Abstract
Among photosynthetic bacteria, strains B10 and E1F1 of Rhodobacter capsulatus photoreduce 2,4-dinitrophenol (DNP), which is stoichiometrically converted into 2-amino-4-nitrophenol by a nitroreductase activity. The reduction of DNP is inhibited in vivo by ammonium, which probably acts at the level of the DNP transport system and/or physiological electron transport to the nitroreductase, since this enzyme is not inhibited by ammonium in vitro. Using the complete genome sequence data for strain SB1003 of R. capsulatus, two putative genes coding for possible nitroreductases were isolated from R. capsulatus B10 and disrupted. The phenotypes of these mutant strains revealed that both genes are involved in the reduction of DNP and code for two major nitroreductases, NprA and NprB. Both enzymes use NAD(P)H as the main physiological electron donor. The nitroreductase NprA is under ammonium control, whereas the nitroreductase NprB is not. In addition, the expression of the nprB gene seems to be constitutive, whereas nprA gene expression is inducible by a wide range of nitroaromatic and heterocyclic compounds, including several dinitroaromatics, nitrofuran derivatives, CB1954, 2-aminofluorene, benzo[a]pyrene, salicylic acid, and paraquat. The identification of two putative mar/sox boxes in the possible promoter region of the nprA gene and the induction of nprA gene expression by salicylic acid and 2,4-dinitrophenol suggest a role in the control of the nprA gene for the two-component MarRA regulatory system, which in Escherichia coli controls the response to some antibiotics and environmental contaminants. In addition, upregulation of the nprA gene by paraquat indicates that this gene is probably a member of the SoxRS regulon, which is involved in the response to stress conditions in other bacteria.
Nitroaromatics are released into the environment almost exclusively as a consequence of anthropogenic activities related to explosives, paints, dyes, and pharmacology industries (23). Microorganisms have developed many different strategies to remove and degrade these xenobiotic compounds, and oxidative or reductive pathways for the degradation of nitroaromatics have been widely studied (19, 23, 29). Polynitroaromatic compounds are usually degraded through the following two major reductive pathways: (i) reduction of the aromatic ring by the addition of hydride ions to produce hydride-Meisenheimer complexes and (ii) reduction of the nitro group(s) to a hydroxylamino or amino group(s) by nitroreductases (3, 10, 15, 25). The oxygen-sensitive nitroreductases catalyze one electron step reaction to produce a nitro radical anion that can be reoxidized by oxygen with the concomitant production of superoxide. However, the best-studied nitroreductases are oxygen insensitive since they do not produce radicals and they catalyze the sequential addition of pairs of electrons donated by NAD(P)H to convert the nitro groups into hydroxylamines or amines, often through nitroso derivatives (15, 23). These enzymes are usually homodimers of a 25-kDa polypeptide and contain flavin mononucleotide as a cofactor. Among these, the nitroreductases of Escherichia coli (16) and Rhodobacter capsulatus E1F1 (4) have been purified and characterized. Nitroreductases participate in environmental detoxification processes, although they are also present in the intestinal microflora, reducing more stable compounds to mutagenic nitro derivatives (24). Interestingly, the NfsB nitroreductase of E. coli has been used in cancer treatments based on the antibody-directed enzyme prodrug therapy technique (7). Photoreduction of nitroaromatics has been described for phototrophic bacteria, such as strains E1F1 and B10 of Rhodobacter capsulatus, which photoreduce 2,4-dinitrophenol (DNP) by use of a nitroreductase. DNP is stoichiometrically reduced to 2-amino-4-nitrophenol (ANP) under anaerobic phototrophic conditions (5). This is clearly a cometabolic process, since DNP is an uncoupler which strongly inhibits nitrogen fixation and since DNP photoreduction enables the bacterium to grow by fixing dinitrogen. However, ANP can be further metabolized by a light-dependent microaerobic pathway that releases nitrite into the medium (28). DNP photoreduction requires the presence of additional carbon and nitrogen sources, and this process is induced by the presence of DNP and repressed by ammonium or glutamine (5, 17, 28). It has been proposed that NtrC, a general regulator in response to ammonium, could regulate DNP reduction in R. capsulatus by controlling the expression of the Rnf proteins, which supply electrons for both nitrogenase and nitroreductase enzymes (17). The nitroreductase enzyme of R. capsulatus E1F1 is composed of two subunits of 27 kDa each, contains flavin mononucleotide as a prosthetic group, and uses NAD(P)H as a physiological electron donor (4). On the other hand, the marRAB operon is a regulatory locus that controls multiple environmental hazard resistances in E. coli and therefore is induced by a variety of chemical agents, such as tetracycline, chloramphenicol, menadione, benzoate, salicylate, and 2,4-dinitrophenol (1). The marRAB operon consists of an operator-promoter region (marO) and the genes marR, coding for a repressor of this operon, marA, encoding a positive transcriptional regulator of unlinked target genes, and marB, of unknown function (1). 2,4-Dinitrophenol and salicylic acid, among others, interact with MarR in vitro, and it is thought that, in vivo, repression is relieved and the activation of marRAB results from the direct interaction of these inducers with the repression complex MarR-marO (1). The mar boxes are often elements of recognition for other regulatory proteins, such as SoxS, which is implicated in the expression of genes related to the response to oxidative stress conditions. The SoxRS regulon in E. coli includes at least 15 genes that are upregulated in response to superoxide formed by redox-cycling compounds such as paraquat (14). The SoxR protein acts as a sensor to detect elevated levels of superoxide within the cells and to activate the transcription of soxS, which codes for a transcriptional activator. More than 50% of genes upregulated by SoxS are also upregulated by the MarA protein (14).
