Abstract
Molecular analysis of bacteria enriched under in situ-like conditions and mechanically isolated by micromanipulation showed that a hitherto-uncultivated microaerophilic bacterium thriving in oxygen-sulfide counter-gradients (R. Thar and M. Kühl, Appl. Environ. Microbiol. 68:6310-6320, 2000) is affiliated with the ɛ-subdivision of the Proteobacteria. The affiliation was confirmed by the use of whole-cell hybridization with newly designed specific oligonucleotide probes. The bacterium belongs to a new genus and received the provisional name “Candidatus Thioturbo danicus.”
The oxic-anoxic interface is a highly fluctuating microbial habitat, and motile microaerophilic bacteria must therefore be well adapted to such variable conditions in order to remain active. Motility coupled with chemotaxis is one of the mechanisms to find the most favorable living conditions. Sulfide-oxidizing bacteria such as Beggiatoa and Thiovulum have a characteristic morphology, and their activity and behavior in oxygen-sulfide counter-gradients are well studied (10, 12, 17, 26). However, microscopic inspection shows the presence of many other microaerophilic bacteria at the oxic-anoxic interface (30). Recently, Thar and coworkers described a bacterium that was able to form conspicuous veils in oxygen-sulfide counter-gradients of sulfidic marine sediments (27, 29, 30). The colorless gram-negative bacterium has a vibrioid-shaped cell with bipolar polytrichous flagella. Free-swimming cells have a unique motility behavior and move in a boomerang-like track, as they rotate and translate along their short axis (27). The bacterium exhibits true chemotaxis towards oxygen and can employ spatial oxygen sensing in its motility (28). When attached to a solid substratum via a mucous stalk, the bacterium forms a cohesive whitish veil, whereon attached cells keep rotating as a means to enhance their oxygen uptake rate (27). Microsensor measurements of oxygen and sulfide gradients indicated that the bacterium's physiology is based on the oxidation of reduced sulfur compounds (27). Attempts to isolate this bacterium in pure culture have not been successful, and hence its phylogenetic affiliation has remained unknown.
Here, we describe a full-cycle rRNA approach (3) to identify this particular bacterium. Denaturing gradient gel electrophoresis (DGGE) of PCR-amplified 16S rRNA gene fragments (19) was used to determine the diversity of enrichments. DNA fragments were excised from the gels and sequenced. Subsequently, the sequences were used to determine the phylogenetic affiliation of the bacterium and to design specific oligonucleotide probes. Whole-cell hybridization with fluorescent oligonucleotide probes (3) was used to confirm the identification of the bacterium.
Sampling site and enrichment culture.
Sediment samples were taken from Nivå Bay (Denmark), a sulfidic habitat which has been studied in great detail for >35 years (9). Samples were transported to the laboratory and placed in an aquarium filled with seawater from the sampling site. Successful enrichment of the bacteria was based on two microenvironmental conditions: (i) the oxygen-sulfide interface should be located above the sediment surface in the diffusive boundary layer, i.e., the sulfide production in the sediment must be sufficiently high, and (ii) the overlaying seawater should be gently flushed with air, causing a slow advective water movement (ca. 1 cm/s) above the sediment. The setup was kept at room temperature and exposed to dim daylight. The enrichments were fuelled with organic material by burying Kleenex tissues in the sediments, a slow degradable source of organic material for the sulfate reducers.
Micromanipulation of bacteria.
About 1 mm2 of the veils was extracted with a Pasteur pipette and transferred into flat glass capillaries (8 by 0.8 by 40 mm inner dimension; VitroCom Inc., Mountain Lakes, N.J.) mounted on a research microscope. Within 20 to 40 min, the inner region of the flat capillaries became anoxic due to the O2-respiring bacteria. Around this region, the bacteria of interest aggregated chemotactically in a circular band at the oxic-anoxic interface. A microcapillary (inner tip diameter ca. 10 μm) was introduced into the flat capillaries with help of a manual micromanipulator (Unisense, Denmark). The microcapillary was connected to a manual microinjector (CellTram oil; Eppendorf, Germany). The position of the microcapillary could be followed with the microscope. Thus, clusters of the bacteria of interest were extracted with the microcapillary and transferred into Eppendorf tubes for further processing.
