Abstract
The effects of the lengths of aeration and nonaeration periods on nitrogen removal and the nitrifying bacterial community structure were assessed in intermittently aerated (IA) reactors treating digested swine wastewater. Five IA reactors were operated in parallel with different aeration-to-nonaeration time ratios (ANA). Populations of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) were monitored using 16S rRNA slot blot hybridizations. AOB species diversity was assessed using amoA gene denaturant gradient gel electrophoresis. Nitrosomonas and Nitrosococcus mobilis were the dominant AOB and Nitrospira spp. were the dominant NOB in all reactors, although Nitrosospira and Nitrobacter were also detected at lower levels. Reactors operated with the shortest aeration time (30 min) showed the highest Nitrosospira rRNA levels, and reactors operated with the longest anoxic periods (3 and 4 h) showed the lowest levels of Nitrobacter, compared to the other reactors. Nitrosomonas sp. strain Nm107 was detected in all reactors, regardless of the reactor's performance. Close relatives of Nitrosomonas europaea, Nitrosomonas sp. strain ENI-11, and Nitrosospira multiformis were occasionally detected in all reactors. Biomass fractions of AOB and effluent ammonia concentrations were not significantly different among the reactors. NOB were more sensitive than AOB to long nonaeration periods, as nitrite accumulation and lower total NOB rRNA levels were observed for an ANA of 1 h:4 h. The reactor with the longest nonaeration time of 4 h performed partial nitrification, followed by denitrification via nitrite, whereas the other reactors removed nitrogen through traditional nitrification and denitrification via nitrate. Superior ammonia removal efficiencies were not associated with levels of specific AOB species or with higher AOB species diversity.
There is increasing interest in biological nitrogen removal technologies that use low levels of oxygen to achieve partial nitrification, the oxidation of ammonia to nitrite by ammonia-oxidizing bacteria (AOB), and subsequent denitrification via nitrite, the reduction of nitrite to dinitrogen gas by heterotrophic denitrifiers. Alkalinity and oxygen demands are lower for partial nitrification, and organic substrate requirements are lower for denitrification via nitrite, than the traditional nitrification/denitrification process, resulting in substantial operational savings (2). Partial nitrification relies on the selection of AOB over nitrite-oxidizing bacteria (NOB), which allows the accumulation of nitrite. Sustained nitrite accumulation can be accomplished by controlling solids retention time, temperature, free ammonia and hydroxylamine concentrations, or dissolved oxygen (DO) conditions (2, 12, 15, 19, 23, 42).
The key to efficient and robust biological wastewater treatment relies on knowing the microorganisms involved and how they respond to different operating conditions (41). Several microbial diversity studies of activated sludge and biofilms based on 16S rRNA gene libraries have been reported in the last decade. Denaturant gradient gel electrophoresis (DGGE) has been used to separate amplified 16S rRNA genes and determine the effects of ammonia and dissolved oxygen concentrations on community compositions of nitrifiers (18, 25, 35). However, commonly used 16S rRNA primers for AOB studies have limited specificity, and the high similarity among 16S rRNA genes of AOB makes it impossible to resolve and identify closely related AOB species (31). Alternatively, the functional gene encoding the alpha subunit of ammonia monooxygenase (amoA), the enzyme responsible for the conversion of ammonia to hydroxylamine found in all AOB, has been used as a specific molecular marker in environmental studies of AOB using DGGE (4, 5, 32, 39) and real-time PCR (17).
Intermittently aerated reactors have been successfully used for nitrogen removal from digested swine manure by achieving complete nitrification during aerated periods, followed by denitrification during nonaerated periods (9, 30). Digested swine manure usually contains high ammonia concentrations and a low carbon/nitrogen ratio. These characteristics impose challenges for the traditional nitrification/denitrification approach due to (i) the high oxygen demand for complete nitrification of ammonia to nitrate and (ii) the relatively low organic substrate content available for complete denitrification. Intermittently aerated reactors can potentially be optimized if used to perform partial nitrification followed by denitrification via nitrite, resulting in reduced oxygen demand for ammonia removal and reduced organic substrate for denitrification. We hypothesized that aeration cycles with sufficiently short aerated periods or sufficiently long nonaerated periods can provide appropriate conditions for partial nitrification and denitrification via nitrite. In the present study, we assessed the effects of different aeration cycles on nitrogen removal performance and the community compositions of AOB and NOB by using amoA DGGE and quantitative slot blot hybridizations based on 16S rRNA.
MATERIALS AND METHODS
Laboratory-scale reactors.
Five identical 6-liter Plexiglas reactors (A, B, C, D, and E) were operated under intermittent aeration conditions, each with a different aeration-to-nonaeration time ratio (ANA): 1 h:1 h (reactor A), 1 h:3 h (reactor B), 0.5 h:1.5 h (reactor C), 0.5 h:2 h (reactor D), and 1 h:4 h (reactor E). The reactors were fed with anaerobically digested swine wastewater with the following average concentrations: 197 ± 111 mg NH3-N liter−1 (total ammonia), 296 ± 150 mg total Kjeldahl nitrogen (TKN) liter−1, 344 ± 83 mg soluble chemical oxygen demand liter−1, and 305 ± 114 mg total organic carbon liter−1. The influent flow rate was 2 liters day−1, with substrate inflow for 20 min every 60 min. The target hydraulic retention time and mean cell residence time were 3 days and 20 days, respectively. All reactors were operated at room temperature (25°C). The mean pH in all reactors ranged from 7.6 to 7.8, and alkali addition was not necessary.