At present, most nitroreductases characterized at the biochemical and/or molecular level belong to enterobacteria. The aim of this work was the identification, isolation, and characterization of the nitroreductase genes, nprA and nprB, involved in 2,4-dinitrophenol reduction in the phototrophic bacterium R. capsulatus B10. These genes were cloned, and the phenotypes of single and double mutants defective in each of or both the nprA and nprB genes were analyzed. The control of the expression of both nitroreductase genes by aromatic acids and ammonium was also investigated.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Rhodobacter capsulatus strains were routinely cultured under diazotrophic conditions at 30°C in RCV medium (26), with 30 mM malate as the carbon source, in the presence of 0.15 mM 2,4-dinitrophenol. Ammonium chloride (5 mM) or 7 mM l-glutamine was used as a nitrogen source when indicated. The E. coli strains were cultured in Luria-Bertani (LB) medium or on LB agar plates at 37°C (18). The vectors pGEM-T, pGEM-T Easy (Promega), and pBluescript (Stratagene) were used for routine gene manipulation, and the pSUP202 and pSUP301 plasmids were used as mobilizable vectors (20).
Analytical determinations and enzyme assays.
Cell growth was followed turbidimetrically by measuring the absorbance of the cultures at 680 nm. 2,4-Dinitrophenol and 2-amino-4-nitrophenol concentrations were determined with a Gold system (Beckman) high-performance liquid chromatograph with a reverse-phase column as previously described (4). The nitrite concentration was determined colorimetrically (21). The ammonium concentration was estimated with phenol-hypochlorite (22). β-Galactosidase activity was determined at 30°C as previously described (12), and activities are expressed in arbitrary units (11). Nitroreductase activity was determined by following 2-amino-4-nitrophenol formation as previously described (4).
DNA manipulations.
DNA manipulations were performed by using standard procedures (18). Insertional mutagenesis of the nprA gene was carried out by using total DNA from R. capsulatus B10 and amplifying by PCR a 1.3-kb fragment containing this gene. The primers used were synthesized according to the database of the genome sequence of R. capsulatus strain SB1003 (6), for which a putative oxygen-insensitive nitroreductase gene has been described (RRCO1791), and their sequences were as follows: primer A, 5′-CGGGATTCGCGAATTCTTTCAGAT-3′; and primer B, 5′-CTGCTTGACTTTCAGCGCGACTTTG-3′. The PCR program was 96°C for 2 min, 96°C for 30 s, 65°C for 30 s, and 69°C for 1.5 min; the last three steps were repeated for a total of 30 cycles, and a final step of 69°C for 10 min was performed. The 1.3-kb PCR fragment was cloned into pGEM-T and subcloned into pBluescript with the restriction enzymes ApaI and SpeI to perform sequence analysis. The kanamycin resistance cassette from the pSUP2021 plasmid (20) was introduced into the nprA gene with the restriction enzymes HindIII and SalI, which also deleted 0.5 kb of the nprA gene. In order to generate EcoRI restriction sites at both ends of this fragment, a new PCR with the forward and reverse primers from pBluescript was developed, and the PCR fragment was cloned into pGEM-T Easy. Finally, the EcoRI fragment was cloned into the mobilizable vector pSUP202 to produce the plasmid pMO8-AKm (nprAΔ::Km), which was further transferred by conjugation from the donor strain E. coli S17-1 to the recipient wild-type strain R. capsulatus B10 (20). To create the transcriptional nprA-lacZ fusion, a 1.3-kb AatII/PstI fragment was isolated from pGEM-T and cloned into pSUP301. The lacZ gene was inserted in both orientations into the nprA gene with the restriction enzyme SalI to generate the plasmids pMO-AL+ (nprA-lacZ+) and pMO-AL− (nprA-lacZ, in reverse orientation). The insertion/deletion nprB mutant strain was generated by PCR amplification of two fragments, of 938 bp (primers 1 and 2) and 680 bp (primers 3 and 4), using total DNA from R. capsulatus B10. Primers 2 and 3 correspond to the 5′ and 3′ ends of the nprB gene, respectively. In the sequence of the nprB gene, both primers are separated by 370 bp. The primers were synthesized according to the database of the genome sequence of R. capsulatus strain SB1003 (6), for which a second putative nitroreductase gene has been described (RRCO3929). The primer sequences were as follows: primer 1, 5′-CCTCGAGGGCTTCACGTTTTCGCG-3′; primer 2 (with BamHI site underlined), 5′-AGGAAGGTGGGATCCGCGGCGTCG-3′; primer 3 (with BamHI site underlined), 5′-GCGGCGGGGATCCAGCTTCTCAAT-3′; and primer 4, 5′-CGAAGAAAACGACGGGCGCCAGGG-3′. The