DNA extraction.
Genomic DNA was extracted from the sediment samples and enrichments using the Ultra Clean soil DNA isolation kit (MoBIO Laboratories) according to the manufacturer's protocol. The quality of the extracted DNA was analyzed by agarose electrophoresis.
PCR amplification of 16S rRNA gene fragments.
Primer pair GM3 and GM4 were used to amplify the nearly complete 16S rRNA gene (18). Subsequently, this PCR product was used as a template in a second PCR to create DNA fragments that could be analyzed by DGGE (23). The primers used in this study are described in Table 1. The PCR products were first inspected on 2% (wt/vol) agarose gels before they were analyzed by DGGE.
TABLE 1.
Oligonucleotides used in this study
| Name | Target organisms | Sequence (5′-3′) | Target site | % Formamidef | Reference |
|---|---|---|---|---|---|
| GM3Fa | Bacteria | AGA GTT TGA TCM TGG C | 16S (8-24) | NA | 18 |
| GM4Ra | Bacteria | TAC CTT GTT ACG ACT T | 16S (1492-1507) | NA | 18 |
| 341F-GCa,b | Bacteria | CC TAC GGG AGG CAG CAG | 16S (341-357) | NA | 23 |
| 907RMa | Bacteria | CCG TCA ATT CMT TTG AGTT T | 16S (907-927) | NA | 23 |
| ALF1bc | α-Proteobacteria | CGT TCG YTC TGA GCC AG | 16S (19-35) | 20 | 15 |
| ALF968c | α-Proteobacteria | GGT AAG GTT CTG CGC GTT | 16S (968-986) | 20 | 20 |
| BET42ac,d | β-Proteobacteria | GCC TTC CCA CTT CGT TT | 23S (1027-1043) | 35 | 15 |
| GAM42ac,d | γ-Proteobacteria | GCC TTC CCA CAT CGT TT | 23S (1027-1043) | 35 | 15 |
| CF319ac | Cytophaga-Flavobacterium cluster | TGG TCC GTG TCT CAG TAC | 16S (319-336) | 35 | 16 |
| FB648c | T. danicus and strain DK4 | ACC TCT CCC ATG GTC TAG TT | 16S (648-668) | 40 | This study |
| FB741c | T. danicus and strain DK4 | CCT CAG CGT CAG CTA TGT TC | 16S (741-761) | 40 | This study |
| FB842c | Strain DK4 | ACT GTG TTA CTG CAG CCT CT | 16S (842-862) | 40 | This study |
| ARC94c | Arcobacter spp. | TGC GCC ACT TAG CTG ACA | 16S (94-112) | 20 | 24 |
| ARC1430c | Arcobacter spp. | TTA GCA TCC CCG CTT CGA | 16S (1430-1448) | 20 | 24 |
| EUB338c | Most bacteria | GCT GCC TCC CGT AGG AGT | 16S (338-356) | 35 | 2 |
| EUB338IIc,e | Planctomycetales | GCA GCC ACC CGT AGG TGT | 16S (338-356) | 35 | 7 |
| EUB338IIIc,e | Verrucomicrobiales | GCT GCC ACC CGT AGG TGT | 16S (338-356) | 35 | 7 |
Oligonucleotide used as primer in the PCR.
The GC clamp (5′-CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCC CCG CCC G-3′) was incorporated at the 5′ end of this primer.
Oligonucleotide used as probe in whole-cell hybridization.
Probe GAM42a was applied together with the unlabeled BET42a to prevent unspecific hybridization to possible β-Proteobacteria.
Probes EUB338II and EUB338III were used together with EUB338 to cover all Bacteria. The combination of the three probes is named EUB338mix.
NA, not applicable.
