Abstract
Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) is a cyclic nitroamine explosive that is a major component in many military high-explosive formulations. In this study, two aerobic bacteria that are capable of using RDX as the sole source of carbon and nitrogen to support their growth were isolated from surface soil. These bacterial strains were identified by their fatty acid profiles and 16S ribosomal gene sequences as Williamsia sp. KTR4 and Gordonia sp. KTR9. The physiology of each strain was characterized with respect to the rates of RDX degradation and [U-14C]RDX mineralization when RDX was supplied as a sole carbon and nitrogen source in the presence and absence of competing carbon and nitrogen sources. Strains KTR4 and KTR9 degraded 180 μM RDX within 72 h when RDX served as the only added carbon and nitrogen source while growing to total protein concentrations of 18.6 and 16.5 μg/ml, respectively. Mineralization of [U-14C]RDX to 14CO2 was 30% by strain KTR4 and 27% by KTR9 when RDX was the only added source of carbon and nitrogen. The addition of (NH4)2SO4 greatly inhibited KTR9's degradation of RDX but had little effect on that of KTR4. These are the first two pure bacterial cultures isolated that are able to use RDX as a sole carbon and nitrogen source. These two genera possess different physiologies with respect to RDX mineralization, and each can serve as a useful microbiological model for the study of RDX biodegradation with regard to physiology, biochemistry, and genetics.
Hexahydro-1,3,5-trinitro-1,3,5-triazine(RDX) is a cyclic nitroamine that is one of the most powerful and commonly used military explosives. RDX has contaminated soils and groundwater at army ammunition plants and other military sites through its manufacture, use on testing and firing ranges, and disposal (22). RDX is a toxic compound that affects the central nervous system of laboratory animals, causing convulsions (10, 37), and it has been proposed as a possible human carcinogen (38). Information on the environmental factors that determine the transport and fate of RDX in soils is needed to accurately assess the risk posed by RDX and to ensure the sustainability of live-fire training exercises.
Anaerobic biodegradation of RDX has been extensively studied (1, 6, 21, 23, 25, 26, 29, 42-45). McCormick et al. (29) proposed a pathway where RDX undergoes sequential reduction of the nitro groups to form hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine; hexahydro-1,3-dinitroso-5-nitro-1,3,5-triazine; and hexahydro-1,3,5-trinitroso-1,3,5-triazine. According to the proposed pathway, this transformation then produces formaldehyde, methanol, hydrazine, and dimethyl hydrazine. Hawari et al. (23) proposed another pathway where ring cleavage occurs before sequential reduction, producing the metabolites methylenedinitramine and bis(hydroxymethyl)nitramine.
Although most of the initial strains isolated could only degrade RDX anaerobically (25, 32, 41, 42), several pure strains have subsequently been isolated that aerobically degrade RDX as a nitrogen source. Binks et al. (8) isolated Stenotrophomonas maltophilia PB1 and tentatively identified an RDX metabolite as methylene-N-(hydroxymethyl)-hydroxylamine-N′-(hydroxymethyl)nitroamine. Rhodococcus sp. strain DN22 was isolated by Coleman et al. (12) and was found to produce nitrite as a metabolite. Fournier et al. (19) found that DN22 also produces the metabolites ammonia, nitrous oxide, formaldehyde, carbon dioxide, and a dead-end product with a molecular weight of 119 that was later identified as 4-nitro-2,4-diazabutanal by Bhushan et al. (7). Fournier et al. (19) also proposed a pathway for aerobic RDX degradation in which denitration occurs, followed by spontaneous ring cleavage. Further experiments determined that the enzyme responsible for the degradation of RDX by DN22 is a cytochrome P450 enzyme (7, 13). Rhodococcus rhodochrous strain 11Y, which was isolated by Seth-Smith et al. (33), was found to produce the metabolites nitrite, formate, and formaldehyde. Seth-Smith et al. (33) also identified the gene responsible for RDX degradation as a cytochrome P450-like gene, xplA, and reported that the mechanism of action was initial denitration, followed by spontaneous ring cleavage and mineralization.
In this study, we report on the isolation of two bacteria that, unlike all previously described bacteria, degrade RDX aerobically as a sole carbon and nitrogen source. These bacteria are able to mineralize RDX when used as a source of carbon, nitrogen, or carbon and nitrogen. Their unique metabolisms, ease of culture, and rapid growth make these strains excellent models for the basic genomic and proteomic studies required for the development of RDX bioremediation systems and potentially green RDX biosynthetic systems.
MATERIALS AND METHODS
Chemicals.
RDX (>95% purity) was purchased from the Indian Head Division of the Naval Surface Warfare Center (Indian Head, MD). 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) was obtained from A. T. K. Thiokol Propulsion (Brigham City, UT) as the ɛ isomer (>99% purity). [U-14C]RDX, with a specific activity of 4.3 mCi/mmol and a radiochemical purity of 98.4%, was purchased from New England Nuclear Research Products (Boston, MA). Unlabeled RDX, CL-20, and [U-14C]RDX were dissolved in acetone, methanol, or ethanol, respectively. 4-Nitro-2,4-diazabutanal was synthesized by Ron Spanggord (SRI International, Menlo Park, CA) by the method described by Fournier et al. (20).
Enrichment cultures.
Initial enrichments were designed to obtain aerobic bacteria that could use RDX as the sole source of nitrogen to support their growth. Surface soil from Site 8 of the Naval Air Warfare Center Weapons Division in China Lake, CA, was used as the inoculum. There were no explosives detected in this soil, although prior exposure is possible. One gram of soil was added to 5 ml of a sterile mineral salts medium (designated MSM1) in a 15-ml screw-cap test tube. Glucose (5.6 mM) and RDX (45 μM) were added aseptically. MSM1 was prepared by a method similar to that described by Shelton and Tiedje (34) and consisted of (per liter): KH2PO4, 0.272 g; K2HPO4, 0.348 g; MgSO4 · 7H2O, 0.2 mg; FeSO4 · 7H2O, 2 mg; CaCl2 · 2H2O, 0.03 mg; MnCl2 · 4H2O, 0.5 mg; H3BO3, 0.05 mg; ZnCl2, 0.05 mg; CuCl2, 0.03 mg; Na2MoO4 · 2H2O, 0.01 mg; CoCl2 · 6H2O, 0.5 mg; NiCl2 · 6H2O, 0.05 mg; and Na2SeO3, 0.5 mg. The NH4Cl, resazurin, NaHCO3, Na2S · 9H2O, and vitamins were omitted and the medium was kept aerobic. Enrichments were incubated at 25°C in the dark with mixing at 30 rpm.
