Abstract
This study reports the effects of long-term elevated atmospheric CO2 on root production and microbial activity, biomass, and diversity in a chaparral ecosystem in southern California. The free air CO2 enrichment (FACE) ring was located in a stand dominated by the woody shrub Adenostoma fasciculatum. Between 1995 and 2003, the FACE ring maintained an average daytime atmospheric CO2 concentration of 550 ppm. During the last two years of operation, observations were made on soil cores collected from the FACE ring and adjacent areas of chaparral with ambient CO2 levels. Root biomass roughly doubled in the FACE plot. Microbial biomass and activity were related to soil organic matter (OM) content, and so analysis of covariance was used to detect CO2 effects while controlling for variation across the landscape. Extracellular enzymatic activity (cellulase and amylase) and microbial biomass C (chloroform fumigation-extraction) increased more rapidly with OM in the FACE plot than in controls, but glucose substrate-induced respiration (SIR) rates did not. The metabolic quotient (field respiration over potential respiration) was significantly higher in FACE samples, possibly indicating that microbial respiration was less C limited under high CO2. The treatments also differed in the ratio of SIR to microbial biomass C, indicating a metabolic difference between the microbial communities. Bacterial diversity, described by 16S rRNA clone libraries, was unaffected by the CO2 treatment, but fungal biomass was stimulated. Furthermore, fungal biomass was correlated with cellulase and amylase activities, indicating that fungi were responsible for the stimulation of enzymatic activity in the FACE treatment.
The rapid increase of carbon dioxide (CO2) in the atmosphere over the last century has led to an increased global ecosystem C storage, at least temporarily, by stimulating photosynthesis (41). However, the fate of this C and its effects on soil microbial communities are uncertain. Predicted changes in atmospheric CO2 are small compared to the relatively high CO2 concentrations in the pore space of active soils, so effects of elevated atmospheric CO2 on soil microbes are generally mediated by plant root production and exudation (22, 30, 50). While plant responses to elevated atmospheric CO2 are fairly well understood, the responses of soil microbial communities are highly variable. In response to elevated CO2, microbial biomass and activity have been observed to decrease (14, 34, 43), increase (15, 48), or remain unchanged (32, 35). No consistent effects of increased CO2 on soil microbial community composition have yet emerged. CO2-induced changes in microbial community composition have been detected in some cases (15, 17, 28, 37), but none were found in others (16, 49).
Effects of global change on soil microbial communities are potentially important in that microbes can control the responses of ecosystems through their effects on C and nutrient cycling, yet little progress has been made in this area. Soil microbial communities remain mysterious mainly because of their extraordinary complexity (10, 13). However, this field of study has recently benefited from the combination of molecular techniques to describe microbial communities (18, 33) with more sophisticated phylogenetic techniques for analyzing and comparing communities (6, 27). The present study investigated the effects of 8 years of elevated CO2 treatment on root growth and on microbial biomass, activity and community structure in a chaparral ecosystem in southern California. The system afforded an opportunity to study how elevated CO2 interacts with a complex, patchy landscape in a natural ecosystem with strong water and nutrient limitations. We tested the hypothesis that elevated CO2 increases root biomass, which in turn increases microbial biomass and activity and alters microbial community structure. This hypothesis was tested by comparing how microbial parameters varied across the landscape inside a free air CO2 enrichment (FACE) treatment ring relative to the surrounding landscape.
MATERIALS AND METHODS
Site description and FACE treatment.
The research was conducted at San Diego State University's Sky Oaks Field Station in northeastern San Diego County, California, (33°23′ N, 116°37′ W; 1,420 m above sea level). The chaparral at Sky Oaks Field Station is dominated by the shrubs Adenostoma fasciculatum H. & A., Adenostoma sparsifolium Torr., and Ceanothus greggii Gray. The soil in the study area is a loamy sand, Ultic Haploxeroll, with a bulk density of 1.04g cm−3, containing 32% rocks, and belonging to the Sheephead series (8) (see Table 1 for other soil properties). The entire area used in this study was burned in July 1992 prior to the establishment of the FACE treatment. The site was dominated by A. fasciculatum, a species which quickly regenerates after fire by resprouting from a lignotuber (9). The purpose of the burning treatment was to minimize historical differences in vegetation and soil properties that might have existed across the landscape. The FACE ring was ∼16 m in diameter, occupying an area of 178 m2 of chaparral. The CO2 concentration was maintained near 550 ppm during the daylight hours by releasing compressed CO2 gas from pipes at the perimeter of the ring. Wind direction was continuously sensed so that gas was released only on the upwind side of the ring. The set point was maintained within 10% for 87% of the time and within 20% for 96% of the time. A detailed description of the construction and operation of the FACE facility is available elsewhere (38). The FACE treatment operated from January 1995 to May 2003. The surrounding landscape (>10 m beyond the ring) served as a control with ambient levels of CO2 (360 ppm).
TABLE 1.