DGGE of PCR products.
DGGE was performed as described by Schäfer and Muyzer (23). Individual bands were excised, reamplified, and run again on a denaturing gradient gel to verify their purity. PCR products for sequencing were purified using the QIAquick PCR purification kit (QIAGEN, Germany). DNA sequencing was carried out by a commercial company (BaseClear, Leiden, The Netherlands).
Phylogenetic analysis.
The sequences were first compared to sequences stored in GenBank using the BLAST algorithm (1; http://www.ncbi.nlm.nih.gov/BLAST). Subsequently, the sequences were imported into the ARB software program (14; http://www.arb-home.de), aligned, and added to a phylogenetic tree using the QUICK_ADD_TO_EXISTING_TREE tool. The alignment was further corrected by eye, and a tree was calculated using the neighbor-joining algorithm with Felsenstein correction. Bootstrap values were determined with the software program PAUP (25).
Probe design and whole-cell hybridization.
Specific oligonucleotide probes were designed using the DESIGN_PROBES function in the ARB software program (14). The in silico specificity of the probes was checked using the MATCH_PROBES function in ARB (14) and the PROBE_MATCH function of the Ribosomal Database Project II (6; http://rdp.cme.msu.edu).
Whole-cell hybridization was performed as described by Pernthaler et al. (21). The specificity of the designed probes was tested by using increasing formamide concentrations in the hybridization buffer. A concentration of 40% (vol/vol) was chosen whereby we found an intense signal with the bacterium of interest but no signal with the other bacteria in the sample. Ten microliters of hybridization buffer including fluorescently labeled oligonucleotide probes (0.5 pmol for Cy3/Cy5 and 0.83 pmol for fluorescein-labeled probes) was added to the cells and incubated for 2 h at 46°C. After washing for 20 min at 48°C, the specimens were dried by compressed air and embedded in Vectashield H-1000 mounting medium for fluorescence (Vector Laboratories, Burlingame, CA). Slides were observed with a Zeiss Axioplan 2 imaging epifluorescence microscope. Images were taken with a Leica D350F black and white charge-coupled device camera and acquired with Leica FW4000 software.
Enrichment and isolation by micromanipulation.
Whitish translucent veils appeared within 1 to 3 days on the sediment surface of the enrichment cultures (27, 29). Microscopic inspection ensured that the veils were formed and mainly consisted of “Candidatus Thioturbo danicus.” The vibrioid cells showed typical dimensions of 2 by 6 μm and could be unequivocally identified by their morphology and unique motility behavior (27, 28). These cells together with other microaerophilic bacteria were extracted by micromanipulation for the subsequent phylogenetic analysis.
PCR-DGGE analysis.
Because of the low number (between 100 and 1,000) of cells isolated by micromanipulation, and hence the low amount of extracted DNA, we used a nested PCR approach (8). First, the nearly complete 16S rRNA gene was amplified using primers GM3F and GM4R. Subsequently, this PCR product was used as a template in a second PCR using primers 341F-GC and 907RM, which generated products suitable for DGGE analysis. DGGE of the PCR products obtained with primers 341F-GC and 907RM showed different patterns with a separated band (results not shown). Sequencing of DGGE-separated gene fragments resulted in sequences with lengths between 488 and 540 nucleotides. Subsequently, these sequences were used to infer the phylogenetic affiliation of the community members.
Phylogenetic analysis.