PCR program was the same as that described above to amplify the nprA gene. The 938-bp and 680-bp fragments were cloned into pGEM-T Easy to generate pPCR12 and pPCR34, respectively. The 680-bp BamHI/SpeI fragment was cloned into pPCR12 to generate the pPCR14 plasmid. In this construct, a deletion of 322 bp was created in the nprB gene (original coding sequence of 603 bp), and a new BamHI restriction site was created to introduce the 4.3-kb gentamicin/spectinomycin resistance cassette from the Tn5B12 plasmid (20). The resulting 5.9-kb NcoI/PstI fragment was cloned into pSUP202 to generate the pMO2-BGmSp (nprBΔ::GmSp) plasmid, which was transferred by conjugation from the donor strain E. coli S17-1 to either the recipient wild-type strain R. capsulatus B10 (to generate the nprB mutant strain) or the previously isolated R. capsulatus nprA mutant strain (to generate the nprA nprB double mutant strain). To create a transcriptional nprB-lacZ fusion, the 1.6-kb EcoRI fragment with the nprB gene was isolated from pPCR14 and cloned into pSUP202. The lacZ gene was inserted in both orientations into the BamHI site of the nprB gene to generate the plasmids pMO-BL+ (nprB-lacZ+) and pMO-BL− (nprB-lacZ, in reverse orientation). The plasmids with the nprA-lacZ+, nprA-lacZ (in reverse orientation), nprB-lacZ+, and nprB-lacZ (in reverse orientation) fusions were transferred by conjugation from E. coli S17-1 to the wild-type strain R. capsulatus B10.
RESULTS AND DISCUSSION
Effect of mutations of the nprA and nprB genes on DNP reduction in R. capsulatus.
The phototrophic bacterium Rhodobacter capsulatus photoreduces DNP to ANP, which is stoichiometrically released into the medium under anaerobic phototrophic conditions, although it is further degraded by light and microaerobiosis (5, 17, 28). The photoreduction of DNP depends on the presence of alternative nitrogen and carbon sources. Thus, the highest rate of this cometabolic process was observed in the presence of malate and dinitrogen gas (not shown).
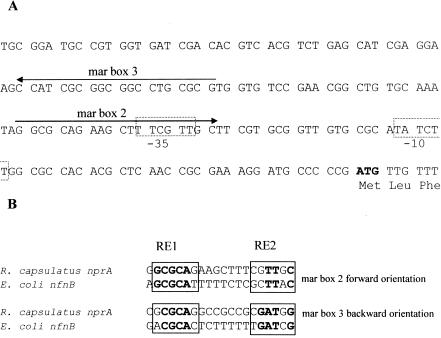
Two putative genes coding for possible nitroreductases are annotated in the genome sequence of strain SB1003 of R. capsulatus: they are a gene for a putative oxygen-insensitive NAD(P)H nitroreductase (RRCO1791) and a gene for a putative protein from the nitroreductase family (RRCO3929) (6). From the DNA sequences of these genes, different primers were synthesized, and PCRs with total DNA isolated from R. capsulatus strain B10 were carried out to clone and sequence the corresponding nitroreductase genes. The sequence of the R. capsulatus B10 nprA gene (RRCO1791 in strain SB1003) showed 27% identity and 50% similarity with the nitroreductase nfnB gene of E. coli and other classical nitroreductase genes of enterobacteria, whereas the R. capsulatus B10 nprB gene (RRCO3929 in strain SB1003) showed 35% identity and 50% similarity with hypothetical nitroreductase genes from Caulobacter crescentus and Pseudomonas aeruginosa. The nprA and nprB genes shared 14% identity with each other (not shown). Activation of the E. coli nitroreductase nfnB (also named nfsB) gene by MarA through a mar box in its promoter region has been described (2). mar boxes are asymmetric degenerate DNA sequences with two conserved recognition elements, RE1 and RE2, which can be classified into two groups depending on their orientation and location with respect to the −10 and −35 hexamers (2) (Fig. 1). Sequence analysis of the promoter region of the nfnB gene of E. coli revealed the presence of four putative mar boxes. However, studies in vitro have revealed that MarA only binds with a high affinity to mar box 2, which belongs to class II and overlaps the −35 hexamer (2). In the possible promoter region of the R. capsulatus B10 nprA gene, two putative mar boxes can be found (Fig. 1A). One of them, mar box 2, lies in a forward orientation and overlaps the −35 hexamer, as described for mar box 2 of the E. coli nfnB gene. Another sequence, mar box 3, is located upstream and in the reverse orientation. The putative mar box 2 of the nprA gene is highly similar to the functional mar box 2 described for the E. coli nfnB gene, and mar box 3 is also very similar to the corresponding mar box 3 of the E. coli nfnB gene (Fig. 1B). Nevertheless, further studies are needed to determine the transcription start site of the nprA gene and the possible interactions between MarA and the two putative mar boxes of the nprA gene of R. capsulatus. On the other hand, the putative promoter region of the nprB gene of R. capsulatus does not contain mar box sequences (not shown).