Properties of soil from plant and interplant samples of FACE and outside control plots
| Parameter | Mean value (SD)
|
na | |||
|---|---|---|---|---|---|
| Face
|
Outside
|
||||
| Plant | Interplant | Plant | Interplant | ||
| % OM | 4.2 (1.3) | 2.8 (0.4) | 5.2 (1.6) | 4.2 (1.5) | 103 |
| % GWC | 9.6 (7.0) | 11.5 (2.4) | 12.0 (7.3) | 13.7 (5.6) | 103 |
| % Sand | 73.32 (0.65) | 74.00 (0.79) | 77.44 (1.93) | 72.03 (4.91) | 17 |
| % Silt | 15.48 (1.54) | 12.85 (0.39) | 14.49 (1.43) | 15.47 (1.60) | 17 |
| % Clay | 11.20 (1.15) | 13.15 (0.89) | 8.07 (2.01) | 12.50 (3.38) | 17 |
n, sample size for analysis.
Soil collection and analysis.
Soil samples were collected on various dates from 2001 to 2003, mainly during spring and summer. On dates in 2003, soil respiration measurements were taken (EGM-4 gas analyzer with SRC-1 chamber; PP Systems, Amesbury MA) before samples were collected from the same area. Soil samples were generally collected with a 5-cm-diameter polyvinylchloride pipe to a depth of 12 to 15 cm, except in March 2003, when samples were collected for root biomass measurements by using a 10-cm-diameter metal soil coring device to a depth of 30 cm. Holes were back-filled to minimize disturbance in the plots. Samples were collected directly under plant canopies and in gaps between plants, from the FACE ring and from the control area. For root biomass measurements, the samples were taken at 10 cm and 30 cm from the bases of plants located within or outside the FACE ring. Soil samples were sieved (2 mm), and roots were separated from soil and sorted into size classes. Roots were rinsed in deionized water and dried at 60°C to constant weight. Soil organic matter (OM) (weight loss on combustion at 500°C for 24 h) and gravimetric water content (GWC) (100°C until constant weight) were determined for all soil samples. Soil texture was measured for a subset of the samples by using sieving and sedimentation. The samples used for molecular analysis of bacterial diversity were kept frozen (−80°C) until analysis. The samples used for measurements of microbial activity and biomass were kept cool (0 to 4°C) until analysis (up to 1 week).
Microbial biomass and activity.
Substrate-induced respiration (SIR) measurements using glucose were done as described earlier (24). Briefly, using sidearm flasks (Bellco Glass, Vineland, NJ), enough glucose was added to soils to maximize respiration (2 mg C g−1 soil), along with [14C]glucose (∼0.1 μCi g−1). Evolved CO2 was trapped in 1 ml NaOH (1 M) in the sidearm portion of each flask, and radioactivity was measured by liquid scintillation counting. Glucose SIR is commonly used to represent general heterotrophic microbial activity and biomass (2, 24, 42). SIR measurement was performed with soils near optimal water content (∼60% of field capacity). Microbial biomass C was measured by the chloroform fumigation-extraction method (21) with modifications (23). The activities of extracellular enzymes in breaking down carboxymethylcellulose (a soluble cellulose analog) and starch (amylase activity) were measured as described earlier (23). Fungal biomass was measured by direct microscopic observation, using the grid-intersection method to estimate fungal length (7). Hyphal length was converted to biomass by using an estimated average hyphal diameter of 5 μm and a factor of 0.26 g biomass cm−3 cell volume (7). Bacteria stained with DAPI (4′,6′-diamidino-2-phenylindole) (Molecular Probes, Eugene, OR) were counted by fluorescence microscopy and converted to biomass by assuming 0.27 pg/cell.
16S rRNA clone libraries.
Soil collected in February 2002 was used for the construction of clone libraries. To obtain a spatially averaged measure of bacterial diversity for each treatment (and because of the high cost of producing and sequencing multiple clone libraries), four spatial replicates from each sample type were pooled, producing four clone libraries (under plants or in gaps from FACE and control plots). Soil was extracted using a modified bead beating protocol (29). Tubes containing approximately 5 g soil samples, 2.0 g zirconia/silica beads (0.1 mm; BioSpec Products, Bartlesville, OK), and 10 ml lysis buffer (Tris-EDTA with 0.2% sodium dodecyl sulfate) were vortexed (Vortex Genie II; Fisher Scientific) at maximum speed for 5 min. To the resultant mixtures, 30 units of proteinase K and 10 units lysozyme (Fisher Bioreagents) were added, and the samples were incubated in a shaking incubator (37°C, 100 rpm) for 1 h. Standard protocols were used to purify DNA by cetyltrimethylammonium bromide extraction (5). DNA was further purified by agarose gel extraction (Qiaex II; QIAGEN). Bacterial 16S rRNA genes were amplified using universal bacterial primers f8-27 (5′-AGAGTTTGATCCTGGCTCAG-3′) and r1510 (5′-GGTTACCTTGTTACGACTT-3′). The PCR mixture consisted of 3.0 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate, 1 μM of each primer, 1 g/liter bovine serum albumin, 50 mM betaine, 1 unit Fisher Taq polymerase, and “buffer A” supplied with the enzyme (Fisher Biosciences). After an initial denaturation step of 4 min at 94°C, reactions were run for 32 cycles (1 min at 94°C, 45 s at 56°C, and 1 min at 72°C), followed by a final 10-min extension step at 72°C. The PCR product was gel purified (Quiex II; QIAGEN). The purified product was cloned using the TOPO TA cloning kit (Invitrogen). Clones from the four libraries were partially sequenced on a Prism 3100 capillary electrophoresis DNA sequencer (ABI) at the San Diego State University Microchemical Core Facility, using universal bacterial primer r1111 (5′-TTGCGCTCGTTGCGGGACT-3′).