Reactors A and B were inoculated with activated sludge from the Neuse River Wastewater Treatment Plant (Raleigh, NC). Waste sludge from reactors A and B was stored at 4°C and used for inoculating reactors C, D, and E. Wastewater was obtained biweekly from a swine lagoon at the North Carolina State University Lake Wheeler Road Field Laboratory and stored at 4°C. The reactor design allowed biomass settling in the clarification zone and recycling to the aeration zone. Air cycling was controlled using a solenoid valve activated by an electronic timer (ChronTrol Corp., San Diego, Calif.). Compressed air was regulated to 10 lb/in2, and airflow was controlled by a gas mass flow controller at 500 ml min−1 (Cole-Parmer Instrument Co., Vernon Hills, Ill.).
Analytical methods.
Grab samples of the influent and the effluent during the aerated phase were collected from each reactor and analyzed for TKN, NH3-N (total ammonia), NO3−-N, NO2−-N, soluble chemical oxygen demand, total organic carbon, pH, total suspended solids, and volatile suspended solids by standard methods (10). DO was measured using a YSI 52 DO meter and a YSI 5739 oxygen probe (YSI Inc., Yellow Springs, Ohio). Oxidation-reduction potential measurements were taken with an Accumet metallic combination platinum/Ag/AgCl electrode (EID Corp., Bridgeport, Connecticut).
Bacterial cultures.
Pure cultures of Nitrosomonas europaea (ATCC 25978 in ATCC medium 2265), Nitrosospira multiformis (ATCC 25196 in ATCC medium 929), and Nitrobacter agilis (ATCC 25384 in ATCC medium 480) were grown aerobically in 0.5-liter flasks at 30°C. The pH was kept at 8.0 by periodic addition of 20% Na2CO3. Cells were harvested by centrifugation at 3,200 × g, and cell pellets were processed for extraction of RNA.
Nucleic acid extraction and in vitro transcription.
Two sets of mixed liquor samples (14 ml each sample) were centrifuged at 3,200 × g for 5 min and stored at −80°C for RNA and DNA extractions. RNA was extracted using a modified low-pH hot-phenol extraction procedure (38). DNA was extracted using a PowerSoil DNA isolation kit (Mo Bio Laboratories, Inc., Carlsbad, CA) according to the manufacturer's instructions. Nucleic acids concentrations were measured spectrophotometrically.
Since pure cultures of Nitrospira were not available, in vitro-transcribed 16S rRNA was used as reference rRNA in membrane hybridizations as previously described (30).
DGGE and cloning.
amoA gene fragments were amplified using a seminested approach in which products of an initial round of PCR with primers AmoA-1F and AmoA-2R-TC (31, 36) were agarose purified and used as template for a second round of PCR using the same primers, except that the forward primer (AmoA-1F) had a GC clamp attached to its 5′ end. Agarose purification of PCR products was performed by coring bands of the correct size using a sterile plastic pipette tip and transferring the excised core into a sterile PCR tube for the second round of PCR. Amplifications were performed using 0.5 μM of each primer, 25 μl of FailSafe PCR system reaction mix E (Epicenter Technologies, Madison, WI), 1 μl DNA extract, and sterile pure water to a total reaction volume of 50 μl. PCR was performed using a thermal cycler (Eppendorf Scientific Inc., Westbury, NY) under the following conditions: 94°C for 5 min; 28 cycles of 92°C for 1.0 min, 58°C for 1.0 min, and 72°C for 1.0 min; and a final extension at 72°C for 45 min.
Amplified amoA gene fragments (491 bp) were separated on 8% (wt/vol) acrylamide-bisacrylamide gels with a denaturant gradient of 20 to 60% urea-formamide at 60°C and 80 V for 16 h using a D-Code System (Bio-Rad Laboratories, Hercules, CA). The gels were stained with SYBR Gold (Molecular Probes, Inc., Eugene, OR), visualized with a Dark Reader transilluminator (Clare Chemical Research, Inc., Dolores, CO), and photographed with a Canon Powershot A70 digital camera (Canon USA, Inc., Chesapeake, VA).
Sterile syringe needles were used to core DGGE bands (approximately one-third of the band width), and excised cores were transferred into sterile PCR tubes for reamplification and subsequent DGGE for checking efficacy of band isolation. This procedure was repeated three to five times. Nonetheless, some bands appeared pure, while others were still “contaminated” with comigrating bands. To resolve band purity and obtain reliable sequences, “isolated” bands were reamplified using unclamped primers, ligated into pCR2.1 vectors, and transformed into Escherichia coli INVaF′ competent cells by using a TA cloning kit (Invitrogen, Carlsbad, CA). White colonies were picked from agar plates (10 colonies from each ligation sample), and plasmid inserts were amplified using primers AmoA-1F-GC clamp and AmoA-2R-TC. A final DGGE was required before sequencing of the bands to confirm that amplified inserts and originally excised bands had the same migrating patterns. To check reproducibility, two pairs of bands with identical migrating patterns but from different environmental samples were also sequenced.
Sequencing.
Selected clones were sequenced at the Duke University DNA Sequencing Facility using a Perkin-Elmer dye terminator cycle sequencing system with AmpliTaq DNA polymerase combined with ABI 3730 and 3100 PRISM DNA sequencing instruments and BigDyeTMv1.1 terminator.
Oligonucleotide probes and slot blot hybridizations.
