Abstract
Members of several bacterial lineages are known only as symbionts of insects and move among hosts through maternal transmission. Such vertical transfer promotes strong fidelity within these associations, favoring the evolution of microbially mediated effects that improve host fitness. However, phylogenetic evidence indicates occasional horizontal transfer among different insect species, suggesting that some microbial symbionts retain a generalized ability to infect multiple hosts. Here we examine the abilities of three vertically transmitted bacteria from the Gammaproteobacteria to infect and spread within a novel host species, the pea aphid, Acyrthosiphon pisum. Using microinjection, we transferred symbionts from three species of natural aphid hosts into a common host background, comparing transmission efficiencies between novel symbionts and those naturally infecting A. pisum. We also examined the fitness effects of two novel symbionts to determine whether they should persist under natural selection acting at the host level. Our results reveal that these heritable bacteria vary in their capacities to utilize A. pisum as a host. One of three novel symbionts failed to undergo efficient maternal transmission in A. pisum, and one of the two efficiently transmitted bacteria depressed aphid growth rates. Although these findings reveal that negative fitness effects and low transmission efficiency can prevent the establishment of a new infection following horizontal transmission, they also indicate that some symbionts can overcome these obstacles, accounting for their widespread distributions across aphids and related insects.
Bacteria and insects have evolved a diverse array of symbiotic interactions which play roles in insect nutrition (5, 7, 20, 22, 38, 76), defense (24, 37, 50, 51), reproduction, and development (11, 29, 35, 56, 66). Symbiotic bacteria harbored by insects are often heritable, i.e., they are transferred directly from mother to offspring (for an example, see references 9 and 62). This vertical (i.e., maternal) route of transmission aligns the interests of symbiotic partners, since symbiont spread depends upon host reproduction (10, 23, 40). As a result, the prevalence of a microbial symbiont will be determined by its net effects on host fitness and the efficiency of transmission. Both fitness benefits and highly efficient transmission will favor the spread of a heritable symbiont throughout a host population (for an example, see reference 71).
Aphids are an ideal group of organisms for studies of symbiosis. Nearly all of these phloem-feeding insects harbor the bacterium Buchnera aphidicola (45, 47, 48), an ancient heritable symbiont that is essential for growth and reproduction (21, 52). Many aphids contain additional heritable bacteria known as accessory, or secondary, symbionts (9, 12, 18, 26, 27, 60, 72). These are often present at intermediate frequencies within aphid species (14, 32, 65, 70) and are generally thought to be nonessential from their hosts' perspective (but see references 26 and 60). Like B. aphidicola, secondary symbionts are passed on from mother to offspring with high efficiency, approaching 100% in the lab (13, 19, 28, 60). Recent studies have identified benefits conferred by these microbes involving resistance to natural enemies (24, 55), heat tolerance (13, 42, 57), and host plant utilization (70) (but see reference 39). These effects provide adaptive explanations for secondary symbiont prevalence, supporting theoretical predictions.
Secondary symbionts of aphids have evolved from a wide range of bacterial clades. Several, including Arsenophonus species, “Candidatus Serratia symbiotica” (also known as R-type, S-sym, PASS), “Candidatus Hamiltonella defensa” (also known as T-type, PABS), and “Candidatus Regiella insecticola” (also known as U-type, PAUS), belong to the gamma-3 subdivision of the Proteobacteria and are related to free-living microbes such as Escherichia coli, Proteus vulgaris, Yersinia pestis, and Serratia species (44, 58, 60). Whereas the “Candidatus Serratia symbiotica” and “Candidatus Regiella insecticola” are currently described from multiple aphid families, Arsenophonus and “Candidatus Hamiltonella defensa” have been found in even more distantly related hosts. Both infect whiteflies and psyllids, phloem-feeding relatives of the aphids; Arsenophonus species have been identified in ticks and wasps (16, 18, 29, 31, 58, 60, 68, 77). Small genetic distances between bacteria infecting phylogenetically distant host species and incongruent host and symbiont phylogenies reveal that these four heritable symbionts have undergone occasional horizontal transmission between host species (58, 60, 77). This suggests that they have retained an ability to colonize and persist in many aphid species and even in other hosts. We currently lack a detailed understanding of the forces shaping symbiont host range and whether symbionts vary in their potential range of hosts.
To examine their capacities to infect novel hosts, we have transferred secondary symbionts from three aphid species into the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). Here, we describe the abilities of Arsenophonus and “Candidatus Regiella insecticola” isolates to undergo vertical transmission in this novel background. We compare the transmission efficiencies of these “novel symbionts” with those of “native” “Candidatus Regiella insecticola” originating from A. pisum. We also compare the effects of novel symbionts (Arsenophonus and “Candidatus Hamiltonella defensa”) on A. pisum fitness to effects induced by native symbionts (“Candidatus Serratia symbiotica,” “Candidatus Hamiltonella defensa,” and “Candidatus Regiella insecticola”). Our results reveal variation in both transmission rates and fitness effects among these heritable bacteria, suggesting two barriers that limit their natural distributions.
MATERIALS AND METHODS
Symbionts and aphids.
A. pisum was reared on synchronously aged, preflowering fava beans (Vicia faba) grown in a greenhouse at the University of Arizona. All cultures were reared on single plants in 4-inch-diameter pots, each enclosed by a clear Solo cup containing a mesh top for ventilation. These cultures were placed in growth chambers which were set for 18 h of light and 6 h of darkness (18L:6D) and a constant temperature of 20°C.
Four clones of A. pisum were used in the transmission efficiency experiment: “recipient” clones 5A and 7a and “donor” clones 2a and 8-10-1, all of which harbor B. aphidicola. Recipient clones lacked the pea aphid Rickettsia symbiont, Arsenophonus, “Candidatus Serratia symbiotica,” “Candidatus Hamiltonella defensa,” and “Candidatus Regiella insecticola,” as confirmed by diagnostic PCR (60; J. A. Russell, unpublished data). Donor clones were naturally infected with “Candidatus Regiella insecticola” and free of the other four secondary symbionts. Each A. pisum clone was started from a single parthenogenetic female collected from alfalfa in the Finger Lakes region of New York in 2000 (7a, 2a, and 8-2b) or 2001 (8-10-1) or from alfalfa (5A) or black medic (2BB) in Arlington, Wisconsin. We occasionally performed diagnostic PCR screens on each of these clones to confirm their secondary symbiont complements (58). Our results resembled those from previous studies (14, 19, 60), revealing stable infections and highly efficient transmission.