Statistical and phylogenetic analyses.
Regression analysis showed that most measured variables were significantly related to OM. To control for variation in OM across the landscape and between treatments, the effect of CO2 on most variables was tested by analysis of covariance (ANCOVA). These analyses included data from several dates, so a full general linear model was first used to test effects of date, CO2 treatment, and OM on microbial activity and biomass. As can be seen by the reasonably good fits of the regressions in the figures discussed below, OM generally accounted for the majority of the variance. The CO2-date interaction was not significant for any of the variables tested. For these reasons and because the primary purpose of this study was not to address seasonal changes, date was not factored into the analysis. When the effect of OM was not significant, a simple analysis of variance (ANOVA) was used to compare FACE and control samples. ANCOVA analysis was used to detect different responses of soil GWC in the FACE and control treatments and to detect differences in fungal biomass per unit microbial biomass. In some cases, data were log transformed to fit the assumptions of the analysis. Statview software (SAS Institute) was used for statistical analyses.
Sequences from the clone libraries were screened for chimeras by using Chimera_Check (http://rdp.cme.msu.edu). A total of 156 sequences (69 from inside the FACE ring and 87 from outside) were produced and identified using BLAST searches. Sequences were aligned using ARB software and placed into a neighbor-joining phylogenetic tree using Phylip software. Sequences with greater than 98% similarity were considered duplicates and were removed from the analysis. This resulted in 129 unique sequences (60 from inside the FACE ring and 69 from outside). The number of sequences that appeared only once versus the number that appeared twice was used to calculate the Chao1 estimate of diversity by using EstimateS software (12). Neighbor-joining analysis used 100 bootstrap replicates and the Jin-Nei method with a gamma factor of 0.1 to allow for different substitution rates between sites. The bacterial communities from the four clone libraries were compared by using permutation tail probability (PTP) and the Fst statistic (27), using PAUP and Arlequin software, respectively. In the PTP test, the hypothesis that phylogeny covaries with community type was tested by generating 10,000 randomly permuted trees and calculating the tree length needed to evolve the different communities. The tree length of the original data set was compared to this frequency distribution to produce a P value. The Fst statistic compares genetic diversity between samples to overall diversity, using the formula Fst = (θT − θW)/θT, where θT is total genetic diversity and θW is average within-sample diversity for all samples This produces values that vary from 0 to 1 and which can be considered pairwise distances. The statistical significance of Fst was determined by comparing the actual Fst value with a distribution created by randomly permuting the communities 1,000 times.
Nucleotide sequence accession numbers.
The sequences used for phylogenetic analysis have been submitted to GenBank (www.ncbi.nlm.nih.gov) under accession numbers DQ201647 through DQ201772.
RESULTS
Plant root biomass and soil respiration.
The concentration of root biomass in soil taken from inside the FACE ring was greater than that in soil from outside the ring (Fig. 1). The higher root biomass in FACE soils could be attributed to increased roots close to the bases of plants, rather than to roots found in canopy gaps, where root biomass was generally lower. This root distribution is characteristic of the patchy nature of the chaparral. While soil texture does not vary widely over the landscape, soil OM is highly variable and is consistently lower in canopy gaps than under plant canopies (Table 1). Most of the microbial biomass and activity parameters were strongly correlated with soil OM, as is commonly observed (4, 19, 46). To control for variability in OM across the landscape and to correct for any systematic biases between the FACE plot and the rest of the landscape, ANCOVA was used to detect differences between FACE and control soils in the relationships between microbes and soil OM. Soil respiration was significantly correlated with soil OM (P = 0.02), but the slightly higher slope in FACE soils was not significant (Fig. 2A). However, respiration in FACE and control soils differed in the response to soil water content (Fig. 2B). Respiration from control soils was significantly related to water content (P = 0.005), while there was no such relationship in FACE soils (P = 0.817). In the ANCOVA of respiration versus GWC and CO2 treatment, the difference in slope was marginally significant (P = 0.055), as was the difference in intercept (P = 0.052).
FIG. 1.
Fine and coarse plant root dry masses in cores from FACE and control soils collected at two distances from bases of plants. Each bar represents the mean and standard error of 9 or 10 measurements.
FIG. 2.
Variation in soil respiration with (A) soil organic matter (grams OM gram−1 soil) and (B) soil water content (grams H2O gram−1 soil) in FACE and outside control plots. The P values are for the interaction terms in the ANCOVA.
Microbial biomass and activity.