The oligonucleotide probes targeting the 16S rRNAs of nitrifiers used in slot dot hybridizations are listed in Table 1. The probes were obtained from Sigma-Genosys (The Woodlands, TX). Probes were 5′ end labeled with [γ-32P]ATP (ICN Radiochemicals, Irvine, California) and T4 polynucleotide kinase (Promega Corp., Madison, Wisconsin) and purified with a Quickspin Oligo column (Roche Molecular Biochemicals, Indianapolis, Indiana). Membranes with immobilized RNA were hybridized as previously described (27) and washed at the appropriate wash temperatures (Table 1). The results were expressed as percentages of the total rRNA as measured with the universal probe.
TABLE 1.
Oligonucleotide probes used in slot blot hybridizations
| Probe
|
Target siteb | Sequence (5′ to 3′) | Wash temp (°C) | Target organisms | Reference | |
|---|---|---|---|---|---|---|
| Namea | Abbreviation | |||||
| S-*-Univ-1390-a-A-18 | Univ1390 | 1390-1407 | GACGGGCGGTGTGTACAA | 44 | All | 43 |
| S-G-Nsm-0156-a-A-19 | Nsm156 | 156-174 | TATTAGCACATCTTTCGAT | 46 | Nitrosomonas spp., Nitrosococcus mobilis | 29 |
| S-G-βAOB-1224-a-A-20 | Nso1225 | 1224-1243 | CGCCATTGTATTACGTGTGA | 51 | Betaproteobacterial ammonia-oxidizing bacteria | 29 |
| S-G-Nbac-1000-a-A-15 | Nb1000 | 1000-1014 | TGCGACCGGTCATGG | 42 | Nitrobacter spp. | 29 |
| S-G-Ntspa-0685-a-A-22 | Ntspa685 | 664-685 | CACCGGGAATTCCGCGCTCCTC | 63 | Nitrospira moscoviensis, Nitrospira marina | 20 |
Probe names have been standardized according to the Oligonucleotide Probe Database (3).
E. coli numbering.
RESULTS
Reactor performance.
The influent wastewater was the same for all reactors. However, biomass was subjected to unique NH3 and oxygen concentrations in each reactor, as influent feeding was semicontinuous and NH3 accumulation occurred during nonaerated periods. The nitrogen, pH, DO, and oxidation-reduction potential profiles for the reactors described in this study have been previously discussed (M. A. Head et al., submitted for publication). A summary of the data for nitrogen and DO profiles is presented in Table 2. Reactors B (ANA, 1 h:3 h) and E (ANA, 1 h:4 h), showed nitrite accumulation of up to 5.4 mg NO2−-N liter−1 and 7.8 mg NO2−-N liter−1, respectively, while the other reactors exhibited negligible nitrite accumulation during aeration periods. Only reactor E, with the longest nonaeration period, showed substantial nitrite reduction during nonaeration periods as indicated by an effluent nitrite concentration of 0.8 mg NO2−-N liter−1 at the end of nonaeration periods. Nitrite reduction in reactor B was minor, as indicated by an effluent nitrite concentration of 4.9 mg NO2−-N liter−1 at the end of nonaeration periods.
TABLE 2.
Summary of data for nitrogen and DO profiles during aeration cycles
| Reactor | ANA (h on:h off) | Maximum DO conc during aeration (mg liter−1)a | NH3 conc (mg N liter−1)
|
NO2− conc (mg N liter−1)
|
||
|---|---|---|---|---|---|---|
| Beginning of aeration | End of aeration | Beginning of nonaeration | End of nonaeration | |||
| A | 1:1 | 5.0 | 3.7 | 0.2 | 0.1 | 0.1 |
| B | 1:3 | 4.2 | 8.0 | 1.2 | 5.4 | 4.9 |
| C | 0.5:1.5 | 4.3 | 10.0 | 6.0 | 2.3 | 3.8 |
| D | 0.5:2 | 3.2 | 6.3 | 0.2 | 0.1 | 0.9 |
| E | 1:4 | 1.4 | 12.0 | 3.6 | 7.8 | 0.8 |
DO concentrations reached levels of below 0.3 mg liter−1 during nonaeration periods in all reactors.
Long-term operation of reactors and membrane hybridizations.
Table 3 shows average effluent concentrations during aeration periods from the five reactors for 1 year of operation. The reactors showed stable performance for most of the days sampled. However, short periods of efficiency instability occurred in all reactors, resulting in relatively high standard deviations for the effluent data shown in Table 3. Reactor C had the highest mean effluent ammonia concentrations (32 mg NH3-N liter−1), and reactor B had the lowest mean effluent ammonia concentrations (22 mg NH3-N liter−1). However, mean effluent ammonia concentrations were not significantly different among reactors (analysis of variance [ANOVA] P value of 0.7212; α = 0.5), indicating that the AOB communities in all reactors could oxidize ammonia to similar levels during aerated periods. Effluent nitrite and nitrate concentrations were significantly different among reactors (ANOVA P values of 0.003 and 0.004 for nitrite and nitrate, respectively; α = 0.5). Reactor A, with the shortest nonaerated period, had the lowest mean effluent nitrite concentration. Reactor E, with the longest nonaerated period, had the highest mean effluent nitrite concentration. These findings suggest that NOB are more sensitive than AOB to longer nonaerated periods. Reactor B had the lowest mean effluent nitrate concentration, and reactor C had the highest mean effluent nitrate concentration.
TABLE 3.
