Abstract
The proteolytic system of Bifidobacterium animalis subsp. lactis was analyzed, and an intracellular endopeptidase (PepO) was identified and characterized. This work reports the first complete cloning, purification, and characterization of a proteolytic enzyme in Bifidobacterium spp. Aminopeptidase activities (general aminopeptidases, proline iminopeptidase, X-prolyl dipeptidylaminopeptidase) found in cell extracts of B. animalis subsp. lactis were higher for cells that had been grown in a milk-based medium than for those grown in MRS. A high specific proline iminopeptidase activity was observed in B. animalis subsp. lactis. Whole cells and cell wall-bound protein fractions showed no caseinolytic activity; however, the combined action of intracellular proteolytic enzymes could hydrolyze casein fractions rapidly. The endopeptidase activity of B. animalis subsp. lactis was examined in more detail, and the gene encoding an endopeptidase O in B. animalis subsp. lactis was cloned and overexpressed in Escherichia coli. The deduced amino acid sequence for B. animalis subsp. lactis PepO indicated that it is a member of the M13 peptidase family of zinc metallopeptidases and displays 67.4% sequence homology with the predicted PepO protein from Bifidobacterium longum. The recombinant enzyme was shown to be a 74-kDa monomer. Activity of B. animalis subsp. lactis PepO was found with oligopeptide substrates of at least 5 amino acid residues, such as met-enkephalin, and with larger substrates, such as the 23-amino-acid peptide αs1-casein(f1-23). The predominant peptide bond cleaved by B. animalis subsp. lactis PepO was on the N-terminal side of phenylalanine residues. The enzyme also showed a post-proline secondary cleavage site.
Bifidobacteria are gram-positive anaerobic bacteria commonly found in the human intestinal tracts of mammals. Many bifidobacterium-containing dairy products have been developed due to their reported health-promoting effects. These organisms are employed to increase the beneficial properties of fermented milks, infant formulas, cheese, and ice cream (10, 28, 31, 43). One of the strains commonly used in the industry is Bifidobacterium animalis subsp. lactis, which is particularly suitable due to its technological properties such as tolerance to oxygen, acid resistance, and ability to grow in milk-based media (20, 30, 33).
Information in the literature regarding the metabolism of bifidobacteria focuses mainly on their glycolytic capabilities, since these organisms have been reported to grow well on oligosaccharide-based substrates (21, 49). Analysis of the genome sequence of Bifidobacterium longum NCC2705 revealed a large number of predicted proteins specialized for oligosaccharide metabolism (45). Genetic and biochemical characterization of Bifidobacterium glycosyl hydrolases has identified several enzymes that utilize nondigestible oligosaccharides as substrates (19, 21, 22, 26). On the other hand, very little is known about the proteolytic enzyme systems of Bifidobacterium spp. However, analysis of the B. longum NCC2705 genome predicted more than 20 peptidases, including general aminopeptidases, peptidases specific for proline-residues, dipeptidases, and endopeptidases, as well as ABC-type transporter systems specific for oligopeptides. No genes encoding proteins similar to PrtP, a cell envelope-associated proteinase, were identified in the NCC2705 genome.
Endopeptidases are of particular interest due to their ability to hydrolyze peptide bonds within an oligopeptide. Several endopeptidases have been identified and characterized in Lactobacillus spp., Streptococcus thermophilus, and Lactococcus lactis (5-8, 13, 37); all of these are metalloproteases. Although their physiological role is unclear, the implication of these enzymes in the degradation of peptides produced during cheese manufacturing and ripening, such as αs1-casein(f1-23), has been described (1, 7, 8). In previous studies, we have reported that the poor growth of B. animalis subsp. lactis in milk could be enhanced by supplementation of the medium with whey peptide fractions (glycomacropeptide [GMP] and whey protein concentrate) (20, 28). Therefore, the present study has focused on the proteolytic ability of B. animalis subsp. lactis to utilize milk proteins and milk-derived peptides. Special attention was given to the endopeptidase PepO, which was cloned, overexpressed, and characterized, particularly for its specificity against αs1-casein(f1-23).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Escherichia coli strains XL1-Blue (4) and BL21(DE3)pLysS (Novagen, Madison, Wis.) were used as cloning and expression hosts, respectively. Cells were routinely grown at 37°C in Luria-Bertani medium (44) supplemented with 12.5 μg/ml tetracycline, 30 μg/ml kanamycin, 34 μg/ml chloramphenicol, and/or 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) when appropriate. Bifidobacterium animalis subsp. lactis DSM 10140T was cultured in MRS broth (Pronadisa, Madrid, Spain) and a milk-based medium, which contained 100 g/liter reconstituted skim milk powder (Scharlau, Barcelona, Spain) and 10 g/liter GMP (Lacprodan; Arla Food Ingredients, Denmark). Cells grown in this milk-based medium were propagated first in MRS broth and then in skim milk enriched with 5 g/liter yeast extract and 5 g/liter glucose. All media were supplemented with 0.5 g/liter l-cysteine hydrochloride to lower the redox potential. B. animalis subsp. lactis cultures were incubated at 37°C under anaerobic conditions (Gas-Pack, Anaerogen; Oxoid, Basingstoke, United Kingdom) for 24 h.
Cell fractionation.
Cells were collected from MRS broth (1 × 109 CFU/ml) and milk-based medium (4 × 108 CFU/ml) by centrifugation (10,000 × g, 10 min, 4°C). Before centrifugation, the milk culture was neutralized at pH 6.5 with 1 M NaOH and cleared by adding 1% trisodium citrate to cause casein micelle dispersion, as previously described (14). Whole cells were washed with 2 mM Ca2+-containing 50 mM Tris buffer, pH 8, and taken to an optical density at 600 nm (OD600) of 20. The cell wall-bound fraction (CWF) was obtained by incubating the cell pellet obtained from milk cultures at 25°C for 1 h in 20 mM Bis-Tris buffer, pH 6.0, containing 10 mM EDTA as described by Fernández de Palencia et al. (16). The protein material of the fraction was precipitated with 80% ammonium sulfate and resuspended in 20 mM sodium phosphate buffer, pH 7, after centrifugation (20,000 × g, 5 min, 4°C). Cell extracts (CFE) were obtained from MRS and milk B. animalis subsp. lactis culture pellets using mechanical disruption by mixing cells (1:1) with glass beads (diameter, 150 to 212 μm; Sigma, St. Louis, Mo.) and vortexing the ice-cooled suspensions four times over 4 min. Cell debris and glass beads were collected by centrifugation (12,000 × g, 5 min, 4°C). Protein concentrations were determined as described by Bradford (3) using a commercial reagent (Bio-Rad Laboratories, Hercules, Calif.) with bovine serum albumin as a standard.