Three aphid species were used as donors of novel symbionts. First, a Myzocallis species feeding on oak (Quercus virginiana) on the University of Arizona campus was found to harbor a symbiont related to Arsenophonus secondary symbionts of psyllids and whiteflies and to the male-killing Arsenophonus nasoniae of the wasp Nasonia vitripennis (58). The symbiont was detected in all surveyed individuals within this population (14/14 collected from 2001 to 2003) according to PCR assays, and no other secondary symbionts were identified in these aphids (0/6 for “Candidatus Serratia symbiotica,” 0/6 for “Candidatus Hamiltonella defensa,” 0/6 for “Candidatus Regiella insecticola,” and 0/5 for Wolbachia) (58; Russell, unpublished). Diagnostic PCR revealed an absence of Arsenophonus from over 100 A. pisum individuals collected from New York and Wisconsin (Russell, unpublished) and a similar absence from Japanese populations of A. pisum (70), indicating that Arsenophonus is not a common associate of this aphid. Second, Macrosiphum euphorbiae aphids feeding on Penstemon species in Tucson, AZ, were infected with “Candidatus Regiella insecticola” at a high frequency (7/7 infected across 1999 and 2001) (58; Russell, unpublished). These aphids harbored “Candidatus Hamiltonella defensa” at lower frequencies (1/7), whereas all individuals were negative for “Candidatus Serratia symbiotica” (0/7) and pea aphid Rickettsia (0/3). The 16S rRNA sequence of the “Candidatus Regiella insecticola” symbiont (GenBank accession no. AY136149) displayed 99.2% similarity to the “Candidatus Regiella insecticola” isolate from A. pisum clone 2a (GenBank accession no. AY136138) (58). Finally, diagnostic PCR demonstrated that Aphis craccivora (from alfalfa in Marana, AZ) harbored a “Candidatus Hamiltonella defensa” isolate with 99.7% similarity (GenBank accession no. AY136136) to the 16S rRNA gene of the isolate from A. pisum clone 8-2b (GenBank accession no. AY136141) (58). Clonal A. craccivora colonies were established in the lab and reared on alfalfa with 16 h of light and 8 h of darkness and at a constant 22°C.
Transmission efficiency. (i) Injections.
Secondary symbionts reside in multiple locations within their aphid hosts, including the bacteriocytes and hemolymph (9, 12, 28, 60). Previous studies have reported the successful transfer of these bacteria between aphids by microinjection of hemolymph from an infected aphid (13, 27, 50). We used a similar approach to transfer symbionts among aphids, first extracting body fluids from late instar juveniles with a glass microcapillary tube pulled into a fine needle. The contents were injected into the abdominal segments of second to fourth instar juveniles which were immobilized on a pipette tip attached to a vacuum on the stage of a dissecting microscope. Injected aphids were placed onto fava bean plants and reared with 18L:6D and at 20°C. Field-collected M. euphorbiae and Myzocallis individuals were used as symbiont donors. Alternatively, A. pisum (2a and 8-10-1) donors were obtained from clonal, lab-reared cultures, as were the recipient A. pisum (5A and 7a).
(ii) Measuring maternal transfer.
To determine the capacity for symbionts to infect novel host species, we compared maternal transmission efficiencies of Arsenophonus and “Candidatus Regiella insecticola” in A. pisum to those of native “Candidatus Regiella insecticola” symbionts originating from other A. pisum clones. Several dozen A. pisum juveniles were injected over a 2- to 4-day period in both March 2002 (83 to 85 per treatment) and April 2003 (116 to 120 per treatment). These individuals were reared on fava beans until 6 days postinjection, when survivors were counted. Wounding from injections led to high mortality that differed between the time blocks (18 to 25% survival for 2002 and 48 to 56% survival for 2003). However, chi-square tests revealed no differences in survival among the different injection treatments.
At day 6, survivors from each treatment were used to start single female lineages. To obtain estimates of transmission over time, we collected and froze offspring (F1) born to these females between 8 to 10, 12 to 14, and 16 to 20 days postinjection. Based on previous findings (14), we expected efficient transmission beginning close to 2 weeks and comparatively inefficient transmission before this time. Data on offspring produced between days 12 and 14 were available only for the March 2002 experiment.
Single female survivors were collected and frozen at 18 or 20 days postinjection. Lineages descended from these aphids were perpetuated for at least 4 generations using single female parents to seed each generation. Lines were subsequently maintained by transferring multiple females to new fava bean plants at approximately 2 week intervals. We froze and screened offspring from several of these lineages over multiple generations to measure transmission efficiency and to aid in our selection of lines for use in future experiments.
DNA extractions were performed on single aphids as previously described (4, 60), and samples were assayed for symbiont infection using diagnostic PCR, in which symbiont-specific primers for the 16S rRNA gene (Ars1015F, 5′-ATCCAGCGAATCCTTTAG-3′, for Arsenophonus; U1279F, 5′-CGAACGTAAGCGAACCTCAT-3′, for “Candidatus Regiella insecticola”) (58) were paired with a more general, or “universal” bacterial primer from the 23S rRNA gene (35R, 5′-CCTTCATCGCCTCTGACTGC-3′). Diagnostic primers were perfect matches to 16S rRNA sequences deposited in GenBank under accession numbers AY136138 and AY136149 (“Candidatus Regiella insecticola” of A. pisum and M. euphorbiae) and AY136153 (Arsenophonus of the Myzocallis sp.). By amplifying across the intergenic spacer between the 16S and 23S rRNA genes, we obtained products of specific and repeatable lengths and excluded the possibility of amplification of B. aphidicola, in which these two genes are not contiguous. Thus, our diagnostic screening assays utilized two types of information: presence or absence of product and product size.
PCR assays were conducted in 10-μl volumes as described previously (58). Briefly, each reaction mixture contained 5.92 μl water, 1 μl of Eppendorf Taq buffer (with 15 mM Mg2+), 1 μl of 10 mM deoxynucleoside triphosphates, 0.8 μl of each primer (at 5 μM), and 0.08 μl of Eppendorf Taq polymerase. The recipe was the same for Arsenophonus screens, except that 5.52 μl of water was added along with 0.4 μl of 25 mM Mg2+, giving a final magnesium concentration of 2.5 mM (versus 1.5 mM for the other reaction mixtures). PCR cycling conditions were as follows: 1 cycle of 94°C for 2 min; 35 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 2 min; and 1 cycle of 72°C for 6 min. Products were electrophoresed in 2% agarose gels and run side-by-side with a 1-kb Plus DNA ladder (Invitrogen), used as a size standard.
To determine the quality of PCR templates, all DNA samples were assayed with the primers Buch 757F (5′-GAGGAATACCYKTGGCGAAA-3′) and 1507R (5′-TACCTTGTTACGACTTCACCCCAG-3′) (60), which amplify a portion of the B. aphidicola 16S rRNA gene. Since all A. pisum lines harbor Buchnera, we excluded samples that failed to amplify with these primers, as these were likely poor in quality.
Fitness experiments.
Aphids are cyclical parthenogens, and can be reared clonally in the laboratory. This enabled us to create several genetically identical lines of A. pisum which differed only in their infection status. The selected recipient clone (5A) was naturally free of secondary symbionts, and through microinjections we obtained a total of 6 lines that were examined in fitness experiments: (i) uninfected, (ii) infected with a native “Candidatus Serratia symbiotica” isolate (“Candidatus Serratia symbiotica” from clone 2BB), (iii) infected with a native “Candidatus Hamiltonella defensa” isolate (“Candidatus Hamiltonella defensa”A. pisum from clone 8-2b), (iv) infected with a native “Candidatus Regiella insecticola” isolate (“Candidatus Regiella insecticola”A. pisum from clone 2a), (v) infected with a novel “Candidatus Hamiltonella defensa” isolate from Aphis craccivora (“Candidatus Hamiltonella defensa”A. craccivora), and (vi) infected with the novel Arsenophonus isolate from the Myzocallis species. The Arsenophonus-infected line was descended from a single female injected with hemolymph from a field-collected Myzocallis donor. All other infected lines were descended from single females injected with hemolymph from a lab-reared donor. PCR indicated that these lines were consistently infected with the expected symbionts, revealing the stability of artificial infections.