Isolation procedure.
Enrichment cultures were monitored for their ability to degrade RDX by high-performance liquid chromatography (HPLC) analysis of the culture medium. Enrichment cultures that degraded the RDX were transferred (10% [vol/vol]) to a second mineral salts medium (MSM2) (10 ml). MSM2 contained (per liter): K2HPO4, 0.38 g; MgSO4 · 7H2O, 0.2 g; and FeCl3 · 6H2O, 0.05 g. Glucose (5 mM), succinate (5 mM), and glycerol (10 mM) were aseptically added as carbon sources, and RDX (180 μM) was added as the nitrogen source. After three sequential growth transfers, cultures were serially diluted with phosphate-buffered saline (NaCl, 8.5 g liter−1; Na2HPO4, 0.6 g liter−1; and KH2PO4, 0.3 g liter−1), and dilutions (0.1 ml) were spread onto the surface of 5% PTYG (4) agar plates. Plates were incubated aerobically at 30°C for 5 days. All of the colonies appearing on the plates were isolated by transferring them to fresh 5% PTYG agar plates to confirm purity and subsequently inoculated into MSM2 containing the carbon sources listed above and 180 μM RDX. RDX degradation was confirmed by HPLC analysis of culture medium.
Growth experiments.
In all growth experiments, isolates were inoculated into MSM2 (50 ml) at an optical density of 600 nm (OD600) of 0.05, and RDX was added at a concentration of 180 μM. Acetone from the RDX stock solution was allowed to evaporate before the addition of MSM2. When applicable, carbon was added in the concentrations listed previously, and (NH4)2SO4 was used at a concentration of 4 mM. For experiments with CL-20, 8 or 23 μM CL-20 was added to MSM2 along with the addition of the carbon sources listed previously. All studies were performed in triplicate, and cultures were incubated at 30°C in the dark with shaking at 120 rpm.
Resting cell study.
Isolates were grown to late log phase in MSM2 as described above with the addition of RDX and the carbon sources listed previously. Cultures were harvested by centrifugation at 2,800 × g for 10 min, washed twice in 40 mM phosphate buffer (pH 7), and resuspended to an OD600 of 1.2 in 40 mM phosphate buffer. RDX was added at a concentration of 180 μM to the resting cells; in some cases, 1 mM (NH4)2SO4 was added to prevent the use of nitrite by the cells.
Growth conditions of other RDX-degrading strains.
Rhodococcus sp. strain DN22, provided by Nicholas V. Coleman, was grown in MSM2 (50 ml) with the addition of RDX (180 μM) as a nitrogen source and succinate (10 mM) as a carbon source. Rhodococcus rhodochrous strain 11Y was purchased from NCIMB (Aberdeen, United Kingdom) and was grown in the same manner but with glucose (5 mM), succinate (5 mM), and glycerol (10 mM) as carbon sources.
HPLC methods.
Soil enrichments (1 g of soil and 5 ml of MSM1) were mixed with 5 ml of acetonitrile, treated for 18 h at 15°C in an ultrasonic bath (Branson, Danbury, CT), centrifuged at 2,800 × g for 10 min, and filtered (0.45-μm Target PTFE syringe filters; National Scientific, Duluth, GA) into autosampler vials. Samples (each, 1 ml) of pure cultures were filtered into autosampler vials. RDX concentrations were determined with an Agilent (Palo Alto, CA) 1100 Series HPLC equipped with a quaternary pump, autosampler, diode array UV absorbance detector (set at 254 nm), and column oven. An Agilent LC-18 reversed-phase column (100 by 4.6 mm; particle size, 5 μm) was used as the primary column along with an ODS-Hypersil C18 guard column (20 by 4.0 mm; particle size, 5 μm). The system operated at 39°C at a flow rate of 1.5 ml/min with 68% (vol/vol) of the mobile phase, consisting of 20 mM NH4Cl, and 32% (vol/vol), consisting of a methanol/butanol mixture (98:2 vol/vol).
Metabolite analysis.
Samples (each, 200 μl) taken from growing cultures and resting cells were filtered to remove cells with 0.45-μm Target PTFE syringe filters (National Scientific, Duluth, GA). Filtrates were analyzed for nitrite using the Griess Reagent system (Promega, Madison, WI) according to the manufacturer's instructions. Formaldehyde was analyzed colorimetrically with the Hantzsch reaction (30). Briefly, samples were treated with an equal volume of acetylacetone reagent (2 M ammonium acetate, 0.05 M acetic acid, and 0.02 M acetylacetone), mixed, incubated at 60°C for 10 min, and read at 412 nm on a UV-1601 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Nitrous oxide was monitored by analysis of headspace samples (100 μl) using a Hewlett-Packard 5890 series II gas chromatograph equipped with a thermal conductivity detector and QPlot column (15-m length, 0.5-mm internal diameter; Restek Corporation, Bellefonte, PA) that was held isothermally at 35°C. Helium was used as the carrier gas at a flow rate of 2.2 ml/min. The injector and detector temperatures were 35°C and 200°C, respectively, and the injector was operated in splitless mode. 4-Nitro-2,4-diazabutanal was analyzed by HPLC under the same conditions described previously.
[U-14C]RDX tracer study.
Cultures were set up in sterile 125-ml serum bottles containing 55 ml of MSM2 with the addition of carbon sources or (NH4)2SO4 when applicable. [U-14C]RDX was added at a final concentration of 5,000 dpm/ml. Unlabeled RDX was also added to reach a final RDX concentration of 180 μM. A glass tube containing 1 ml of a 1 N KOH solution was inserted into the culture bottle to absorb 14CO2. The bottles were sealed with Teflon-coated butyl septa and crimped with aluminum caps. The KOH solution was removed daily, added to 15 ml of Ultima Gold scintillation cocktail (Perkin-Elmer, Boston, MA), and counted on a Beckman Coulter (Fullerton, CA) scintillation counter. To perform 14C mass balances, aqueous samples that included cells (100 μl; unfiltered) were also taken daily and counted in the same manner.