FIG. 1.
Locations of hypothetical mar boxes in the possible promoter region of the R. capsulatus nprA gene. (A) Sequence of the putative promoter region of the nprA gene. Arrows indicate the localization and orientation of the two hypothetical mar boxes. The putative −10 and −35 heptamers are indicated by dotted lines, and the translation start codon is shown in bold. (B) Sequence alignment of the hypothetical mar boxes of R. capsulatus nprA and E. coli nfnB. The recognition elements RE1 and RE2 for MarA binding are indicated in squares, and the internal conserved nucleotides are shown in bold.
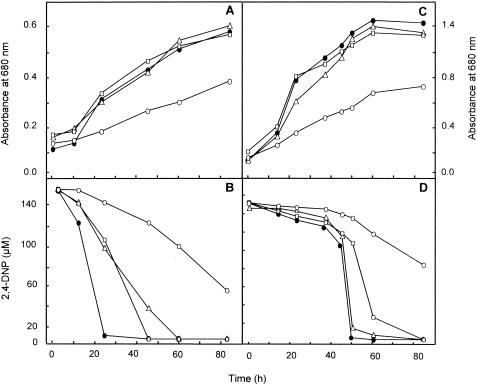
To determine if the nprA and nprB genes of R. capsulatus B10 code for nitroreductases, three defective strains, the nprA, nprB, and nprA nprB mutants, were constructed by the insertion of antibiotic resistance cassettes into these genes. Bacterial growth and DNP uptake were analyzed in diazotrophic cultures of the wild-type and mutant strains (Fig. 2). The diazotrophic growth rates with DNP of the nprA (in which nitroreductase NprB is functional), nprB (with nitroreductase NprA being functional), and wild-type (with both NprA and NprB being functional) strains were similar, but the growth of the double nprA nprB mutant was considerably affected (Fig. 2A), indicating that simultaneous mutations in both genes impair diazotrophic growth in the presence of DNP, an uncoupler that must be removed to allow dinitrogen fixation. DNP was completely consumed under diazotrophic conditions by the wild-type strain, but in the nprA and nprB mutant strains this process was delayed for 45 and 60 h, respectively. DNP was even more slowly consumed in the double mutant strain (Fig. 2B). The low rate of DNP reduction observed in the nprA nprB mutant strain of R. capsulatus can be explained by the presence of other nonspecific nitroreductases that may be present in this bacterium. The presence of several nitroreductases in a single organism is not unusual; for example, in E. coli there are at least two oxygen-insensitive (type I) nitroreductases (NfsB and NfsA) in addition to oxygen-sensitive (type II) nitroreductases (27). The E. coli NfsA and NfsB proteins have similar enzymatic properties, although they share only 7% identity (8). In summary, sequence and mutational analyses indicate that the nprA and nprB genes of R. capsulatus B10 code for the major oxygen-insensitive nitroreductases involved in DNP reduction.
FIG. 2.
Bacterial growth (A and C) and 2,4-dinitrophenol uptake (B and D) in wild-type and mutant strains of R. capsulatus B10 growing under diazotrophic conditions (A and B) or with 5 mM ammonium chloride (C and D) in the presence of 150 μM 2,4-dinitrophenol. Bacterial growth and 2,4-dinitrophenol uptake were determined as indicated in Materials and Methods at the times indicated in the figure. •, wild-type strain; ▵, nprA mutant strain; □, nprB mutant strain; ○, nprA nprB mutant strain. Data correspond to a representative experiment which was repeated three times with variations of <10%.
In the presence of ammonium and DNP, cell growth was stimulated in all of the strains, but as observed in medium without ammonium, the nprA nprB double mutant showed a lower growth rate than the single mutants and the wild-type strain (Fig. 2C and D). With regard to DNP uptake in the presence of ammonium, the process showed two phases. The initial DNP uptake took place very slowly, corresponding to the presence in the medium of high ammonium concentrations (between 5 and 1.5 mM). This result agrees with that previously reported about the inhibition of DNP uptake by ammonium in R. capsulatus E1F1 (5, 17, 28). The second phase took place when the remaining ammonium concentration was lower than 1.5 mM. In this second phase, strong differences in DNP uptake were observed for the wild-type and mutant strains. As shown in Fig. 2D, DNP uptake in the wild-type strain paralleled that in the nprA strain (where NprB is functional), whereas the nprB strain (where NprA is present) consumed DNP at a lower rate. This result suggests that NprA could be negatively regulated by ammonium. Thus, if the NprA enzyme is not functional in the wild-type strain in the presence of ammonium, the phenotypes of the wild-type strain and the nprA mutant strain should be similar. In both strains, only the nitroreductase NprB is active, since this protein seems to not be affected by the presence of ammonium.
Characterization of in vitro nitroreductase activities of R. capsulatus B10.