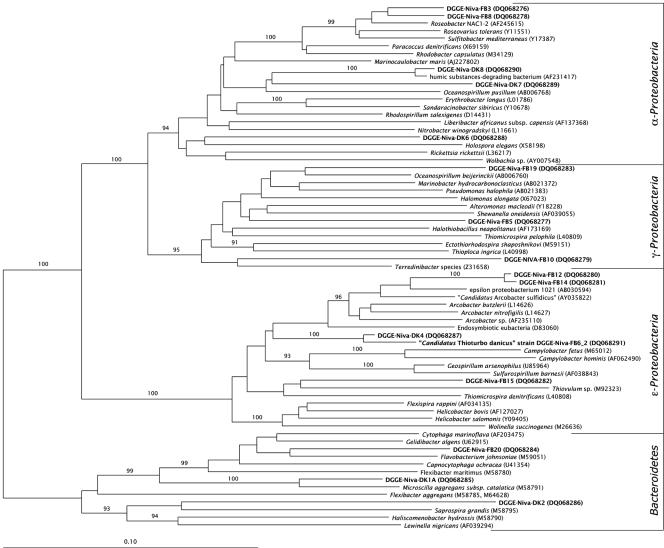
The bacteria found in our enrichments were affiliated with different groups within the Proteobacteria and the Bacteroidetes (Fig. 1). Five sequences, i.e., DGGE-Niva-FB3, DGGE-Niva-FB8, DGGE-Niva-DK6, DGGE-Niva-DK7, and DGGE-Niva-DK8, fell within the α-subdivision of the Proteobacteria. The sequences DGGE-Niva-FB3 and DGGE-Niva-FB8 formed a coherent group affiliated with Roseobacter sp. strain NAC1-2, Roseovarius tolerans, and Sulfitobacter mediterraneus. Sequence DGGE-Niva-DK8 was closely related to a bacterium that could degrade humic substances, while sequence DGGE-Niva-DK7 was only loosely related to this cluster. The sequence of DGGE-Niva-DK6 was distantly related to Holospora elegans.
FIG. 1.
Neighbor-joining tree based on 16S rRNA gene sequences, showing the phylogenetic affiliation of bacteria obtained from enrichments and micromanipulations. Names of the sequences determined in this study are in bold. The sequence accession numbers are within parentheses. The bar indicates 10% sequence variation. The numbers on the branches are bootstrap values of 1,000 iterations; only those greater than 90% are given.
Three sequences (i.e., DGGE-Niva-FB5, DGGE-Niva-FB10, and DGGE-Niva-FB19) were affiliated with the γ-subdivision of the Proteobacteria. Sequence DGGE-Niva-FB5 clustered together with the cluster consisting of Alteromonas macleodii and Shewanella oneidensis. DGGE-Niva-FB10 was related to a Terredinibacter species, and sequence DGGE-Niva-FB19 was affiliated with Oceanospirillum beijerinckii (22).
Five sequences (i.e., DGGE-Niva-FB6_2, DGGE-Niva-FB15, DGGE-Niva-FB12, DGGE-Niva-FB14, and DGGE-Niva-DK4) were grouped within the ɛ-subdivision of the Proteobacteria. Sequence DGGE-Niva-FB15 grouped together with Thiovulum sp., which was also observed microscopically in the veils (30). Sequences DGGE-Niva-FB12 and DGGE-Niva-FB14 formed a tight cluster, affiliated with members of the genus Arcobacter, most closely related to the ɛ-proteobacterium 1021. Sequences DGGE-Niva-FB6_2 and DGGE-Niva-DK4 formed a tight cluster (bootstrap value of 100%) in between the genera Arcobacter, Campylobacter, and Sulfurospirillum.
Three sequences (i.e., DGGE-Niva-FB20, DGGE-Niva-DK1, and DGGE-Niva-DK2) were affiliated with the Bacteroidetes. Sequence DGGE-Niva-FB20 was most closely related to Flavobacterium johnsoniae. Sequence DGGE-Niva-DK1 grouped tightly with Microscilla aggregans, and sequence DGGE-Niva-DK2 grouped loosely with Saprospira grandis.
The bacteria in our enrichments represented typical members of microbial communities of coastal sediments. α- and γ-Proteobacteria are frequently detected both by molecular (e.g., reference 11) as well as by cultivation approaches (e.g., reference 5). Also, bacteria affiliated with the ɛ-Proteobacteria, such as Thiomicrospira denitrificans (18, 31) and “Candidatus Arcobacter sulfidicus” (32), have been isolated from these habitats. Members of the Bacteroidetes that are able to degrade polymers and other macromolecules are commonly found in marine sediments (13).
