberry mixture at 25%/75%, wt/wt. The pH was 7.0. Each experiment consisted of three replicates of various phytochemical concentrations. Each experiment was repeated three times. The error bars represent ±1 standard deviation from the mean. Bars with the same letters are not significantly different. Statistical analysis was done by analysis of variance; P < 0.05.
Urease activity assay.
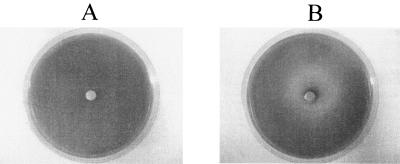
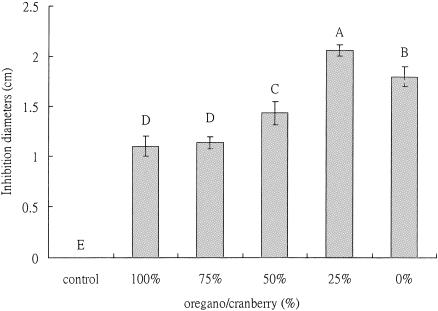
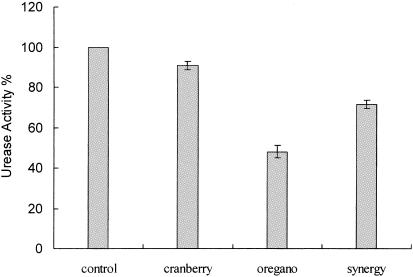
Figures 3 and 4 show that the urease activity was inhibited in the plates containing phytochemical mixtures. The transition range of bromocresol purple is pH 5.2 to 6.8. The pH of the plates was 6.0 and the color was yellowish prior to inoculation with H. pylori. After inoculation and 48 h of incubation at 37°C, the area with bacterial growth turned into purple, indicating that the bacteria were able to counter the pH of the medium, likely by producing ammonia through urease activity. When the paper disks containing extract mixtures were placed on the medium, the area around the paper disks did not change to purple, indicating that the bacteria did not counter the pH of medium and urease was likely inhibited. The diameter of the yellow zone was measured and is shown in Fig. 4. The mixture of oregano (25%) and cranberry (75%) gave a larger yellow zone, indicating a higher inhibition of urease activity.
FIG. 3.
Examples of urease inhibition in agar diffusion assay. (A) Control. One hundred microliters of sterile water was added to the paper disk. (B) One hundred microliters of phytochemical extracts was added into the paper disk. All extract mixtures were tested at the level of 0.05 mg phenolics per disk for urease inhibition. The plate medium was adjusted to pH 6.0. The yellow zone indicates the area where there is no urease activity. The purple zone indicates the area where urease is active.
FIG. 4.
Synergistic effect of oregano and cranberry extracts on inhibition of urease. The plate medium was adjusted to pH 6.0. The total phenolic concentration was 0.05 mg/disk, and inhibition diameter was monitored after incubation at 37°C for 48 h. The control was a bacterial culture under the same conditions in which same volume of water instead of phytochemical was placed. Each experiment consisted of three replicates of various phytochemical concentrations. Each experiment was repeated three times. The error bars represent ±1 standard deviation from the mean. Bars with same letters are not significantly different. Statistical analysis was done by analysis of variance; P < 0.05.
The urease activity assay was also performed in liquid culture to determine whether the phytochemical extracts inhibited the urease activity. H. pylori was treated with potential phytochemical urease inhibitors prior to determining the urease activity. Cells treated with 0.05 mg oregano phenolics/ml showed a marked decrease in the urease activity (52% inhibition compared to the control). The mixture of phenolics in extract also resulted in 29% inhibition. Cranberry phenolics individually resulted in 9% urease inhibition compared to the control (Fig. 5.) These results suggested that the phytochemicals inhibited the urease activity. However, unlike in plate assays, oregano alone had the most potent inhibitory activity, which may be a reflection of differences in the types of phenolics in cranberry and oregano and their behavior in a liquid system compared to a solid system in a plate assay. It is likely that oregano being enriched in partially hydrophobic phenolics could effectively affect enzymes such as urease that are located on the plasma membrane. As opposed to oregano, cranberry has good acidifying and soluble phenolics that enter the cytosol and are likely less effective on urease but more effective at the proline dehydrogenase level.