Determination of cell growth.
Cell growth was monitored by protein concentration using the Coomassie Plus Protein Assay kit (Pierce, Rockford, IL) according to the manufacturer's specifications with bovine serum albumin as a standard. Additionally, growth was measured by OD600 values with a UV-1601 spectrophotometer (Shimadzu Corp., Kyoto, Japan).
Identification of isolates.
DNA was extracted from each isolate using the QIAGEN (Hilden, Germany) DNeasy tissue kit as instructed by the manufacturer. The 16S rRNA gene was PCR amplified with 27f (17) and 1492r (40) primers by standard protocols (3). PCR products were purified with Microcon 100 spin columns (Millipore, Billerica, MA). Amplified DNA was quantified with the Picogreen dsDNA quantitation assay by Molecular Probes (Eugene, OR). The purified PCR products were sequenced with an ABI 3100 Genetic Analyzer (Foster City, CA) using the Big Dye sequencing kit (Perkin-Elmer, Boston, MA), according to the manufacturer's specifications. The sequencing primers were 27f, H complement (1075f), H (1075r), G complement (790f), and G (790r) (5, 27, 31). National Center for Biotechnology Information BLAST database searches were performed to determine 16S rRNA gene sequence homology.
The 16S rRNA gene sequences determined above were aligned with 16S rRNA sequences from selected species within the suborder Corynebacterineae. Sequences of the reference strains were obtained from the GenBank/EMBL database and initially aligned with the Ribosomal Database Project, release 8.1 Sequence Aligner (11). In preparation for input to PHYLIP phylogenetic analyses (18), all 27 sequences were uniformly truncated to begin at position 32 and end at position 1501, corresponding to positions in the 16S rRNA sequence for Escherichia coli (9), and further manually aligned to 1,463 bp. The DNAPARS program of the PHYLIP package was used to estimate an unrooted phylogeny by the parsimony method, and the DRAWGRAM program was used to construct the phenogram initiated with the Dietzia maris sequence.
Total cellular fatty acids were analyzed by MIDI Laboratories using the Sherlock Microbial Identification system (Microbial ID, Inc., Newark, DE).
Nucleotide sequence accession numbers.
The nucleotide sequences from the 16S rRNA genes of KTR4 and KTR9 were deposited in GenBank (http://www.ncbi.nlm.nih.gov/GenBank/) and given the accession numbers DQ068382 and DQ068383, respectively.
RESULTS
Enrichment and isolation of KTR4 and KTR9.
For the enrichment of RDX-degrading bacteria, surface soil from the China Lake Naval Air Warfare Center Weapons Division was used as the inoculum in mineral salts medium with RDX as the nitrogen source. This soil has been shown to degrade RDX and CL-20 under aerobic conditions (16) and anaerobic conditions (F. H. Crocker, K. T. Thompson, J. E. Szecsody, and H. L. Fredrickson, unpublished data). RDX was not detectable in enrichment cultures after 8 days. After the enrichments were transferred twice, the disappearance of RDX occurred at a faster rate, with no RDX detected after 6 days. Seven different colony types were isolated from the 5% PTYG agar plates. Two of these, strains KTR4 and KTR9, were found to degrade RDX. KTR4 formed smooth, pink colonies and KTR9 formed smooth, orange colonies on complex media. Cells of KTR4 were coccoid shaped, and cells of KTR9 were short rods. Neither strain formed granules or clumps when grown on RDX in liquid medium. Both organisms were found to be gram-positive, aerobic, oxidase-negative, catalase-positive, and nonmotile bacteria.
Identification of KTR4 and KTR9.
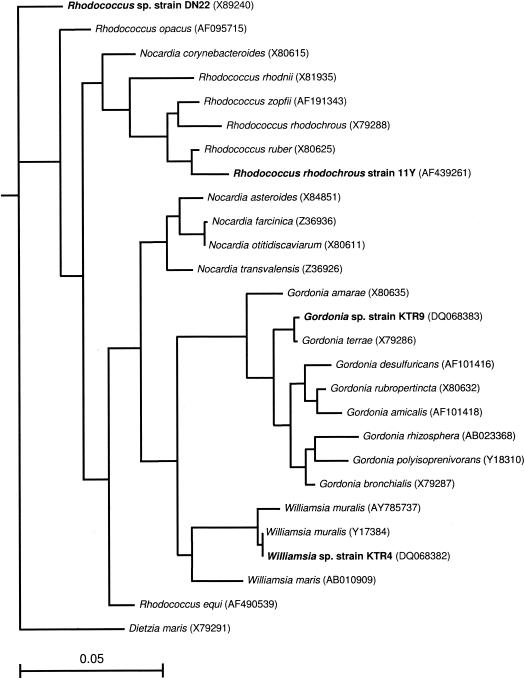
Nearly complete sequences of the 16S rRNA genes of strains KTR4 (1,487 bp) and KTR9 (1,488 bp) were determined. The results of the BLAST search indicated that the sequence of KTR4 was 99% similar to Williamsia muralis and the 16S rRNA gene sequence of strain KTR9 was 99% similar to that of Gordonia terrae. The relationships of the 16S rRNA gene sequences of KTR4 and KTR9 to those of 16S rRNA gene sequences from selected species within the suborder Corynebacterinieae are shown in Fig. 1. The sequence of strain KTR9 clustered on a branch containing a Gordonia terrae sequence. Strain KTR4 clustered with Williamsia muralis and Williamsia maris strains at a similarity of 99% and 97%, respectively. Comparisons of the total cellular fatty acid profile of KTR4 to the environmental database matched most closely to that of Nocardia asteroides with a similarity index of 0.634. KTR9 matched most closely to Gordonia sputi with a similarity index of 0.903. Tuberculostearic acid (d-10-methyloctadecanoic acid) was a major component of the fatty acid trace of each organism, with strain KTR4 containing 20% of this fatty acid and strain KTR9 containing 12%. The level of tuberculostearic acid present in strain KTR4 is consistent with levels found in other Williamsia species, while Gordonia species have been found to contain less (24).
FIG. 1.