Probe design and whole-cell hybridization.
We first used published probes specific for the α-Proteobacteria (15, 20), the γ-Proteobacteria (15), the Bacteroidetes (16), and for the genus Arcobacter (24). None of these probes, however, gave a positive hybridization signal with the bacterium of interest. Because no probe has been described for the ɛ-Proteobacteria in general, we designed two probes (i.e., FB648 and FB741) for the sequences DGGE-Niva-DK4 and DGGE-Niva-FB6 that formed a small, but coherent group within the ɛ-subdivision of the Proteobacteria (Fig. 1). A third probe was designed for DGGE-Niva-DK4 only. No specific probe could be designed for DGGE-Niva-FB6_2.
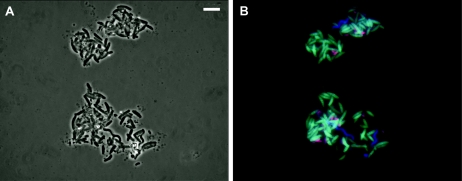
Comparative sequence analysis using the PROBES_MATCHfunction in ARB and the MATCH_PROBES function in RDP showed that the probes had at least two mismatches with all other 16S rRNA gene sequences. Subsequently, these probes were tested with the bacterial samples that were extracted by the micromanipulation procedure. Positive hybridization results were obtained with probe FB648 (Fig. 2B) and FB741 (results not shown). No signal was found with probe FB842, which is specific for the sequence DGGE-Niva-DK4 only. This indicates that the bacterium of interest is represented by sequence DGGE-Niva-FB6_2 and that sequence DGGE-Niva-DK4 probably belongs to a close relative, although we cannot exclude the possibility that the absence of hybridization reaction with probe FB842 is due to the inaccessibility of the target rRNA (4). In addition, the mixture of cells was hybridized with probes specific for members of the genus Arcobacter (i.e., probes ARC94 and ARC1430). Both probes hybridized with small vibrioid-shaped cells (Fig. 2B), indicating the presence of cells affiliated with the genus Arcobacter, which may be those represented by the sequences DGGE-Niva-FB12 and DGGE-Niva-FB14 (Fig. 1). Unfortunately, the target site of these probes cannot be checked on the sequences, because they fall outside the amplified fragment. The combined hybridization with the EUB338mix probe (2, 7), specific for all Bacteria (Fig. 2B), showed the presence of a third, spirillum-like bacterium for which the identity is unknown.
FIG. 2.
Identification of “Candidatus Thioturbo danicus” by whole-cell fluorescent in situ hybridization. (A) Phase-contrast image of all cells. (B) Cells hybridized with a mixture of three probes, i.e., probe FB648 labeled with fluorescein (green) and specific for “Candidatus Thioturbo danicus,” probe ARC1430 labeled with Cy3 (red) and specific for members of the genus Arcobacter, and probe EUB338mix, which is labeled with Cy5 (blue) and specific for all Bacteria. Note the presence of three different bacterial populations, i.e., cells of “Candidatus Thioturbo danicus” stained light blue (green plus blue), cells affiliated with Arcobacter stained pink (red plus blue), and spirillum-shaped cells stained blue. Bar, 10 μm.
The rRNA approach showed that the uncultivated sulfur-oxidizing bacterium is a member of a new genus within the ɛ-subdivision of the Proteobacteria, for which we propose the provisional name “Candidatus Thioturbo danicus,” the “Danishsulfur whirl.” Now that FISH probes are available for this bacterium, its distribution and ecological significance can be studied. In addition, we will continue our quest for the identification of other motile bacteria that live at the oxic-anoxic interface of sulfidic marine sediments. Our study illustrates how enrichment in gradient cultures, which can be described, e.g., by microsensor measurements (27), can be combined with cell micromanipulation and subsequent molecular analysis in order to identify the phylogeny of yet-uncultivated bacteria, which can then be quantified by fluorescence in situ hybridization analysis with probes designed on basis of the molecular data. This seems a promising approach for future studies of environmentally relevant microorganisms from natural habitats without the need of previous cultivation.