FIG. 5.
Synergistic effect of oregano and cranberry extracts on inhibition of urease in broth. The total phenolic concentration was 0.05 mg/ml. The control was a bacterial culture under the same conditions in which same volume of water instead of phytochemical was placed. The data are means and standard deviations from triplicate experiments.
Previous studies have indicated the antimicrobial potential of phytochemical extracts (6, 7, 11). Oregano and cranberry are useful botanicals which are generally recognized as safe for food flavoring and as potential functional ingredients, which are known for their antimicrobial activity linked to the phenolic moiety. Phenolic phytochemicals such as ellagic acid and rosmarinic acid have the potential to interact with proteins and alter their conformation. These phytochemicals can directly interact with the receptors on the cell membrane and could affect normal functioning of ion pumps (17, 27, 28, 32, 39, 41). Also, the partially hydrophobic nature of phenolic constituents allows for accumulation and attachment in the bacterial cytoplasmic membrane, where inhibitory effects may eventually lead to cell death. Recent evidence has also indicated that altering multidrug resistance pumps on the cytoplasmic membrane of bacteria by inhibitors or genetic knockout can enhance the antimicrobial function of phytochemicals (36). Therefore, in oregano and cranberry phenolic profiles, specific phenolics may inhibit multidrug resistance pumps, allowing other phenolics in a synergistic profile to inhibit the bacterium.
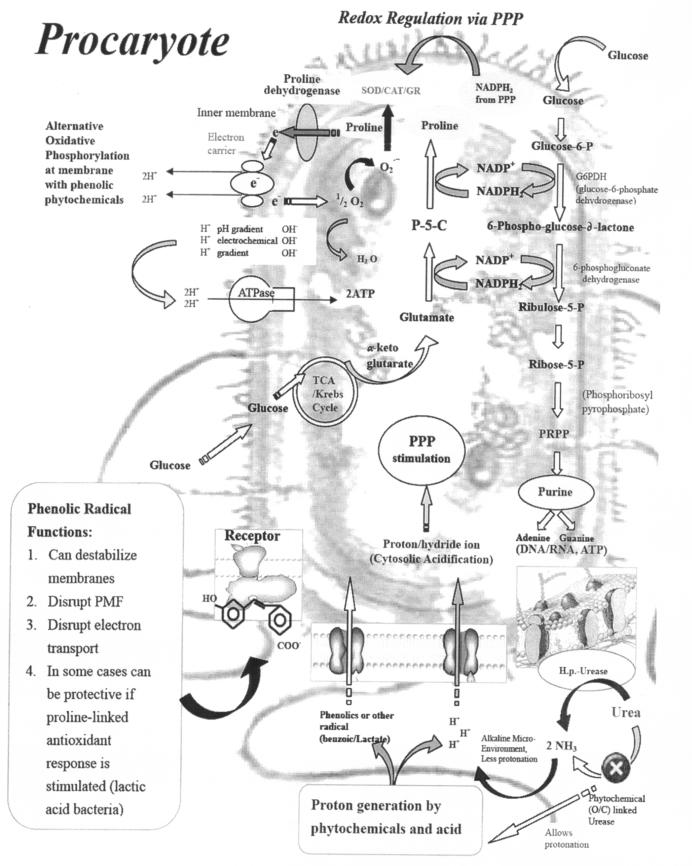
Plate assay results indicated that the oregano and cranberry extract mixture was superior in inhibiting H. pylori than individual extracts at the same phenolic concentration. When different extract ratios based on phenolic content were used, a larger inhibition zone was observed, indicating higher susceptibility to a specific ratio (25% oregano and 75% cranberry) of extract mixture. This may be due to one (or more than one) specific phenolic present in the extract that damages the membrane first, making cells more sensitive to the other phenolics (36). As a consequence, impairment of proton pumps and loss of H+-ATPase in damaged membranes can cause disruption in the normal cellular function of the microorganism and therefore lead to cell death (Fig. 6). Further, the acidic nature of phenolic-containing extracts themselves at higher concentrations may create a low-pH microenvironment due to proton donation and cell membrane disruption due to stacking (32), which is likely more effective than low pH alone. Clues from this study indicated that the mechanism of action for regulating membrane-linked energy production could be through proline dehydrogenase, based on the studies with A2C and phenolics as well as combinations. These studies indicated that phenolics in phytochemical extracts behaved similarly to the proline analog A2C and that the inhibitory effect could be overcome by proline. This provided clues that proline dehydrogenase at the plasma membrane could likely be the site of action for phenolic phytochemicals.