To determine the outcomes of interactions with novel and native symbionts, we measured and compared mean relative growth rates (MRGR) between these aphid lines. This measure provided an estimate of aphid fitness by incorporating data on adult and birth weights along with development time. We also compared survival among these lineages, estimating this fitness component as the proportion of aphids reaching adulthood. All fitness experiments were conducted after at least 10 generations postinjection, preventing any confounding effects of wounds received in the injection treatment.
In August 2002, we conducted our first set of fitness experiments, examining MRGR and survival in three separate time blocks for five lines of A. pisum clone 5A (uninfected or infected with “Candidatus Serratia symbiotica,” “Candidatus Hamiltonella defensa”A. pisum, “Candidatus Regiella insecticola”A. pisum, or Arsenophonus) which had been maintained synchronously and under identical conditions for at least two generations. Adult aphids of similar age were used to produce experimental insects which were weighed in a tin foil boat on a microbalance (Cahn 29 Automatic Electrobalance) within 90 min of birth. These aphids were placed onto fava beans (one aphid per plant) and reared at 20°C with 18L:6D. We checked cultures for adults daily, beginning on day 5, distinguishing mature aphids from juveniles through morphological features, including a prominent increase in cauda length. Aphids were weighed, for a second time, on their first day of adulthood. Using our estimates of development time, along with birth and adult weights, MRGR was calculated as (ln adult weight − ln birth weight)/days to adulthood.
In the summer of 2003, we conducted another experiment to examine the effects of “Candidatus Hamiltonella defensa”A. craccivora on A. pisum hosts. MRGR was estimated at both 20°C and 25°C for A. pisum 5A lines which were free of secondary symbionts (N) or infected with either the native (“Candidatus Hamiltonella defensa”A. pisum) or novel (“Candidatus Hamiltonella defensa”A. craccivora) isolate. The design of this experiment was identical to that for the 2002 experiments, except that cultures were checked for adults at 12 (versus 24)-hour intervals to increase resolution.
Statistics.
All statistical analyses were performed using JMPIN, version 3.2.6. Transmission efficiency in the first generation was compared between symbionts using logistic regression, treating the broods of single females as replicate units. Individual offspring of single females were scored as infected or uninfected based on the presence or absence of properly sized products obtained through diagnostic PCR assays. Logistic regression compared the odds of transmission of both the “Candidatus Regiella insecticola” isolate from M. euphorbiae (“Candidatus Regiella insecticola”M. euphorbiae) and the Arsenophonus symbiont to those for the native “Candidatus Regiella insecticola” of A. pisum (“Candidatus Regiella insecticola”A. pisum). In all analyses, we pooled the results of transmission in clones 7a and 5A and of the “Candidatus Regiella insecticola” isolates from clones 8-10-1 and 2a, having found no effects of the A. pisum genotype or “Candidatus Regiella insecticola”A. pisum isolate on transmission efficiency (data not shown). Separate analyses were conducted on offspring born at different times postinjection (8 to 10 days, 12 to 14 days, and 16 to 20 days postinjection). Coefficients from logistic regression equations represented the log odds of the difference in transmission success (proportion of infected offspring) between the novel symbionts versus the native “Candidatus Regiella insecticola”A. pisum. These estimates were back transformed (by taking the inverse natural log), and we refer to these values for all discussions in the text to clarify differences in transmission.
In estimating the effects of symbionts on aphid performance, we compared survival between lines using a chi-square test. We also performed analysis of variance to compare MRGR among aphids harboring different symbionts, assessing statistical differences between pairs of A. pisum lines through contrast tests.
RESULTS
Maternal transmission of novel and native symbionts.
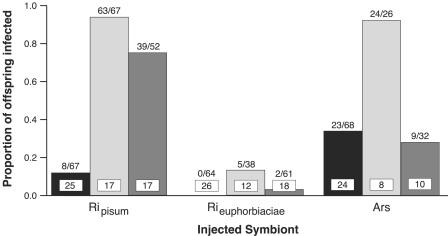
Symbionts were detected in offspring produced 8 to 10 days postinjection for 7/24 parents injected with “Candidatus Regiella insecticola”A. pisum and 14/23 parents treated with the novel Arsenophonus symbiont. In contrast, “Candidatus Regiella insecticola” donated from M. euphorbiae was not detected in any of the 26 broods produced by injected A. pisum during this same period. At 12 to 14 days postinjection, nearly all aphids injected with “Candidatus Regiella insecticola”A. pisum (16/17) or with Arsenophonus (7/8) gave birth to infected offspring, while only 33% (4/12) of aphids injected with “Candidatus Regiella insecticola”M. euphorbiae passed the symbiont on to their progeny. Transmission of the novel “Candidatus Regiella insecticola” remained rare during the 16 to 20 day interval: infected offspring were detected in 2/18 broods, compared to 4/10 broods for Arsenophonus and 16/17 broods for “Candidatus Regiella insecticola”A. pisum. Comparisons of the proportions of infected offspring largely mirrored the above trends (Fig. 1), again revealing the rarity of “Candidatus Regiella insecticola”M. euphorbiae in A. pisum offspring.
FIG. 1.
Maternal transmission efficiencies of three heritable symbionts in A. pisum. The proportions of offspring (of injected females) in which symbionts were detected are presented for 8 to 10 (black), 12 to 14 (light gray), or 16 to 20 (dark gray) days postinjection. Fractions above bars represent the number of positive offspring/the total number of screened offspring. Values within bars represent the number of injected parents whose offspring (F1 generation) were screened. Ars, Arsenophonus; Ripisum, A. pisum isolate of “Candidatus Regiella insecticola”; Rieuphorbiaciae, M. euphorbiae isolate of “Candidatus Regiella insecticola.” The results of statistical analyses of these data are presented in Table 1.
Logistic regression was used to compare transmission efficiency among the three injected symbionts at each sampled time point in this first generation (Table 1), treating broods of different females as replicates. This approach allowed us to account for different numbers of broods among treatments and different sample sizes among broods: factors that would not be considered under a simple chi-square test. At 8 to 10 days postinjection, Arsenophonus transmission was twice as likely as “Candidatus Regiella insecticola”A. pisum transmission (P = 0.0036). Efficiencies were statistically indistinguishable between Arsenophonus and “Candidatus Regiella insecticola”A. pisum at days 12 to 14 (P = 0.7622), whereas the odds of “Candidatus Regiella insecticola”A. pisum transmission were 2.8 times greater than those for the novel Arsenophonus isolate during the 16- to 20-day period (P < 0.0001).
TABLE 1.
Logistic regression analyses of symbiont transmission
| Days postinjection | Logistic regression equation (statistics for whole-model test) |
P value (95% confidence interval) forc:
|
|
|---|---|---|---|
| β1 | β2 | ||
| 8-10 | Y = 1.33 + 0.66 Arsa (chi-square = 9.45 [df = 1]; P = 0.0021) | 0.0036 (0.22 to 1.11) | NAd |
| 12-14 | Y = −0.289 − 0.14 Ars − 2.32 Rieb (chi-square = 85.54 [df = 2]; P < 0.0001) | 0.7622 (−0.74 to 1.02) | <0.0001 (−1.63 to −3.01) |
| 16-20 | Y = 2.21 − 1.02 Ars − 2.24 Rieb (chi-square = 72.70 [df = 2]; P < 0.0001) | <0.0001 (−0.52 to −1.52) | <0.0001 (−1.47 to −3.01) |
Regression equation is Y=β0+β1 Ars. Y represents the natural log of the odds ratio, ln[p/(1−p)], with p equal to the odds of symbiont transmission(the probability that an aphid is infected). β0 represents the intercept, β1 represents the ln(natural log) of the difference in the odds of transmission between “Candidatus Regiella insecticola”A.pisum versus Arsenophonus(Ars).
