Abstract
Using the genes encoding the 2,4-dinitrotoluene degradation pathway enzymes, the nonpathogenic psychrotolerant rhizobacterium Pseudomonas fluorescens ATCC 17400 was genetically modified for degradation of this priority pollutant. First, a recombinant strain designated MP was constructed by conjugative transfer from Burkholderia sp. strain DNT of the pJS1 megaplasmid, which contains the dnt genes for 2,4-dinitrotoluene degradation. This strain was able to grow on 2,4-dinitrotoluene as the sole source of carbon, nitrogen, and energy at levels equivalent to those of Burkholderia sp. strain DNT. Nevertheless, loss of the 2,4-dinitrotoluene degradative phenotype was observed for strains carrying pJS1. The introduction of dnt genes into the P.fluorescens ATCC 17400 chromosome, using a suicide chromosomal integration Tn5-based delivery plasmid system, generated a degrading strain that was stable for a long time, which was designated RE. This strain was able to use 2,4-dinitrotoluene as a sole nitrogen source and to completely degrade this compound as a cosubstrate. Furthermore, P. fluorescens RE, but not Burkholderia sp. strain DNT, was capable of degrading 2,4-dinitrotoluene at temperatures as low as 10°C. Finally, the presence of P. fluorescens RE in soils containing levels of 2,4-dinitrotoluene lethal to plants significantly decreased the toxic effects of this nitro compound on Arabidopsis thaliana growth. Using synthetic medium culture, P. fluorescens RE was found to be nontoxic for A.thaliana and Nicotiana tabacum, whereas under these conditions Burkholderia sp. strain DNT inhibited A.thaliana seed germination and was lethal to plants. These features reinforce the advantageous environmental robustness of P. fluorescens RE compared with Burkholderia sp. strain DNT.
The xenobiotic compound 2,4-dinitrotoluene (2,4-DNT) is an important intermediate in the polyurethane and explosive industries. Because of its toxicity (8, 11), carcinogenicity (14), and widespread environmental occurrence, this compound is a U.S. Environmental Protection Agency priority pollutant (17), and mineralization of it has become a main objective in bioremediation.
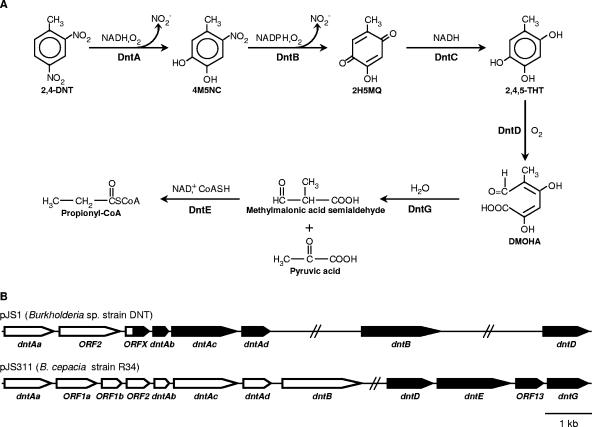
So far, several 2,4-DNT-degrading strains have been isolated from 2,4-DNT-contaminated sites (22, 25). Although apparently all degrading strains that have been isolated use the same degradation pathway (Fig. 1A) (22), at present genetic characterization of the 2,4-DNT degradation pathway has been carried out only for Burkholderia sp. strain DNT and Burkholderia cepacia strain R34. In these strains, the degradative genes are organized into an upper pathway and a lower pathway and are located in plasmids pJS1 and pJS311, respectively (Fig. 1B) (15, 27). The upper pathway dntAaAbAcAd and dntB genes, encoding 2,4-DNT dioxygenase and 4-methyl-5-nitrocathecol (4M5NC) monooxygenase, respectively, are responsible for hydroxylation of the aromatic ring and elimination of nitro substituents (12, 16, 27, 28). The resulting compound, 2-hydroxy-5-methylquinone (2H5MQ), is converted to 2,4,5-trihydroxytoluene (2,4,5-THT) by a nonspecific reductase. The gene encoding this enzyme is designated dntC and has not been isolated so far (16, 27). Finally, the lower pathway operon, encoding a 2,4,5-THT oxygenase (dntD), a coenzyme A (CoA)-dependent methylmalonate semialdehyde dehydrogenase (dntE), and a bifunctional isomerase/hydrolase (dntG), is responsible for extradiol fission of the aromatic ring, catalyzing the production of 2,4-dihydroxy-5-methyl-6-oxo-2,4-hexadienoic acid (DMOHA) and subsequent degradation of this compound to pyruvate and propionyl-CoA (13, 15, 16).