FIG. 6.
Proposed mechanism of antimicrobial effects of phenolic phytochemicals in prokaryotic cells. PPP, pentose phosphate pathway; SOD, superoxide dismutase; CAT, catalase; GR, glutathione reductase; P-5-C, pyrroline-5-carboxylate; TCA, tricarboxylic acid; PRPP, phosphoribosylpyrophosphate; PMF, proton motive force; H.p., H. pylori; O/C, oregano or cranberry.
Our results indicated that the activity of urease was inhibited in the presence of phytochemical extracts. The mechanism of urease inhibition is not clear. It could be due to the conformational change of urease by phenolics. In plate assay, combinations of cranberry and oregano were effective, indicating that higher-molecular-weight phenolics from oregano may operate at the plasma membrane level and subsequently, upon urease inhibition, small phenolics from cranberry may affect cytosolic functions. On the other hand, in the liquid system oregano phenolics were most effective, as it is possible that partial hydrophobicity of phenolics of oregano may allow attachment and inhibition at the plasma membrane level.
In this study, the effects of a combined treatment with an oregano and cranberry extract mixture on the inhibition of H. pylori were demonstrated in plate assays and specific urease inhibition was demonstrated in plate and broth assays. These combinations of beneficial plant extracts provide a natural and dietary solution, as well as an additional strategy to inhibit the growth of H. pylori. Synergistic effects of combinations of plant extracts provide a wide range of phenolic diversity, significantly increasing antimicrobial efficacy. If the antimicrobial mechanisms at the cellular level are further confirmed based on clues from this study, then this is an excellent strategy to design the right plant extract with a specific phenolic profile to prevent H. pylori infection. Also, the diversity of phenolic types from different botanical sources greatly increases the functionality for health and wellness (e.g., antioxidants for oxidation-linked diseases or for chronic infectious diseases). Such phenolic profiles also have the added benefit of enhancing host tissue and cellular responses through enhanced antioxidant enzyme activity (32).
The exact mechanisms of cellular damage by phenolics at the urease or proline dehydrogenase level will be further investigated in our laboratory. If these mechanisms are further elucidated, designing specific phenolics profiles to inhibit H. pylori will become feasible and also provide additional health benefits.
REFERENCES
- 1.Akiyama, H., K. Fujii, O. Yamasaki, T. Oono, and K. Iwatsuki. 2001. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 48:487-491. [DOI] [PubMed] [Google Scholar]
- 2.Akyon, Y., and G. Hascelik. 1999. The effect of Helicobacter pylori on neutrophil chemotaxis is independent of caga. FEMS Immunol. Med. Microbiol. 24:209-213. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Cammarota, G., V. Pasceri, A. Papa, R. Cianci, A. Gasbarrini, P. Fedeli, F. Cremonini, G. Fedeli, A. Maseri, and G. Gasbarrini. Helicobacter pylori infection and ischaemic heart disease. J. Gastroenterol. Hepatol. 30:304-306. [PubMed]
- 5.Chandler, S. F., and J. H. Dodds. 1983. The effect of phosphate nitrogen and sucrose on the production of phenolics and socosidine in callus cultures of Solanum tuberosum. Plant Cell Rep. 2:105-108. [DOI] [PubMed] [Google Scholar]
- 6.Chun, S. S., D. A. Vattem, Y.-T. Lin, and K. Shetty. 2005. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 40:809-816. [Google Scholar]
- 7.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creno, R. J., R. E. Wenk, and P. Bohlig. 1970. Automated micromeasurement of urea using urease and the Berthelot reaction. Am. J. Clin. Pathol. 54:828-832. [DOI] [PubMed] [Google Scholar]