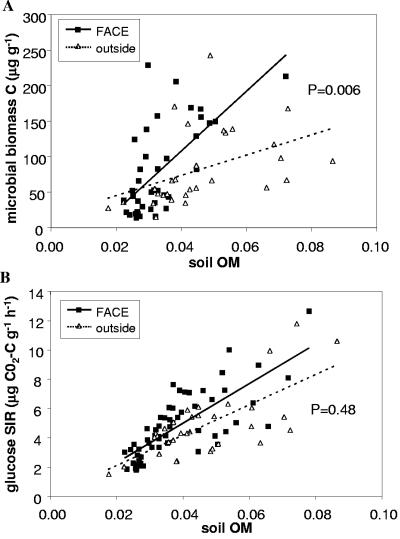
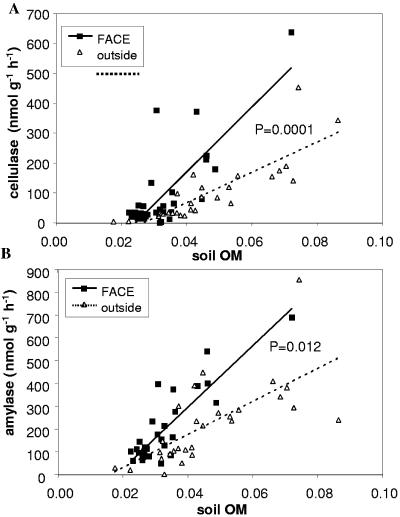
Microbial biomass C (measured by chloroform fumigation-extraction) increased more rapidly with soil OM in FACE soils than in controls (Fig. 3A). However, the corresponding trend for glucose SIR was smaller and nonsignificant (Fig. 3B). Soil cellulase and amylase activities both increased more steeply with OM in FACE soils than in controls (Fig. 4). These results show that elevated CO2 stimulated microbial biomass production and activity, particularly in high-OM areas (i.e., under plants).
FIG. 3.
Variation in (A) microbial biomass C and (B) glucose SIR with soil OM (grams OM gram−1 soil) in FACE and control plots. The P values are for the CO2-OM interaction term in the ANCOVA.
FIG. 4.
Variation in the activities of the soil extracellular enzymes (A) cellulase and (B) amylase with soil OM (grams OM gram−1 soil) in FACE and control plots. The P values are for the CO2-OM interaction term in the ANCOVA.
The ratio of soil respiration to glucose SIR was significantly higher in FACE soils than in controls (Table 2). This parameter is similar to a “metabolic quotient” and to “specific respiration” used to indicate the metabolic state of microbial biomass (3, 20, 47). However, it should be noted that soil respiration was measured in the field and included root respiration, while SIR was measured in the laboratory without roots. The glucose SIR/microbial biomass ratio also describes the metabolic state of microbial biomass. These variables were both log transformed before the analysis in Table 2, which shows that this ratio is lower in FACE soils. These respiratory parameters were not correlated with soil OM, and so both were analyzed by one-way ANOVA.
TABLE 2.
Means (and standard errors) of selected respiratory, bacterial and fungal biomass parameters in soils from FACE and control plots
| Parameter (units) | Mean value (SE)
|
Pa | nb | |
|---|---|---|---|---|
| Control | FACE | |||
| Respiration/SIR (unitless) | 0.291 (0.026) | 0.419 (0.052) | 0.032 | 68 |
| Log SIR/log MBCc (h−1) | 0.363 (0.022) | 0.297 (0.013) | 0.0095 | 59 |
| Bacterial biomass (μg g−1) | 607 (114) | 546 (67) | 0.640 | 30 |
| Bacteria/fungi (unitless) | 3.57 (1.09) | 2.46 (0.44) | 0.329 | 30 |
| Fungi/MBC (unitless) | 3.84 (0.62) | 6.73 (1.37) | 0.0024 | 55 |
The P value listed is for the one-way ANOVA, performed on log- or square-root-transformed data in some cases.
n, total sample size for analysis.
MBC, microbial biomass C determined by chloroform fumigation-extraction.
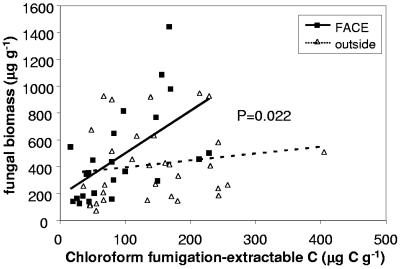
The ratio of fungal biomass to total microbial biomass C was higher in FACE soils than in control soils (Table 2), and this differential between FACE and control plots increased with increasing biomass (Fig. 5), indicating that the stimulatory effect of elevated CO2 on microbial biomass disproportionately affected fungi. Bacterial biomass was not significantly different in FACE and control plots (Table 2). Fungal biomass correlated significantly with the activities of amylase and cellulase enzymes in the soil (Fig. 6), whereas bacterial biomass did not (data not shown).
FIG. 5.
Variation of fungal biomass with microbial biomass C (chloroform fumigation-extraction) in FACE and control plots. The P value is for the interaction term in the ANCOVA.
FIG. 6.
Correlations of extracellular enzymatic activities with fungal biomass measurements in soils. The P values are given for both regressions.
Bacterial diversity.
