Abstract
In a recent study we demonstrated that a high-hydrostatic-pressure-tolerant isolate of Listeria monocytogenes lacks a codon in the class 3 heat shock regulator gene ctsR. This mutation in the region that encodes four consecutive glycines was directly responsible for the observed piezotolerance, increased stress resistance, and reduced virulence. The aim of the present study was to determine whether mutations in ctsR are frequently associated with piezotolerance in L. monocytogenes. Wild-type cultures of L. monocytogenes were therefore exposed to 350 MPa for 20 min, and the piezotolerance of individual surviving isolates was assessed. This rendered 33 isolates with a stable piezotolerant phenotype from a total of 84 survivors. Stable piezotolerant mutants were estimated to be present in the initial wild-type population at frequencies of >10−5. Subsequent sequencing of the ctsR gene of all stable piezotolerant isolates revealed that two-thirds of the strains (i.e., n = 21) had mutations in this gene. The majority of the mutations (16 of 21 strains) consisted of a triplet deletion in the glycine-encoding region of ctsR, identical to what was found in our previous study. Interestingly, 2 of 21 mutants contained a codon insertion in this repeat region. The remaining three stable piezotolerant strains showed a 19-bp insertion in the glycine repeat region, a 16-bp insertion downstream of the glycine repeat area (both leading to frameshifts and a truncated ctsR), and an in-frame 114-bp deletion encoding a drastically shortened carboxy terminus of CtsR. In four instances it was not possible to generate a PCR product. A piezotolerant phenotype could not be linked to mutations in ctsR in 8 of 33 isolates, indicating that other thus-far-unknown mechanisms also lead to stable piezotolerance. The present study highlights the importance of ctsR in piezotolerance and stress tolerance of L. monocytogenes, and it demonstrates that short-sequence repeat regions contribute significantly to the occurrence of a piezotolerant and stress-tolerant subpopulation within L. monocytogenes cultures, thus playing an important role in survival.
Listeria monocytogenes is a gram-positive facultative anaerobic bacterium, which can cause severe food-borne illness in humans, known as listeriosis. This disease occurs at a relatively low incidence of 11 cases per million persons in Europe, but high mortality rates of ca. 30% make the organism a public health concern (17). Listeriosis occurs mostly in immunocompromised individuals, elderly, pregnant women, and newborns, while 5 to 10% of the general population is estimated to be carrier of the organism usually without developing listeriosis (14, 17). The microorganism can be present in a broad range of foods from animal or plant origin, and it can grow at pH values ranging from pH 5 to 9, at NaCl concentrations of up to 12%, and at temperatures from −0.4 to 44°C (10, 24). These characteristics make it very difficult, but necessary, to eliminate L. monocytogenes from foods.
Efficient inactivation of the organism in foods is achieved by conventional heat inactivation procedures such as pasteurization. During the last decade an increased consumers' demand for minimally processed products has led to the development of new methods of food preservation in the food industry. As a result, nonthermal preservation processes such as high hydrostatic pressure treatment have received increasing attention. Pressures within the range of 200 to 700 MPa are used to inactivate vegetative cells of microorganisms, including pathogens such as L. monocytogenes, whereas inactivation of bacterial spores requires higher pressures of around 1,000 MPa. Spore inactivation can also be achieved by combining other inactivation treatments (e.g., heat) with somewhat lower pressures (500 to 700 MPa), depending on the intensity of the other treatment (6, 8, 9, 25).
In a previous study we isolated a piezotolerant strain of L. monocytogenes, named AK01 (12). This strain demonstrated increased stress resistance to pressurization, heat, acid, and hydrogen peroxide treatment. AK01 also showed morphological differences compared to the wild-type strain, namely, increased cells size and lack of flagella, resulting in a nonmotile phenotype (12). The observed phenotypic differences were attributed to a single codon deletion a glycine-encoding repeat region in the ctsR gene, which encodes the class 3 heat shock response regulator (11). CtsR interacts with DNA through an amino-terminal helix-turn-helix (HTH) motif and has been shown to act as a dimer with a dimerization domain encoded in the region upstream of the HTH region (5). The highly conserved glycine-encoding repeat domain is located downstream of the HTH domain and is thought to play an important role in the conformational stability of the regulator, indirectly influencing DNA binding (5). The role of the amino-terminal domain is less clear; the study by Derré et al. (5) suggests that truncations in this region lead to unstable CtsR proteins that are rapidly degraded. In our previous study we demonstrated that a single triplet contraction in the glycine-encoding repeat region results in high expression levels of inactive CtsRΔGly (11). The deletion occurred in a short sequence repeat (SSR) region of three GGT triplets, encoding for three of the four consecutive glycines.