FIG. 1.
(A) Proposed pathway for 2,4-DNT degradation in B. cepacia strain R34. The pathway includes the following enzymes: DntA, 2,4-DNT dioxygenase; DntB, 4M5NC monooxygenase; DntC, 2H5MQ reductase; DntD, 2,4,5-THT oxygenase; DntG, DMOHA isomerase/4-hydroxy-2-keto-5-methyl-6-oxo-3-hexenoate hydrolase; and DntE, methylmalonate semialdehyde dehydrogenase. NO2−, nitrite; CoASH, coenzyme A (16). (B) dnt genes for 2,4-DNT degradation in Burkholderia sp. strain DNT and B. cepacia strain R34. The schematic diagrams of the pJS1 and pJS311 plasmids show the dnt genes isolated from strains DNT and R34, respectively. The solid regions represent the dnt genes transferred to P. fluorescens RE.
The use of 2,4-DNT-degrading bacteria in the design of in situ bioremediation processes is an interesting approach for cleaning up contaminated sites. However, the possible emergence of Burkholderia species as plant pathogens and opportunistic human pathogens (21, 30), which could hamper the in situ environmental biotechnological applications of these organisms (5), remains to be resolved. Moreover, it has been observed that in the absence of 2,4-DNT, the degradative phenotype of Burkholderia sp. strain DNT and B. cepacia R34 is unstable (15, 27), a phenomenon that is undesirable for in situ bioremediation (29). Taking this into account, in this work we investigated the bacterial intergeneric transfer of the 2,4-DNT degradative phenotype by introduction of dnt genes into the nonpathogenic receptor strain Pseudomonas fluorescens ATCC 17400 (26). It has been hypothesized that this strain, referred to below as P. fluorescens WT, is a plant growth-promoting bacterium that could be a biocontrol agent (10, 31) that is suitable for rhizoremediation. It is a psychrotolerant bacterium (26), which is important for conducting biodegradation at relatively low temperatures. In addition to its environmental aspects and its genetic tractability, we determined that this Pseudomonas strain does not biotransform 2,4-DNT, which not only indicated that it does not contain a degradation pathway but also eliminated the possibility that there is any reductive misrouting to dead-end products that would not allow complete 2,4-DNT degradation. We approached our goal by using two basic techniques, conjugative transfer of the pJS1 megaplasmid from Burkholderia sp. strain DNT to obtain P.fluorescens MP and chromosomal insertion of the catabolic genes dntAbAcAd, dntB, and the lower pathway operon with a suicide integration plasmid system to obtain P. fluorescens RE. Although P. fluorescens MP acquired the ability to grow on 2,4-DNT, its degradative phenotype was highly unstable in 2,4-DNT-free medium. In contrast, P. fluorescens RE was a degrading strain that was stable for a long time and was able to cometabolically degrade 2,4-DNT at low temperatures and to reduce the toxicity of this pollutant for plant growth without any harmful effects on plant development.
MATERIALS AND METHODS
Chemicals.