Neighbor-joining tree based on nearly complete 16S rRNA gene sequences of Gordonia and other mycolic acid-containing actinomycetes (after Arenskötter et al.) (2) showing the relationships of Gordonia sp. KTR9 and Williamsia sp. KTR4. The sequence of D. maris served as the outgroup. The scale bar indicates 0.05 substitution per nucleotide position.
Physiological characterization of KTR4 and KTR9.
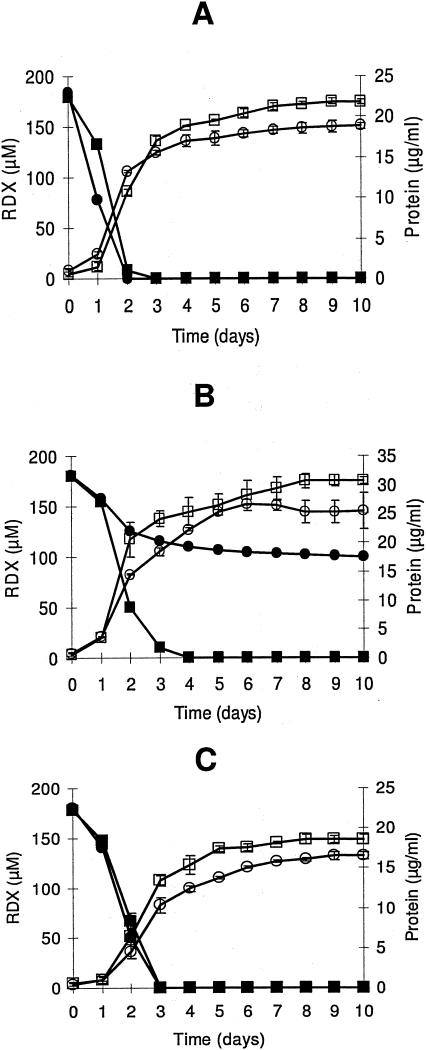
Strains KTR4 and KTR9 grew aerobically at the expense of RDX as their sole carbon, nitrogen, or carbon and nitrogen source (Fig. 2). RDX removal from the culture medium was followed by HPLC analysis. KTR4 degraded RDX faster when it was used as a nitrogen source (half-life = 0.89 days) than when it was used as a carbon source (half-life = 1.14 days) or as a carbon and nitrogen source (half-life = 1.16 days). Degradation of RDX by KTR9 also occurred fastest when RDX served as the nitrogen source (half-life = 0.63 days) than when it was a carbon source (half-life = 11.20 days) or a carbon and nitrogen source (half-life = 1.07 days). Neither organism was able to degrade the explosive CL-20 as a nitrogen source at a concentration of 8 or 23 μM. Control experiments were performed on each strain to determine if CO2 or N2 from the atmosphere was being used as a source of carbon or nitrogen. Cells were given (NH4)2SO4 as the nitrogen source without the addition of a carbon source, and no growth was observed. Alternately, cells were given glucose, glycerol, and succinate as carbon sources without the addition of a nitrogen source. This also resulted in no growth of either strain (data not shown). It was determined from these experiments that strains KTR4 and KTR9 were not able to grow in the absence of an added carbon source or the absence of an added nitrogen source.
FIG. 2.
Growth of KTR4 and KTR9 on RDX as a nitrogen source (A), a carbon source (B), or a carbon and nitrogen source (C). For KTR4, ▪, RDX concentration; □, protein concentration. For KTR9, •, RDX concentration; ○, protein concentration. Error bars indicate standard deviations of triplicate results.
Cell growth was monitored by protein levels in the growth medium (Fig. 2). KTR4 grew to a final protein concentration of 21.8 μg/ml, 30.7 μg/ml, and 18.6 μg/ml (OD600 values of 0.66, 1.06, and 0.64) when RDX was the nitrogen, carbon, or carbon and nitrogen source, respectively. Growth of KTR9 was slightly less under similar growth conditions, with the final protein concentrations reaching 18.9 μg/ml, 25.5 μg/ml, and 16.5 μg/ml (OD600 values of 0.58, 1.04, and 0.74). Growth yields based on the amount of carbon or nitrogen added to growing cultures are listed in Table 1. Yields with respect to carbon were lowest for both strains when glucose, glycerol, and succinate were added as carbon sources, indicating that there was an excess of carbon present. Similarly, the yields with respect to nitrogen were lowest when (NH4)2SO4 was added in excess. Both strains produced slightly more biomass based on nitrogen content when glucose, glycerol, and succinate were added as carbon sources and RDX was the nitrogen source than when RDX served as the carbon and nitrogen source. In this case, the nitrogen content stayed the same (180 μM RDX), but more carbon was added, which changed the conditions from carbon limiting to nitrogen limiting.
TABLE 1.
Rate constants and growth yields for strains KTR4 and KTR9 when RDX is used as a source of carbon, nitrogen, or carbon and nitrogen
| Isolate | Carbon source | Nitrogen source | C:N | Degradation rate k (mg RDX/day)a | Growth rate (μ day−1)b | Growth yield (g protein/mol C) | Growth yield (g protein/mol N) |
|---|---|---|---|---|---|---|---|
| KTR4 | RDX | RDX | 1:2 | 0.60 | 0.27 | 34.40 | 17.20 |
| Glucose, glycerol, succinate | RDX | 75:1 | 0.78 | 0.38 | 0.27 | 20.15 | |
| RDX | Ammonium sulfate | 1:17 | 0.61 | 0.26 | 56.93 | 3.84 | |
| KTR9 | RDX | RDX | 1:2 | 0.65 | 0.21 | 30.63 | 15.28 |
| Glucose, glycerol, succinate | RDX | 75:1 | 1.10 | 0.66 | 0.24 | 17.48 | |
| RDX | Ammonium sulfate | 1:17 | 0.06 | 0.37 | 47.14 | 3.18 |
k, degradation rate constant.
μ, growth rate constant.
One key difference between KTR4 and KTR9 was seen in the RDX degradation rate when RDX was used as a carbon source and (NH4)2SO4 was added. With KTR4, degradation occurred 22% more slowly when RDX and (NH4)2SO4 were added than when RDX served as the sole nitrogen source. But with KTR9, there was a 94% decrease in degradation rate under the same conditions (Table 1). Also, the growth yield with respect to carbon was lower for strain KTR9 (47.14 g protein/mol of C) than the yield observed for strain KTR4 (56.93 g of protein/mol of C) under the same conditions (Table 1).