Various studies indicate that regions with short tandem repeats show increased rates of spontaneous mutations through strand slippage (22) and that tandem repeats are very common in stress response genes (19). Repetitive DNA sequences can consist of homopolymeric tracts of a single nucleotide type [poly(A), poly(G), poly(C), or poly(T)] or of small or large numbers of several multimeric classes of repeats (22). It has been postulated that the relatively short unit repeats, like the glycine-encoding region of ctsR, are usually involved in regulatory processes that are affected by slipped-strand mispairing (22). In Haemophilus influenzae and Neisseria meningitidis there is an abundance of contingency loci, which seem to have a major effect on fitness, survival and pathogenicity. They are located in the genes encoding for evasins, lipopolysaccharide biosynthesis proteins, adhesins, iron acquisition proteins, and restriction modification systems (16, 22, 23). A strategy to increase versatility may be crucial for the survival of at least few cells under adverse conditions, since it allows organisms to achieve phenotypic variation.
The deletion of a triplet in the glycine-encoding region of CtsR leads to piezotolerance and stress tolerance of a single L. monocytogenes isolate (11, 12) and, given the nature of this mutation, variation in length of this triplet repeat is expected to occur at a relatively high rate. The aim of the present study was therefore to assess whether this mechanism indeed leads to increased survival of a subpopulation of cells within clonal populations of wild-type L. monocytogenes and at what frequencies it occurs.
MATERIALS AND METHODS
Bacterial strains and culturing conditions.
L. monocytogenes Scott A (Department of Food Science, Wageningen Agricultural University, Wageningen, The Netherlands) and L. monocytogenes Scott A AK01 (12) were used in the present study. Stock cultures of these strains and new isolates (see below) were kept at −80°C in 15% (vol/vol) glycerol. Stock cultures were transferred to sterile brain heart infusion (BHI) broth (Oxoid, Hampshire, England) and incubated twice at 30°C overnight (0.3% [vol/vol] inoculum) before use. Cultures were routinely incubated with shaking (160 rpm).
Selection of piezotolerant mutants.
The first objective was the isolation of piezotolerant mutants derived from wild-type L. monocytogenes Scott A. Therefore, we first purified the stock culture on BHI agar plates and inoculated three individual colonies from different plates into BHI broth. The cultures of the three selected colonies were subcultured at 30°C (0.3% inocula) before a 0.3% (vol/vol) inoculum of stationary-phase culture (∼5 × 109 CFU/ml) was added to 100 ml of BHI broth. Cells from this subculture were harvested at mid exponential phase (optical density at 660 nm [OD660] of ∼0.3), washed twice in 50 mmol of ACES buffer (Sigma-Aldrich, Steinheim, Germany) liter−1 (pH 7.0), and resuspended in this buffer to an OD660 of ∼0.1. Suspensions were sealed into small sterile plastic pouches which were submerged in glycol (the fluid medium through which the pressure was transferred). Pressurization of 350 MPa for 20 min was performed by using 9-ml pressure chambers (Resato, Roden, The Netherlands). The pressure was applied within 1 min, and temperature recorders indicated a maximum temperature of 30°C during pressurization. Once 350 MPa was reached, the temperature returned to 20°C within 2 min (see references 11 and 12 for additional experimental details). The L. monocytogenes cell suspensions were then plated onto BHI agar plates, and CFU counts from the surviving cells were determined. Importantly, these cultures showed identical inactivation by pressurization compared to the initial −80°C wild-type stock culture. From each of the three independent experiments, 28 surviving isolates were randomly selected and stored at −80°C in 15% (vol/vol) glycerol. The total of 84 individual isolates were subsequently assessed for stable piezotolerant phenotypes. Isolates were subcultured during five consecutive days using 0.3% (vol/vol) inocula in fresh BHI medium. On day 5 (equivalent to ∼70 generations), cultures were inoculated with a 0.3% (vol/vol) inoculum in 100 ml of BHI broth, incubated at 30°C under shaking (160 rpm), and harvested in mid-exponential phase (OD660 of ∼0.2). These cells were washed, and viable numbers were determined before and after pressure treatment (350 MPa for 20 min) to determine the piezotolerance of individual cultures. L. monocytogenes wild type and strain AK01 were used as controls in each experiment. For details on pressure treatments and procedures please see above and references 11 and 12.