2,4-DNT (99% pure) was obtained from Fluka, Buchs, Switzerland.4M5NC and 2,4,5-THT were kindly provided by Jim C. Spain, Tyndall Air Force Base, Florida. 2H5MQ was obtained from 2,4,5-THT samples based on spontaneous conversion of the hydroxytoluene to quinone at pH 6.7 (12). DMOHA wasobtained after incubation of Escherichia coli JM109(pJS76) (Table 1) with 2,4,5-THT.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | Host strain for DNA manipulation | Stratagene, La Jolla, CA |
| E. coli S17-1 (λpir) | Tpr Smr; recipient for conjugal transfer of pUT mini-Tn5 | 6 |
| Burkholderia sp. strain DNT | 2,4-DNT-degrading strain carrying pJS1 megaplasmid | 25 |
| P. fluorescens ATCC 17400 | Cmr; wild type | 26 |
| P. fluorescens MP | Cmr; wild type carrying pJS1 megaplasmid | This study |
| P. fluorescens RE | Cmr Hgr Kmr Gmr; wild type with dntABDEG chromosomal insertion | This study |
| P. syringae pv. tomato DC3000 | Phytopathogen | 1 |
| Plasmids | ||
| pGEM-T Easy | Apr; PCR product cloning vector | Promega, Madison, WI |
| pGEMEX-1 | Apr; cloning vector | Promega, Madison, WI |
| pJS1 | Burkholderia sp. strain DNT megaplasmid containing dnt genes | 27 |
| pJS37 | Apr; dntAaAbAcAd inserted into pGEM7Zf(+) | 27 |
| pJS53 | Apr; dntB inserted into pGEM7Zf(+) | 27 |
| pJS76 | Apr; dntD inserted into pGEM7Zf(+) | 27 |
| pJS325 | Apr; lower pathway operon inserted into pGEM7Zf(+) | 15 |
| pUT mini-Tn5 Hgr | Apr Hgr; delivery plasmid for mini-Tn5 Hgr | 6 |
| pUT mini-Tn5 Kmr | Apr Kmr; delivery plasmid for mini-Tn5 Kmr | 6 |
| pUT mini-Tn5 Gmr | Apr Gmr; delivery plasmid for mini-Tn5 Gmr | 32 |
| pMRM1 | Apr; dntAbAcAd inserted into pGEM-T Easy | This study |
| pMRM2 | Apr; dntD inserted into pGEM-T Easy | This study |
| pMRM3 | Apr; lower pathway operon inserted into pGEM-T Easy | This study |
| pMRM4 | Apr; dntD inserted into pGEMEX-1 | This study |
| pMRM5 | Apr; dntB and dntD inserted into pGEMEX-1 | This study |
| pUT-MRM1 | Apr Hgr; dntAbAcAd inserted into pUT mini-Tn5 Hgr | This study |
| pUT-MRM2 | Apr Kmr; dntB and dntD inserted into pUT mini-Tn5 Kmr | This study |
| pUT-MRM3 | Apr Gmr; lower pathway operon inserted into pUT mini-Tn5 Gmr | This study |
Resistance markers: Tpr, trimethoprim; Smr, streptomycin; Cmr, chloramphenicol; Apr, ampicillin; Hgr, mercury; Kmr, kanamycin; Gmr, gentamicin.
Bacterial strains, plasmids, and culture media.
The bacterial strains and plasmids used in this study are listed in Table 1. Burkholderia sp. strain DNT and plasmids pJS37, pJS53, pJS76, and pJS325 were supplied by Jim C. Spain, Tyndall Air Force Base, Florida. P. fluorescens ATCC 17400 (= WT), which was first isolated from a hen's egg (26), was provided by Carlos Domenech, Universidad Nacional de Río IV, Argentina. The nitrogen-free minimal medium was identical to that described by Bruhn et al. (3), except that ferric chloride was used instead of ferric citrate (Bruhn medium) and (when necessary) 1.5 mM sodium nitrite was added (BN medium). The P. fluorescens and Burkholderia sp. strain DNT cultures were incubated with agitation (225 rpm) at 28°C unless indicated otherwise. E. coli strains used as cloning hosts were cultured in Luria-Bertani (LB) medium (23) and shaken at 37°C.
Megaplasmid pJS1 conjugation assay.
Biparental mating was performed as previously described (6) using Burkholderia sp. strain DNT as the donor and the chloramphenicol-resistant organism P. fluorescens WT as the recipient strain. P.fluorescens transconjugants were isolated by plating cultures on chloride-free agar medium (19) supplemented with 60 μg/ml chloramphenicol and 0.5 mM 2,4-DNT.
PCR amplification and subcloning of dnt genes.