Description of “Candidatus Thioturbo danicus.”
“Thioturbo danicus” (Thi.o.tur′bo. Gr. N. thion sulfur, L. masc. n. turbo thing that spins, whirl, N.L. masc. n. Thioturbo, the sulfur whirl. Da′ni.cus. M.L. masc. adj. danicus, Danish). Cells are vibrioid-shaped with a length between 4 and 10 μm and a diameter between 1.3 and 2.5 μm. They contain several spherical inclusions of poly-β-hydroxybutyric acid. The bacterium is microaerophilic and its physiology is presumably based on the oxidation of reduced sulfur compounds. The cells have bipolar polytrichous flagella and exhibit a unique boomerang-like swimming pattern, rotating and translating along their short axis, with a motility speed of ca. 75 μm s−1. They aggregate chemotactically in oxygen-sulfide counter-gradients at a preferred oxygen concentration of ca. 2 μM, where they can attach with a mucous stalk to solid substrata, forming a cohesive whitish veil at the oxic-anoxic interface. Attached cells can increase their oxygen uptake rate by their joint flagellar action, causing advective water transport towards the veil. The stalk length can be dynamically adapted in response to changing oxygen conditions. The species was enriched from an organic-rich, sulfidic marine sediment in Nivå Bay, Denmark. The bacterium is a member of a new genus within the ɛ-subdivision of the Proteobacteria. The accession number of the partial 16S rRNA gene sequence is DQ068291.
Nucleotide sequence accession numbers.
All sequences determined in this study were deposited in GenBank under accession numbers DQ068276 to DQ068291.
Acknowledgments
We acknowledge the kind advice of Hans Trüper in identifying the name “Thioturbo danicus.” We thank Dimitri Sorokin for helpful discussions.
This study was funded by the Danish Natural Science Research Council (R.T. and M.K.).
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann, R. I., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrens, S., C. Rühland, J. Inácio, H. Huber, Á. Fonseca, I. Spencer-Martins, B. M. Fuchs, and R. Amann. 2003. In situ accessibility of small-subunit rRNA of members of the domains Bacteria, Archaea, and Eucarya to Cy3-labeled oligonucleotide probes. Appl. Environ. Microbiol. 69:1748-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkhoff, T., G. Muyzer, C. O. Wirsen, and J. Kuever. 1999. Thiomicrospira kuenenii sp. nov. and Thiomicrospira frisia sp. nov., two mesophilic obligately chemolithoautotrophic sulfur-oxidizing bacteria isolated from an intertidal mud flat. Int. J. Syst. Bacteriol. 49:385-392. [DOI] [PubMed] [Google Scholar]
- 6.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 1:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daims, H., A. Brühl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 8.Dar, S. A. J. G. Kuenen, and G. Muyzer. 2005. Nested PCR-denaturing gradient gel electrophoresis approach to determine the diversity of sulfate-reducing bacteria in complex microbial communities. Appl. Environ. Microbiol. 71:2325-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenchel, T. 1969. The ecology of marine microbenthos. IV. Structure and function of the benthic ecosystem, its chemical and physical factors and the microfauna communities with special reference to the ciliated protozoa. Ophelia 6:1-182. [Google Scholar]
- 10.Fenchel, T. 1994. Motility and chemosensory behaviour of the sulphur bacterium Thiovulum majus. Microbiology 140:3109-3116. [Google Scholar]
- 11.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jørgensen, B. B., and N. P. Revsbech. 1983. Colorless sulfur bacteria, Beggiatoa spp. and Thiovulum spp., in O2 and H2S microgradients. Appl. Environ. Microbiol. 45:1261-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchman, D. L., L. Yu, and M. L. Cottrell. 2003. Diversity and abundance of uncultured Cytophaga-like bacteria in the Delaware Estuary. Appl. Environ. Microbiol. 69:6587-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligonucleotide probes for major subclasses of proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 16.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K.-H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 17.Møller, M. M., L. P. Nielsen, and B. B. Jørgensen. 1985. Oxygen response and mat formation by Beggiatoa spp. Appl. Environ. Microbiol. 50:373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muyzer, G., A. Teske, C. O. Wirsen, and H. W. Jannasch. 1995. Phylogenetic relationships of Thiomicrospira species and their indentification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 164:165-172. [DOI] [PubMed] [Google Scholar]
- 19.Muyzer, G. 1999. DGGE/TGGE: a method for identifying genes from natural environments. Curr. Opin. Microbiol. 2:317-322. [DOI] [PubMed] [Google Scholar]
- 20.Neef, A. 1997. Anwendung der in situ Einzell-Identifizierung von Bakterien zur Populationsanalyse in komplexen mickrobiellen Biozönosen. Ph.D. thesis. Technical University München, München, Germany.