Mean effluent concentrations of nitrogen species during aeration cycles
| Reactor | ANA (h on:h off) | Mean effluent conc (mg liter−1)a
|
|||
|---|---|---|---|---|---|
| NH3-N | NO2-N | NO3-N | TKN | ||
| A | 1:1 | 22.8 (39.5) | 0.7 (0.7) | 14.9 (20.1) | 40.5 (45.4) |
| B | 1:3 | 22.0 (33.0) | 1.9 (1.8) | 10.3 (6.3) | 51.5 (41.9) |
| C | 0.5:1.5 | 31.9 (43.5) | 2.5 (2.4) | 22.7 (22.0) | 55.4 (50.8) |
| D | 0.5:2 | 23.7 (36.5) | 2.5 (2.3) | 16.9 (10.9) | 48.0 (43.6) |
| E | 1:4 | 23.9 (37.4) | 2.8 (4.6) | 15.7 (10.8) | 49.3 (45.2) |
| ANOVA P valueb | 0.721 | 0.003 | 0.004 | 0.597 | |
A total of at least 40 data points were used to calculate mean effluent concentrations. Standard deviations are in parentheses.
Single-factor ANOVA.
The fractions of AOB (total β-AOB) and NOB (including Nitrobacter and Nitrospira) in the biomass were also monitored, and the average values are shown in Table 4. The mean fraction of AOB was not significantly different among reactors (ANOVA P value of 0.376; α = 0.5). Nitrosomonas and Nitrosoccocus were the dominant AOB in all reactors, accounting for more than 70% of the total AOB fraction. Reactors C and D, both with the shortest aeration period (0.5 h), showed the lowest Nitrosomonas-Nitrosoccocus mean percentage of total β-AOB among the reactors. Nitrospira was the dominant group of NOB in all reactors, accounting for more than 73% of total NOB. Reactors B and E, both with the longest nonaeration periods (3 and 4 h), showed the lowest mean Nitrobacter, Nitrospira, and total NOB (Nitrobacter plus Nitrospira) fractions among the reactors, suggesting that long nonaeration periods have a strong effect on the biomass levels of NOB. Reactors C and D, both operated with 0.5 h of aeration, did not show significantly different total NOB biomass fractions than reactors with longer aeration periods, suggesting that short aeration periods did not have considerable impacts on the NOB populations in the reactors.
TABLE 4.
Mean fraction of nitrifying bacteria measured with slot blot hybridizations and results of ANOVA
| Target microorganism(s) (probes) | Avg levels of nitrifiers (% total rRNA)a in reactor:
|
P valueb | ||||
|---|---|---|---|---|---|---|
| A (1 h:1 h) | B (1 h:3 h) | C (0.5 h:1.5 h) | D (0.5 h:2 h) | E (1 h:4 h) | ||
| AOB | ||||||
| Nitrosomonas + N. mobilis (Nsm156/Univ1390) | 9.1 (3.3) | 11.3 (5.1) | 12.9 (5.0) | 10.0 (4.1) | 13.6 (5.1) | 0.130 |
| Total AOB (Nso1225/Univ1390) | 11.4 (4.4) | 13.2 (9.0) | 17.2 (9.7) | 14.4 (5.7) | 15.2 (6.1) | 0.376 |
| Nitrosomonas + N. mobilis/β-AOB | 83.3 (11.6) | 85.9 (15.7) | 78.9 (18.2) | 71.9 (18.7) | 89.2 (15.1) | 0.095 |
| NOB | ||||||
| Nitrospira (Ntspa685/Univ1390) | 7.0 (1.6) | 5.3 (2.1) | 5.9 (2.9) | 6.4 (2.8) | 4.8 (1.8) | 0.168 |
| Nitrobacter (Nb1000/Univ1390) | 2.6 (2.3) | 1.6 (0.9) | 2.2 (1.3) | 2.2 (2.1) | 1.1 (0.4) | 0.158 |
| Total NOB (Ntspa685 + Nb1000/Uni) | 9.5 (3.1) | 6.9 (2.5) | 8.1 (3.7) | 8.6 (4.0) | 5.8 (2.0) | 0.049 |
| Nitrospira/Total NOB | 75.5 (14.1) | 77.0 (9.7) | 73.2 (12.5) | 75.0 (13.4) | 81.4 (5.5) | 0.497 |
A total of 12 data points were used to calculate mean rRNA levels. Standard deviations are in parentheses. All membrane hybridization measurements were performed in triplicate.
P values from single-factor ANOVA.
amoA DGGE profiles.
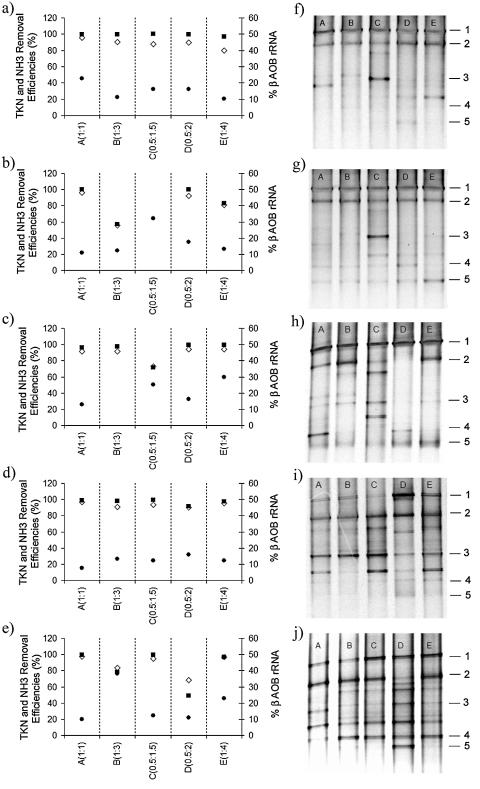
The community structure of AOB and removal efficiencies of TKN and ammonia were monitored in each reactor throughout the experiment (Fig. 1). This approach was used to (i) visually assess the stability of AOB community structure and (ii) investigate whether ammonia and TKN removal efficiencies could be related to AOB diversity or to specific AOB species. amoA DGGE profiles showed frequent shifts in the composition and diversity of AOB communities, as indicated by the appearance and disappearance of certain bands with time. Five bands were successfully isolated and sequenced. BLAST analysis of the sequences revealed that the sequence of band 1 (Fig. 1) shared 99% sequence similarity to the amoA gene of Nitrosomonas sp. strain Nm107 (Table 5). The sequences of bands 2 and 3 shared 91 and 95% sequence similarity to the amoA sequence of Nitrosomonas europaea, respectively. The sequence of band 4 was 91% similar to that of Nitrosomonas sp. strain ENI-11, and the sequence of band 5 shared 92% sequence similarity to the amoA gene of Nitrosospira multiformis.