The dntAbAcAd genes were obtained from plasmid pJS37 (Table 1) by PCR amplification of a 2.7-kb fragment encoding the enzyme components DntAbAcAd without the ferredoxin oxidoreductase DntAa, which has been shown to be unnecessary for 2,4-DNT dioxygenase activity (28). The primers used were dntA-for (5′-AACTTCGAGCAGGACTTGCC-3′) and dntA-rev (5′-CTCACAGGAAGATCATCAGG-3′). The dntD gene (1.2 kb) was amplified from plasmid pJS76 (Table 1) using the following primers: dntD-for (5′-ATGAATTCTCCGAAATGCGGCCGCCTGATGAGG-3′) (an engineered NotI site is underlined) and dntD-rev (5′-TAGATCTCGAGCCAGGACATCTGTGTC-3′). The lower pathway operon (4.7 kb), which includes dntD, dntE, ORF13 (encoding a putative NADH-dependent reductase with an unknown role in 2,4-DNT degradation), and dntG, was amplified from plasmid pJS325 (Table 1) using primers dntLP-for (5′-CGAGATGACCGAGCGTGCCTAC-3′) and dntLP-rev (5′-CTGGATCGGCTGGACTAACTCG-3′). For further manipulation, all PCR products were initially cloned into the pGEM-T Easy vector, generating plasmids pMRM1 (dntAbAcAd), pMRM2 (dntD), and pMRM3 (lower pathway operon) (Table 1).
It should be mentioned that the promoter region of the dnt genes has not been characterized so far. All dnt gene constructions used in this work except the dntAbAcAd construction included a 5′ untranslated sequence which could contain a promoter region.
Construction of pUT mini-Tn5 delivery vectors.
To obtain pUT-MRM1, the 2.7-kb dntAbAcAd genes present in plasmid pMRM1 were isolated by digestion with NotI and introduced into the unique NotI site of pUT mini-Tn5 Hgr (6). pUT-MRM2 was constructed in two steps. First, a 1.2-kb EcoRI-XhoI fragment from pMRM2 containing the dntD gene was ligated with EcoRI-XhoI-digested vector pGEMEX-1, producing pMRM4. Then the dntB gene (2.2 kb) obtained by ApaI digestion of plasmid pJS53 (Table 1) was cloned into an ApaI restriction site of pMRM4, generating pMRM5. Subsequently, a 3.4-kb NotI fragment containing the dntB and dntD genes from pMRM5 was cloned into a NotI restriction site of pUT mini-Tn5 Kmr (6) to obtain pUT-MRM2. To construct pUT-MRM3, a 4.7-kb NotI fragment including the lower pathway operon was obtained from pMRM3 and introduced into the NotI restriction site of pUT mini-Tn5 Gmr (32).
pUT-MRM vector conjugation assays.
Transfer of each pUT-MRM plasmid to P. fluorescens WT was performed with the donor E. coli S17-1 strain by biparental conjugation using the filter mating method (6). P. fluorescens pUT-MRM1, pUT-MRM2, and pUT-MRM3 transconjugants were selected on M9 minimal salt (23) agar plates containing 1.25 μg/ml mercuric chloride, 150 μg/ml kanamycin, and 5 μg/ml gentamicin, respectively. In the first step, we transferred dntAbAcAd and dntB-D by successive pUT-MRM1 and pUT-MRM2 conjugation events. The P. fluorescens recombinant strain was able to transform 2,4-DNT, but it accumulated the DntD product DMOHA. In order to obtain complete degradative capability, this strain was then used as a recipient for transfer of the dnt lower operon (pUT-MRM3). Chromosomal integration of dnt genes was confirmed by Southern blotting and PCR amplification, and the DntA, DntB, and DntD enzyme activities were confirmed by the microplate activity assay (27). To confirm authentic transposition of the mobile elements, the transconjugants were tested for sensitivity to piperacillin (6).
Analytical methods.
2,4-DNT and the degradation products were analyzed by reversed-phase high-performance liquid chromatography. Cells were removed from the culture medium by centrifugation, and the supernatant fluid was injected into an Allsphere ODS-1 column (5 μm; 250 by 4.6 mm; Alltech) with 13.5 mM trifluoroacetic acid-acetonitrile (50:50) as the mobile phase. To analyze 2,4-DNT and 4M5NC, the flow rate was 1.0 ml/min, and the compounds were detected by UV absorbance at 254 nm with a Spectra-Physics variable-wavelength detector (Westshore Technologies). To analyze 2H5MQ, 2,4,5-THT and DMOHA, the flow rate was 0.5 ml/min; these compounds were detected at 268 nm.