Frequency determination of piezotolerant mutants.
The frequency (F) at which the piezotolerant mutants occur in a wild-type population can be calculated by dividing the number of piezotolerant cells in the initial population (Np) before pressure treatment by the total number of cells of the initial population in ACES buffer (Nt) before pressurization: F = Np Nt−1. In three independent experiments, cultures were grown to mid-exponential phase at 30°C and washed in buffer. A sample was taken to determine the initial number of cells (Nt) in duplicate before the same sample was challenged with pressure treatment (350MPa for 20 min at 20°C). After pressure treatment, the total number of survivors (Ntotal surv) were determined in duplicate. Subsequently, the piezotolerance of 28 randomly selected surviving isolates (Nselect) was determined by challenging mid-exponential-phase cultures individually with pressure treatment (350 MPa for 20 min at 20°C). The pressure treatment used to assess piezotolerance of individual cultures was therefore identical to the treatment used to select for surviving colonies from the wild-type population. This is important in relation to the calculations and assumptions that follow.
An isolate was characterized as stable piezotolerant when the reduction in viable numbers was at least 100-fold (i.e., 2 log units) higher than the average reduction in viable numbers of the wild type. This rendered the number of stable piezotolerant isolates (Nstable select) in the randomly selected surviving isolates from which the actual fraction of stable piezotolerant mutants can be derived (fraction = Nstable select Nselect−1). By extrapolation, we estimated the number of total stable piezotolerant isolates in the surviving population per experiment (Ntotal stable surv) as follows: Ntotal stable surv = (Nstable select Nselect−1) × Ntotal surv. The number of piezotolerant cells in the initial population (Np) can be determined by using the assumption that the piezotolerant mutants form a distinct population in the initial population, with an average log reduction (a) that is calculated for each of the individual experiments. The assumption that piezotolerant mutants preexisted in the initial wild-type population before the application of pressure is based on the findings that DNA replication, RNA transcription, and protein translation are disabled above 77 MPa (25). In addition, DNA damage as a result of the pressure treatment is highly unlikely, since DNA is extremely stable even at pressures above the one used in this experiment (8). The number of stable piezotolerant cells at the onset of the experiment can now be calculated as: Np = 10a Ntotal stable surv. This then allowed for the estimation of the frequency of occurrence of piezotolerant cells in the initial wild-type population as follows: F = Np Nt−1 = 10a Ntotal stable surv Nt−.
Motility tests.
The motility of all 84 isolates that survived the initial pressure treatment was tested by using semisolid motility test medium. The medium consisted of 10 g of peptone (Oxoid), 5 g of NaCl (Merck, Darmstadt, Germany), 4 g of agar (Oxoid), 3 g of beef extract (Oxoid), and 0.05 g of 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich) liter−1. The medium was boiled for 1 to 2 min with agitation before pouring 8 to 9 ml into screw-cap glass tubes, followed by autoclaving (15 min at 121°C).
Stock cultures of the isolates, kept at −80°C in 15% (vol/vol) glycerol, were transferred to 9 ml of sterile BHI broth and incubated twice at 30°C overnight (0.3% [vol/vol] inoculum). Subsequently, each culture was inoculated by stabbing a sterile needle into a tube with motility test medium and incubated at 25°C for 5 days, since L. monocytogenes shows the highest mobility at this temperature (13).
Bacterial metabolism reduces 2,3,5-triphenyltetrazolium chloride to formazan, which is red in color. Only the isolates that were motile were able to swarm into the semisolid motility test medium, showing a red cloudy pattern away from the initial stab.
Sequence analysis of the ctsR gene.