Regression equation is Y=β0+β1 Ars+β2 Rie. Again, Y represents the natural log of the odds ratio, ln[p/(1−p)], with p equal to the odds of symbiont transmission(the probability that an aphid is infected). β0 represents the intercept. β1 represents the ln of the difference in the odds of transmission between “Candidatus Regiella insecticola”A.pisum versus Arsenophonus(Ars). β2 represents the ln of the difference in the odds of transmission between “Candidatus Regiella insecticola”A.pisum versus “Candidatus Regiella insecticola”M.euphorbiae(Rie).
Statistics reveal whether β parameters differed significantly from zero and, thus, whether the ln of the odds of transmission differed between the native symbiont and the novel symbionts.
NA, not applicable.
In contrast to Arsenophonus, novel “Candidatus Regiella insecticola” fared poorly in A. pisum. Though estimates of regression coefficients were unstable (due to the complete absence of “Candidatus Regiella insecticola”M. euphorbiae transmission at the 8- to 10-day time span), “Candidatus Regiella insecticola”A. pisum transmission efficiency clearly exceeded that of “Candidatus Regiella insecticola”M. euphorbiae at 8 to 10 days: 8/67 offspring of aphids treated with the native symbiont were infected compared to 0/64 offspring of those injected with the novel “Candidatus Regiella insecticola” (chi-square = 8.98, df = 1, P < 0.01) (Fig. 1). At 12 to 14 days postinjection, the odds of “Candidatus Regiella insecticola”A. pisum transmission were 10.2 times greater than those for “Candidatus Regiella insecticola”M. euphorbiae, according to logistic regression (P < 0.0001). This was comparable to the 9.4-fold difference observed for the 16- to 20-day time interval (P < 0.0001).
The rarity of “Candidatus Regiella insecticola”M. euphorbiae in the offspring of injected aphids could reflect failed persistence within injected A. pisum hosts or low transmissibility of surviving symbionts. To discriminate between these possibilities, we conducted logistic regression analyses on only those offspring of injected females testing positive for the transferred symbionts. Although the magnitudes of transmission differences between the novel and native “Candidatus Regiella insecticola” were slightly lower in these analyses, the results were qualitatively similar to the previous analysis on offspring from all parents. Specifically, the odds of transmission for the naturally occurring “Candidatus Regiella insecticola” were over 7 times greater than for “Candidatus Regiella insecticola”M. euphorbiae at both the 12- to 14- and 16- to 20-day time points (data not shown). Since infection was confirmed in the parents of these offspring, the lower transmission efficiency for the novel symbiont suggests an inability to colonize developing aphid embryos. However, we cannot rule out the possibility that “Candidatus Regiella insecticola”M. euphorbiae were present at lower densities than “Candidatus Regiella insecticola”A. pisum.
Maternal transmission in subsequent generations.
To monitor transmission over a longer time course, we screened subsequent generations for “Candidatus Regiella insecticola” and Arsenophonus symbionts, using descendants of single females that transmitted symbionts to the first generation. First, we screened second and third generation aphids from eight lines that descended from five single females injected with “Candidatus Regiella insecticola”A. pisum. Transmission was nearly perfect within these lines, with only one instance of failure (Table 2). We similarly screened five lineages that descended from four single females injected with Arsenophonus. Incomplete transmission was found within 2/5 lineages, with both failures taking place during passage from the second to third generation. Despite imperfect transmission at this stage, “Candidatus Regiella insecticola”A. pisum and Arsenophonus have been stably maintained for over 20 generations, each within a single A. pisum lineage.
TABLE 2.
Transmission efficiencies for multiple A. pisum lineages in the second (F2) and third (F3) generations postinjection
| Symbiont | Proportion of infected generation
|
|
|---|---|---|
| F2 | F3 | |
| “Candidatus Regiella insecticola”A. pisuma | 8/8 | 7/8 |
| 9/9 | 8/8 | |
| 7/7 | 8/8 | |
| 6/6 | 8/8 | |
| 8/8 | 7/7 | |
| 8/8 | 8/8 | |
| 8/8 | 8/8 | |
| 9/9 | 8/8 | |
| Arsenophonusa | 8/8 | 6/6 |
| 8/8 | 4/5 | |
| 8/8 | 7/7 | |
| 8/8 | 7/7 | |
| 7/7 | 0/3 | |
Each row represents a distinct lineage, descended from an aphid injected with one of the two described symbionts. Fractions of infected offspring are confined to aphids produced by a single female from each lineage.
In contrast, “Candidatus Regiella insecticola”M. euphorbiae was rarely transmitted to progeny beyond the first generation. Of 39 F2 to F5 aphids belonging to lineages that descended from 23 different injected females, only one individual (an F3) tested positive. We also examined pooled samples containing the DNA of 8 aphids descended from one of four single females that transmitted “Candidatus Regiella insecticola”M. euphorbiae to the first generation of offspring. No “Candidatus Regiella insecticola”M. euphorbiae was detected beyond the second (F2) generation in these lines (data not shown). This symbiont thus appears incapable of utilizing A. pisum as a host. We must also mention that our results could be partially explained by low densities of “Candidatus Regiella insecticola” within M. euphorbiae and, thus, low titers in our inoculum. This would imply another barrier to symbiont spread, whereby insufficient symbiont numbers indirectly reduce the likelihood of transmission in a novel host.
Fitness effects of novel and native symbionts.