The nitrite concentration in culture fluid was analyzed spectrophotometrically using Griess reagents (Britania, Buenos Aries, Argentina). Briefly, cell-free culture medium (200 μl) was mixed with each Griess reagent (100 μl), and the absorbance at 550 nm was determined after incubation in the dark for 15 min.
Evaluation of 2,4-DNT toxicity for plant growth in the presence of bacteria.
Arabidopsis thaliana (Col-0 ecotype) and Nicotiana tabacum cv. petit havana seeds were surface sterilized by soaking them in 2.5% (vol/vol) sodium hypochloride, rinsed four times with sterilized water, and vernalized at 4°C for 24 h on 0.1% phytoagar (33). To analyze the effect of bacteria on in vitro germination, plates of Gamborg's medium (33) with or without 2,4-DNT (200 μM) were inoculated with 2 × 108 CFU/ml of P. fluorescens WT, P. fluorescens RE, or Burkholderia sp. strain DNT (previously grown in BN medium with 0.1% yeast extract and 0.2% succinate) and incubated at 28°C for 2 h. Vernalized seeds were transferred to these plates, incubated at 22°C, and maintained in plant growth chambers with a photoperiod consisting of 9 h of light and 15 h of darkness. To analyze the effect of bacteria on in vitro A. thaliana plant growth, 2.5 × 107 CFU/ml of Burkholderia sp. strain DNT, Pseudomonas syringae pv. tomato, or P.fluorescens RE (previously grown in LB media and washed twice with distilled water) was added to Murashige and Skoog basal medium plates containing 15-day-old plants grown in vitro (1, 33).
For soil assays, flasks containing nonsterile planting mixture (ratio of soil to vermiculite, 1:1 [wt/wt]) were supplemented with sterile double-distilled water or 2,4-DNT (final concentration, 500 μM) and shaken at 200 rpm for 20 min. Bacterial cultures (P. fluorescens WT, P. fluorescens RE, and Burkholderia sp. strain DNT) were added at a final concentration of 2 × 108 CFU/ml, and the flasks were shaken at 28°C for an additional 20 min. Then the planting mixtures were placed in plastic pots and allowed to stand overnight. Sterile vernalized A.thaliana seeds were sown (45 to 50 seeds/pot), and the pots were incubated at 22°C with cycles consisting of 14 h of light and 10 h of darkness. Alternatively, seeds were coated with bacteria by soaking them in a bacterial suspension (5 × 109 CFU/ml) for 20 h at 28°C. The seeds were then sown on soil to which bacteria were not added and which contained or did not contain 2,4-DNT (500 μM), and the pots were maintained under the conditions described above. Under these conditions germination started 5 days after sowing. Phenotypes were scored daily. The assay was performed in duplicate.
RESULTS
Construction of P. fluorescens strain MP by conjugative transfer of megaplasmid pJS1.
As a first approach, we carried out conjugative transfer of the pJS1 megaplasmid, which contained the genes encoding the 2,4-DNT degradation enzymes, from Burkholderia sp. strain DNT to P. fluorescens WT. A selected P. fluorescens transconjugant, designated P. fluorescens MP, acquired the ability to degrade 2,4-DNT and to grow on 2,4-DNT as the sole source of carbon, nitrogen, and energy to an extent similar to that of Burkholderia sp. strain DNT (see Fig. S1 in the supplemental material). Then we compared the stability of the 2,4-DNT degradative phenotype of P. fluorescens MP with the stability of the 2,4-DNT degradative phenotype of Burkholderia sp. strain DNT strain by examination of the pJS1-encoded DntA and DntB activities. To do this, serial dilutions (1/200) in LB liquid medium without 2,4-DNT were made every 48 h, and colonies were isolated from the batch cultures to examine both enzyme activities. After 18 days of consecutive growth cycles, 90% of the P. fluorescens MP cells had lost the DntA+ DntB+ phenotype and no Burkholderia sp. strain DNT cells with DntA and DntB activities were detected (not shown).