We previously demonstrated that a single codon deletion in a repeat region of ctsR resulted in increased piezotolerance, tolerance to other stresses, and a loss of motility (11). In the present study, we investigated the involvement of mutations in ctsR in piezotolerance by analyzing the sequence of ctsR and the upstream region of ctsR (∼170 bp) in stable piezotolerant mutants. PCR amplification of the ctsR gene was performed by using standard methods (20). Chromosomal DNA from all stable piezotolerant isolates was isolated by using the method described by Pospiech and Neumann (18) and used as a template in PCRs. To serve as controls, DNA was isolated from the wild-type strain and from four isolates that survived the pressure treatment but did not display a stable piezotolerant phenotype. Primers CtsRfw (5′-GAGAGCGTCGACCGTAGCACAATTCTCGCAT) and CtsRrv (5′-AAGCTTGAATTCGCCAATGGTAGTTGGGGGC) were used for PCR amplification and DNA sequence analysis (BaseClear, Leiden, The Netherlands, and Lark Technologies, United Kingdom) was performed in duplicate.
RESULTS
Isolation of piezotolerant strains and their motility phenotype.
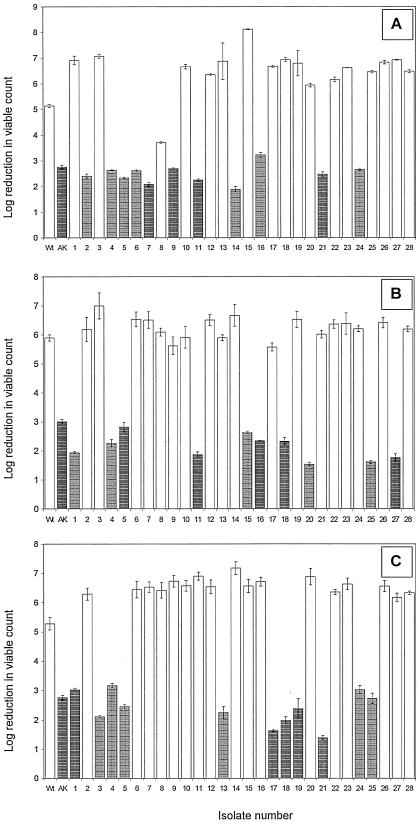
In three independent experiments, cultures of L. monocytogenes Scott A wild-type cells were exposed to a single pressure treatment of 350 MPa for 20 min. From each of these experiments, 28 strains were isolated that survived the pressure treatment. The total of 84 isolates were cultured individually in the absence of pressure during ∼70 generations before their piezotolerance was assessed once again. This yielded a total of 33 isolates with stable piezotolerant phenotypes, evenly distributed among the three groups, namely, 12 of 28 in group A, 10 of 28 in group B, and 11 of 28 in group C (Fig. 1). Strikingly, all stable piezotolerant isolates except isolate A8 were immotile, as determined in the swarming assay (Fig. 1). All other isolates displayed normal motility.
FIG. 1.
Log reduction in viable counts of individual L. monocytogenes isolates obtained from the three independent repetitive experiments A, B, and C, after exposure to 350 MPa for 20 min at 20°C. Shaded bars mark strains that are nonmotile. Reductions in viable number were determined in triplicate for each isolate, and error bars represent standard deviations of log reductions. Wt represents the log reduction of the viable counts of the wild-type culture from which all isolates were obtained. AK represents the log reduction of the viable counts of the AK01 mutant tested in parallel.
Sequence analysis of ctsR of piezotolerant isolates.