Screening for proteolytic activity of B. animalis subsp. lactis with chromogenic substrates.
Whole cells, CFE, and CWFs were tested for proteolytic activity with chromogenic substrates. Proteinase activity was examined in whole cells (OD600, 20) and CWFs (30 mg/ml). Endopeptidase, X-prolyl-dipeptidyl aminopeptidase, and aminopeptidase activities were determined in CFE (2 mg/ml). The substrates employed (all purchased from Sigma, except for succinyl-Ala-Glu-Pro-Phe-p-nitroanilide [Bachem, Bubendorf, Switzerland] and Universal Protease Substrate [Roche Diagnostics, Mannheim, Germany]) and references for the corresponding methods are listed in Table 1. All enzymatic reactions were performed in 50 mM sodium phosphate buffer, pH 7, at 37°C. Results are expressed as specific activity (units per milligram of protein). One unit of enzyme activity is defined as the amount of enzyme required to release 1 μmol chromogenic substrate per min under the enzyme assay conditions.
TABLE 1.
Chromogenic substrates and corresponding references to the methods employed for the determination of proteolytic activities in Bifidobacterium animalis subsp. lactis
| Activity | Substrate | Reference |
|---|---|---|
| Proteinase | Azocasein | 46 |
| Universal Protease Substrate | 42 | |
| Succinyl-Ala-Glu-Pro-Phe- p-nitroanilide | 29 | |
| Endopeptidase | N-Glutaryl-Ala-Ala-Phe- 4-methoxy-β-naphthylamide | 25 |
| N-Benzoyl-Val-Gly-Arg- p-nitroanilide | 6 | |
| N-Benzoyl-Pro-Phe-Arg- p-nitroanilide | ||
| N-Succinyl-Ala-Ala-Pro- Phe-p-nitroanilide | ||
| X-prolyl-dipeptidyl aminopeptidase | Arg-Pro-p-nitroanilide | 11 |
| Proline iminopeptidase | Pro-p-nitroanilide | 11 |
| General aminopeptidases | Leu-p-nitroanilide | 11 |
| Lys-p-nitroanilide |
Analysis of galactosidase activity.
The α- and β-galactosidase activities were measured by employing o-nitrophenyl-α-d-galactopyranoside and o-nitrophenyl-β-d-galactopyranoside (Sigma), respectively. The reaction mixture contained 50 mM sodium phosphate buffer, pH 6.5, 1 mM magnesium chloride, 1 mM 2-mercaptoethanol, 2.32 mM substrate, and CFE (2 mg/ml). The liberation of o-nitrophenol was observed at 410 nm and 37°C, and activity is expressed as units per milligram of protein. One unit is defined as the amount of enzyme that can release 1 μmol of o-nitrophenol per min under the given conditions.
Hydrolysis of milk proteins and casein-derived peptides by B. animalis subsp. lactis.
Activities against caseins, whey proteins, GMP, and αs1-casein(f1-23) were studied with whole cells, CWFs, CFE, and purified recombinant PepO. Whole cells were employed in the reaction mixture at a final OD600 of 20, CFE at a final concentration of 2 mg/ml protein, CWFs at 30 mg/ml, and recombinant PepO at 10 μg/ml. Pure α-, β-, and κ-caseins (Sigma) were suspended in 50 mM sodium phosphate buffer, pH 7, and used at a final concentration of 1 mg/ml, while α-lactoalbumin and β-lactoglobulin (Sigma) were used at a final concentration of 0.5 mg/ml. Aliquots were taken from the reaction mixtures after 2, 4, and 24 h of incubation and centrifuged (12,000 × g, 5 min), and the supernatants were mixed 1:1 with solubilization buffer (125 mM Tris buffer, pH 7.5, 20% glycerol, 4% sodium dodecyl sulfate [SDS], 0.01% bromophenol blue, and 10% mercaptoethanol). Samples were denatured (100°C, 5 min), and the hydrolysis products were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) on 15% polyacrylamide gels as described by Laemmli (24). Activities against GMP (Sigma) and αs1-casein(f1-23) (a gift from P. Fernández de Palencia [15]) were assayed in 50 mM sodium phosphate buffer, pH 6, with 1 mg/ml and 4 mg/ml substrate, respectively. After a 50-min reaction, samples were centrifuged (12,000 × g, 5 min), and the supernatants were heat inactivated (100°C, 5 min). Hydrolysis products were analyzed by reverse-phase high-performance liquid chromatography (RP-HPLC) following the procedures described by Exterkate et al. (12) for αs1-casein(f1-23) and by Martín-Diana et al. (27) for GMP. Peptides generated by enzymatic hydrolysis were collected and freeze-dried, and the N-terminal amino acid sequence was determined by automated Edman degradation analysis at the Protein Synthesis Laboratory in the Centro de Investigaciones Biológicas (CSIC, Madrid, Spain).
DNA manipulations.
B. animalis subsp. lactis chromosomal DNA was obtained as described by Meile et al. (34). E. coli plasmid isolation was done with the GFX Micro Plasmid Prep kit (Amersham Biosciences, Uppsala, Sweden). DNA manipulations and electrotransformation of E. coli cells were carried out by standard methods (44).
Cloning and expression of the endopeptidase gene (pepO).