FIG. 1.
Comparison between composition of AOB populations and ammonia and TKN removal efficiencies in intermittently aerated reactors. (a to e) ⋄, TKN removal efficiency; ▪, ammonia removal efficiency; •, rRNA fraction of AOB in the Betaproteobacteria subgroup. (f to j) amoA DGGE profiles. Sequence analysis of bands 1 to 5 is discussed in the text and summarized in Table 5. Panels a and f correspond to day 78, b and g correspond to day 170, c and h correspond to day 226, d and i correspond to day 281, and e and j correspond to day 310.
TABLE 5.
Nearest GenBank relatives of amoA gene fragments retrieved in this study
| DGGE band | GenBank accession no. | Phylotype with highest sequence similarity | Accession no. of phylotype | % Sequence similarity |
|---|---|---|---|---|
| 1 | DQ088390 | Nitrosomonas sp. strain Nm107 | AF272407 | 99 |
| 2 | DQ088391 | Nitrosomonas europaea ATCC 19718 | BX321863 | 91 |
| 3 | DQ088392 | Nitrosomonas europaea ATCC 19718 | BX321863 | 95 |
| 4 | DQ088393 | Nitrosomonas sp. strain ENI-11 | AB079055 | 91 |
| 5 | DQ088394 | Nitrosospira multiformis | AY177933 | 92 |
Comparison between sequences of band pairs with identical migrating patterns but from different environmental samples resulted in identical sequences for one pair and a total of two mismatches for the other pair of bands (data not shown).
Although environmental conditions in the reactors were considerably different, practically all amoA DGGE profiles showed the same two dominant bands (bands 1 and 2). Band 3 was particularly strong in virtually all amoA DGGE profiles of reactor C (0.5 h:1.5 h) and was occasionally present in the other reactors. Bands 4 and 5 were only occasionally detected in all reactors and usually with faint signals, except for Fig. 1h and j, which show band 4 as a major band. Throughout the experiment, amoA DGGE profiles showed a total of nine different bands, with increasing AOB species diversity (based on the number of bands) toward the end of the experiment.
High ammonia and TKN removal efficiencies were observed for samples with various amoA DGGE profiles, including samples with high and low AOB species diversity. Similarly, amoA DGGE profiles showing high AOB diversity, as well as amoA DGGE profiles with low AOB diversity, were shown for days with poor ammonia and TKN removal performance.
DISCUSSION
Nitrite accumulation is a result of the ammonia oxidation rate exceeding the rate of nitrite oxidation. Differences in oxidation rates can be attributed to AOB outcompeting NOB due to a number of factors, including free ammonia inhibition, dissolved oxygen concentration, organic matter and volatile fatty acid concentrations, temperature, and pH (2, 12, 34, 42). Accumulation of nitrite at low DO is usually attributed to the difference in the saturation constant for DO between AOB and NOB (16). Values in the literature for the saturation constants of AOB and NOB in activated sludge range from 0.25 to 0.5 mg O2 liter−1 and from 0.34 to 2.5 mg O2 liter−1, respectively (6). Hanaki et al. (16) reported that AOB remained unaffected in a suspended-growth reactor operated at low DO (<0.5 mg liter−1), while NOB were strongly inhibited, resulting in accumulation of nitrite to 60 mg liter−1. In addition, AOB have been found to more readily recover from periods of substrate starvation than NOB, resulting in occasional nitrite accumulation after ammonia becomes available again (13, 40). Peng et al. (33) reported that AOB developed an ability to endure DO fluctuations but that NOB did not. In this study, NOB and AOB were found to adjust well to varying DO levels when nonaeration cycles were below 2 h. NOB were affected only by long nonaeration periods of 3 and 4 h, resulting in lower NOB biomass fractions and nitrite accumulation during aeration periods. Short aeration periods (0.5 h) did not affect NOB populations significantly, and the nitrification process was carried all the way to nitrate during aeration. Therefore, not only the dissolved oxygen concentration but also the length of nonaeration periods is important in the selection of AOB over NOB in intermittently aerated reactors.
High ammonia concentrations have been shown to select AOB over NOB, resulting in nitrite accumulation. Generally, AOB have lower ammonia inhibition constants and can survive at elevated ammonia concentrations (42). Since influent feeding of the reactors was semicontinuous, ammonia accumulation occurred during nonaeration periods and biomass in reactors with different aeration cycles was subjected to unique ammonia concentrations. However, ammonia inhibition of NOB was likely not important, since usually only free ammonia is taken into consideration in the context of inhibition and free ammonia is negligible at the pH range (7.6 to 7.8) and total ammonia concentrations (1.2 to 64.0 mg NH3-N) maintained in this study.