Construction of the recombinant P. fluorescens strain RE by chromosomal insertion of dnt genes.
Subsequently, we ascertained whether chromosomal integration of dnt genes into P.fluorescens WT was sufficient to completely degrade 2,4-DNT and led to a stable degradation capacity. To do this, we constructed three mini-Tn5 transposon delivery vectors, pUT-MRM1, pUT-MRM2, and pUT-MRM3, to perform chromosomal insertion of the structural genes encoding the enzymes described for the 2,4-DNT pathway from Burkholderia sp. strain DNT and B. cepacia R34 (dntAbAcAd, dntB, and the lower pathway operon) (Fig. 1B). It is important to point out that the nondegrading P. fluorescens WT strain catalyzed the conversion of 2H5MQ to 2,4,5-THT, indicating that it had a nonspecific 2H5MQ reductase activity (DntC).
In this way, a recombinant strain, designated P. fluorescens RE, was developed by successive dnt gene transposition events. After this, we analyzed the effect of dnt gene integration on P.fluorescens RE metabolism by comparing the growth rate of this strain with that of the P. fluorescens WT strain in different media, such as LB medium and BN medium supplemented with succinate, valine, or pyruvate. No differences in the growth rate were observed in any of the media analyzed (not shown), suggesting that no major metabolic changes were caused by chromosomal insertion of the dnt modules.
Characterization of the 2,4-DNT-degrading ability of P. fluorescens RE.
The P. fluorescens RE recombinant strain was then investigated with respect to the 2,4-DNT degradative phenotype. In 2,4-DNT degradation assays, in which cells from stationary-phase cultures were used, net disappearance of 2,4-DNT was observed (not shown). Moreover, the intermediates 4M5NC, 2H5MQ, and 2,4,5-THT were not detected, and only a small amount of DMOHA was found (≤3% of the initial 2,4-DNT concentration), indicating that there was expression of the complete 2,4-DNT degradation pathway in P. fluorescens RE, which was clearly sufficient to completely degrade 2,4-DNT in this heterologous system.
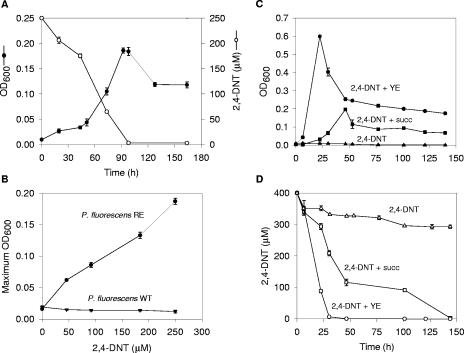
Then we asked whether P. fluorescens RE was capable of growing with 2,4-DNT as the sole source of nitrogen (Fig. 2A). Following batch culture incubation in nitrogen-free medium supplemented with 2,4-DNT and succinate, we observed that 2,4-DNT degradation was accompanied by concomitant growth on the nitroaromatic compound as the nitrogen source (Fig. 2A). No nitrite accumulation was detected under these conditions (not shown). In addition, in an equivalent nitrogen-free medium with a fixed succinate concentration, clear increases in growth were observed with increases in the 2,4-DNT concentration up to 250 μM (Fig. 2B), but no additional increases in growth were observed at concentrations above 250 μM, probably due to an inhibitory effect of 2,4-DNT or to limiting carbon source availability under these conditions. In contrast, the P. fluorescens WT strain was not able to grow under any of these conditions (Fig. 2B).
FIG. 2.
Growth and 2,4-DNT degradation properties of P. fluorescens RE. (A) Optical density at 600 nm (OD600) (•) and 2,4-DNT concentration (○) in a culture of P. fluorescens RE containing nitrogen-free Bruhn medium supplemented with 0.1% succinate and 250 μM 2,4-DNT. (B) Maximum optical densities at 600 nm reached by the P. fluorescens RE (•) and P. fluorescens WT (▾) strains in cultures containing Bruhn medium supplemented with 0.1% succinate and different initial concentrations of 2,4-DNT. (C and D) Optical densities at 600 nm (C) and concentrations of 2,4-DNT (D) in cultures of P. fluorescens RE containing BN medium with 400 μM 2,4-DNT (▵) supplemented with 0.1% succinate (succ) (□) or 0.1% yeast extract (YE) (○). The symbols indicate averages of triplicate cultures, and the error bars indicate standard deviations.