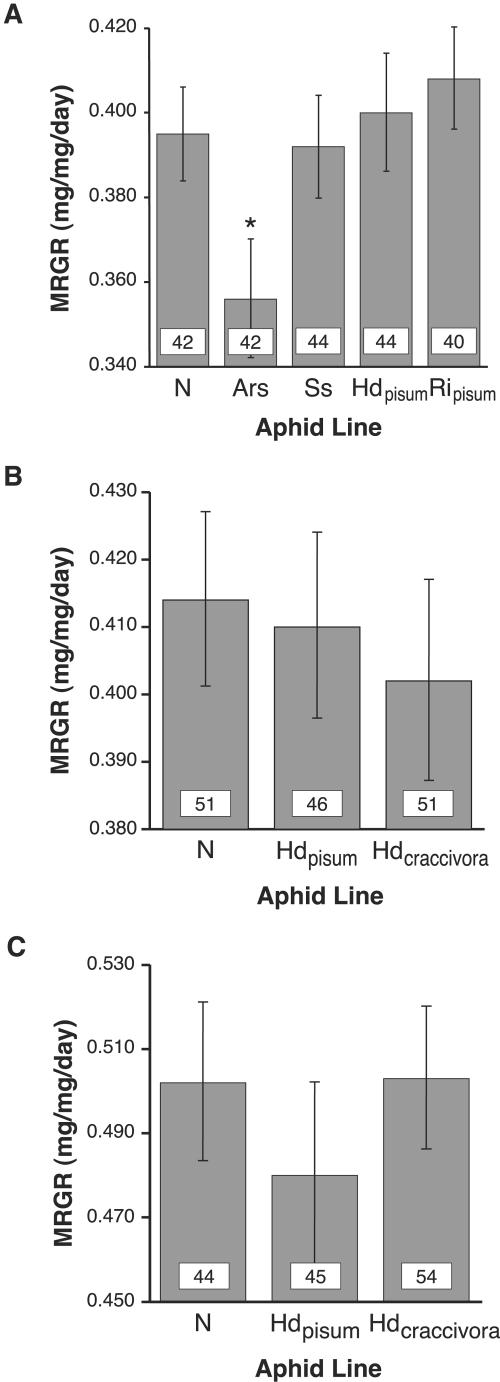
We compared fitness measures between genetically identical aphids which were either free of secondary symbionts or artificially infected with novel or native symbionts. In the first experiment, we reared aphids of A. pisum clone 5A at 20°C, comparing MRGR and survival between an uninfected line, and lines harboring either “Candidatus Serratia symbiotica,” “Candidatus Hamiltonella defensa”A. pisum, “Candidatus Regiella insecticola”A. pisum, or Arsenophonus. The proportions of aphids reaching adulthood ranged from 0.88 to 0.91, and aphids infected with Arsenophonus survived at rates comparable to those for each of the other aphid lines (data not shown). In contrast, Arsenophonus-infected hosts suffered a 9 to 13% reduction in MRGR compared to uninfected aphids and to those harboring any of the three native symbionts (Fig. 2A). This reduction was due to both reduced growth from birth to adulthood (i.e., a smaller value for ln adult weight − ln birth weight) and prolonged development time (extended by over 0.6 days compared to all other lines). In contrast, there were no significant differences in MRGR among the remaining lines, revealing negligible fitness effects of the three native symbionts in this clonal A. pisum background. Fitness costs were a consistent trait of Arsenophonus-infected A. pisum across time and rearing temperatures, as Arsenophonus-infected aphids suffered from prolonged development when reared at 25°C in experiments performed several months later (Russell, unpublished). Future work is required to determine whether fitness costs stemmed from high bacterial densities, differential tissue tropism, or the secretion of virulent toxins.
FIG. 2.
Effects of secondary symbionts on MRGR of A. pisum. (A) Experiment performed at 20°C. Aphid lines: N, uninfected; Ars, infected with Arsenophonus from a Myzocallis sp.; Ss, infected with “Candidatus Serratia symbiotica” from A. pisum; Hdpisum, infected with “Candidatus Hamiltonella defensa” from A. pisum; Ripisum, infected with “Candidatus Regiella insecticola” from A. pisum. (B) Experiment performed at 20°C. Aphid lines: N, uninfected; Hdpisum, infected with “Candidatus Hamiltonella defensa” from A. pisum; Hdcraccivora, infected with “Candidatus Hamiltonella defensa” from A. craccivora. (C) Experiment performed at 25°C. Abbreviations are the same as for panel b. For all parts of this figure, numbers within bars represent the numbers of aphids for which MRGR was measured. Statistical differences are indicated above bars by an asterisk, i.e., Arsenophonus-infected lines had significantly lower MRGR than each of the other lines according to contrast tests (P < 0.0001 in all cases). MRGR were calculated as described in Materials and Methods. Error bars span 95% confidence intervals.
After demonstrating the stable maintenance of a novel “Candidatus Hamiltonella defensa” symbiont in the A. pisum background (Russell, unpublished), we examined its effects on aphid fitness. MRGR was measured for three lineages of A. pisum clone 5A in this experiment: uninfected, infected with “Candidatus Hamiltonella defensa”A. pisum, and infected with “Candidatus Hamiltonella defensa”A. craccivora. We found no significant differences in MRGR among lines at either 20°C or 25°C (Fig. 2B and C). However, those infected with the novel “Candidatus Hamiltonella defensa” experienced elevated survival at 25°C compared to the other lines: 54/56 aphids harboring “Candidatus Hamiltonella defensa”A. craccivora reached adulthood, compared to 44/53 and 45/55 for the “Candidatus Hamiltonella defensa”A. pisum-infected and uninfected lines, respectively (χ2 = 5.40, df = 1, P < 0.025 for “Candidatus Hamiltonella defensa”A. craccivora versus uninfected lines; χ2 = 6.14, df = 1, P < 0.025 for novel versus native “Candidatus Hamiltonella defensa”-infected lines).
DISCUSSION
Generalist symbionts.
Closely related heritable bacteria are often distributed across distantly related insect hosts, revealing histories of horizontal transfer or host switching (2, 43). This pattern suggests that the symbionts retain a general ability to survive, reproduce, and undergo efficient transmission in novel hosts. Here, we have examined this ability in three secondary symbionts of aphids, providing further insight into the generalist nature of heritable microbes.
Both Arsenophonus and “Candidatus Hamiltonella defensa”A. craccivora symbionts were shown to be capable of persisting in a novel species, A. pisum, after transfer from their natural aphid hosts. We have stably maintained these bacteria within A. pisum for several dozen generations. Moreover, the transmission efficiency of Arsenophonus was found to be similar to that of an isolate of “Candidatus Regiella insecticola” occurring naturally in A. pisum. Combined, these findings indicate that A. pisum is a physiologically suitable host for these symbiont isolates.
Our findings are not the first to document a multihost capacity of heritable symbionts. Some Wolbachia and Spiroplasma isolates can infect multiple arthropod hosts, efficiently colonizing the offspring of novel species and persisting across multiple generations after experimental transfer (8, 55, 59, 61). In addition, other isolates of secondary symbionts can persist in more than one aphid species. After artificial infection, both “Candidatus Serratia symbiotica” (also known as PASS or R-type) and “Candidatus Hamiltonella defensa” (also known as PABS or T-type) of A. pisum were efficiently transmitted in the aphids Acyrthosiphon kondoi and Aphis fabae, respectively (14, 18). These findings are consistent with observations of incongruent host and symbiont phylogenies, revealing that several heritable symbionts are, indeed, generalists. However, their host ranges and degrees of generalism vary, as discussed below.
A transmission barrier to horizontal transfer.
In contrast to the novel “Candidatus Hamiltonella defensa” and Arsenophonus symbionts, “Candidatus Regiella insecticola”M. euphorbiae was rarely transmitted to A. pisum offspring despite surviving inside injected aphids. This failure to propagate in progeny suggests that an interaction between genotypes of host and symbiont limits the range of suitable hosts, though low density in our inoculum could partially account for our results. The donor of the poorly transmitted “Candidatus Regiella insecticola,” M. euphorbiae, is more closely related to A. pisum than are either A. craccivora or Myzocallis sp., the donors of efficiently transmitted symbionts examined in this experiment. Thus, we note that the ability to persist in a new species is not a simple reflection of phylogenetic distance from the ancestral host.
Inefficient maternal transmission has been documented previously for other heritable bacteria of hexapods. For example, after experimental transfer between insect species, several Wolbachia isolates are passed onto offspring at levels insufficient for sustained maintenance over multiple generations (6, 15, 34, 54, 74). Similarly, Pseudomonas-like symbionts of Paederus beetles vary in their abilities to colonize the progeny of novel beetle species, with some exhibiting transmission failure and ephemeral persistence (36). Inefficient transmission, therefore, appears to be a common barrier to horizontal transfer, constraining the distributions of heritable symbionts across the insects. Yet given the documented instances of symbiont persistence after cross-species transfer, it is clear that the effectiveness of this barrier varies across hosts and symbionts.