DGGE based on amoA genes has been previously used to study the AOB communities in soil, biofilm, and activated sludge (4, 28, 32). For most groups of AOB, a high consistency has been found when comparing phylogenetic trees based on 16S rRNA and amoA sequences (1). We used methods targeting two AOB genes: 16S rRNA membrane hybridizations to estimate the biomass fraction of AOB at the genus level and amoA DGGE to monitor AOB community structure at the species level. 16S rRNA membrane hybridizations and amoA DGGE results corroborated each other for the ammonia- oxidizing bacteria, as both methods indicated the dominance of Nitrosomonas and N. mobilis in the AOB populations assessed. Although using DGGE has many advantages and applications, problems may arise due to PCR bias, heterogeneity of copy number of 16S rRNA among species, and the fact that single DGGE bands do not always represent a single bacterial strain (37). However, it was shown in this study that sequences of bands from different environmental samples and identical migrating patterns were identical or differed by only two base pairs. Avrahami and Conrad (5) have shown that sequences of amoA bands that migrated identically but originated from different environmental samples were always found to be identical by amino acid sequence and only occasionally showed differences at no more than two base pairs. Bias caused by multiple copies of amoA within one organism can be excluded, because only nondegenerate amoA primers were used in this study. In addition, previous amplification of the amoA genes from 31 pure cultures with the same primers resulted in unambiguous sequences (1, 5, 31).
Membrane hybridizations showed that the biomass fraction of Nitrosomonas and N. mobilis far exceeded that of Nitrosospira in all reactors, suggesting that the former were responsible for most of the ammonia oxidation. These results concur with a number of previous studies that have suggested that Nitrosomonas can outcompete Nitrosospira in environments with high nitrogen loads (14, 17, 21). amoA DGGE profiles showed that a close relative to Nitrosomonas sp. strain Nm107 corresponded to the dominant band in virtually all samples analyzed. Nitrosomonas sp. strain Nm107 was first isolated from an activated sludge rendering plant and is believed to be a strain of Nitrosococcus mobilis based on its 16S rRNA (35). In addition, the amoA sequences of Nitrosomonas sp. strain Nm107 and Nitrosococcus mobilis were shown to be identical (35). Nitrosococcus mobilis was isolated from a sample of brackish water and is characterized by an obligate salt requirement, being considered a moderate halophilic AOB (24). The maximum growth rate of Nitrosococcus mobilis reported for pure cultures is close to that of N. europaea/N. oligotropha (14, 24). Juretschko et al. (21) used fluorescent in situ hybridization to confirm that N. mobilis was the numerically dominant AOB in the nitrifying activated sludge of an industrial wastewater treatment plant receiving sewage with high ammonia concentrations. Gieseke et al. (14) detected coexistence of Nitrosococcus mobilis and Nitrosomonas europaea/N. oligotropha in a biofilm from a nitrifying pilot-scale sequencing batch reactor treating nitrogen-rich wastewater loads. Although Nitrosomonas sp. strain Nm107 corresponded to the dominant band in practically all amoA DGGE profiles, it may not have been the numerically dominant or the most active AOB in the reactors due to the inherent qualitative rather than quantitative feature of PCR-DGGE.
Regardless of the significant differences in the environments created by each of the aeration cycles, comparison of amoA DGGE profiles showed that AOB species were rather similar among the reactors. This suggests that factors other than the operating parameters tested had a stronger influence on AOB species. The concomitant increase in AOB species diversity in all reactors during the last phase of the investigation suggests that AOB species diversity was affected by an environmental factor common to all reactors. Given that temperature, pH, and aeration cycles remained unchanged, influent characteristics are probably the strongest factor affecting AOB species diversity. Since the influent was collected from an anaerobic lagoon treating swine wastewater, it was impossible to control numerous potential factors affecting AOB species, such as ammonia concentrations, wastewater salinity, metal concentrations, organic matter, and solids content. Previous studies have shown that the microbial community in identically operated laboratory-scale reactors can differ significantly while showing similar performances (22). Therefore, when assessing the effect of environmental parameters on the microbial communities in laboratory-scale experiments, it is desirable to test reproducibility by either running duplicates or operating the reactors for long periods (a total of 1 year in this study).
In the process of denitrification in intermittently aerated reactors, the lengths of the sequential aeration and nonaeration periods within the residence time are also of significance (34). As the induction of the different denitrification enzymes proceeds sequentially, the different intermediates of denitrification accumulate temporarily during nonaeration periods. Oxygen inhibits the synthesis of nitrate reductase only partially, while the synthesis of nitrite reductase is completely suppressed (34). At the start of anoxic conditions after an aerobic period in intermittently aerated reactors, only the nitrate reduction takes place, leading to a temporary nitrite accumulation (26). Therefore, the length of nonaeration periods in intermittently aerated reactors is also important to achieve complete denitrification. Baumann et al. (7) assessed mRNA and the enzymes involved in denitrification in activated sludge reactors operated with intermittent aeration and concluded that denitrifying bacteria were not able to completely synthesize the enzymes for the denitrification process in appropriate amounts when subjected to nonaerated stages shorter than 3 h, resulting in the accumulation of nitrite during nonaeration periods. In the current study, only the reactor with a 4-h nonaeration period showed considerable nitrite reduction during nonaeration periods.