B. animalis subsp. lactis pepO was identified by sequence similarity within a fragment of chromosomal B. animalis subsp. lactis DNA obtained and sequenced in the course of a random genome sampling of B. animalis subsp. lactis (unpublished results). To express the predicted B. animalis subsp. lactis endopeptidase gene in E. coli, a 2.28-kb DNA fragment was amplified by PCR using B. animalis subsp. lactis chromosomal DNA as a template and the primer pair NcoI_for (GAAGCCATGGATACCACTTTGCAATCAGGC)-HindIII_rev (TATCAAGCTTCCAGATGCGCACGCGTTC) (underlined sequences indicate NcoI and HindIII restriction sites, respectively). DNA amplification was performed with Pwo DNA polymerase (Roche) according to the manufacturer's instructions. The NcoI/HindIII-digested PCR product was inserted into the Novagen pET-28a(+) vector. The recombinant plasmid, pET-pepO, encoding the complete endopeptidase gene as well as a C-terminal His6 tag, was transformed into E. coli BL21(DE3)pLysS. The entire pepO sequence was confirmed by DNA sequencing.
Expression of the B. animalis subsp. lactis pepO gene was induced by adding IPTG (final concentration, 1 mM) to E. coli BL21(DE3)pLysS cells containing pET-pepO and growing them to an OD600 of approximately 0.5. For optimal expression, 0.8 mM glucose was also added to the culture medium. After a further 4 h of incubation, the cells were harvested by centrifugation (10,000 × g, 10 min). The production and the cellular localization of the target protein were verified by analysis of total proteins, soluble and insoluble cytoplasmic fractions, and the medium fraction according to the pET System Manual (39). IPTG-induced cultures of E. coli BL21(DE3)pLysS harboring pET-28a(+) were used as a negative control. The protein pattern of cell fractions was monitored by SDS-PAGE (24).
Purification of recombinant PepO.
All steps for recombinant protein purification were carried out at 4°C. The induced cell pellet was frozen at −80°C, resuspended in 2 ml/g (wet weight) of binding buffer (0.02 M sodium phosphate, pH 7.4, 0.5 M NaCl, 0.01 M imidazole), and then mixed (1:1, wt/vol) with glass beads to obtain CFE. These CFE were used to purify the recombinant PepO by immobilized metal affinity chromatography (IMAC), employing a 1-ml HiTrap Chelating HP column (Amersham) according to the manufacturer's instructions. The enzyme was eluted with 0.1 M imidazole, and the solution was desalted (PD-10 column; Amersham) and stored in 0.02 M sodium phosphate buffer, pH 6, at −80°C. The molecular mass and purity of the recombinant enzyme were confirmed by native PAGE on 7.5% polyacrylamide gels using a nondenatured protein molecular weight marker (Sigma) as a standard.
Effects of reagents, pH, and temperature.
All enzymatic reactions were carried out with bradykinin as the substrate. The purified recombinant PepO was preincubated with various metals and reagents at a 1 mM concentration in 50 mM sodium phosphate buffer, pH 6. The temperature optimum was determined by performing the enzymatic reactions at different temperatures (5 to 55°C), and the pH optimum was measured over pH ranges from 3 to 9 by employing different buffers, namely, sodium acetate (pH 3 to 5), sodium phosphate (pH 6 to 7), and Tris-HCl (pH 8 to 9). Thermostability was analyzed by incubating the enzyme at temperatures between 5 and 55°C and measuring the residual activity under standard conditions. pH stability was determined by incubating the enzyme at different pH values (pH 3 to 9) before measuring residual activity under standard conditions.
Substrate specificity and identification of hydrolysis products.
The activity of the recombinant enzyme was measured toward bradykinin, angiotensin, substance P, methyl enkephalin, and methyl enkephalinamide (Sigma) as peptide substrates. Standard enzyme assays in 100 μl were carried out in 50 mM sodium phosphate buffer, pH 6, containing 4 μg/ml recombinant PepO and 0.16 mM peptide. After incubation for 50 min at 37°C, the reaction was stopped with 50 μl 3% trifluoroacetic acid, and the hydrolysis products were analyzed by RP-HPLC as described by Pritchard et al. (40). Hydrolysis of caseins, whey proteins, GMP, and αs1-casein(f1-23) by the recombinant enzyme was also analyzed as described above. Hydrolysis products were collected, freeze-dried, and identified by amino acid sequencing.
Nucleotide sequence accession number.
The entire pepO sequence was confirmed by DNA sequencing and deposited in the GenBank and EMBL databases (accession no. AJ844608).
RESULTS
Screening for proteolytic activity of B. animalis subsp. lactis with chromogenic substrates.
Proteinase activity was not detected in CWFs or in whole cells with chromogenic substrates. The same negative result was obtained for endopeptidase activity measured with chromogenic substrates in CFE. However, X-prolyl-dipeptidyl aminopeptidase and general aminopeptidase activities could be detected in CFE (Table 2). CFE obtained from cells grown in MRS medium showed no detectable activity, whereas the same experiments performed with CFE obtained from cells grown in milk-based medium showed measurable activities, among which proline iminopeptidase activity was remarkably high. Analysis of galactosidase activities in B. animalis subsp. lactis CFE (Table 2) showed 100-fold-higher α- and β-galactosidase activities in cells grown in milk than in cells grown in MRS. The results indicate that the activities in CFE from MRS-grown cells were below the lowest quantifiable level (0.005 U/mg).
TABLE 2.
Aminopeptidase and galactosidase activities in CFE of Bifidobacterium animalis subsp. lactis cells cultured in MRS or milk supplemented with 10 g/liter GMP
| Substrate | Mean sp act (U/mg)a ± SD in CFE from the following medium:
|
|
|---|---|---|
| MRS | Milk + GMP | |
| Arg-Pro-p-nitroanilide | NDb | 0.057 ± 0.004 |
| Pro-p-nitroanilide | ND | 0.129 ± 0.002 |
| Leu-p-nitroanilide | ND | 0.060 ± 0.004 |
| Lys-p-nitroanilide | ND | 0.026 ± 0.002 |
| o-Nitrophenyl-α-d-galactopyranoside | 0.005 ± 0.000 | 0.350 ± 0.018 |
| o-Nitrophenyl-β-d-galactopyranoside | 0.019 ± 0.001 | 2.330 ± 0.086 |
One unit is defined as the amount of enzyme required to release 1 μmol of p-nitroaniline or o-nitrophenol per min.
ND, not detected; activity was lower than the lowest quantifiable level (0.005 U/mg).