- 9.Danesh, J., and R. Peto. 1998. Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. Br. Med. J. 316:1130-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daroch, F., M. Hoeneisen, and C. L. Gonzalez. 2001. In vitro antibacterial activity of Chilean red wines against Helicobacter pylori. Microbios 104:79-85. [PubMed] [Google Scholar]
- 11.Dean, S. G., and G. Richie. 1987. Antimicrobial properties of plant essential oils. Int. J. Food Microbiol. 5:165-180. [Google Scholar]
- 12.De Boer, W. A., and G. N. Tytgat. 2000. Treatment of Helicobacter pylori infection. Br. Med. J. 320:31-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gene, E., X. Calvet, R. Azagra, and J. P. Gisbert. 2003. Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta-analysis. Aliment. Pharmacol. Ther. 17:1137-1143. [DOI] [PubMed] [Google Scholar]
- 15.Hu, L. T., and H. L. T. Mobley. 1990. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect. Immun. 58:992-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, N. L., S. Shabib, and S. M. Sherman. 1997. Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS Microbiol. Lett. 146:223-227. [DOI] [PubMed] [Google Scholar]
- 17.Kim, H. J., K. S. Yum, J. H. Sung, D. J. Rhie, M. J. Kim, D. S. Min, S. J. Hahn, M. S. Kim, Y. H. Jo, and S. H. Yoon. 2004. Epigallocatechin-3-gallate increases intracellular [Ca2+] in U87 cells mainly by influx of extracellular Ca2+ and partly by release of intracellular stores. Naunyn Schmiedebergs Arch. Pharmacol. 369:260-267. [DOI] [PubMed] [Google Scholar]
- 18.Kouassi, Y., and L. A. Shelef. 1998. Inhibition of Listeria monocytogenes by cinnamic acid—possible interaction of the acid with cysteinyl residues. J. Food Saf. 18:231-242. [Google Scholar]
- 19.Larson, A. E., R. R. Y. Yu, O. A. Lee, S. Price, G. J. Haas, and E. A. Johnson. 1996. Antimicrobial activity of hop extracts against Listeria monocytogenes in media and in food. Int. J. Food Microbiol. 33:195-207. [DOI] [PubMed] [Google Scholar]
- 20.Lin, Y.-T., R. G. Labbe, and K. Shetty. 2004. Inhibition of Listeria monocytogenes in fish and meat systems using oregano and cranberry synergies. Appl. Environ. Microbiol. 70:5672-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendall, M. A., P. M. Goggin, N. Molineaux, J. Levy, T. Toosy, D. Stachan, A. J. Camm, and T. C. Northfield. 1994. Relation of Helicobacter pylori infection and coronary heart disease. Br. Heart J. 71:437-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moayyedi, P., S. Soo, J. Deeks, D. Forman, J. Mason, and M. Innes. 2000. Systematic review and economic evaluation of Helicobacter pylori eradication treatment for non-ulcer dyspepsia. Br. Med. J. 321:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray, L. J. 1997. Helicobacter pylori infection and ischaemic heart disease. Cardiologia 42:1027-1035. [PubMed] [Google Scholar]
- 24.Murray, L. J., A. J. Lane, I. M. Harvey, J. L. Donovan, P. Nair, and R. F. Harvey. 2002. Inverse relationship between alcohol consumption and active Helicobacter pylori infection: the Bristol Helicobacter project. Am. J. Gastroenterol. 97:2750-2755. [DOI] [PubMed] [Google Scholar]
- 25.Nagata, K., T. Mizuta, Y. Tonokatu, Y. Fukuda, H. Okamura, T. Hayashi, T. Shimoyama, and T. Tamura. 1992. Monoclonal antibodies against the native urease of Helicobacter pylori: synergistic inhibition of urease activity by monoclonal antibody combinations. Infect. Immun. 60:4826-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura, H., H. Yoshiyama, H. Takeuchi, T. Mizote, K. Okita, and T. Nakazawa. 1998. Urease plays an important role in the chemotactic motility of Helicobacter pylori in a viscous environment. Infect. Immun. 66:4832-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan, C. Y., Y. H. Kao, and A. P. Fox. 2002. Enhancement of inward Ca (2+) currents in bovine chromaffin cells by green tea polyphenol extracts. Neurochem. Int. 40:131-137. [DOI] [PubMed] [Google Scholar]