The soil bacterial community was dominated by the Acidobacteria and Proteobacteria phyla (Fig. 7 and 8), as is typical for soils (18). Based on the Chao1 parameter, there were 445 ± 225 distinct bacterial ribotypes in this community. There was no obvious clustering of sequences from the same sample type in Fig. 7, and PTP analysis confirms that the bacterial community is not different between the FACE and control plots (P = 0.310) or between plant and gap samples (P = 0.561). Similarly, the Fst statistic showed that the FACE and control communities were very similar in composition (distance of 0.0027 on a scale of 0 to 1) and not significantly different from each other (P = 0.223). The PTP and Fst analyses address whether two communities are phylogenetically distinct and are not sensitive to the abundance of species. However, there was also remarkable similarity in the frequency of major bacterial taxa in the four clone libraries (Fig. 8). The only group that showed consistent differences between the two FACE and two control libraries was the Gammaproteobacteria, and this change, if real, was relatively minor in terms of the whole community.
FIG. 7.
Neighbor-joining phylogenetic tree of 16S rRNA sequences in clone libraries from plant and interplant samples of FACE and outside control plots. Firmi, Firmicutes; CFB, Cytophaga-Bacteroides-Flexibacter; Actino, Actinobacteria; BD, Gemmatimonadetes/BD; Verruco, Verrucomicrobia; NS, Nitrospira; GNS, green nonsulfur bacteria.
FIG. 8.
Frequencies of major bacterial taxa in (A) all four clone libraries and (B) weighted means and standard errors of the plant and interplant (gap) libraries from FACE and control plots. Acido, Acidobacteria; Alpha, Beta, Gamma, and Delta, classes of Proteobacteria; CFB, Cytophaga-Bacteroides-Flexibacter; Firmi, Firmicutes; Verruco, Verrucomicrobia.
DISCUSSION
The patchy plant distribution in the study area gave rise to a similar pattern in soil OM, with highest levels occurring directly under plants. While there was high variability in microbial biomass and activity across the landscape, soil OM explained most of the variation in these data. This allowed us to overcome the lack of replicate FACE rings (the cost in compressed CO2 gas alone was about $40,000 per year for a single ring). Because the variation across the landscape was well understood and controlled for by using ANCOVA with soil OM as a covariate, differences between FACE and control samples could be attributed to CO2 effects rather than random landscape effects. From a microbial perspective, the 178-m2 region within the FACE ring represents a vast landscape that includes similar extremes in soil OM found elsewhere across the landscape. The FACE ring soil also did not differ in texture or pH from other areas in the landscape where control samples were collected. Hence, the elevated CO2 treatment is the most likely explanation for the different relationships between soil OM and microbial activity in the FACE and control plots. Furthermore, an increase in root biomass and microbial activity in response to elevated CO2 is consistent with the literature discussed below.
Whereas soil OM significantly affected microbial biomass and activity, its effect on bacterial diversity was minimal. The frequency of the bacterial taxa in clone libraries varied somewhat between higher-OM samples under plants and lower-OM samples in gaps (Fig. 8A), but the PTP analysis showed that these communities were not phylogenetically distinct. A more spatially intensive study of bacterial diversity would be required to address whether the abundance of these groups truly varies with OM, but we can conclude from the PTP analysis that similar species exist at both extremes.
The compositions of the clone libraries of FACE and control plots were strikingly similar, both in the abundance of major taxa (Fig. 8B), and in the phylogenetic placement of species (Fig. 7). The only group that possibly showed a consistent response to elevated CO2 was the Gammaproteobacteria, a relatively minor component of the community. A spatially intensive approach would be required to see if this increase from 5% to 9% was a consistent effect of elevated CO2 on the Gammaproteobacteria of this ecosystem. The PTP and Fst analyses verified that the bacterial communities in the FACE and control plots were not phylogenetically distinct. The insignificant PTP and Fst results can best be understood by noting that most major branches on the phylogenetic tree (Fig. 7) have representatives from all four libraries.
The 16S rRNA clone libraries were designed to represent the spatially averaged bacterial communities within each treatment block, and therefore spatial replicates were combined. This design does not provide enough replicates to compare the abundances of each bacterial taxon with statistical rigor, but it does provide well-mixed samples of diversity from each treatment type that can be compared with phylogenetic tools, such as the PTP and Fst tests. It is generally not practical to exhaustively sequence all 16S rRNA ribotypes in a soil community. However, the 129 unique sequences generated in this study represent a significant subsample of the total diversity: about 29%, based on the Chao1 estimate of 445 ± 225 distinct ribotypes for this community. In order for further sequencing to alter the results of the PTP or Fst analyses, entirely new clades would have to emerge, consisting solely of FACE or outside sequences. The phylogenetic tree (Fig. 7) represents the most common phyla found in soils (18) and has sequences from both FACE and outside plots in most clades. Therefore, it is unlikely that the remaining unsequenced ribotypes would drastically change the results.