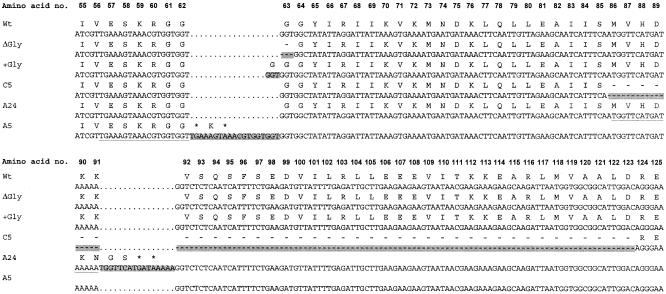
The sequence of the ctsR gene could be determined for 29 of the 33 stable piezotolerant mutants; in four cases PCR products could not be generated. The majority of stable piezotolerant isolates (i.e., 21 isolates) had mutations in their ctsR gene. The previously observed codon deletion in the region that encodes the glycine repeat in CtsR (11) was predominantly found, namely, in 16 of 21 isolates (Fig. 2). Interestingly, codon insertions in the glycine repeat region were observed in 2 of 21 strains with mutations in ctsR. The remaining three mutations in ctsR constituted (i) a 19-bp insertion of a repeat sequence in the glycine repeat region, leading to a frameshift and a truncated CtsR; (ii) a 16-bp insertion of a repeat sequence further downstream, also leading to a frameshift and truncated CtsR; and (iii) a large 114-bp in-frame deletion, encoding a variant of CtsR that lacks 38 amino acids in its C-terminal region (amino acids 86 to 123) (Fig. 2). A total of 8 of 33 stable piezotolerant strains did not show mutations in their ctsR gene and the 170 basepair upstream regions, indicating that other, as-yet-unknown mechanisms underlie piezotolerance in these strains. In four instances, we were unable to amplify the ctsR gene of isolates with a stable piezotolerant phenotype. This is possibly due to deletion of the ctsR region or poor hybridization of primers as a result of mutations. Finally, the wild-type strains and four strains that survived the initial pressure treatment but did not display stable piezotolerance showed no mutations in ctsR and the upstream region (data summarized in the legend to Fig. 2).
FIG. 2.
Sequence analysis of the ctsR genes of stable piezotolerant mutants. The total length of CtsR is 152 amino acids. Sequence analysis of ctsR of 33 stable piezotolerant mutants revealed that the majority of strains had mutations in ctsR. Approximately half of the stable piezotolerant isolates contained a triplet deletion in the glycine-encoding repeat region. ΔGly = A2, A4, A6, A7, A11, A14, A16, A21, B1, B4, B11, B15, B16, C4, C21, and C24; +Gly = A9 and B5; A5, 19-bp insert; A24, 16-bp insert; C5, 114-bp deletion. The following had no mutations in ctsR: B18, B20, B25, B27, C13, C17, C18, and C25. The following had no PCR product: C1, A8, C3, and C19.
Frequency determination of stable piezotolerant mutants in initial population. (i) Experiment A.
The initial overall population before pressure treatment was determined: Nt = 4.63 × 108 CFU ml-1. The overall surviving population was Ntotal surv = 3.38 × 103 CFU ml-1. In experiment A, Nstable select Nselect−1 = 12/28 = 0.43. Extrapolation to the overall surviving population gives Ntotal stable surv = 1.45 × 103 CFU ml-1. The average log reduction of piezotolerant mutants in experiment 1 is a = 2.58 (calculated from data presented in Fig. 1). The frequency of naturally occurring piezotolerant mutants in the initial wild-type population of experiment A is therefore FA = 10a Ntotal stable surv Nt−1 = 102.58 × 1.45 × 103/4.63 × 108 = 1.2 × 10−3.
(ii) Experiment B.
With Nt = 1.08 × 109 CFU ml-1, Nstable select Nselect−1 = 10/28 = 0.36, Ntotal surv = 1.41 × 103 CFU ml-1, and a = 2.11, the frequency of naturally occurring piezotolerant mutants in the initial wild-type population of experiment B is FB = 6.0 × 10−5.
(iii) Experiment C.
With Nt = 3.39 × 108 CFU ml-1, Nstable select Nselect−1 = 11/28 = 0.39, Ntotal surv = 1.73 × 103 CFU ml-1, and a = 2.37, the frequency of naturally occurring piezotolerant mutants in the initial wild-type population of experiment C is FC = 4.7 × 10−4.
Approximately half of the mutants are expected to have a codon deletion in the glycine repeat region of ctsR. The frequencies vary significantly per experiment, with an average frequency of 5.7 × 10−4, i.e., ∼6 per 10,000.