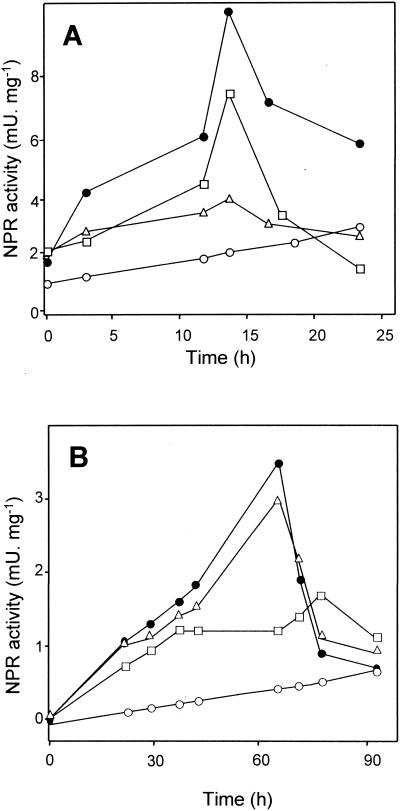
Nitroreductase activities were assayed with NADPH or NADH as the electron donor and with DNP as the substrate in crude extracts from wild-type and mutant strains grown under diazotrophic conditions with DNP. In all cases, NADH-dependent nitroreductase activities were lower than NADPH-dependent nitroreductase activities (not shown), thus indicating that R. capsulatus nitroreductases preferably use NADPH as the physiological electron donor. It is worth nothing that NprA and NprB nitroreductase activities can be determined in crude extracts in the presence of oxygen, which indicates that both nitroreductases belong to the oxygen-insensitive type of enzymes and that their activity was unaffected in vitro by the addition of ammonium, as previously described for R. capsulatus E1F1 (4). A higher level of nitroreductase activity was found in the wild-type strain, corresponding to the presence of both the NprA and NprB nitroreductases (Fig. 3A). The nitroreductase activity of the nprB mutant strain was lower than that found in the wild-type strain but higher than the activity showed by the nprA defective strain (Fig. 3A). This result indicates that NprA is more effective than NprB under diazotrophic conditions. Finally, the nitroreductase activity of the nprA nprB mutant strain was very low (Fig. 3A), confirming that NprA and NprB are the major 2,4-dinitrophenol nitroreductases of R. capsulatus B10.
FIG. 3.
Nitroreductase (NPR) activities in crude extracts from wild-type and mutant strains of R. capsulatus B10 grown diazotrophically (A) or with 5 mM ammonium chloride (B) in the presence of 150 μM 2,4-dinitrophenol. Samples were collected at the indicated times, and crude extracts were obtained as described in Materials and Methods. •, wild-type strain; ▵, nprA mutant strain; □, nprB mutant strain; ○, nprA nprB mutant strain. Data correspond to a representative experiment which was repeated three times with variations of <12%.
When nitroreductase activities were assayed in crude extracts from cells grown with 5 mM ammonium chloride and in the presence of DNP, the nitroreductase activities were lower than those in the absence of ammonium (Fig. 3B). In all cases, the highest nitroreductase activities were observed when the remaining ammonium concentration in the medium was in the range of 0.3 to 0.6 mM (not shown). Nitroreductase activities were similar in the wild-type and nprA strains, suggesting that in the presence of ammonium, NprA is inactive or absent in the wild-type strain (Fig. 3B). On the other hand, the NprA activity of the nprB mutant strain was very low in cells grown in the presence of ammonium. This result, together with the lack of inhibition of NprA by ammonium in vitro, suggests a negative effect of ammonium on nprA gene expression.
Analysis of nprA and nprB gene expression.