- 21.Pernthaler, J., F. O. Glöckner, W. Schönhuber, and R. Amann. 2001. Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. Methods Microbiol. 30:207-226. [Google Scholar]
- 22.Satomi, M., B. Kimura, T. Hamada, S. Harayama, and T. Fujii. 2002. Phylogenetic study of the genus Oceanospirillum based on 16S rRNA and gyrB genes: emended description of the genus Oceanospirillum, description of Pseudospirillum gen. nov., Oceanobacter gen. nov. and Terasakiella gen. nov. and transfer of Oceanospirillum jannaschii and Pseudomonas stanieri to Marinobacterium as Marinobacterium jannaschii comb. nov. and Marinobacterium stanieri comb. nov. Int. J. Syst. Evol. Microbiol. 52:739-747. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer, H., and G. Muyzer. 2001. Denaturing gradient gel electrophoresis in marine microbial ecology. Methods Microbiol. 30:425-468. [Google Scholar]
- 24.Snaidr, J., R. Amann, I. Huber, W. Ludwig, and K.-H. Schleifer. 1997. Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl. Environ. Microbiol. 63:2884-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swofford, D. L. 2002. PAUP. Phylogenetic analysis using parsomony, version 4. Sinauer Asssociates, Sunderland, Mass.
- 26.Thar, R., and T. Fenchel. 2001. True chemotaxis in oxygen gradients of the sulfur-oxidizing bacterium Thiovulum majus. Appl. Environ. Microbiol. 67:3299-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thar, R., and M. Kühl. 2002. Conspicuous veils formed by vibrioid bacteria on sulfidic marine sediments. Appl. Environ. Microbiol. 68:6310-6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thar, R., and M. Kühl. 2003. Bacteria are not too small for spatial sensing of chemical gradients: an experimental evidence. Proc. Nat. Acad. Sci. USA 100:5748-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thar, R., and M. Kühl. 2005. Complex pattern formation of marine gradient bacteria explained by a simple computer model. FEMS Microbiol. Lett. 246:75-79. [DOI] [PubMed] [Google Scholar]
- 30.Thar, R., and T. Fenchel. 2005. Survey of motile microaerophilic bacterial morphotypes in the oxygen-gradient above a marine sulfidic sediment. Appl. Environ. Microbiol. 71:3682-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timmer-ten Hoor, A. 1975. A new type of thiosulphate-oxidizing, nitrate-reducing microorganism: Thiomicrospira denitrificans sp. nov. Neth. J. Sea Res. 9:343-351. [Google Scholar]
- 32.Wirsen, C. O., S. M. Sievert, C. M. Cavanaugh, S. J. Molyneaux, A. Ahamad, L. T. Taylor, E. F. deLong, and C. D. Taylor. 2002. Characterization of an autotrophic sulfide-oxidizing marine Arcobacter sp. that produces filamentous sulfur. Appl. Environ. Microbiol. 68:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]