DISCUSSION
While more genomes sequences of species become available, an increasing number of SSRs are being identified. These sequences can lead to the occurrence of relatively high rates of reversible mutations, giving rise to phenotypic variation. This phenomenon is thought to play a crucial role in adaptation to (sudden) changes in environmental conditions, thereby ultimately contributing to survival. Various studies stress the significance of these sequences; however, only in a few instances has a role for SSRs in specific cellular processes been demonstrated experimentally (1, 22). We have previously demonstrated that piezotolerant L. monocytogenes strain AK01 contains a 5′-(GGT)3-3′ triplet contraction in a glycine-encoding repeat region of the ctsR gene, rendering CtsR inactive as a repressor of class III shock genes. As a direct consequence, this mutant showed increased resistance to high hydrostatic pressure, heat, acid, and H2O2; a lack of motility; and reduced virulence (11, 12).
The present study clearly demonstrates that the occurrence of such mutations are not isolated incidents; variation in length of a SSR in ctsR occurs at relatively high frequencies (>10−5), leading to enhanced survival of a clonal population of L. monocytogenes upon sudden exposure to stress. After a single pressure treatment of three independent wild-type L. monocytogenes cultures (grown from a single purified colony), survivors were isolated, and a relatively high percentage of these strains (nearly 40%) showed a stable piezotolerant phenotype. DNA sequence analysis of the ctsR genes of these stable piezotolerant strains revealed that their majority (i.e., two-thirds) contained mutations in ctsR, while the remaining stable isolates (i.e., one-third) likely contain mutations that have thus far not been identified. Interestingly, these stable piezotolerant isolates were also nonmotile except for isolate 8A.
The most common mutation in ctsR constituted a triplet deletion in the glycine-encoding repeat region of ctsR, identical to the one described previously (11). In two instances a GGT triplet expansion was found in the glycine repeat region, resulting in five contiguous glycines instead of four. Mutants containing this insertion in CtsR show a piezotolerant phenotype and loss of motility, which may be indicative that CtsR+Gly has also lost its repressor function. The tertiary structure of repetitive DNA sequences allows for mismatching of neighboring repeats, leading to insertion or deletion during DNA polymerase-mediated DNA duplication (4, 7, 21). Expansion and contraction of the repeat tracts by one or more repeat units is a well-known characteristic of contingency loci, leading to multiple possible “on” and “off” states for the gene containing these loci (1-3, 5, 22). The occurrence of CtsRΔGly and CtsR+Gly is likely due to slippage of the DNA polymerase during replication, and our data suggest that contraction of the glycine rich region occurs more frequently than expansion (16 versus 2 isolates, respectively). The characterized piezotolerant isolates in the present study showed a stable phenotype but, given the nature of the mutations, reversion to the wild-type genotype is anticipated albeit possibly at lower frequencies. When reversion to wild type occurs under conditions that favor growth of the wild type, these cells are expected to outgrow the mutants and eventually dominate the overall population, since the growth rate of the wild type is slightly (5 to 10%) higher than those of the mutants (12).
It is interesting that insertions of relatively large repeats were found in two instances: the 16- and 19-bp insertions in strains A24 and A5, respectively, are exact duplicates of 16- and 19-bp sequences directly upstream (see Fig. 2). These mutations render truncated CtsR proteins which are either inactive or unstable given the piezotolerant phenotype of the strains, in line with results of Derré et al. (5), who found that truncations in this region lead to unstable proteins that are rapidly degraded. This also seems to be the case in piezotolerant isolate C5, which lacks 38 amino acids in its carboxy terminus.
Thus far, the characterization of piezotolerant food-borne pathogens has been restricted to a limited number of incidental isolates of Escherichia coli (6) and L. monocytogenes (12). At a population level, Metrick et al. (15) described the existence of a piezotolerant subpopulation of Salmonella enterica serovar Typhimurium upon pressurization at 340 MPa, resulting in so-called “tailing” effects; however, individual isolates derived from the surviving subpopulation did not show increased hydrostatic pressure resistance. The present study is the first to demonstrate the presence of a stable piezotolerant subpopulation in a wild-type clonal population at frequency of at least 10−5 and highlights a mechanism that contributes significantly to population heterogeneity with regard to piezotolerance and stress tolerance, namely, variations in repeat length of the glycine-encoding region of ctsR in ∼50% of cases. The observed frequencies were rather variable between independent experiments (from ∼10−3 to 10−5) and are inherent to the essay. Since the three independent experiments were performed with cultures that were grown from a single wild-type colony (i.e., cultures were derived from single cells), the final level of mutant cells in each population is thought to vary, depending on the moment and frequency at which strand-slippage occurs. This is a stochastic event, and the fraction of mutants in the final population is likely higher when codon expansions or contractions in ctsR are generated in the early growth phase.