Hydrolysis of milk proteins and casein-derived peptides by B. animalis subsp. lactis.
Whole cells, CWFs, and CFE of B. animalis subsp. lactis grown in milk-based medium were incubated with pure α-, β-, and κ-caseins to analyze caseinolytic activity. CFE could hydrolyze casein fractions completely after 4 h of incubation at 37°C as observed by SDS-PAGE analysis. Both β- and κ-caseins were hydrolyzed more rapidly than α-casein (results not shown). CWFs could not degrade caseins even after 24 h of incubation. Whole cells started to degrade caseins only after a 24-h incubation, which could be a result of cell lysis and leakage of intracellular material into the medium. To assess whether the cells were damaged, the corresponding supernatants were analyzed for β-galactosidase activity. The results showed that β-galactosidase activity was detected in the supernatant only in the 24-h-incubated samples. Whey proteins (α-lactoalbumin and β-lactoglobulin) could not be hydrolyzed by any of the cellular fractions analyzed.
Cloning and expression of the endopeptidase gene (pepO) in E. coli and purification of the recombinant protein.
The endopeptidase gene of B. animalis subsp. lactis was identified by sequence similarity to the putative B. longum NCC2705 pepO (accession no. NC_004307) in the course of a random sampling of the genome of B. animalis subsp. lactis. The predicted B. animalis subsp. lactis endopeptidase gene (deposited under accession no. AJ844608) encodes a 693-amino-acid protein with a calculated molecular mass of 78.3 kDa, an isoelectric point of 4.6, and no predicted signal peptide or transmembrane domains, suggesting an intracellular location of the enzyme. The protein shared 67.4% amino acid sequence identity with the predicted endopeptidase of B. longum NCC2705 (accession no. NP_696878). A high degree of similarity was also found with predicted metalloendopeptidases from Propionibacterium acnes KPA171202 (YP_056588) (45.7% amino acid identity) and Corynebacterium glutamicum ATCC 13032 (NP_599406) (43.8% amino acid identity). However, the B. animalis subsp. lactis gene displayed relatively low identity with PepO enzymes from lactic acid bacteria (LAB), namely, 31.0%, 29.4%, and 28.5% identity with the PepO enzymes of S. thermophilus A (CAC14579), Lactobacillus helveticus CNRZ32 (O52071), and Lactococcus lactis subsp. lactis (F53290), respectively. The PepO-like endopeptidase of B. animalis subsp. lactis belongs to the M13 peptidase family of zinc metallopeptidases, since it contains all the catalytic signatures that are common to all the enzymes of this family, namely, 502HExxH506, 574ExxxD578, and 460vNAfY464 (indices correspond to residue numbering in the B. animalis subsp. lactis PepO amino acid sequence).
Overexpression of the pepO gene in E. coli BL21(DE3)pLysS occurred only when glucose was added to the growth medium along with IPTG. SDS-PAGE protein patterns of cellular fractions obtained from the IPTG-induced culture of E. coli BL21(DE3)pLysS cells bearing pET-pepO showed a strong signal at a molecular mass of about 74 kDa within the total protein and the soluble cytoplasmic fractions (Fig. 1). This size is in agreement with the calculated molecular mass (78 kDa) deduced from the predicted PepO sequence. The medium and insoluble cytoplasmic fractions contained only marginal amounts of protein, suggesting that most of the recombinant enzyme was available in a soluble form and thus probably in an active state (results not shown). Analysis of induced cells bearing pET-28a(+) showed no signal at 74 kDa within any fraction, confirming that the strong bands identified above originate from the expression of the cloned gene. As shown in Fig. 1, the C-terminal His6 tag facilitated the purification to homogeneity of the recombinant PepO by a single IMAC step. Native PAGE of the recombinant protein showed one band with an apparent molecular mass of 74 kDa (results not shown), indicating that B. animalis subsp. lactis PepO is a monomeric enzyme.