Fitness effects can mediate symbiont host range.
Fitness effects of heritable symbionts are predicted to play a large role in shaping their prevalence in host populations (10, 23, 40). Therefore, studies on the outcomes of interactions between novel host and symbiont partners provide an opportunity to decipher whether these effects may promote or limit the spread of microbes across species.
Based on our measures of aphid fitness, we conclude that “Candidatus Hamiltonella defensa”A. craccivora is better equipped to persist within natural A. pisum populations than Arsenophonus, despite the fact that both were stably transmitted in the laboratory. This latter microbe reduced aphid growth and prolonged development, curbing mean relative growth rates. Thus, barring an unforeseen fitness benefit or a greater capacity for horizontal transmission of Arsenophonus, A. pisum infected with this symbiont would be outperformed by other lineages in the field, resulting in their elimination. In contrast, “Candidatus Hamiltonella defensa”A. craccivora had no negative effects on the fitness parameters we measured and even conferred a survival advantage when aphids were reared at 25°C. Furthermore, this same isolate has been shown to protect novel A. pisum hosts from parasitic wasps (K. M. Oliver, N. A. Moran, and M. S. Hunter, submitted for publication), resembling a defensive phenotype documented for naturally occurring “Candidatus Hamiltonella defensa” isolates (50). These benefits would promote the spread of “Candidatus Hamiltonella defensa” within host populations and could account for the broad host range of this symbiont.
It is worth noting that secondary symbionts have not been shown to manipulate the sexual reproduction of aphids. And experiments in our laboratory have revealed that the symbionts examined here induce neither parthenogenesis nor male-killing in sexual generations of A. pisum (J. A. Russell and A. C. C. Wilson, unpublished data). Though some heritable bacteria employ reproductive manipulation to facilitate their spread (for examples, see references 11, 35, 56, and 66), we reason that this strategy would have limited effects in aphids, which reproduce asexually for most or all of the annual life cycle. Instead, fitness effects in these asexual generations are likely the driving force behind symbiont prevalence in aphid populations.
In addition to Arsenophonus, several other heritable symbionts reduce the fitness of novel arthropod hosts, providing further evidence for a fitness barrier that limits horizontal transfer. For example, Acyrthosiphon kondoi suffers from prolonged development times and reduced fecundity and longevity when infected with “Candidatus Serratia symbiotica” (also known as PASS) (13), which confers conditional benefits upon its natural A. pisum hosts (42, 50). Alternatively, there is little evidence for beneficial effects of novel symbionts on their arthropod hosts.
The degree to which benefits can be transported across species boundaries deserves further investigation, especially for the secondary symbionts of aphids. As mentioned above, these bacteria play roles in heat tolerance (42, 57), defense against natural enemies (24, 50), and host plant utilization (69), illustrating their potential significance in aphid ecology and evolution. If these benefits prove transferable, they may help to explain the prevalence of secondary symbionts across the aphids and their sap-feeding relatives.
Further questions on the interactions between insects and maternally transmitted symbionts.
The results of this study have provided further insight into the generalist nature of heritable symbionts of insects. We have revealed that inefficient transmission and detrimental fitness effects can impede the spread of secondary bacterial symbionts across species. Yet we have also demonstrated that the effectiveness of these barriers will depend on the identity of the microbe. Despite our findings and those of previous studies, many intriguing questions on the interactions between insects and heritable microorganisms remain unanswered. For example, we are still unaware of the means by which symbionts are naturally transferred among species. Previous laboratory and phylogenetic studies suggest potential roles for parasitism and oral acquisition (19, 33, 34, 36, 53, 63, 75). Though the natural importance of these routes and the frequencies at which they permit lateral movement among insects are unknown, it is likely that barriers acting at this level, imposed by ecological associations, will play an important role in shaping symbiont distributions.
Our findings also raise the question of how the patterns of specialization within symbiont lineages relate to trends observed in bacterial genomes. Secondary symbionts are entirely dependent on their aphid hosts and have not been cultivated in cell-free media. Such host dependence is correlated with irreversible gene loss in other bacteria (3, 17, 25, 41, 46, 49), including several ancient and highly specialized microbes of insects (1, 30, 64, 67, 73). But in contrast to these specialized microbes, secondary symbionts retain general abilities to infect multiple hosts. Future research will reveal whether their genomes have also evolved along degenerative trajectories and whether they have retained a capacity to acquire DNA through lateral gene transfer. These studies will elucidate the genetic bases of specialization for microbes that play important roles in insect ecology and evolution.
Acknowledgments
We thank Kim Hammond, Phat Tran, Helen Dunbar, Becky Nankivell, Michelle Hoffman, Jean Tsai, and Rachelle Bond for technical assistance. We also thank Susannah Elwyn, Molly Hunter, and Alex Wilson for helpful comments on the manuscript.
This research was supported by NSF grant no. 0313737. J.R. was supported by the NSF IGERT program in genomics, the Center for Insect Science, and the NSF/USDA/DOE Plant-Insect Interaction training program at the University of Arizona.
REFERENCES
- 1.Akman, L., A. Yamashita, H. Watanabe, K. Oshima, T. Shiba, M. Hattori, and S. Aksoy. 2002. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genetics 32:402-407. [DOI] [PubMed] [Google Scholar]
- 2.Aksoy, S., X. Chen, and V. Hypša. 1997. Phylogeny and potential transmission routes of midgut-associated endosymbionts of tsetse (Diptera: Glossinidae). Insect Mol. Biol. 6:183-190. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, S. G. E., and C. G. Kurland. 1998. Reductive evolution of resident genomes. Trends Microbiol. 6:263-268. [DOI] [PubMed] [Google Scholar]
- 4.Bender, W., P. Spierer, and D. S. Hogness. 1983. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J. Mol. Biol. 168:17-33. [DOI] [PubMed] [Google Scholar]
- 5.Bernays, E. A., and B. A. Klein. 2002. Quantifying the symbiont contribution to essential amino acids in aphids: the importance of tryptophan for Uroleucon ambrosiae. Physiol. Entomol. 27:275-284. [Google Scholar]
- 6.Boyle, L., S. L. O'Neill, H. M. Robertson, and T. L. Karr. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796-1799. [DOI] [PubMed] [Google Scholar]
- 7.Bracho, A. M., D. Martinez-Torres, A. Moya, and A. Latorre. 1995. Discovery and molecular characterization of a plasmid localized in Buchnera sp. bacterial endosymbiont of the aphid Rhopalosiphum padi. J. Mol. Evol. 41:67-73. [DOI] [PubMed] [Google Scholar]
- 8.Braig, H. R., H. Guzman, R. B. Tesh, and S. L. O'Neill. 1994. Replacement of the natural Wolbachia symbiont of Drosophila simulans with a mosquito counterpart. Nature 367:453-455. [DOI] [PubMed] [Google Scholar]
- 9.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, N.Y.