To study the transcriptional control of and ammonium effect on the expression of the nprA and nprB nitroreductase genes, transcriptional fusions of the nprA or nprB gene with the β-galactosidase (lacZ) reporter gene were performed. Whereas the expression of the nprB gene was constitutive, the expression of the nprA gene was induced by a wide range of nitroaromatic and heterocyclic compounds (Table 1). The highest β-galactosidase activity was obtained for cells grown under diazotrophic conditions with 2,4-dinitrophenol, and β-galactosidase activity was not detected in the absence of the inducer (Table 1). When cells of R. capsulatus B10 carrying the nprA-lacZ fusion were grown in the presence of DNP with ammonium or glutamine, only a 30% β-galactosidase activity was observed. In contrast, other nitrogen sources, such as nitrate, did not show an effect on the expression of the nprA gene (not shown). The low expression of the nprA gene in the presence of ammonium or glutamine could be explained if these compounds act as repressors and/or if they block the transport of the inducer (DNP) inside the cell. Ammonium or glutamine probably does not affect nprA gene expression directly because the putative promoter region of the nprA gene does not show sequences typical for binding of the σ54 factor or the NtrC regulator, as in most Ntr-controlled promoters. More likely, high concentrations of these two compounds could inhibit DNP uptake, as previously described for R. capsulatus E1F1 (5, 17, 28). In agreement with this, in cultures with an initial ammonium concentration of 10 mM, β-galactosidase activity was only detected when the ammonium concentration decreased below 3 mM, showing that a sufficient amount of DNP could be transported inside the cells to induce nprA gene expression (not shown). In addition to DNP, expression of the R. capsulatus nprA gene was induced in the presence of several nitrofuran derivatives (Table 1). E. coli oxygen-insensitive NfsA and NfsB nitroreductase activities are responsible for most of the nitrofuran reduction activity under aerobic conditions (27). The nprA gene was also induced by the NfsB substrate 5-[azaridin-1-yl]-2,4-dinitrobenzamide (CB1954), a prodrug used in cancer therapy. This result suggests that CB1954 could be a substrate for the R. capsulatus NprA nitroreductase. In addition, two substrates of the Cnr nitroreductase of Salmonella enterica serovar Typhimurium used in the Ames test for potential carcinogens (13), 2-aminofluorene and benzo[a]pyrene, are also inducers of the expression of the R. capsulatus nprA gene (Table 1). β-Galactosidase activity was not detected when mononitroaromatic compounds were tested (not shown), but expression of the nprA gene was induced in the presence of several dinitroaromatic compounds, as well as by salicylic acid and paraquat. Salicylic acid induced expression of the nprA gene to the same extent within the range of 0.1 to 3 mM. The induction of nprA gene expression with salicylic acid and 2,4-dinitrophenol and the presence of two putative mar boxes in its possible promoter region (Fig. 1) suggest that the regulatory MarRA system could be implicated in controlling nprA expression in response to environmental hazards, as occurs with the nfsB gene of E. coli (2).
TABLE 1.
β-Galactosidase activities of a transcriptional nprA-lacZ gene fusion in R. capsulatus B10a
| Compound | β-Galactosidase activity (Miller units)b
|
|
|---|---|---|
| 0.1 mM | 0.2 mM | |
| None | 1 | 1 |
| Nitrofurazone | 10 | 30 |
| Nitrofurantoine | 18 | ND |
| Furazolidone | 9 | 48 |
| CB1954 | 21 | 75 |
| 2-Aminofluorene | 19 | 116 |
| Benzo[a]pyrene | 18 | 25 |
| 2,4-Dinitrotoluene | 54 | 90 |
| 3,5-Dinitrobenzoate | 52 | 62 |
| 2,4-Dinitrophenol | 105 | 144 |
| Salicylate | 30 | 33 |
| Paraquat | ND | 25 |
Cells were harvested at an A680 of about 0.5, and β-galactosidase activity was assayed as described in Materials and Methods. Cells were cultured under diazotrophic conditions with 30 mM malate as the carbon source and in the presence of different compounds at a final concentration of 0.1 mM or 0.2 mM. The compounds used were nitrofuran derivatives that are substrates of NfsA and NfsB of E. coli (27); the prodrug CB1954, which is a substrate of NfsB of E. coli (7); 2-aminofluorene and benzo[a]pyrene, substrates of SnrA of Salmonella enterica (13); several dinitroaromatics, substrates of the nitroreductase of R. capsulatus E1F1 (4); salicylate, a MarR repressor, as 2,4-dinitrophenol (2); and paraquat, a SoxR activator (9).
ND, not determined. The β-galactosidase activity data are means of the values obtained in three independent experiments with variations of <15%.
Effect of cellular redox status on nprA gene expression.
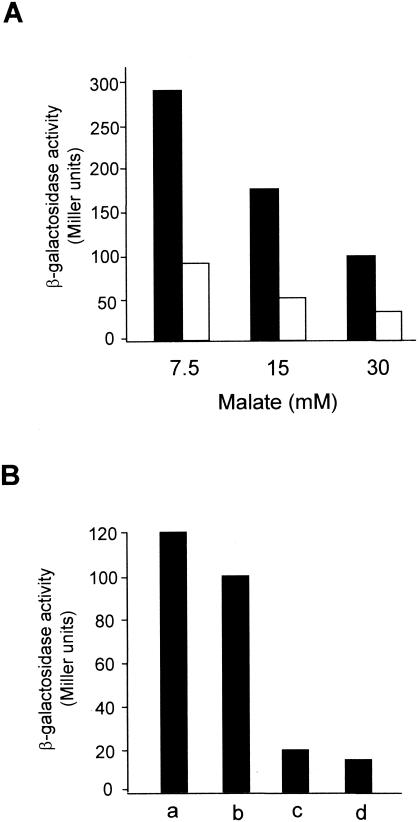
Expression of the nprA gene was induced in the presence of the superoxide-generating compound paraquat (Table 1). The oxygen-insensitive nitroreductases of E. coli and Salmonella, which are under the control of the MarRA system, are also induced in the presence of paraquat via the soxRS system (13, 14). However, it has been postulated that nitroreductases can regulate the accumulation of electron donors in the bacterial cytosol (9). Therefore, many oxidants could trigger induction of the soxRS regulon simply by draining the cellular electron source. Thus, the presence of nitroreductases in E. coli is not required for regulation of the soxRS response, although a depletion of the electron source could contribute to SoxR activation (9). To investigate the possible effect of the cellular redox status on nprA gene expression, cells of R. capsulatus B10 carrying the nprA-lacZ fusion were cultured with different malate concentrations or with different oxidized or reduced carbon sources. The expression of the nprA gene was upregulated under carbon-limiting conditions (Fig. 4A). This upregulation was more evident in cells cultured under diazotrophic conditions than in cultures grown in the presence of glutamine since this compound inhibits the transport of the inducer (DNP) inside the cells (Fig. 4A). In addition, electron-rich carbon sources, such as butyrate or caproate, downregulated nprA gene expression (Fig. 4B), suggesting that a role of the SoxRS system in the control of this nitroreductase could not be discarded. Thus, in the presence of highly reduced carbon sources or high carbon source concentrations, the redox status of the cells becomes more reduced through NAD(P)H accumulation, and under these conditions, SoxR could probably be reduced in its inactive form.