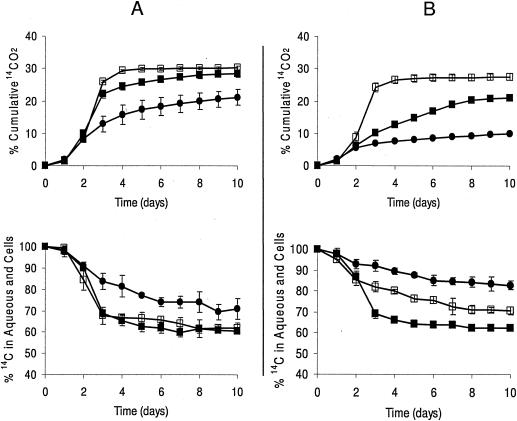
Rates of RDX mineralization were monitored by determining the rate of formation of 14CO2 from [U-14C]RDX. Both isolates were able to mineralize RDX (Fig. 3) with KTR4 mineralizing 28% with RDX as a nitrogen source, 21% with RDX as a carbon source, and 30% with RDX as a carbon and nitrogen source. With KTR9, 21% was mineralized with RDX as a nitrogen source, 10% with RDX as the carbon source, and 27% with RDX as a carbon and nitrogen source. The low mineralization rate of RDX by KTR9 with RDX as the carbon source was consistent with the slower rate of RDX degradation by KTR9 under similar conditions. After 10 days of growth, KTR4 contained 60%, 70%, and 61% 14C in the aqueous phase (including cells) when RDX was the sole nitrogen, carbon, or carbon and nitrogen source, respectively. In the case of KTR9, 70%, 82%, and 62% 14C remained in the aqueous phase after 10 days.
FIG. 3.
Total 14CO2 evolved and 14C content of the aqueous phase (including cells) in KTR4 (A) and KTR9 (B) when RDX is used as a nitrogen source (▪), carbon source (•), or carbon and nitrogen source (□). Error bars indicate standard deviations of triplicate results.
Metabolite identification.
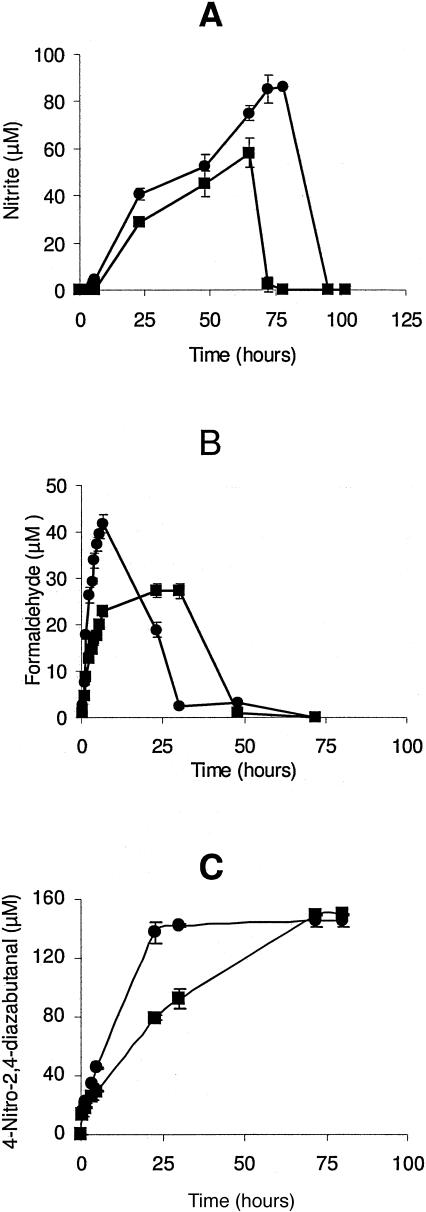
Actively growing cultures were monitored for the production of nitrite, formaldehyde, and nitrous oxide, which have been determined to be metabolites of aerobic RDX degradation by Rhodococcus sp. strain DN22 (19). Nitrite and nitrous oxide production was below the detection limits of 2.5 μM and 6.8 mM, respectively, and formaldehyde was detected at very low concentrations and quickly disappeared. To promote the accumulation of these metabolites, resting cell studies were performed with washed cells suspended in phosphate buffer and 180 μM RDX. Under these conditions, formaldehyde was produced by both isolates, with strain KTR4 producing 27.3 μM in 30 h and strain KTR9 producing 41.6 μM in 6.5 h (Fig. 4). Formaldehyde was not detectable in either culture after 72 h of incubation. Very little nitrite was produced under resting cell conditions, so (NH4)2SO4 (1 mM) was added to prevent nitrite utilization, with the expectation that the cells would use ammonium over nitrite (19). Under these conditions, strain KTR4 produced 58.0 μM of nitrite in 65 h and strain KTR9 produced 86.1 μM of nitrite in 78 h (Fig. 4). Nitrous oxide was not detected above its detection limit of 6.8 mM under any of these culture conditions.
FIG. 4.
Production of the metabolites nitrite (A), formaldehyde (B), and 4-nitro-2,4-diazabutanal (C) by strain KTR4 (▪) and strain KTR9 (•). Error bars indicate standard deviations of triplicate results.
An unknown peak was detected by HPLC that appeared to increase as RDX was degraded. This peak eluted at 0.82 min, which is earlier than RDX (retention time of 2.3 min). This unknown metabolite was compared to a compound identified as 4-nitro-2,4-diazabutanal, which is a soluble metabolite produced from RDX degradation by Rhodococcus sp. strain DN22 (7). A standard of this compound was analyzed by HPLC and found to elute at the same time as the unknown metabolite. In addition, the UV absorption spectra of the unknown metabolite and 4-nitro-2,4-diazabutanal were similar (data not shown). This compound was not degraded by strains KTR4 and KTR9 during growth experiments and resting cell studies. Strain KTR4 produced 148.9 μM of the metabolite in 72 h and strain KTR9 produced 145.6 μM in 30 h as a result of RDX degradation (180 μM) by actively growing cultures (Fig. 4). This metabolite was produced in similar amounts regardless of whether RDX was used as the sole nitrogen or sole carbon source or as the nitrogen and carbon source. RDX degradation by Rhodococcus sp. strain DN22 and Rhodococcus rhodochrous strain 11Y also produced 4-nitro-2,4-diazabutanal. In our laboratory, R. rhodochrous strain 11Y produced a concentration of this compound of 158.7 μM and Rhodococcus strain DN22 produced 134.6 μM from 180 μM RDX. No other metabolite peaks were detected by HPLC as a result of RDX degradation by strains KTR4 and KTR9 or by Rhodococcus sp. strain DN22 and R. rhodochrous strain 11Y.