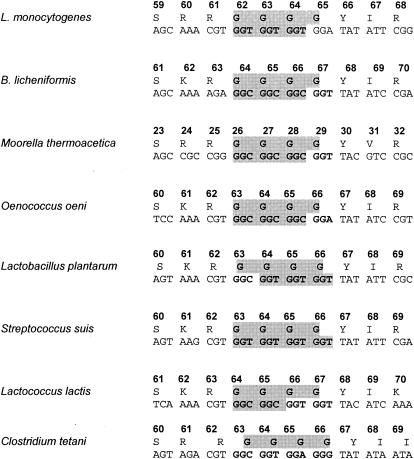
CtsR proteins, their glycine-rich region, and their target sequences are highly conserved among gram-positive bacteria (2, 5), indicating that the phenomenon described above might also play a role in a number of other bacteria. An alignment of the glycine repeat region of CtsR protein sequences and ctsR DNA sequences showed a very high conservation at the protein level but less conservation at the DNA sequence level, possibly depending on the codon usage of the particular organism (Fig. 3). In most cases, a minimum number of three triplet repeats were found, but there were cases with two or no repeats (Fig. 3). Whereas this indicates that DNA strand slippage due to SSRs might occur, experimental verification for individual organisms is required to confirm this hypothesis.
FIG. 3.
Protein and DNA sequences of the glycine-rich region of the ctsR of several gram-positive bacteria. DNA sequences presented might contain a number of hypermutable short sequence repeats (e.g., L. monocytogenes), or no hypermutable regions (e.g., L. lactis and C. tetani).
Overall, the present study demonstrates that hypermutability of repeat sequences in the glycine-encoding region of ctsR of L. monocytogenes leads to high numbers of piezotolerant and stress-tolerant cells within clonal populations. Mutations in this region render CtsR inactive, leading to constitutive expression of the Clp stress proteins in the cells. This strategy ensures the presence of a certain number of stress resistant cells within a wild-type population, allowing for the survival of a subpopulation upon an insult such as pressure or heat treatment. This phenomenon differs from adaptation responses that require de novo synthesis of mRNA and proteins and thereby offers an alternative survival strategy upon exposure to sudden unfavorable conditions. CtsR has been found to play a similar role with a number of other gram-positive bacteria, such as B. subtilis and S. aureus (2). Since CtsR proteins, their glycine-rich region, and their target sequences are highly conserved among gram-positive bacteria, it remains to be established how widespread SSRs in ctsR contribute to stress resistance in other gram-positive bacteria. Furthermore, we isolated various stable piezotolerant isolates without mutation in ctsR, which is a clear indication that other unidentified mechanisms are contributing significantly to piezotolerance.
Acknowledgments
This research was supported by the Wageningen Centre of Food Sciences.
V.P.V. was supported by the DG XXII-Leonardo da Vinci Programme of EU.