FIG. 4.
Effect of carbon source on nprA gene expression. (A) R. capsulatus cells harboring the transcriptional fusion nprA-lacZ+ were cultured with 150 μM 2,4-dinitrophenol either under diazotrophic conditions (black bars) or with 7 mM l-glutamine (white bars) in the presence of several concentrations of malate. (B) R. capsulatus cells harboring the transcriptional fusion nprA-lacZ+ were cultured with 150 μM 2,4-dinitrophenol under diazotrophic conditions in the presence of the following carbon sources: a, 50 mM acetate; b, 30 mM malate; c, 10 mM butyrate; and d, 5 mM caproate. When cultures reached an absorbance at 680 nm of 0.5, cells were harvested, and the β-galactosidase activity was determined as indicated in Materials and Methods. Data are means of the values obtained in three independent experiments with variations of <15%.
In conclusion, this work shows that R. capsulatus B10 presents two major nitroreductases involved in 2,4-dinitrophenol reduction. NprA is the main oxygen-insensitive NAD(P)H-dependent nitroreductase, whereas NprB is a second oxygen-insensitive NAD(P)H-dependent nitroreductase involved in DNP reduction. The nitroreductases behave in different manners; whereas the expression of the nprB gene is constitutive, nprA gene expression is induced by DNP and a variety of nitroaromatic and nitroheterocyclic compounds, including the prodrug CB1954 used in cancer therapy. Ammonium and glutamine have a negative effect, probably due to an inhibition of DNP uptake and consequently of nprA gene induction. The presence of putative mar/sox boxes in the possible promoter region of the nprA gene and the induction of nprA gene expression by 2,4-dinitrophenol and salicylic acid suggest the involvement of the two-component regulatory MarRA system in controlling nprA gene expression. In addition, induction of the nprA gene by paraquat and by an oxidized redox status in the cell suggests that the SoxRS system could also be implicated in controlling nprA gene expression.
Acknowledgments
This work was funded by Ministerio de Ciencia y Tecnología (grant BMC 2002-04126-CO3-03) and Junta de Andalucía (grant CVI-0117). E.P.R. was the recipient of a fellowship from the Ministerio de Ciencia y Tecnología, and M.D.R. holds a postdoctoral contract from Junta de Andalucía, Spain.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1999. Alteration of the repressor activity of MarR, the negative regulator of the Escherichia coli marRAB locus, by multiple chemicals in vitro. J. Bacteriol. 181:4669-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbosa, T. M., and S. B. Levy. 2002. Activation of the Escherichia coli nfnB gene by MarA through a highly divergent marbox in a class II promoter. Mol. Microbiol. 45:191-202. [DOI] [PubMed] [Google Scholar]
- 3.Blasco, R., E. Moore, V. Wray, D. Pieper, K. Timmis, and F. Castillo. 1999. 3-Nitroadipate, a metabolic intermediate for mineralization of 2,4-dinitrophenol by a new strain of a Rhodococcus species. J. Bacteriol. 181:149-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco, R., and F. Castillo. 1993. Characterization of a nitrophenol reductase from the phototrophic bacterium Rhodobacter capsulatus E1F1. Appl. Environ. Microbiol. 59:1774-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasco, R., and F. Castillo. 1992. Light-dependent degradation of nitrophenols by the phototrophic bacterium Rhodobacter capsulatus E1F1. Appl. Environ. Microbiol. 58:690-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haselkorn, R., A. Lapidus, Y. Kogan, C. Vlcek, J. Paces, V. Paces, P. Ulbrich, T. Pecenkova, D. Rebrekov, A. Milgram, M. Mazur, R. Cox, N. Kyrpides, N. Ivanova, V. Kapatral, T. Los, A. Lykidis, N. Mikhailova, G. Reznik, O. Vasieva, and M. Fonstein. 2001. The Rhodobacter capsulatus genome. Photosynth. Res. 70:43-52. [DOI] [PubMed] [Google Scholar]
- 7.Knox, R. J., and T. A. Connors. 1997. Prodrugs in cancer chemotherapy. Pathol. Oncol. Res. 3:309-324. [DOI] [PubMed] [Google Scholar]
- 8.Kobori, T., H. Sasaki, W. C. Lee, S. Zenno, K. Saigo, M. E. P. Murphy, and M. Tanokura. 2001. Structure and site-directed mutagenesis of a flavoprotein from Escherichia coli that reduces nitrocompounds. J. Biol. Chem. 276:2816-2823. [DOI] [PubMed] [Google Scholar]