Comparisons of amoA DGGE profiles and TKN and ammonia removal efficiencies suggest that superior ammonia and TKN removal efficiencies did not seem to be associated with specific AOB species or with higher AOB species diversity. These results seem to contradict the generally accepted concept that higher functional diversity is beneficial for performance stability (8, 11). However, this concept is necessarily related to the diversity of the microorganisms that are metabolically active in the process and not merely the species diversity of microorganisms present in the biomass. Although amoA is one of the functional genes involved in the oxidation of ammonia, PCR amplification of environmental amoA genes does not reveal gene expression or microbial activity. Rather, amplification of the amoA gene was used only as an alternative method for identifying AOB and hence can give an indication only of species diversity and not of functional diversity. A more appropriate approach for testing correlation of functional diversity and performance stability would involve methods that can determine which processes are catalyzed by which microorganisms and to what extents.
Acknowledgments
We thank the U.S. Department of Agriculture National Research Initiative Program (grant 2001-35102-10783) for funding this research.
REFERENCES
- 1.Aakra, A., J. B. Utaker, and I. F. Nes. 2001. Comparative phylogeny of the ammonia monooxygenase subunit A and 16S rRNA genes of ammonia-oxidizing bacteria. FEMS Microbiol. Lett. 205:237-242. [DOI] [PubMed] [Google Scholar]
- 2.Abeling, U., and C. F. Seyfried. 1992. Anaerobic-aerobic treatment of high-strength ammonium wastewater—nitrogen removal via nitrite. Water Sci. Technol. 26:1007-1015. [Google Scholar]
- 3.Alm, E. W., D. B. Oerther, N. Larsen, D. A. Stahl, and L. Raskin. 1996. The oligonucleotide probe database. Appl. Environ. Microbiol. 62:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avrahami, S., R. Conrad, and G. Braker. 2002. Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers. Appl. Environ. Microbiol. 68:5685-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avrahami, S., and R. Conrad. 2003. Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl. Environ. Microbiol. 69:6152-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes, D., and P. J. Bliss. 1983. Biological control of nitrogen in wastewater treatment. E. & F.N. Spon, New York, N.Y.
- 7.Baumann, B., M. Snozzi, J. R. van der Meer, and A. J. B. Zehder. 1997. Development of stable denitrifying cultures during repeated aerobic-anaerobic transient periods. Water Res. 31:1947-1954. [Google Scholar]
- 8.Briones, A., and L. Raskin. 2003. Diversity and dynamics of microbial communities in engineered environments and their implications for process stability. Curr. Opin. Biotechnol. 14:270-276. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, J., and B. Liu. 2001. Nitrification/denitrification in intermittent aeration process for swine wastewater treatment. J. Environ. Eng. 127:705-711. [Google Scholar]
- 10.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton. 1995. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 11.Dejonghe, W., N. Boon, D. Seghers, E. M. Top, and W. Verstraete. 2001. Bioaugmentation of soils by increasing microbial richness: missing links. Environ. Microbiol. 3:649-657. [DOI] [PubMed] [Google Scholar]
- 12.Garrido, J. M., W. A. J. Van Benthum, M. C. M. Van Loosdrecht, and J. J. Heijnen. 1997. Influence of dissolved oxygen concentration on nitrite accumulation in a biofilm airlift suspension reactor. Biotechnol. Bioeng. 53:168-178. [DOI] [PubMed] [Google Scholar]
- 13.Gerards, S., H. Duyts, and H. J. Laanbroek. 1998. Ammonium-induced inhibition of ammonium-starved Nitrosomonas europaea cells in soil and sand slurries. FEMS Microbiol. Ecol. 26:269-280. [Google Scholar]
- 14.Gieseke, A., L. Bjerrum, M. Wagner, and R. Amann. 2003. Structure and activity of multiple nitrifying bacterial populations co-existing in a biofilm. Environ. Microbiol. 5:355-369. [DOI] [PubMed] [Google Scholar]
- 15.Han, D. W., J. S. Chang, and D. J. Kim. 2002. Nitrifying microbial community analysis of nitrite accumulating biofilm reactor by fluorescence in situ hybridization. Water Sci. Technol. 47:97-104. [PubMed] [Google Scholar]
- 16.Hanaki, K., W. Chalermraj, and O. Shinichiro. 1990. Nitrification at low levels of dissolved oxygen with and without organic loading in a suspended-growth reactor. Water Res. 24:297-302. [Google Scholar]
- 17.Harms, G., A. Layton, H. Dionisi, I. Gregory, V. Garret, S. Hawkins, K. Robinson, and B. Sayler. 2003. Real-time PCR quantification of nitrifying bacteria in a municipal wastewater treatment plant. Environ. Sci. Technol. 37:343-351. [DOI] [PubMed] [Google Scholar]
- 18.Hastings, R. C., J. R. Saunders, G. H. Hall, R. W. Pickup, and A. J. McCarthy. 1998. Application of molecular biological techniques to a seasonal study of ammonia oxidation in a eutrophic freshwater lake. Appl. Environ. Microbiol. 64:3674-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellinga, C., A. A. J. C. Schellen, J. W. Mulder, M. C. M. Van Loosdrecht, and J. J. Heijnen. 1998. The Sharon process: an innovative method for nitrogen removal from ammonium-rich wastewater. Water Sci. Technol. 37:135-142. [Google Scholar]