- 10.Bull, J. J., I. J. Molineux, and W. R. Rice. 1991. Selection of benevolence in a host-parasite system. Evolution 45:875-882. [DOI] [PubMed] [Google Scholar]
- 11.Caspari, E., and G. S. Watson. 1959. On the evolutionary importance of cytoplasmic sterility in mosquitos. Evolution 13:568-570. [Google Scholar]
- 12.Chen, D. Q., B. C. Campbell, and A. H. Purcell. 1996. A new Rickettsia from a herbivorous insect, the pea aphid Acyrthosiphon pisum (Harris). Curr. Microbiol. 33:123-128. [DOI] [PubMed] [Google Scholar]
- 13.Chen, D. Q., C. B. Montllor, and A. H. Purcell. 2000. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 95:315-323. [Google Scholar]
- 14.Chen, D. Q., and A. H. Purcell. 1997. Occurrence and transmission of facultative endosymbionts in aphids. Curr. Microbiol. 34:220-225. [DOI] [PubMed] [Google Scholar]
- 15.Clancy, D. J., and A. A. Hoffmann. 1997. Behavior of Wolbachia endosymbionts from Drosophila siumulans in Drosophila serrata, a novel host. Am. Nat. 149:975-988. [DOI] [PubMed] [Google Scholar]
- 16.Clark, M. A., L. Baumann, M. A. Munson, P. Baumann, B. C. Campbell, J. E. Duffus, L. S. Osborne, and N. A. Moran. 1992. The eubacterial endosymbionts of whiteflies (Homoptera: Aleyrodoidea) constitute a lineage distinct from the endosymbionts of aphids and mealybugs. Curr. Microbiol. 25:119-123. [Google Scholar]
- 17.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 18.Darby, A. C., L. M. Birkle, S. L. Turner, and A. E. Douglas. 2001. An aphid-borne bacterium allied to the secondary symbionts of whitefly. FEMS Microbiol. Ecol. 36:43-50. [DOI] [PubMed] [Google Scholar]
- 19.Darby, A. C., and A. E. Douglas. 2003. Elucidation of the transmission patterns of an insect-borne bacterium. Appl. Environ. Microbiol. 69:4403-4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas, A. E. 1988. Sulfate utilization in an aphid symbiosis. Insect. Biochem. 18:599-605. [Google Scholar]
- 21.Douglas, A. E. 1992. Requirement of pea aphids (Acyrthosiphon pisum) for their symbiotic bacteria. Entomol. Exp. Appl. 65:195-198. [Google Scholar]
- 22.Douglas, A. E., and W. A. Prosser. 1992. Synthesis of the essential amino acid tryptophan in the pea aphid (Acyrthosiphon pisum) symbiosis. J. Insect Physiol. 38:565-568. [Google Scholar]
- 23.Ewald, P. 1983. Transmission modes and the evolution of the parasitism-mutualism continuum. Ann. N. Y. Acad. Sci. 503:295-306. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari, J., A. C. Darby, T. J. Daniell, H. C. J. Godfray, and A. E. Douglas. 2004. Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol. Entomol. 29:60-65. [Google Scholar]
- 25.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischman, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, J. L. Fritchman, J. F. Weidman, K. V. Small, M. Sandusky, J. Fuhrmann, D. Nguyen, T. R. Utterback, D. M. Saudek, C. A. Phillips, J. M. Merrick, J. F. Tomb, B. A. Dougherty, K. F. Bott, P. C. Hu, T. S. Lucier, S. N. Peterson, H. O. Smith, C. A. Hutchison, and J. C. Venter. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 26.Fukatsu, T. 2001. Secondary intracellular symbiotic bacteria in aphids of the genus Yamatocallis (Homoptera: Aphididae: Drepanosiphinae). Appl. Environ. Microbiol. 67:5315-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukatsu, T. 2001. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 67:1284-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukatsu, T., N. Nikoh, R. Kawai, and R. Koga. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gherna, R. L., J. H. Werren, W. Weisburg, R. Cote, C. R. Woese, L. Mandelco, and D. J. Brenner. 1991. Arsenophonus nasoniae gen. nov., sp. nov., the causative agent of the son-killer trait in the parasitic wasp Nasonia vitripennis. Int. J. Syst. Bacteriol. 41:563-565. [Google Scholar]
- 30.Gil, R., F. J. Silva, E. Zientz, F. Delmotte, F. Gonzalez-Candelas, A. Latorre, C. Rausell, J. Kamerbeek, J. Gadau, B. Holldobler, R. C. van Ham, R. Gross, and A. Moya. 2003. The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc. Natl. Acad. Sci. USA 100:9388-9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grindle, N., J. J. Tyner, K. Clay, and C. Fuqua. 2003. Identification of Arsenophonus-type bacteria from the dog tick Dermacentor variabilis. J. Invertebr. Pathol. 83:264-266. [DOI] [PubMed] [Google Scholar]
- 32.Haynes, S., A. C. Darby, T. J. Daniell, G. Webster, F. J. F. van Veen, H. C. J. Godfray, J. L. Prosser, and A. E. Douglas. 2003. Diversity of bacteria associated with natural aphid populations. Appl. Environ. Microbiol. 69:7216-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heath, B. D., R. D. J. Butcher, W. G. F. Whitfield, and S. F. Hubbard. 1999. Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr. Biol. 9:313-316. [DOI] [PubMed] [Google Scholar]
- 34.Huigens, M. E., R. P. de Almaida, P. A. H. Boons, R. F. Luck, and R. Stouthamer. 2004. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. Lond. Ser. B 271:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurst, G. D. D., F. M. Jiggins, J. H. G. von der Schulenburg, D. Bertrand, S. A. West, I. I. Goriacheva, I. A. Zakharov, J. H. Werren, R. Stouthamer, and M. E. N. Majerus. 1999. Male-killing Wolbachia in two species of insect. Proc. R. Soc. Lond. Ser. B 266:735-740. [Google Scholar]
- 36.Kellner, R. L. L. 2002. Interspecific transmission of Paederus endosymbionts: relationship to the genetic divergence among the bacteria associated with pederin biosynthesis. Chemoecology 12:133-138. [Google Scholar]
- 37.Kellner, R. L. L., and K. Dettner. 1996. Differential efficacy of toxic pederin in deterring potential arthropod predators of Paederus (Coleoptera: Staphylinidae) offspring. Oecologica 107:293-300. [DOI] [PubMed] [Google Scholar]
- 38.Lai, C. Y., L. Baumann, and P. Baumann. 1994. Amplification of trpEG; adaptation of Buchnera aphidicola to an endosymbiotic association with aphids. Proc. Natl. Acad. Sci. USA 91:3819-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leonardo, T. E. 2004. Removal of a specialization-associated symbiont does not affect aphid fitness. Ecol. Lett. 7:461-468. [Google Scholar]
- 40.May, R. M., and R. M. Anderson. 1982. Coevolution of hosts and parasites. Parasitology 85:411-426. [DOI] [PubMed] [Google Scholar]
- 41.Mira, A., H. Ochman, and N. A. Moran. 2001. Deletional bias and the evolution of bacterial genomes. Trends Genet. 17:589-596. [DOI] [PubMed] [Google Scholar]
- 42.Montllor, C. B., A. Maxmen, and A. H. Purcell. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27:189-195. [Google Scholar]
- 43.Moran, N., and P. Baumann. 1994. Phylogenetics of cytoplasmically inherited microorganisms of arthropods. Trends Ecol. Evol. 9:15-20. [DOI] [PubMed] [Google Scholar]