FIG. 1.
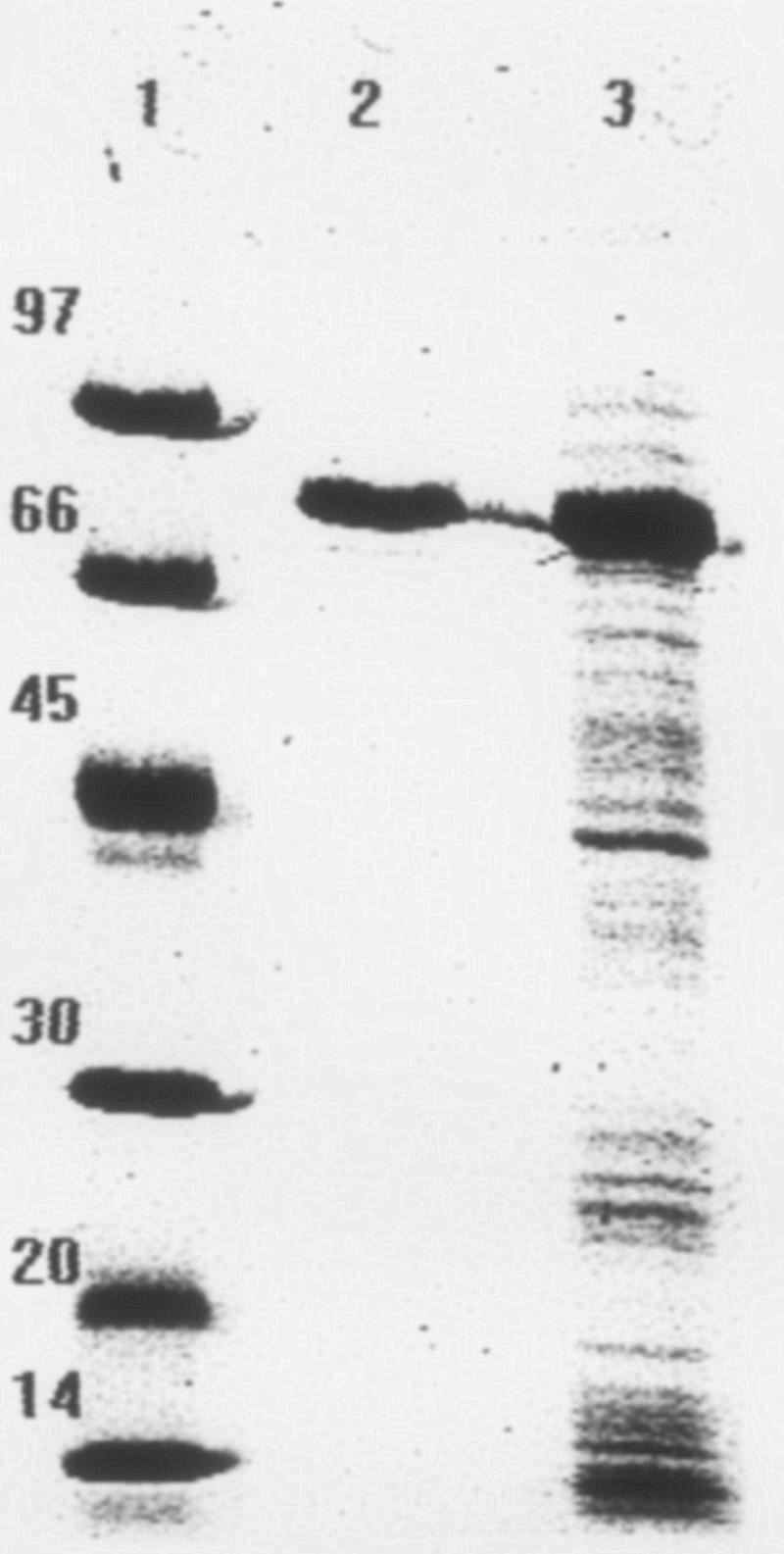
SDS-PAGE analysis of purified protein fraction of IPTG-induced E. coli BL21(DE3)pLysS harboring the recombinant PepO. Lanes: 1, protein standard; 2, soluble cytoplasmic fraction eluted by IMAC with 0.1 M imidazole; 3, cell extract of 0.1 mM IPTG-induced E. coli BL21(DE3)pLysSpET-pepO cells.
Biochemical characterization of the recombinant B. animalis subsp. lactis PepO.
For biochemical characterization, B. animalis subsp. lactis recombinant PepO activity toward bradykinin was measured. Optimal temperature and pH were 37°C and 6.5, respectively (results not shown). Residual activity decreased below 35% at pH values lower than 6.5, and no activity was measured below pH 5, while at pH 9 the residual activity was 48% (results not shown). The PepO was significantly inhibited by EDTA and 1,10-phenanthroline (Table 3), both of which are metalloenzyme inhibitors, but was only partially inhibited by phosphoramidon, an agent known to completely inhibit other metalloendopeptidases (48). Thiorphan, another specific inhibitor of endopeptidases, was even less effective at the inhibition of B. animalis subsp. lactis PepO than phosphoramidon (Table 3). Diethyl pyrocarbonate, which inhibits activity by reactivity with the active-site histidine (25), and the serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF) also caused partial inhibition. Dithiothreitol was the only agent that acted as an activator (Table 3), which could be due to its reducing character, whereas p-(hydroxymercuri)benzoic acid, which has an oxidizing effect, reduced the activity.
TABLE 3.
Effects of inhibitors and metal ions (1 mM) on the activity of recombinant PepO of Bifidobacterium animalis subsp. lactis
| Substance | Mean residual activitya (±SD) |
|---|---|
| Phosphoramidon | 23.46 (±5.92) |
| Diethyl pyrocarbonate | 50.77 (±5.29) |
| Thiorphan | 80.77 (±8.16) |
| EDTA | 5.77 (±6.53) |
| 1,10-Phenanthroline | 0.00 (±0.86) |
| p-(Hydroxymercuri)benzoic acid | 43.85 (±5.77) |
| PMSF | 30.58 (±2.45) |
| Dithiothreitol | 110.12 (±15.37) |
| Zn2+ | 15.77 (±1.33) |
| Mn2+ | 115.77 (±5.45) |
| Cu2+ | 5.77 (±3.26) |
Expressed as a percentage of the activity on bradykinin in the absence of metal ions and chemical reagents, taken as 100% (0.064 U/mg).
Since the enzyme was found to have no activity with synthetic chromogenic substrates, the hydrolysis of natural peptides was assessed (Table 4). Relative activities toward the different substrates were determined by following substrate disappearance as a function of time using RP-HPLC. In addition, the hydrolysis products were identified in order to determine the cleavage bond specificity. The predominant peptide bond cleaved by B. animalis subsp. lactis PepO was on the N-terminal side of phenylalanine residues. Main secondary cleavage sites were also at Pro-Xxx bonds. The specific activity of the enzyme was calculated on the basis of the primary cleavage site and before secondary products were released. Regardless of the preferential cleavage bonds, specific activity increased with peptide length, indicating that the enzyme functions as an endopeptidase (Table 4).
TABLE 4.
Substrate specificity and cleavage sites of recombinant PepO of Bifidobacterium animalis subsp. lactis
| Substrate | Relative activitya | Cleavage sitesb |
|---|---|---|
| Substrate P | 100.0 (±0.0) | R-P-2K-P-Q-Q-1F-F-G-L-M-NH2 |
| Angiotensin | 93.8 (±3.3) | D-R-V-Y-2I-H-P-1F-H-L |
| Bradykinin | 38.8 (±7.8) | R-P-2P-G-2F-S-P-1F-R |
| Met-enkephalinamide | 10.5 (±2.7) | Y-G-G-1F-M-NH2 |
| Met-enkephalin | 10.2 (±4.8) | Y-G-G-1F-M |
| αs1-Casein(f1-23) | 2.2 (±0.06) | R-P-K-H-P-I-K-H-Q-G-L-P-Q-1E-V-L-N-E-N-L-L-R-F |
Expressed as a percentage of the activity toward substance P (1.7 U/mg), taken as 100%. Values are means (±standard deviations).
1, primary cleavage site; 2, secondary cleavage site.