- 20.Hovanec, T. A., L. T. Taylor, A. Blakis, and E. F. DeLong. 1998. Nitrospira-like bacteria associated with nitrite oxidation in freshwater aquaria. Appl. Environ. Microbiol. 64:258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juretschko, S., G. Timmermann, M. Schmid, K.-H. Schleifer, A. Pommerening-Röser, H.-P. Koops, and M. Wagner. 1998. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl. Environ. Microbiol. 64:3042-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaewpipat, K., and C. P. L. Grady, Jr.. 2002. Microbial population dynamics in laboratory-scale activated sludge reactors. Water Sci. Technol. 46:19-27. [PubMed] [Google Scholar]
- 23.Kim, D. J., J. S. Chang, D. I. Lee, D. W. Han, I. K. Yoo, and G. C. Cha. 2003. Nitrification of high strength ammonia wastewater and nitrite accumulation characteristics. Water Sci. Technol. 47:45-51. [PubMed] [Google Scholar]
- 24.Koops, H.-P., and H. Harms. 1985. Deoxyribonucleic acid homologies among 96 strains of ammonia-oxidizing bacteria. Arch. Microbiol. 141:214-218. [DOI] [PubMed] [Google Scholar]
- 25.Kowalchuk, G. A., P. L. E. Bodelier, G. H. J. Heilig, J. R. Stephen, and H. J. Laanbroek. 1998. Community analysis of ammonia-oxidizing bacteria in relation to oxygen availability in soils and root-oxygenated sediments using PCR, DGGE, and oligonucleotide probe hybridization. FEMS Microbiol. Ecol. 27:339-350. [Google Scholar]
- 26.Krul, J. M., and R. Veeningen. 1977. The synthesis of the dissimilatory nitrate reductase under aerobic conditions in a number of denitrifying bacteria, isolated from activated sludge and drinking water. Water Res. 11:39-43. [Google Scholar]
- 27.Liao, J., I. Lou, and F. L. de los Reyes III. 2004. Relationship of species-specific filament levels to filamentous bulking in activated sludge. Appl. Environ. Microbiol. 70:2420-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendum, T. A., and P. R. Hirsch. 2002. Changes in the population structure of β-group autotrophic ammonia oxidizing bacteria in arable soils in response to agricultural practice. Soil Biol. Biochem. 34:1479-1485. [Google Scholar]
- 29.Mobarry, B. K., M. Wagner, V. Urbain, B. E. Rittmann, and D. A. Stahl. 1996. Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl. Environ. Microbiol. 62:2156-2162. (Erratum, 63: 815, 1997.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mota, C. R., J. Ridenoure, J. Cheng, and F. L. de los Reyes III. 2005. High levels of nitrifying bacteria in intermittently-aerated reactors treating high ammonia wastewater. FEMS Microbiol. Ecol. 54:391-400. [DOI] [PubMed] [Google Scholar]
- 31.Nicolaisen, M. H., and N. B. Ramsing. 2002. Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria. J. Microbiol. Methods 50:189-203. [DOI] [PubMed] [Google Scholar]
- 32.Oved, T., A. Shaviv, T. Goldrath, R. T. Mandelbaum, and D. Minz. 2001. Influence of effluent irrigation on community composition and function of ammonia-oxidizing bacteria in soil. Appl. Environ. Microbiol. 67:3426-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng, D. C., N. Bernet, J. P. Delgenes, and R. Moletta. 2000. Effects of oxygen supply methods on the performance of a sequencing batch reactor for high ammonium nitrification. Water Environ. Res. 72:195-200. [Google Scholar]
- 34.Philips, S., H. J. Laanbroek, and W. Verstraete. 2002. Origin, causes and effects of increased nitrite concentrations in aquatic environments. Rev. Environ. Sci. Biotechnol. 1:115-141. [Google Scholar]
- 35.Purkhold, U., A. Pommerening-Röser, S. Juretschko, M. C. Schmid, H.-P. Koops, and M. Wagner. 2000. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl. Environ. Microbiol. 66:5368-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotthauwe, J.-H., K.-P. Witzel, and W. Liesack. 1997. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63:4704-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekiguchi, H., N. Tomioka, T. Nakahara, and H. Uchiyama. 2001. A single band does not always represent single bacterial strains in denaturing gradient gel electrophoresis analysis. Biotechnol. Lett. 23:1205-1208. [Google Scholar]
- 38.Stahl, D. A., B. Flesher, H. Mansfield, and R. Montgomery. 1988. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl. Environ. Microbiol. 54:1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephen, J. R., G. A. Kowalchuk, M. A. Bruns, A. E. McCaig, C. J. Phillips, T. M. Embley, and J. I. Prosser. 1998. Analysis of the β-subgroup ammonia oxidizer populations in soil by DGGE analysis and hierarchical phylogenetic probing. Appl. Environ. Microbiol. 64:2958-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tappe, W., A. Laverman, M. Bohland, M. Braster, S. Rittershaus, J. Groeneweg, and H. W. van Verseveld. 1999. Maintenance energy demand and starvation recovery dynamics of Nitrosomonas europaea and Nitrobacter winogradsky cultivated in a retentostat with complete biomass retention. Appl. Environ. Microbiol. 65:2471-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner, M., and A. Loy. 2002. Bacterial community composition and function in sewage treatment systems. Curr. Opin. Biotechnol. 13:218-227. [DOI] [PubMed] [Google Scholar]
- 42.Wyffels, S., P. Boeckx, K. Pynaert, W. Verstraete, and O. Van Cleemput. 2003. Sustained nitrite accumulation in a membrane-assisted bioreactor (MBR) for the treatment of ammonium-rich wastewater. J. Chem. Technol. Biotechnol. 78:412-419. [Google Scholar]
- 43.Zheng, D., E. W. Alm, D. A. Stahl, and L. Raskin. 1996. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl. Environ. Microbiol. 62:4504-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]