REFERENCES
- 1.Bayliss, C. D., D. Field, and E. R. Moxon. 2001. The simple sequence contingency loci of Haemophilus influenzae and Neisseria meningitidis. J. Clin. Investig. 107:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chastanet, A., J. Fert, and T. Msadek. 2003. Comparative genomics reveal novel heat shock regulatory mechanisms in Staphylococcus aureus and other gram-positive bacteria. Mol. Microbiol. 47:1061-1073. [DOI] [PubMed] [Google Scholar]
- 3.Chiurazzi, P., L. Kozak, and G. Neri. 1994. Unstable triplets and their mutational mechanism: size reduction of the CGG repeat versus germline mosaicism in the fragile X syndrome. Am. J. Med. Genet. 51:517-521. [DOI] [PubMed] [Google Scholar]
- 4.Coggins, L. W., and M. O'Prey. 1989. DNA tertiary structures formed in vitro by misaligned hybridization of multiple tandem repeat sequences. Nucleic Acids Res. 17:7417-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derré, I., G. Rapoport, and T. Msadek. 2000. The CtsR regulator of stress response is active as a dimer and specifically degraded in vivo at 37°C. Mol. Microbiol. 38:335-347. [DOI] [PubMed] [Google Scholar]
- 6.Hauben, K. J. A., D. H. Bartlett, C. C. F. Soontjens, K. Cornelis, E. Y. Wuytack, and C. W. Michiels. 1997. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl. Environ. Microbiol. 63:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauge, X. Y., and M. Litt. 1993. A study of the origin of “shadow bands” seen when typing dinucleotide repeat polymorphisms by the PCR. Nucleic Acids Res. 2:411-415. [DOI] [PubMed] [Google Scholar]
- 8.Heremans, K. 1982. High pressure effects on proteins and other biomolecules. Annu. Rev. Biophys. Bioeng. 11:1-21. [DOI] [PubMed] [Google Scholar]
- 9.Iwahashi, H., S. Fujii, K. Obuchi, S. C. Kaul, A. Sato, and Y. Komatsu. 1993. Hydrostatic pressure is like high temperature and oxidative stress in the damage it causes to yeast. FEMS Microbiol. Lett. 108:53-58. [DOI] [PubMed] [Google Scholar]
- 10.Kallipolitis, B. H., and H. Ingmer. 2001. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol. Lett. 204:111-115. [DOI] [PubMed] [Google Scholar]
- 11.Karatzas, K. A. G., J. A. Wouters, G. M. C. Gahan, C. Hill, T. Abee, and M. H. J. Bennik. 2003. The CtsR regulator of Listeria monocytogenes contains a variant glycine repeat region that affects piezotolerance, stress resistance, motility, and virulence. Mol. Microbiol. 49:1227-1238. [DOI] [PubMed] [Google Scholar]
- 12.Karatzas, K. A. G., and M. H. J. Bennik. 2002. Characterization of a Listeria monocytogenes Scott A isolate with high tolerance to high hydrostatic pressure. Appl. Environ. Microbiol. 68:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudsen, G. M., J. E. Olsen, and L. Dons. 2004. Characterization of DegU, a response regulator in Listeria monocytogenes, involved in regulation of motility and contributes to virulence. FEMS Microbiol. Lett. 240:171-179. [DOI] [PubMed] [Google Scholar]
- 14.Lou, Y., and A. E. Yousef. 2000. Characteristics of Listeria monocytogenes important to food processors, p. 131-224. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis, and food safety, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 15.Metrick, C., D. G. Hoover, and D. F. Farkas. 1989. Effects of high hydrostatic pressure on heat-resistant and heat-sensitive strains of Salmonella. J. Food Sci. 54:1547-1549. [Google Scholar]
- 16.Moxon, E. R., and D. S. Thaler. 1997. The tinkerer's evolving toolbox. Nature 387:659-662. [DOI] [PubMed] [Google Scholar]
- 17.Notermans, S., and E. Hoornstra. 2000. Risk assessment of Listeria monocytogenes in fish products: some general principles, mechanism of infection, and the use of performance standards to control human exposure. Int. J. Food Microbiol. 62:223-229. [DOI] [PubMed] [Google Scholar]
- 18.Pospiech, A., and B. Neumann. 1995. Isolation of genomic DNA from gram-positive bacteria. Trends Genet. 11:217-218. [DOI] [PubMed] [Google Scholar]
- 19.Rocha, E. P. C., I. Matic, and F. Taddei. 2002. Over-representation of repeats in stress response genes: a strategy to increase versatility under stressful conditions? Nucleic Acids Res. 30:1886-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Snyder, L., and W. Champness. 1997. Molecular genetics of bacteria. American Society for Microbiology, Washington, D.C.
- 22.van Belkum, A., S. Scherer, L. van Alpen, and H. Verburg. 1998. Short-sequence DNA repeats in prokaryotic genomes. Microbiol. Mol. Biol. Rev. 62:275-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Woude, M. W., and A. J. Bäumler. 2004. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17:581-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker, S. J., P. Archer, and J. G. Banks. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157-162. [DOI] [PubMed] [Google Scholar]
- 25.Yayanos, A. A., and E. C. Pollard. 1969. A study of the effects of hydrostatic pressure on macromolecular synthesis in Escherichia coli. Biophys. J. 9:1464-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]