DISCUSSION
Williamsia strain KTR4 and Gordonia strain KTR9 are among the first bacteria isolated that degrade RDX aerobically. Most previous work has focused on anaerobic degradation using inocula such as activated sludge (29) or explosive-contaminated soil (25). This study used surface soil that did not contain detectable levels of explosive contamination via HPLC analysis. Initially, the objective of this study was to isolate aerobic bacteria that degraded RDX as a source of nitrogen. The ability of these strains to degrade RDX as a sole source of carbon and nitrogen was also explored. Both of these studies were successful, resulting in the first report of aerobic bacteria able to use RDX as a sole carbon and nitrogen source. Other aerobic RDX degraders such as Rhodococcus sp. strain DN22 (12) and Rhodococcus rhodochrous strain 11Y (33) have been able to use RDX as a nitrogen source, but not as a carbon source. Seth-Smith et al. (33) reported that this ability would only be present in a methylotroph because RDX is a one-carbon substrate.
These strains degrade RDX slightly slower than the recently published Rhodococcus sp. strain DN22 (12) and R. rhodochrous strain 11Y (33). While using RDX as a nitrogen source, Rhodococcus sp. strain DN22 degraded 160 μM in 20 h and R. rhodochrous strain 11Y degraded 250 μM RDX in 21 h. Strains KTR4 and KTR9 degraded 180 μM within 48 h when RDX was the nitrogen source and within 72 h when RDX was the carbon and nitrogen source. When RDX was used as a carbon source with (NH4)2SO4 as the nitrogen source, strain KTR4 degraded 180 μM in 96 h while strain KTR9 degraded only 80 μM in 240 h. This inhibition of RDX degradation when ammonium is present has also been observed with Rhodococcus strain DN22 (12), Acetobacterium paludosum (35), and three strains of Corynebacterium (41).
RDX mineralization by other strains is higher than what was seen with strains KTR4 and KTR9. Rhodococcus sp. strain DN22 (19) mineralized 30% in 4 days while using RDX as a source of nitrogen and Methylobacterium sp. strain BJ001 (39) mineralized 58% in 40 days through the cometabolism of RDX. Strain KTR4 mineralized 29% when RDX was the nitrogen source and 30% when RDX was the carbon and nitrogen source, while KTR9 mineralized 21% with RDX serving as the nitrogen source and 28% when serving as carbon and nitrogen source after 10 days of growth. These slightly lower levels of mineralization could be due in part to 14C-labeled metabolites remaining in the aqueous phase. The aqueous 14C data reported (Fig. 3) for strains KTR4 and KTR9 included cells that could contain 14C as biomass. It has been conclusively determined that both strains are using RDX as their source for carbon and nitrogen, since neither can grow in the absence of an added carbon source or nitrogen source.
The compounds nitrite and formaldehyde were detected in this study as metabolites from RDX degradation by resting cells of strains KTR4 and KTR9. These metabolites have also been reportedly formed from RDX degradation by Rhodococcus sp. strain DN22 (19) and R. rhodochrous strain 11Y (33). RDX degradation by strain KTR4 produced 0.32 mol of nitrite per mol of RDX and strain KTR9 produced 0.48 mol of nitrite per mol of RDX before it was quickly consumed by the cells, while Rhodococcus sp. strain DN22 reportedly produced 2 mol of nitrite per mol of RDX (19). The smaller amounts of nitrite measured in our study could be due to its uptake by the resting cells. We did have difficulty in accumulating enough nitrite to measure and therefore added (NH4)2SO4 to inhibit its uptake by the cells. However, nitrite production was still followed by rapid consumption by the cells, so some amount of uptake may have occurred when nitrite measurements were taken. The formation of formaldehyde was also followed by its degradation, probably through mineralization to CO2.
The formation of a third metabolite, 4-nitro-2,4-diazabutanal, occurred in growing and resting cells as a result of RDX degradation. This compound, unlike nitrite and formaldehyde, was stable in all of the cultures and therefore is concluded to be a dead-end metabolite. This compound was produced by strain KTR4, strain KTR9, Rhodococcus sp. strain DN22, and R. rhodochrous strain 11Y in similar concentrations. The ratio of 4-nitro-2,4-diazabutanal produced to RDX degraded was about 0.8 for all four bacterial strains, indicating that 1 mol of this metabolite was formed per mol of RDX consumed. This metabolite accumulated without being taken up by the cells and was produced in almost identical concentrations by all four bacteria.
Fatty acid patterns and 16S rRNA gene analysis indicate that strain KTR9 is a member of the genus Gordonia and that strain KTR4 is a member of the genus Williamsia. The genus Gordonia includes many species that degrade xenobiotics and environmental pollutants and is already considered potentially useful for bioremediation (2). Bacteria in this genus also catalyze a wide range of enzymatic reactions that are being exploited for biotechnological applications. This ability may be due in part to the mycolic acid composition of their cell wall and their ability to produce biosurfactants. The genus Gordonia is distinguished from the genus Nocardia by an ability to reduce nitrate and by the absence of a true mycelium (2). The enzyme S-triazine hydrolase from Gordonia rubropertincta (DSM 10347) can dechlorinate atrazine and deaminate melamine (14, 15). Gordonia nitida LE31 degrades 3-methylpyridine and 3-ethylpyridine through formamide to produce formic acid and ammonia (28). This pathway is similar to the proposed pathway for RDX biodegradation by Rhodococcus sp. strain DN22, which involves the production of formaldehyde and ammonia via formamide (19).