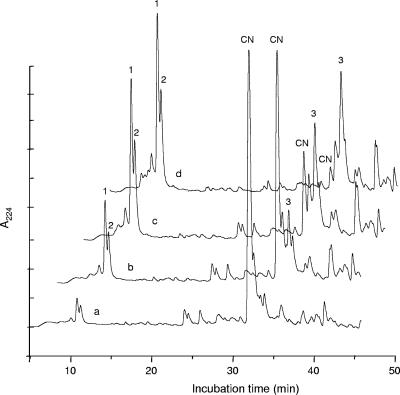
With respect to degradation of milk proteins and derived peptides, the pure recombinant PepO was not able to hydrolyze either α-, β-, or κ-casein, α-lactoalbumin, β-lactoglobulin, or GMP. However, αs1-casein(f1-23), a 23-amino-acid peptide that is present in cheese whey as a consequence of enzymatic hydrolysis of αs1-casein, was rapidly hydrolyzed by the recombinant enzyme. Hydrolysis of αs1-casein(f1-23) was recorded during 20-, 40-, and 60-min incubations with PepO at 37°C (Fig. 2). The preferential cleavage site of PepO in αs1-casein(f1-23) (Table 4) released peptide 3 (f14-23) and peptides 1 and 2, both of which started with the amino-terminal residues of αs1-casein(f1-23). After 1 h of incubation with the recombinant PepO, little or none of the initial αs1-casein(f1-23) could be detected, and the peptides originated underwent no further hydrolysis by this enzyme.
FIG. 2.
Reverse-phase HPLC patterns of (a) pure αs1-casein(f1-23) [CN] and its hydrolysis products (peaks 1, 2, and 3) after (b) 20-min, (c) 40-min, and (d) 60-min incubations with Bifidobacterium animalis subsp. lactis recombinant PepO.
DISCUSSION
This study was conducted to characterize the proteolytic system of B. animalis subsp. lactis with special attention to the identification and characterization of an intracellular endopeptidase. For the first time, we report the cloning, purification, and characterization of a proteolytic enzyme from a Bifidobacterium sp.
To gain insight into the proteolytic system of B. animalis subsp. lactis, cells were grown in a peptide-rich medium (MRS) or a milk-based medium with a low peptide content. By using chromogenic substrates, proteolytic activity was found only for intracellular aminopeptidases (Table 2) and the highest activity was measured in cells that had been grown in the milk-based medium. The increase in proteolytic activities observed when B. animalis subsp. lactis was grown in a milk-based medium is similar to reported findings for LAB, whose proteolytic activities are generally lower in cells grown in a peptide-rich medium, such as M17 or MRS, than in cells grown in milk (18, 23, 32). Interestingly, the specific proline iminopeptidase activity found in B. animalis subsp. lactis intracellular fractions is remarkably high (Table 2). This high activity with a substrate that contains an N-terminal proline residue is a distinctive characteristic of B. animalis subsp. lactis compared with LAB, since most LAB intracellular aminopeptidases have been reported to have a greater affinity for substrates containing lysine or leucine residues at the N-terminal position than for those containing proline (23, 41). Caseins are proteins rich in proline, and specialized peptidases are required to hydrolyze peptide bonds involving this imino acid (9).
No proteinase or endopeptidase activities were detected with specific chromogenic substrates in B. animalis subsp. lactis whole cells grown in MRS or milk-based medium or with their respective CFE. Therefore, these activities were assayed using milk proteins: caseins and whey proteins. Neither whole cells grown in milk nor CWFs were able to hydrolyze these proteins, indicating the absence of an extracellular proteinase activity under these conditions. However, the combined action of intracellular proteolytic enzymes could degrade caseins. Since some of the intracellular endopeptidases (PepO) identified in LAB have been reported to hydrolyze peptides derived from αs1- and β-caseins (1, 7, 8), this enzyme was chosen for further characterization in B. animalis subsp. lactis.
The deduced amino acid sequence of B. animalis subsp. lactis PepO identified it as a member of the M13 family within the zinc metallopeptidase superfamily, whose members are also known as metzincins and whose main representatives are neprilysin (NEP), endothelin-converting enzyme 1 (ECE-1), and peptidase O (PepO). Phylogenetic analysis of B. animalis subsp. lactis PepO showed closer relationships with Propionibacterium and Corynebacterium PepO sequences than with LAB PepO sequences. This fact reflects the grouping of Bifidobacterium spp. as gram-positive bacteria with a high GC content in the class Actinobacteria (17). B. animalis subsp. lactis PepO contains the specific Zn2+-binding domain and a conserved substrate binding and catalysis motif characteristic of these enzymes (2). In this sense, the enzyme was strongly inhibited by the divalent metal chelators EDTA and 1,10-phenanthroline. Specific inhibitors described for mammalian NEP and ECE-1, such as thiorphan and phosphoramidon (47), did not show a significant effect. The decrease in activity found with Zn2+ and PMSF (Table 3) has also been described for L. lactis PepO (47).
Activity of B. animalis subsp. lactis PepO was found toward substrates of at least 5 amino acid residues, such as met-enkephalin, as well as toward larger substrates, such as αs1-casein(f1-23). However, the peptidase was not active with GMP, which is a 64-amino-acid-peptide. To a certain extent, enzyme specificity increased with peptide length (Table 4), as has been described for lactococcal PepO (25), but in contrast with mammalian NEP specificity. The enzyme has no proteinase activity.
The primary cleavage site of B. animalis subsp. lactis PepO (Table 4) is in agreement with the preferential cleavage site described for most LAB PepO enzymes (1, 5, 8, 25). However, B. animalis subsp. lactis PepO also showed a post-proline secondary cleavage site preference. The affinity of B. animalis subsp. lactis PepO for post-proline bonds and its inability to hydrolyze the chromogenic peptide substrates used to identify LAB PepO enzymes are also characteristics described for the PepO2 from L. helveticus (7).
Several duplicated endopeptidase activities have been described for L. lactis (PepO, PepF1, and PepF2 [35, 37, 38]) and for L. helvetivus (PepO, PepO2, and PepE [6, 7, 13]). However, the physiological role of PepO enzymes in LAB has not been clearly elucidated yet, since the growth of L. lactis or L. helveticus pepO deletion mutants in milk or chemically defined media was indistinguishable from that of the wild-type strains (6, 35, 36). The ability of B. animalis subsp. lactis PepO to hydrolyze αs1-casein(f1-23) suggests that the enzyme may play a role in the increase in the growth of B. animalis subsp. lactis in milk when it is supplemented with whey peptide fractions (20, 28).