Interestingly, although P. fluorescens RE was able to completely degrade 2,4-DNT and utilize this compound as the sole source of nitrogen, no growth on 2,4-DNT as a sole carbon and energy source could be detected (Fig. 2C). The inability of this microorganism to grow on 2,4-DNT may not be attributed to disruption of endogenous genes involved in the catabolism of the DntG and DntE products since this recombinant strain grew on pyruvate and valine at the same levels as the parental strain (valine metabolism generates propionyl-CoA, the DntE reaction product). Also, other transconjugants that had dnt lower pathway gene insertions at different positions on the chromosome were not able to utilize 2,4-DNT as a carbon growth substrate, so we concluded that there was not a dnt lower operon chromosomal insertion effect (not shown). There are at least two possible reasons that could explain the lack of growth of P. fluorescens RE in the presence of 2,4-DNT as a sole carbon source: chromosomal insertion of dnt modules may decrease the level of expression of the genes required to carry out efficient degradation (18, 24), or an extra plasmid-encoded factor could be required.
When tested in the presence of different cosubstrates, P.fluorescens RE was capable of growing (Fig. 2C) and degrading the nitroaromatic compound more efficiently (Fig. 2D). This phenomenon was observed with succinate (Fig. 2C and D), as well as with other simple carbon sources, such as glucose, pyruvate, and glutamate (not shown). Moreover, notably improved degradation was obtained when a complex carbon and nitrogen source, such as yeast extract (Fig. 2D) or soybean peptone, was added (not shown). Yeast extract was the most efficient cosubstrate on a g/liter basis, indicating that there was a correlation between cosubstrate complexity and the velocity of 2,4-DNT degradation.
Stability of 2,4-DNT degradative phenotype of P. fluorescens RE.
In addition, the 2,4-DNT-degrading ability acquired after chromosomal insertion of the dnt genes was analyzed by long-term incubation in LB medium without 2,4-DNT and selective markers. All P. fluorescens RE cells exhibited the chromosomally encoded DntA and DntB activities, as well as resistance to the markers included in minitransposons, for more than 18 days (not shown). These results indicate that the 2,4-DNT degradative phenotype in P. fluorescens RE is very stable, an advantage over the unstable plasmid-borne 2,4-DNT-degrading microorganisms P. fluorescens MP and Burkholderia sp. strain DNT.
2,4-DNT degradative abilities of P. fluorescens RE and Burkholderia sp. strain DNT at low temperatures.
One limitation of the use of bacterial strains as degradative vehicles for in situ bioremediation processes is the reduced metabolic activity at low temperatures. In order to investigate whether P. fluorescens RE exhibits cold resistance similar to that of strain WT, we monitored the development of colonies on LB agar plates that were incubated for 8 days at a wide range of temperatures. We observed that the optimal growth temperature of P. fluorescens strain RE was 28°C and that this strain clearly exhibited cold resistance since it was capable of growing at temperatures as low as 5°C (see Fig. S2 in the supplemental material). It should be remarked that the growth of P. fluorescens strain RE at 37°C was totally inhibited and that no colony growth was observed when plates maintained at 37°C were transferred to 28°C, indicating that the exposure to 37°C killed the P. fluorescens RE cells. In contrast, the optimal growth temperature of Burkholderia sp. strain DNT was 37°C, and development of colonies was drastically inhibited at temperatures lower than 16°C (see Fig. S2 in the supplemental material). No Burkholderia sp. strain DNT colony growth was observed after incubation at 37°C of plates that were previously maintained for 8 days at 5°C, showing the susceptibility of this bacterium to low temperatures under the conditions tested.