Williamsia is a recently described genus that is very similar to the genus Gordonia. Both are members of the suborder Corynebacterineae, which also includes the genera Corynebacterium, Nocardia, and Rhodococcus. Williamsia is differentiated from Gordonia by its lack of signature nucleotides present in the family Gordoniaceae and differing chain lengths of mycolic acids (24). Kämpfer et al. (24) also differentiated Williamsia and Gordonia by their tuberculostearic acid content, which is present at higher levels in Williamsia muralis (23%) than Gordonia strains that have been described. This is consistent with the present study, where the proposed Williamsia sp. KTR4 contains 20% of this fatty acid and Gordonia sp. KTR9 contains only 12%. Currently, only two isolates, Williamsia muralis (24) and Williamsia maris (36), have been assigned to the genus. Strain KTR4 represents the first bacterial species within this genus to be isolated from soil. Despite its high sequence similarity to W. muralis, strain KTR4 produces pink colonies instead of yellow colonies (24) and thus could represent a new species of Williamsia.
In summary, strains KTR4 and KTR9 are novel bacterial strains that are capable of using RDX aerobically as a sole source of carbon and nitrogen for growth. RDX metabolites produced by these bacteria were limited to structurally simple compounds, (nitrite and formaldehyde) and the dead-end metabolite 4-nitro-2,4-diazabutanal. These simple products are similar to those produced by previously published RDX degraders. The lack of more-complex intermediates hinders the deduction of RDX biochemical degradation pathways and comparisons of these pathways to ones inferred for other RDX-degrading microorganisms. The ability of these strains to use the carbon from RDX for growth indicates that they have a C1 pathway that the previously isolated strains lack because they are unable to use RDX as a source for both carbon and nitrogen. The two gram-positive strains described here are good models for studying nitrogen-nitrogen bond-cleaving mechanisms and C1 metabolism. They may prove useful for RDX bioremediation that does not require the addition of other carbon or nitrogen sources. They may also serve as models for the biological recognition of xenobiotic explosive molecules.
Acknowledgments
We thank John Furey for the alignment of the 16S rRNA gene sequences, homology analysis, and generation of Fig. 1. We are grateful to Margaret Richmond for the RDX HPLC analysis.
This work was supported by the Strategic Environmental Research and Development Program and the Corps of Engineers Environmental Quality research program.
REFERENCES
- 1.Adrian, N. R., and T. Chow. 2001. Identification of hydroxylaminodinitroso-1,3,5-triazine as a transient intermediate formed during the anaerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine. Environ. Toxicol. Chem. 20:1874-1877. [PubMed] [Google Scholar]
- 2.Arenskötter, M., D. Bröker, and A. Steinbüchel. 2004. Biology of the metabolically diverse genus Gordonia. Appl. Environ. Microbiol. 70:3195-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. Greene Publishing Associates-Wiley Interscience, New York, N.Y.
- 4.Balkwill, D. L., and W. C. Ghiorse. 1985. Characterization of subsurface bacteria associated with two shallow aquifers in Oklahoma. Appl. Environ. Microbiol. 50:580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill, D. L., R. H. Reeves, G. R. Drake, J. Y. Reeves, F. H. Crocker, M. B. King, and D. R. Boone. 1997. Phylogenetic characterization of bacteria in the subsurface microbial culture collection. FEMS Microbiol. Rev. 20:201-216. [DOI] [PubMed] [Google Scholar]
- 6.Beller, H. R. 2002. Anaerobic biotransformation of RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) by aquifer bacteria using hydrogen as the sole electron donor. Water Res. 36:2533-2540. [DOI] [PubMed] [Google Scholar]
- 7.Bhushan, B., S. Trott, J. C. Spain, A. Halasz, L. Paquet, and J. Hawari. 2003. Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a rabbit liver cytochrome P450: insight into the mechanism of RDX biodegradation by Rhodococcus sp. strain DN22. Appl. Environ. Microbiol. 69:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binks, P. R., S. Nicklin, and N. C. Bruce. 1995. Degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Stenotrophomonas maltophilia PB1. Appl. Environ. Microbiol. 61:1318-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosius, J., M. L. Palmer, P. J. Kennedy, and H. R. Noller. 1979. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4801-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdette, L. J., L. L. Cook, and R. S. Dyer. 1988. Convulsant properties of cyclotrimethylenetrinitramine (RDX): spontaneous, audiogenic, and amygdaloid kindled seizure activity. Toxicol. Appl. Pharmacol. 92:436-444. [DOI] [PubMed] [Google Scholar]
- 11.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The ribosomal database project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman, N. V., D. R. Nelson, and T. Duxbury. 1998. Aerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biol. Biochem. 30:1159-1167. [Google Scholar]
- 13.Coleman, N. V., J. C. Spain, and T. Duxbury. 2002. Evidence that RDX biodegradation by Rhodococcus strain DN22 is plasmid-borne and involves a cytochrome p-450. J. Appl. Microbiol. 93:463-472. [DOI] [PubMed] [Google Scholar]
- 14.Cook, A. M., and R. Hütter. 1984. Deethylsimazine: bacterial dechlorination, deamination, and complete degradation. J. Agric. Food Chem. 32:581-585. [Google Scholar]
- 15.Cook, A. M., and R. Hütter. 1986. Ring dechlorination of deethylsimazine by hydrolases from Rhodococcus corallinus. FEMS Microbiol. Lett. 34:335-338. [Google Scholar]
- 16.Crocker, F. H., K. T. Thompson, J. E. Szecsody, and H. L. Fredrickson. 2005. Biotic and abiotic degradation of hexanitrohexaazaisowurtzitane (CL-20) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in soils. J. Environ. Qual. 34:2208-2216. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, U., T. Rogall, H. Blocker, M. Emde, and E. C. Bottger. 1989. Isolation and direct complete determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package), version 3.5c. Department of Genetics, University of Washington, Seattle.
- 19.Fournier, D., A. Halasz, J. Spain, P. Fiurasek, and J. Hawari. 2002. Determination of key metabolites during biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine with Rhodococcus sp. strain DN22. Appl. Environ. Microbiol. 68:166-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier, D., A. Halasz, J. Spain, R. J. Spanggord, J. C. Bottaro, and J. Hawari. 2004. Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine ring cleavage product 4-nitro-2,4-diazabutanal by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 70:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halasz, A., J. Spain, L. Paquet, C. Beaulieu, and J. Hawari. 2002. Insights into the formation and degradation mechanisms of methylenedinitramine during the incubation of RDX with anaerobic sludge. Environ. Sci. Technol. 36:633-638. [DOI] [PubMed] [Google Scholar]
- 22.Hawari, J. 2000. Biodegradation of RDX and HMX: from basic research to field application, p. 277-310. In J. C. Spain, J. B. Hughes, and H. J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. Lewis Publishers, Boca Raton, Fla.