Acknowledgments
This work was financed by the Spanish National Research Project (AGL2004-07285-C02-01). C. Janer is the recipient of a grant from the Spanish Ministry of Education and Science.
REFERENCES
- 1.Baankreis, R., S. van Schalkwijk, A. C. Alting, and F. A. Exterkate. 1995. The occurrence of two intracellular oligoendopeptidases in Lactococcus lactis and their significance for peptide conversion in cheese. Appl. Microbiol. Biotechnol. 44:386-392. [DOI] [PubMed] [Google Scholar]
- 2.Bianchetti, L., C. Oudet, and O. Poch. 2002. M13 endopeptidases: new conserved motifs correlated with structure, and simultaneous phylogenetic occurrence of PHEX and the bony fish. Proteins 47:481-488. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Bullock, W. O., J. M. Fernández, and J. M. Short. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques 4:376-379. [Google Scholar]
- 5.Chavagnat, F., J. Meyer, and M. G. Casey. 2000. Purification, characterisation, cloning and sequencing of the gene encoding oligopeptidase PepO from Streptococcus thermophilus A. FEMS Microbiol. Lett. 191:79-85. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y.-S., and J. M. Steele. 1998. Genetic characterization and physiological role of endopeptidase O from Lactobacillus helveticus CNRZ32. Appl. Environ. Microbiol. 64:3411-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Y.-S., J. E. Christensen, J. R. Broadbent, and J. L. Steele. 2003. Identification and characterization of Lactobacillus helveticus PepO2, an endopeptidase with post-proline specificity. Appl. Environ. Microbiol. 69:1276-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensson, C., H. Bratt, L. J. Collins, T. Coolbear, R. Holland, M. W. Lubbers, P. W. O'Toole, and J. R. Reid. 2002. Cloning and expression of an oligopeptidase, PepO, with novel specificity from Lactobacillus rhamnosus HN001 (DR20). Appl. Environ. Microbiol. 68:245-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham, D. F., and B. O'Connor. 1997. Proline specific peptidases. Biochim. Biophys. Acta 1343:160-186. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, R. H., S. E. Duncan, C. R. Hackney, W. N. Eigel, and J. W. Boling. 2000. Probiotic culture survival and implications in fermented frozen yogurt characteristics. J. Dairy Sci. 83:666-673. [DOI] [PubMed] [Google Scholar]
- 11.Exterkate, F. A. 1984. Location of peptidases outside and inside the membrane of Streptococcus cremoris. Appl. Environ. Microbiol. 47:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Exterkate, F. A., A. C. Alting, and C. J. Slangen. 1991. Specificity of two genetically related cell-envelope proteinases of Lactococcus lactis subsp. cremoris towards αs1-casein-(1-23)-fragment. Biochem. J. 273:135-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenster, K. M., K. L. Parkin, and J. L. Steele. 1997. Characterization of a thiol-dependent endopeptidase from Lactobacillus helveticus CNRZ32. J. Bacteriol. 179:2529-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández de Palencia, P., C. Peláez, T. Requena, and M. C. Martín-Hernández. 1995. Release and partial characterization of cell-envelope proteinases from Lactococcus lactis subsp. lactis IFPL 359 and Lactobacillus casei subsp. casei IFPL 731 isolated from raw goat's-milk cheese. Z. Lebensm. Unters. Forsch. 201:87-90. [Google Scholar]
- 15.Fernández de Palencia, P., C. Peláez, and M. C. Martín-Hernández. 1997. Specificity of the bound and free forms of the cell-envelope proteinase of Lactobacillus casei subsp. casei IFPL 731 towards the αs1-casein-(1-23) fragment. Lett. Appl. Microbiol. 25:388-392. [DOI] [PubMed] [Google Scholar]
- 16.Fernández de Palencia, P., C. Peláez, C. Romero, and M. C. Martín-Hernández. 1997. Purification and characterization of the cell wall proteinase of Lactobacillus casei subsp. casei IFPL 731 isolated from raw goat's milk cheese. J. Agric. Food Chem. 45:3401-3405. [Google Scholar]
- 17.Garrity, G. M., J. A. Bell, and T. G. Lilburn. 2004. Taxonomic outline of the prokaryotes. Bergey's manual of systematic bacteriology, 2nd ed. [Online.] doi: 10.1007/bergeysoutline200405. [DOI]
- 18.Guedon, E., P. Renault, S. D. Ehrlich, and C. Delorme. 2001. Transcriptional pattern of genes coding for the proteolytic system of Lactococcus lactis and evidence for coordinated regulation of key enzymes by peptide supply. J. Bacteriol. 183:3614-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung, M. N., N. T. Hu, and B. H. Lee. 2001. Molecular and biochemical analysis of β-galactosidase from Bifidobacterium infantis HL96. Appl. Environ. Microbiol. 67:4253-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janer, C., C. Peláez, and T. Requena. 2004. Caseinomacropeptide and whey protein concentrate enhance Bifidobacterium lactis growth in milk. Food Chem. 86:263-267. [Google Scholar]
- 21.Janer, C., L. M. Rohr, C. Peláez, M. Laloi, V. Cleusix, T. Requena, and L. Meile. 2004. Hydrolysis of oligofructoses by the recombinant β-fructofuranosidase from Bifidobacterium lactis. Syst. Appl. Microbiol. 27:279-285. [DOI] [PubMed] [Google Scholar]
- 22.Katayama, T., A. Sakuma, T. Kimura, Y. Makimura, J. Hiratake, K. Sakata, T. Yamanoi, H. Kumagai, and K. Yamamoto. 2004. Molecular cloning and characterization of Bifidobacterium bifidum 1,2-α-l-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J. Bacteriol. 186:4885-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenny, O., R. J. FitzGerald, G. O'Cuinn, T. Beresford, and K. Jordan. 2003. Growth phase and growth medium effects on the peptidase activities of Lactobacillus helveticus. Int. Dairy J. 13:509-516. [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Lian, W., D. Wu, W. N. Konings, I. Mierau, and L. B. Hersh. 1996. Heterologous expression and characterization of recombinant Lactococcus lactis neutral endopeptidase. Arch. Biochem. Biophys. 333:121-126. [DOI] [PubMed] [Google Scholar]