The lack of response of the bacterial community to elevated CO2 was consistent with the finding that bacterial biomass was not significantly different between FACE and control plots, despite the significant effects on total microbial biomass. In contrast, fungal biomass responded markedly. Whether the fungal community also changed in composition and the relative responses of mycorrhizal and saprotrophic fungi are beyond the scope of this study. Stimulation of arbuscular mycorrhizae (AM) and changes in AM species composition by elevated CO2 have been reported for A. fasciculatum and other chaparral plants (36, 44), and higher plant allocation to mycorrhizae is consistent with the observed increase in root growth. The data strongly suggest that saprotrophic fungi are stimulated as well. The increased extracellular enzymatic activities under elevated CO2 correlated with fungal biomass, indicating that saprotrophic fungi responded to increased root biomass with growth and exoenzyme production. Other researchers have found that fungi increase in response to inputs of live and dead plant roots (51). Dark septate fungal hyphae were commonly observed in soil samples (unpublished observation). Based on morphology, this type is clearly not an AM fungus, although such morphotypes have also been observed in possible associations with A. fasciculatum roots (1). Increased root biomass probably stimulated fungi that thrive on both dead and live roots.
The stimulation of root growth by elevated CO2 is consistent with the increased photosynthesis and leaf and stem area index observed in the FACE treatment by others (11) and has been widely reported for many ecosystems (22, 31). Additionally, leaf tissue chemistry was altered in the FACE plots (26). It follows that microbial biomass and extracellular enzymatic activity would respond to increased root growth and altered leaf chemistry, as observed in this study, although this is not always the case. Occasionally a smaller, more active pool of microbial biomass has been reported to result from elevated CO2 (15, 40, 45, 50). In the present study, the respiratory physiology of the microbial community shifted in response to elevated CO2. The increased ratio of soil respiration to glucose SIR could have been caused by a better-fed microbial community functioning closer to its respiratory potential (3, 20, 47). This effect could also be caused by stimulated root respiration: although soil respiration in the FACE treatment was not significantly increased in the present study, higher respiration rates were observed in a more temporally intensive study (11). The microbial community in the FACE samples had a markedly lower ratio of glucose SIR to microbial biomass C. This could be attributed to the higher proportion of fungi in FACE samples. Filamentous fungi have the ability to shift resources throughout their mycelial network to exploit areas of high resources, while maintaining viable but inactive hyphae elsewhere in the soil. Higher levels of viable, inactive biomass in fungus-dominated soils would result in lower SIR activity per unit microbial biomass C as measured by fumigation-extraction. Bacteria and fungi generally have different growth kinetics (25), and bacterium/fungus ratios have been linked to variations in specific respiration (respiration per unit biomass) across soil types (39).
This study strongly suggests that fungi, and not bacteria, respond to increased root growth under elevated CO2 and that fungi and bacteria differ significantly in their respiratory properties. These results may also have implications for the C balance of the chaparral ecosystem under elevated CO2. While root biomass was stimulated, this potential sink is likely to be offset by increased decomposition activity, as demonstrated by higher cellulase and amylase activities in the FACE plot. Furthermore, soil respiration under elevated CO2 appeared to be less sensitive to drought than control soil respiration, possibly extending ecosystem C loss during dry periods. On the other hand, a continued shift toward a fungus-dominated microbial community with lower potential respiration rates per unit biomass could lead to a decreased ability of the microbial community to respond to C inputs and could change the relationship between soil respiration and microbial biomass in this ecosystem. This result emphasizes the need to understand the relationship between microbial community structure and soil respiration under current and future conditions.
Acknowledgments
Thanks go to Michelle Blair, Yufu Cheng, Steve Hastings, Pablo Bryant, and Joe Verfaillie for field, laboratory, and logistical assistance and to Scott Kelley for assistance with the phylogenetic analyses. Thanks also go to the anonymous reviewers, who provided many detailed and helpful comments.
REFERENCES
- 1.Allen, M. F., L. M. Egerton-Warburton, E. B. Allen, and O. Karen. 1999. Mycorrhizae in Adenostoma fasciculatum Hook. & Arn.: a combination of unusual ecto- and endo-forms. Mycorrhiza 8:225-228. [Google Scholar]
- 2.Anderson, J. P. E., and K. H. Domsch. 1978. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 10:215-221. [Google Scholar]
- 3.Anderson, T.-H., and K. H. Domsch. 1985. Determination of eco-physiological maintenance requirements of soil microorganisms in a dormant state. Biol. Fert. Soils. 1:81-89. [Google Scholar]
- 4.Anderson, T-H., and K. H. Domsch. 1989. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 21:471-479. [Google Scholar]
- 5.Ausubel, F. M. (ed.) 1994. Current protocols in molecular biology, John Wiley and Sons, New York, N.Y.
- 6.Bohannan, B. J. M., and J. Hughes. 2003. New approaches to analyzing microbial biodiversity data. Curr. Opin. Microbiol. 6:282-287. [DOI] [PubMed] [Google Scholar]
- 7.Bottomley, P. J. 1994. Light microscopic methods for studying soil microorganisms, p. 81-104. In S. H. Mickelson (ed.), Methods of soil analysis, part 2. Microbiological and biochemical properties. Soil Science Society of America, Madison, Wis.
- 8.Bowman, R. H. 1973. Soil survey of the San Diego area, California, part I. USDA Soil Conservation Service and Forest Service, Washington, D.C.