- 9.Krapp, A. R., R. E. Rodríguez, H. O. Poli, D. H. Paladini, J. F. Palatnik, and N. Carrillo. 2002. The flavoenzyme ferredoxin (flavodoxin)-NADP(H) reductase modulates NADP(H) homeostasis during the soxRS response of Escherichia coli. J. Bacteriol. 184:1474-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenke, H., D. H. Pieper, C. Bruhn, and H. J. Knackmuss. 1992. Degradation of 2,4-dinitrophenol by two Rhodococcus erythropolis strains, HL 24-1 and HL 24-2. Appl. Environ. Microbiol. 58:2928-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 12.Moreno-Vivián, C., S. Hennecke, A. Pühler, and W. Klipp. 1989. Open reading frame 5 (ORF5), encoding a ferredoxin-like protein, and nifQ are cotranscribed with nifE, nifN, nifX, and ORF4 in Rhodobacter capsulatus. J. Bacteriol. 171:2591-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nokhbeh, M. R., S. Boroumandi, N. Pokorny, P. Koziarz, E. S. Paterson, and I. B. Lambert. 2002. Identification and characterization of SnrA in Salmonella enterica serovar Typhimurium TA1535. Mutat. Res. 508:59-70. [DOI] [PubMed] [Google Scholar]
- 14.Paterson, E. S., S. E. Boucher, and I. B. Lambert. 2002. Regulation of the nfsA gene in Escherichia coli by SoxS. J. Bacteriol. 184:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson, F. J., R. P. Mason, J. Hovsepian, and J. L. Holtzman. 1979. Oxygen-sensitive and -insensitive nitroreduction by Escherichia coli and rat hepatic microsomes. J. Chem. Biol. 254:4009-4014. [PubMed] [Google Scholar]
- 16.Rau, J., and A. Stolz. 2003. Oxygen-insensitive nitroreductases NfsA and NfsB of Escherichia coli function under anaerobic conditions as lawsone-dependent azo reductases. Appl. Environ. Microbiol. 69:3448-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sáez, L. P., P. García, M. Martínez-Luque, W. Klipp, R. Blasco, and F. Castillo. 2001. Role for draTG and rnf genes in reduction of 2,4-dinitrophenol by Rhodobacter capsulatus. J. Bacteriol. 183:1780-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Schmidt, S. K., K. M. Scow, and M. Alexander. 1987. Kinetics of p-nitrophenol mineralization by a Pseudomonas sp.: effects of second substrates. Appl. Environ. Microbiol. 53:2617-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. BioTechnology 1:784-791. [Google Scholar]
- 21.Snell, F. D., and C. T. Snell. 1949. Colorimetric methods of analysis, 3rd ed., p. 804-805. van Nostrand Reinhold, Princeton, N.J.
- 22.Solórzano, L. 1969. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 14:225-253. [Google Scholar]
- 23.Spain, J. C. 1995. Biodegradation of nitroaromatic compounds. Annu. Rev. Microbiol. 49:523-555. [DOI] [PubMed] [Google Scholar]
- 24.Venitt, S., and C. Crofton-Sleigh. 1987. The toxicity and mutagenicity of the anti-tumor drug 5-aziridino-2,4-dinitrobenzamide (CB1954) is greatly reduced in a nitroreductase-deficient strain of Escherichia coli. Mutagenesis 2:375-381. [DOI] [PubMed] [Google Scholar]
- 25.Vorbeck, C., H. Lenke, P. Fisher, J. C. Spain, and H.-J. Knackmuss. 1998. Initial reductive reactions in aerobic microbial metabolism of 2,4,6-trinitrotoluene. Appl. Environ. Microbiol. 64:246-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver, P. F., J. D. Wall, and H. Gest. 1975. Characterization of Rhodopseudomonas capsulatus. Arch. Microbiol. 105:207-216. [DOI] [PubMed] [Google Scholar]
- 27.Whiteway, J., P. Koziarz, J. V. Veall, N. Sandhu, P. Kumar, B. Hoecher, and I. B. Lambert. 1998. Oxygen-insensitive nitroreductases: analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J. Bacteriol. 180:5529-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witte, C.-P., R. Blasco, and F. Castillo. 1998. Microbial photodegradation of aminoarene metabolism of 2-amino-4-nitrophenol by Rhodobacter capsulatus. Appl. Biochem. Biotechnol. 69:191-202. [DOI] [PubMed] [Google Scholar]
- 29.Zeyer, J., H. P. Kocher, and K. N. Timmis. 1986. Influence of para-substituents on the oxidative metabolism of o-nitrophenols by Pseudomonas putida B2. Appl. Environ. Microbiol. 52:334-339. [DOI] [PMC free article] [PubMed] [Google Scholar]