- 26.Margolles, A., and C. G. de los Reyes-Gavilan. 2003. Purification and functional characterization of a novel α-l-arabinofuranosidase from Bifidobacterium longum B667. Appl. Environ. Microbiol. 69:5096-5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Diana, A. B., M. J. Fraga, and J. Fontecha. 2002. Isolation and characterization of casein macropeptide from bovine, ovine and caprine cheese whey. Eur. Food Res. Technol. 214:282-286. [Google Scholar]
- 28.Martín-Diana, A. B., C. Janer, C. Peláez, and T. Requena. 2003. Development of a fermented goat's milk containing probiotic bacteria. Int. Dairy J. 13:827-833. [Google Scholar]
- 29.Martín-Hernández, M. C., A. C. Alting, and F. A. Exterkate. 1994. Purification and characterization of the mature, membrane-associated cell-envelope proteinase of Lactobacillus helveticus L89. Appl. Microbiol. Biotechnol. 40:828-834. [Google Scholar]
- 30.Masco, L., M. Ventura, R. Zink, G. Huys, and J. Swings. 2004. Polyphasic taxonomic analysis of Bifidobacterium animalis and Bifidobacterium lactis reveals relatedness at the subspecies level: reclassification of Bifidobacterium animalis as Bifidobacterium animalis subsp. animalis subsp. nov. and Bifidobacterium lactis as Bifidobacterium animalis subsp. lactis subsp. nov. Int. J. Syst. Evol. Microbiol. 54:1137-1143. [DOI] [PubMed] [Google Scholar]
- 31.McBrearty, S., R. P. Ross, G. F. Fitzgerald, J. K. Collins, J. M. Wallace, and C. Stanton. 2001. Influence of two commercially available bifidobacteria cultures on cheddar cheese quality. Int. Dairy J. 11:599-610. [Google Scholar]
- 32.Meijer, W., J. D. Marugg, and J. Hugenholtz. 1996. Regulation of proteolytic enzyme activity in Lactococcus lactis. Appl. Environ. Microbiol. 62:156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meile, L., W. Ludwig, U. Rueger, C. Gut, P. Kaufmann, G. Dasen, S. Wenger, and M. Teuber. 1997. Bifidobacterium lactis sp. nov., a moderately oxygen tolerant species isolated from fermented milk. Syst. Appl. Microbiol. 20:57-64. [Google Scholar]
- 34.Meile, L., L. M. Rohr, T. A. Geissmann, M. Herensperger, and M. Teuber. 2001. Characterisation of the d-xylulose 5-phosphate/d-fructose 6-phosphate phosphoketolase gene (xfp) from Bifidobacterium lactis. J. Bacteriol. 183:2929-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mierau, I., P. S. Tan, A. J. Haandrikman, B. Mayo, J. Kok, K. J. Leenhouts, W. N. Konings, and G. Venema. 1993. Cloning and sequencing of the gene for a lactococcal endopeptidase, an enzyme with sequence similarity to mammalian enkephalinase. J. Bacteriol. 175:2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mierau, I., E. R. S. Kunji, K. J. Leenhouts, M. A. Hellendoorn, A. J. Haandrikman, B. Poolman, W. N. Konings, G. Venema, and J. Kok. 1996. Multiple-peptidase mutants of Lactococcus lactis are severely impaired in their ability to grow in milk. J. Bacteriol. 178:2794-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monnet, V., M. Nardi, A. Chopin, M. C. Chopin, and J. C. Gripon. 1994. Biochemical and genetic characterization of PepF, an oligopeptidase from Lactococcus lactis. J. Biol. Chem. 269:32070-32076. [PubMed] [Google Scholar]
- 38.Nardi, M., P. Renault, and V. Monnet. 1997. Duplication of the pepF gene and shuffling of DNA fragments on the lactose plasmid of Lactococcus lactis. J. Bacteriol. 179:4164-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novagen User Protocols. 2004. pET system manual, 10th ed. [Online.] http://www.merckbiosciences.co.uk/docs/docs/PROT/TB055.pdf.
- 40.Pritchard, G. G., A. D. Freebairn, and T. Coolbear. 1994. Purification and characterization of an endopeptidase from Lactococcus lactis subsp. cremoris SK11. Microbiology 140:923-930. [DOI] [PubMed] [Google Scholar]
- 41.Requena, T., C. Peláez, and P. F. Fox. 1993. Peptidase and proteinase activity of Lactococcus lactis, Lactobacillus casei and Lactobacillus plantarum. Z. Lebensm. Untersuch. Forsch. 196:351-355. [Google Scholar]
- 42.Roche Applied Science. 2004. Universal Protease Substrate: casein, resorufin-labeled. Product instructions. [Online.] http://www.roche-applied-science.com/pack-insert/1080733a.pdf.
- 43.Saavedra, J. M., A. Abi-Hanna, N. Moore, and R. H. Yolken. 2004. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am. J. Clin. Nutr. 79:261-267. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M.-C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin, J. Y., W. M. Jeon, G.-B. Kim, and B. H. Lee. 2004. Purification and characterization of intracellular proteinase from Lactobacillus casei ssp. casei LLG. J. Dairy Sci. 87:4097-4103. [DOI] [PubMed] [Google Scholar]
- 47.Tan, P. S., K. M. Pos, and W. N. Konings. 1991. Purification and characterization of an endopeptidase from Lactococcus lactis subsp. cremoris Wg2. Appl. Environ. Microbiol. 57:3593-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner, A. J., R. E. Isaac, and D. Coates. 2001. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays 23:261-269. [DOI] [PubMed] [Google Scholar]
- 49.van Laere, K. J. M., T. Abee, H. A. Schols, G. Beldman, and A. G. J. Voragen. 2000. Characterization of a novel β-galactosidase from Bifidobacterium adolescentis DSM20083 active towards transgalactooligosaccharides. Appl. Environ. Microbiol. 66:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]