- 9.Canadell, J., and P. H. Zedler. 1995. Underground structures of woody plants in Mediterranean regions of California, Chile, and Australia, p. 177-210. In M. T. Kalin-Arroyo, P. H. Zedler, and M. D. Fox (ed.), Ecology and biogeography of Mediterranean ecosystems in Chile, California, and Australia. Springer-Verlag, New York, N.Y.
- 10.Chatzinotas, A., R. A. Sandaa, W. Schoenhuber, R. Amann, F. L. Daae, V. Torsvik, J. Zeyer, and D. Hahn. 1998. Analysis of broad-scale differences in microbial community composition of two pristine forest soils. Syst. Appl. Microbiol. 21:579-587. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, Y., W. C. Oechel, P. J. Bryant, S. J. Hastings. The impacts of elevated CO2 on carbon flux of southern California chaparral using free-air CO2 enrichment. Submitted for publication.
- 12.Colwell, R. K. 1997. EstimateS: statistical estimation of species richness and shared species from samples, version 5. User's guide and application. [Online.] http://viceroy.eeb.uconn.edu/estimates.
- 13.Curtis, T. P., W. T. Sloan, and J. W. Scannall. 2002. Estimating prokaryotic diversity and its limits. Proc. Natl. Acad. Sci. USA 99:10494-10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz, S., J. P. Grime, J. Harris, and E. McPherson. 1993. Evidence of a feedback mechanism limiting plant response to elevated carbon dioxide. Nature 364:616-617. [Google Scholar]
- 15.Grayston, S. J., C. D. Campbell, J. L Lutze, and R. M. Gifford. 1998. Impact of elevated CO2 on the metabolic diversity of microbial communities in N limited grass swards. Plant Soil 203:289-300. [Google Scholar]
- 16.Griffiths, B. S., K. Ritz, N. Ebblewhite, E. Paterson, and K. Killham. 1998. Ryegrass rhizosphere microbial community structure under elevated carbon dioxide concentrations, with observations on wheat rhizosphere. Soil Biol. Biochem. 30:315-321. [Google Scholar]
- 17.Horz, H. P., A. Barbrook, C. B. Field, and B. J. M. Bohannan. 2004. Ammonia-oxidizing bacteria respond to multifactorial global change. Proc. Natl. Acad. Sci. USA 101:15136-15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insam, H., and K. H. Domsch. 1988. Relationship between soil organic C and microbial biomass on chronosequences of reclamation sites. Microb. Ecol. 15:177-188. [DOI] [PubMed] [Google Scholar]
- 20.Insam, H., and K. Haselwandter. 1989. Metabolic coefficient of the soil microflora in relation to plant succession. Oecologia 79:174-178. [DOI] [PubMed] [Google Scholar]
- 21.Jensen, L. S., and J. Sorensen. 1994. Microscale fumigation-extraction and substrate-induced respiration methods for measuring microbial biomass in barley rhizosphere. Plant Soil 162:151-161. [Google Scholar]
- 22.Körner, C., M. Diemer, B. Schäppi, P. Niklaus, and J. Arnone, III. 1997. The responses of alpine grassland to four seasons of CO2 enrichment: a synthesis. Acta Oecologica 18:165-175. [Google Scholar]
- 23.Lipson, D. A., C. W. Schadt, and S. K. Schmidt. 2002. Changes in microbial community structure and function following snow melt in an alpine soil. Microb. Ecol. 43:307-314. [DOI] [PubMed] [Google Scholar]
- 24.Lipson, D. A., S. K. Schmidt, and R. K. Monson. 1999. Links between microbial population dynamics and N availability in an alpine ecosystem. Ecology 80:1623-1631. [Google Scholar]
- 25.Lipson, D. A., and S. K. Schmidt. 2002. Kinetics of microbial processes and population growth in soil, p. 1748-1757. In G. Bitton (ed.) The encyclopedia of environmental microbiology. Wiley and Sons, New York, N.Y.
- 26.Marriott, A. M. 2003. Effects of elevated carbon dioxide on Adenostoma fasciculatum leaf nutrients. M.S. thesis. San Diego State University, San Diego, Calif.