- 23.Hawari, J., A. Halasz, T. Sheremata, S. Beaudet, C. Groom, L. Paquet, C. Rhofir, G. Ampleman, and S. Thiboutot. 2000. Characterization of metabolites during biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) with municipal anaerobic sludge. Appl. Environ. Microbiol. 66:2652-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kämpfer, P., M. A. Andersson, F. A. Rainey, R. M. Kroppenstedt, and M. Salkinoja-Salonen. 1999. Williamsia muralis gen. nov., sp. nov., isolated from the indoor environment of a children's day care centre. Int. J. Syst. Bacteriol. 49:681-687. [DOI] [PubMed] [Google Scholar]
- 25.Kitts, C. L., D. P. Cunningham, and P. J. Unkefer. 1994. Isolation of three hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading species of the family Enterobacteriaceae from nitramine explosive-contaminated soil. Appl. Environ. Microbiol. 60:4608-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitts, C. L., C. E. Green, R. A. Otley, M. A. Alvarez, and P. J. Unkefer. 2000. Type I nitroreductases in soil enterobacteria reduce TNT (2,4,6-trinitrotoluene) and RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine). Can. J. Microbiol. 46:278-282. [DOI] [PubMed] [Google Scholar]
- 27.Lane, D. J., G. Pace, G. J. Olsen, D. A. Stahl, M. L. Sogin, and N. R. Pace. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 82:6955-6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, J. J., S.-K. Rhee, and S.-T. Lee. 2001. Degradation of 3-methylpyridine and 3-ethylpyridine by Gordonia nitida LE31. Appl. Environ. Microbiol. 67:4342-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick, N. G., J. H. Cornell, and A. M. Kaplan. 1981. Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine. Appl. Environ. Microbiol. 42:817-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nash, T. 1953. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 55:416-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves, R. H., J. Y. Reeves, and D. L. Balkwill. 1995. Strategies for phylogenetic characterization of subsurface bacteria. J. Microbiol. Methods 21:235-251. [Google Scholar]
- 32.Regan, K. M., and R. L. Crawford. 1994. Characterization of Clostridium bifermentans and its biotransformation of 2,4,6-trinitrotoluene (TNT) and 1,3,5-triaza-1,3,5-trinitrocyclohexane (RDX). Biotechnol. Lett. 16:1081-1086. [Google Scholar]
- 33.Seth-Smith, H. M. B., S. J. Rosser, A. Basran, E. R. Travis, E. R. Dabbs, S. Nicklin, and N. C. Bruce. 2002. Cloning, sequencing, and characterization of the hexahydro-1,3,5-trinitro-1,3,5-triazine degradation gene cluster from Rhodococcus rhodochrous. Appl. Environ. Microbiol. 68:4764-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelton, D. R., and J. M. Tiedje. 1984. General method for determining anaerobic biodegradation potential. Appl. Environ. Microbiol. 47:850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherburne, L. A., J. D. Shrout, and P. J. J. Alvarez. 2005. Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) degradation by Acetobacterium paludosum. Biodegradation 16:539-547. [DOI] [PubMed] [Google Scholar]
- 36.Stach, J. E. M., L. A. Maldonado, A. C. Ward, A. T. Bull, and M. Goodfellow. 2004. Williamsia maris sp. nov., a novel actinomycete isolated from the Sea of Japan. Int. J. Syst. Bacteriol. 54:191-194. [DOI] [PubMed] [Google Scholar]
- 37.Talmage, S. S., D. M. Opresko, C. J. Maxwell, C. J. E. Welsh, F. M. Cretella, P. H. Reno, and F. B. Daniel. 1999. Nitroaromatic munition compounds: environmental effects and screening values. Rev. Environ. Contam. Toxicol. 161:1-156. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Environmental Protection Agency. 2004. 2004 edition of the drinking water standards and health advisories. Publication EPA 822-R-04-005. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 39.Van Aken, B., J. M. Yoon, and J. L. Schnoor. 2004. Biodegradation of nitro-substituted explosives 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine, and octahydro-1,3,5,7-tetranitro-1,3,5-tetrazocine by a phytosymbiotic Methylobacterium sp. associated with poplar tissues (Populus deltoides x nigra DN43). Appl. Environ. Microbiol. 70:508-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson, K. H., R. B. Blitchington, and R. C. Green. 1990. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J. Clin. Microbiol. 28:1942-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, Y. X., X. Wang, P. Yin, W. H. Li, and P. J. Zhou. 1983. Studies on three strains of Corynebacterium degrading cyclotrimethylene-trinitroamine (RDX). Acta Microbiol. Sin. 23:251-256. [Google Scholar]
- 42.Young, D. M., P. J. Unkefer, and K. L. Ogden. 1997. Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a prospective consortium and its most effective isolate, Serratia marcescens. Biotechnol. Bioeng. 53:515-522. [DOI] [PubMed] [Google Scholar]
- 43.Zhao, J.-S., A. Halasz, L. Paquet, C. Beaulieu, and J. Hawari. 2002. Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine and its mononitroso derivative hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine by Klebsiella pneumoniae strain SCZ-1 isolated from an anaerobic sludge. Appl. Environ. Microbiol. 68:5336-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, J.-S., L. Paquet, A. Halasz, and J. Hawari. 2003. Metabolism of hexahydro-1,3,5-trinitro-1,3,5-triazine through initial reduction to hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine followed by denitration in Clostridium bifermentans HAW-1. Appl. Microbiol. Biotechnol. 63:187-193. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, J.-S., J. Spain, and J. Hawari. 2003. Phylogenetic and metabolic diversity of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX)-transforming bacteria in strictly anaerobic mixed cultures enriched on RDX as nitrogen source. FEMS Microbiol. Ecol. 46:189-196. [DOI] [PubMed] [Google Scholar]