- 28.Papadopoulou, A., and R. A. Frazier. 2003. Characterization of protein—polyphenol interactions. Trends Food Sci. Technol. 5:186-190. [Google Scholar]
- 29.Parente, F., G. Maconi, O. Sangaletti, M. Minguzzi, L. Vago, E. Rossi, and P. G. Bianchi. 1996. Prevalence of Helicobacter pylori infection and related gastroduodenal lesions in spouses of Helicobacter pylori positive patients with duodenal ulcer. Gut 39:629-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seaberg, A., R. L. Labbe, and K. Shetty. 2003. Inhibition of Listeria monocytogenes by elite clonal extracts of oregano (Origanum vulgare). Food Biotechnol. 17:129-149. [Google Scholar]
- 31.Shetty, K., O. F. Curtis, R. E. Levin, R. Witkowsky, and W. Ang. 1995. Prevention of vitrification associated with in vitro shoot culture of oregano (Origanum vulgare) by Pseudomonas spp. J. Plant Physiol. 147:447-451. [Google Scholar]
- 32.Shetty, K., and M. L. Wahlqvist. 2004. A model for the role of proline-linked pentose phosphate pathway in phenolic phytochemical biosynthesis and mechanism of action for human health and environmental applications. Asia Pacific J. Clin. Nutr. 13:1-24. [PubMed] [Google Scholar]
- 33.Smoot, D. T., H. L. T. Mobley, G. R. Chippendale, J. F. Lewison, and J. H. Resau. 1990. Helicobacter pylroi urease activity is toxic to human gastric epithelial cells. Infect. Immun. 58:1992-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somal, A. N., K. E. Coley, P. C. Molan, and B. M. Hancock. 1994. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J. R. Soc. Med. 87:9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson, T. H., L. M. Lucia, and G. R. Acuff. 2000. Development of a selective medium for isolation of Helicobacter pylori from cattle and beef samples. Appl. Environ. Microbiol. 66:723-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tegos, G., F. R. Stermitz, O. Lomovskaya, and K. Lewis. 2002. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 46:3133-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tougas, G., Y. Chen, P. Hwang, M. M. Liu, and A. Eggleston. 1999. Prevalence and impact of upper gastrointestinal symptoms in the Canadian population: findings from the digest study. Am. J. Gastroenterol. 94:2845-2854. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya, H. 2001. Biphasic membrane effects of capsaicin, an active component in Capsicum species. J. Ethnopharmacol. 75:295-299. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchiya, H., M. Sato, Y. Kameyama, N. Takagi, and I. Namikawa. 1987. Effect of lidocaine on phospholipid and fatty acid composition of bacterial membranes Lett. Appl. Microbiol. 4:141-144. [Google Scholar]
- 40.Tsuda, M., M. Karita, M. G. Morshed, K. Okita, and T. Nakazawa. 1994. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect. Immun. 62:3586-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vattem, D. A., Y.-T. Lin, R. Ghaedian, and K. Shetty. 2005. Cranberry synergies for dietary management of Helicobacter pylori infections. Process Biochem. 40:1583-1592. [Google Scholar]
- 42.Xu, J. K., C. S. Goodwin, M. Cooper, and J. Robinson. 1990. Intracellular vacuolization caused by the urease of Helicobacter pylori. J. Infect. Dis. 161:1302-1304. [DOI] [PubMed] [Google Scholar]
- 43.Zheng, Z., and K. Shetty. 2000. Solid-state bioconversion of phenolics from cranberry pomace and role of Lentinus edodes β-glucosedase. J. Agric. Food Chem. 48:895-900. [DOI] [PubMed] [Google Scholar]