- 27.Martin, A. P. 2002. Phylogenetic approaches for describing and comparing the diversity of microbial communities. Appl. Environ. Microbiol. 68:3673-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayr, C., M. Miller, and H. Insam. 1999. Elevated CO2 alters community level physiological profiles and enzyme activities in alpine grassland. J. Microbiol. Methods 36:35-43. [DOI] [PubMed] [Google Scholar]
- 29.Miller, D. N., J. E. Bryant, E. L. Madsen, and W. C. Ghiorse. 1999. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl. Environ. Microbiol. 65:4715-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montealegre, C. M., C. van Kessel, M. P. Russele, and M. J. Sadowsky. 2002. Changes in microbial activity and composition in a pasture ecosystem exposed to elevated atmospheric carbon dioxide. Plant Soil 243:197-207. [Google Scholar]
- 31.Norby, R. J., and R. B. Jackson. 2000. Root dynamics and global change: seeking an ecosystem perspective. New Phytol. 147:3-12. [Google Scholar]
- 32.O'Neill, E. G. 1994. Response of soil biota to elevated atmospheric carbon dioxide. Plant Soil 165:55-65. [Google Scholar]
- 33.Pace, N. R. 1997. A molecular view of microbial diversity and the biosphere. Science 276:734-740. [DOI] [PubMed] [Google Scholar]
- 34.Prior, S. A., H. A. Torbert, G. B. Runion, H. H. Rogers, C. W. Wood, B. A. Kimball, R. L. Lamorte, P. J. Winter, and G. W. Wall. 1997. Free air carbon dioxide enrichment of wheat: soil carbon and nitrogen dynamics. J. Environ. Qual. 26:1161-1166. [Google Scholar]
- 35.Randlett, D. L., D. R. Zak, K. S. Pregitzer, and P. S. Curtis. 1996. Elevated atmospheric carbon dioxide and leaf litter chemistry: influences on microbial respiration and net nitrogen mineralization. Soil Sci. Soc. Am. J. 60:1571-1577. [Google Scholar]
- 36.Rillig, M. C., and M. F. Allen. 1998. Arbuscular mycorrhizae of Gutierrezia sarothrae and elevated carbon dioxide: evidence for shifts in C allocation to and within the mycobiont. Soil Biol. Biochem. 30:2001-2008. [Google Scholar]
- 37.Rillig, M. C., K. M. Scow, J. N. Klironomos, and M. F. Allen. 1997. Microbial carbon substrate utilization in the rhizosphere of Gutierrezia sarothrae grown in elevated atmospheric carbon dioxide. Soil Biol. Biochem. 29:1387-1394. [Google Scholar]
- 38.Roberts, S. W., W. C. Oechel, P. J. Bryant, S. J. Hastings, J. Major, and V. Nosov. 1998. A field fumigation system for elevated carbon dioxide exposure in chaparral shrubs. Func. Ecol. 12:708-719. [Google Scholar]
- 39.Sakamoto, K., and Y. Oba. 1994. Effect of fungal to bacterial biomass ratio on the relationship between CO2 evolution and total soil microbial biomass. Biol. Fertil. Soils 17:39-44. [Google Scholar]
- 40.Santruckova, H., and M. Simek. 1994. Soil microorganisms at different CO2 and O2 tensions. Folia Microbiol. 39:225-230. [Google Scholar]
- 41.Schimel, D. S., J. Melillo, H. Tian, A. D. McGuire, D. Kicklighter, T. Kittel, N. Rosenbloom, S. Running, P. Thornton, D. Ojima, W. Parton, R. Kelly, M. Sykes, R. Neilson, and B. Rizzo. 2000. Contribution of increasing CO2 and climate to carbon storage by ecosystems in the United States. Science 287:2004-2006. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, S. K. 1992. A substrate-induced growth-response (SIGR) method for estimating the biomass of microbial functional groups in soil and aquatic systems. FEMS Microbiol. Ecol. 101:197-206. [Google Scholar]
- 43.Schortemeyer, M., U. A. Hartwig, G. R. Hendrey, and M. J. Sadowsky. 1996. Microbial community changes in the rhizospheres of white clover and perennial ryegrass exposed to free air carbon dioxide enrichment (FACE). Soil Biol. Biochem. 28:1717-1724. [Google Scholar]
- 44.Treseder, K. K., L. M. Egerton-Warburton, M. F. Allen, Y. F. Cheng, and W. C. Oechel. 2003. Alteration of soil carbon pools and communities of mycorrhizal fungi in chaparral exposed to elevated carbon dioxide. Ecosystems 6:786-796. [Google Scholar]
- 45.Van Ginkel, J. H., A. Gorissen, and J. A. van Veen. 1996. Long term decomposition of grass roots as affected by elevated atmospheric carbon dioxide. J. Environ. Qual. 25:1122-1128. [Google Scholar]
- 46.Wardle, D. A. 1992. A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soils. Biol. Rev. 67:321-358. [Google Scholar]
- 47.Wardle, D. A., and A. Ghani. 1995. A critique of the microbial metabolic quotient (qC02) as a bioindicator of disturbance and ecosystem development. Soil Biol. Biochem. 27:1601-1610. [Google Scholar]
- 48.Williams, M. A., C. W. Rice, and C. E. Owensby. 2000. Carbon dynamics and microbial activity in tallgrass prairie exposed to elevated CO2 for 8 years. Plant Soil 227:127-137. [Google Scholar]
- 49.Zak, D. R., K. S. Pregitzer, P. S. Curtis, W. E. Holmes. 2000. Atmospheric CO2 and the composition and function of soil microbial communities. Ecol. Appl. 10:47-59. [Google Scholar]
- 50.Zak, D. R., K. S. Pregitzer, J. S. King, and W. E. Holmes. 2000. Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol. 147:201-222. [Google Scholar]
- 51.Zhu, W., J. G. Ehrenfeld, R. W. Parmelee, W. F. J. Parsons, X. Han. 1996. The effects of live and dead roots on soil fungi in spodosolic soils of the New Jersey Pinelands. Biol. Fertil. Soils 21:215-226. [Google Scholar]