- 44.Moran, N. A., J. A. Russell, R. Koga, and T. Fukatsu. 2005. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 71:3302-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moran, N. A., and A. Telang. 1998. The evolution of bacteriocyte-associated endosymbionts in insects. Bioscience 48:295-304. [Google Scholar]
- 46.Moran, N. A., and J. J. Wernegreen. 1999. Lifestyle evolution in symbiotic bacteria: insights from genomics. Trends Ecol. Evol. 15:321-326. [DOI] [PubMed] [Google Scholar]
- 47.Munson, M. A., P. Baumann, M. A. Clark, L. Baumann, N. A. Moran, D. J. Voegtlin, and B. C. Campbell. 1991. Evidence for the establishment of aphid-eubacterial endosymbiosis in an ancestor of four aphid families. J. Bacteriol. 173:6321-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munson, M. A., P. Baumann, and M. G. Kinsey. 1991. Buchnera gen. nov. and Buchnera aphidicola sp. nov., a taxon consisting of the mycetocyte-associated, primary endosymbionts of aphids. Int. J. Syst. Microbiol. 41:566-568. [Google Scholar]
- 49.Ochman, H., and N. A. Moran. 2001. Genes lost and genes found: evolution of bacterial pathogenesis and symbiosis. Science 292:1096-1098. [DOI] [PubMed] [Google Scholar]
- 50.Oliver, K. M., J. A. Russell, N. A. Moran, and M. S. Hunter. 2003. Facultative bacteria in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 100:1803-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piel, J. 2002. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc. Natl. Acad. Sci. USA 99:14002-14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prosser, W. A., and A. E. Douglas. 1991. An analysis of chlorotetracycline-treated pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 37:713-719. [Google Scholar]
- 53.Purcell, A. H., K. G. Suslow, and M. Klein. 1994. Transmission via plants of an insect pathogenic bacterium that does not multiply or move in plants. Microb. Ecol. 27:19-26. [DOI] [PubMed] [Google Scholar]
- 54.Riegler, M., S. Charlat, C. Stauffer, and H. Merçot. 2004. Wolbachia transfer from Rhagoletis cerasi to Drosophila simulans: investigating the outcomes of host-symbiont coevolution. Appl. Environ. Microbiol. 70:273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rigaud, T., P. S. Pennings, and P. Juchault. 2001. Wolbachia bacteria effects after experimental interspecific transfers in terrestrial isopods. J. Invertebr. Pathol. 77:251-257. [DOI] [PubMed] [Google Scholar]
- 56.Rousset, F., D. Bouchon, B. Pintureau, P. Juchault, and M. Solignac. 1992. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc. R. Soc. Lond. Ser. B 250:91-98. [DOI] [PubMed] [Google Scholar]
- 57.Russell, J. A. 2004. Ph.D. thesis. University of Arizona, Tucson.
- 58.Russell, J. A., A. Latorre, B. Sabater-Muñoz, A. Moya, and N. A. Moran. 2003. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 12:1061-1075. [DOI] [PubMed] [Google Scholar]
- 59.Sakaguchi, B., and D. F. Poulson. 1963. Interspecific transfer of the “sex-ratio” condition from Drosophila willistoni to D. melanogaster. Genetics 48:841-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandström, J. P., J. A. Russell, J. P. White, and N. A. Moran. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217-228. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki, T., T. Kubo, and H. Ishikawa. 2002. Interspecific transfer of Wolbachia between two Lepidopteran insects expressing cytoplasmic incompatibility: a Wolbachia variant naturally infecting Cadra cautella causes male killing in Ephestia kuehniella. Genetics 162:1313-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sauer, C., E. Stackebrandt, J. Gadau, B. Holldobler, and R. Gross. 2000. Systematic relationships and cospeciation of bacterial endosymbionts and their carpenter ant host species: proposal of new taxon Candidatus Blochmannia gen. nov. Int. J. Syst. Evol. Microbiol. 50:1877-1886. [DOI] [PubMed] [Google Scholar]
- 63.Schilthuizen, M., and R. Stouthamer. 1997. Horizontal transmission of parthenogenesis-inducing microbes in Trichogramma wasps. Proc. R. Soc. Lond. Ser. B 264:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shigenobu, S., H. Watanabe, M. Hattori, Y. Sasaki, and H. Ishikawa. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407:81-86. [DOI] [PubMed] [Google Scholar]
- 65.Simon, J. C., S. Carré, M. Boutin, N. Prunier-Leterme, B. Sabater-Muñoz, A. Latorre, and R. Bournoville. 2003. Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts. Proc. R. Soc. Lond. Ser. B 270:1703-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stouthamer, R., J. A. J. Breeuwer, R. F. Luck, and J. H. Werren. 1993. Molecular identification of microorganisms associated with parthenogenesis. Nature 361:66-68. [DOI] [PubMed] [Google Scholar]
- 67.Tamas, I., L. Klasson, B. Canback, A. K. Naslund, A. S. Eriksson, J. J. Wernegreen, J. P. Sandström, N. A. Moran, and S. G. Andersson. 2002. 50 million years of genomic stasis in endosymbiotic bacteria. Science 296:2376-2379. [DOI] [PubMed] [Google Scholar]
- 68.Thao, M. L., M. A. Clark, L. Baumann, E. B. Brennan, N. A. Moran, and P. Baumann. 2000. Secondary endosymbionts of psyllids have been acquired multiple times. Curr. Microbiol. 41:300-304. [DOI] [PubMed] [Google Scholar]
- 69.Tsuchida, T., R. Koga, and T. Fukatsu. 2004. Host plant specialization governed by facultative symbiont. Science 303:1989. [DOI] [PubMed] [Google Scholar]
- 70.Tsuchida, T., R. Koga, H. Shibao, T. Matsumoto, and T. Fukatsu. 2002. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 11:2123-2135. [DOI] [PubMed] [Google Scholar]
- 71.Turelli, M. 1994. Evolution of incompatibility-inducing microbes and their hosts. Evolution 48:1500-1513. [DOI] [PubMed] [Google Scholar]
- 72.Unterman, B. M., P. Baumann, and D. L. McLean. 1989. Pea aphid symbiont relationships established by analysis of 16S rRNAs. J. Bacteriol. 171:2970-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Ham, R. C., J. Kamerbeek, C. Palacios, C. Rausell, F. Abascal, U. Bastolla, J. M. Fernandez, L. Jiminez, M. Postigo, F. J. Silva, J. Tamames, E. Viguera, A. Latorre, A. Valencia, F. Moran, and A. Moya. 2003. Reductive evolution in Buchnera aphidicola. Proc. Natl. Acad. Sci. USA 100:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Meer, M. M. M., and R. Stouthamer. 1999. Cross-order transfer of Wolbachia from Muscidifurax uniraptor (Hymenoptera: Pteromalidae) to Drosophila simulans (Diptera: Drosophilidae). Heredity 82:163-169. [DOI] [PubMed] [Google Scholar]
- 75.Werren, J. H., W. Zhang, and L. R. Guo. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. Ser. B 261:55-63. [DOI] [PubMed] [Google Scholar]
- 76.Wicker, C. 1983. Differential vitamin and choline requirements of symbiotic and aposymbiotic S. oryzae (Coleoptera: Curculionidae). Comp. Biochem. Physiol. 76A:177-182. [Google Scholar]
- 77.Zchori-Fein, E., and J. K. Brown. 2002. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 95:711-718. [Google Scholar]