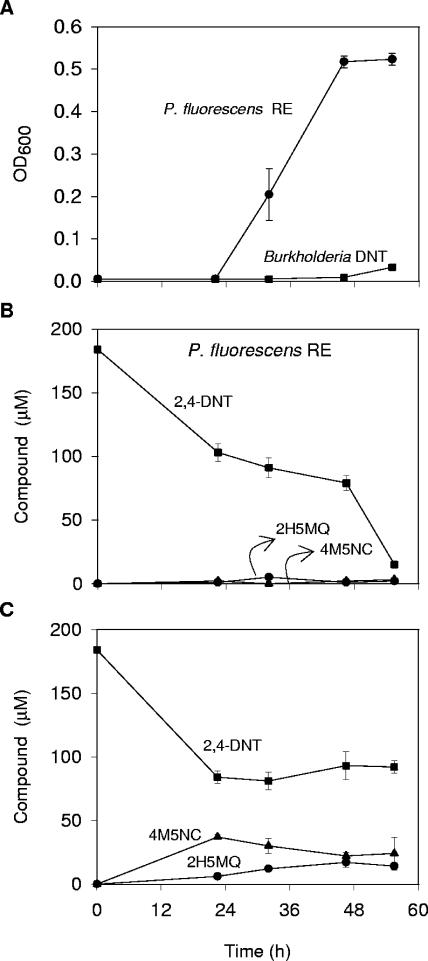
Next, we tested the 2,4-DNT degradative abilities of P. fluorescens RE and Burkholderia sp. strain DNT at 10°C in liquid cultures containing 2,4-DNT. As shown in Fig. 3A and B, P.fluorescens RE not only was capable of growing at this temperature but was also able to progressively degrade 2,4-DNT during incubation (90% of the initial 2,4-DNT concentration) with no accumulation of intermediates. In contrast, Burkholderia sp. strain DNT growth was drastically reduced at this temperature (Fig. 3A), and there was a decrease in its degradation performance (Fig. 3C). During the initial 20 h of incubation, Burkholderia sp. strain DNT-mediated degradation might have been due to the inoculated cells, which consumed approximately 50% of the 2,4-DNT (Fig. 3C). However, after this time Burkholderia sp. strain DNT lost the capacity to degrade 2,4-DNT, and there was a concomitant accumulation of intermediates. After 56 h of incubation, 92 μM 2,4-DNT, 24 μM 4M5NC, and 15 μM 2H5MQ remained in the medium (Fig. 3C). Considering the initial concentration of 2,4-DNT (185 μM), only 30% of the 2,4-DNT was mineralized. These results demonstrate that at low temperatures 2,4-DNT degradation by P. fluorescens RE is greater than 2,4-DNT degradation by Burkholderia sp. strain DNT.
FIG. 3.
Growth of and 2,4-DNT degradation by P. fluorescens RE and Burkholderia sp. strain DNT at 10°C. (A) Optical densities at 600 nm (OD600) of cultures of P. fluorescens RE (•) and Burkholderia sp. strain DNT (▪) in nitrogen-free Bruhn medium supplemented with 0.1% yeast extract and 185 μM 2,4-DNT incubated at 10°C. (B and C) Concentrations of 2,4-DNT (▪) and the degradation intermediates 4M5NC (▴) and 2H5MQ (•) in cultures of P. fluorescens RE (B) and Burkholderia sp. strain DNT (C). The symbols indicate averages of triplicate cultures, and the error bars indicate standard deviations.
P. fluorescens RE-mediated relief of 2,4-DNT toxicity for plant growth.
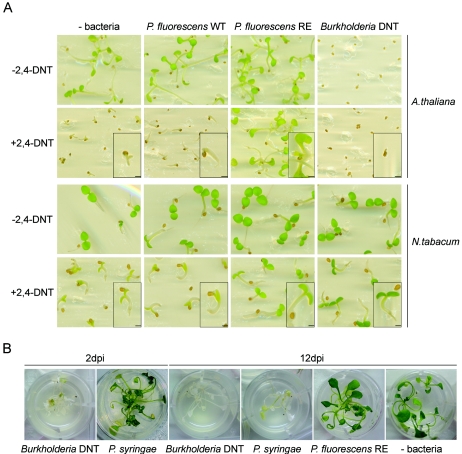
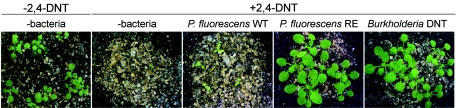
Since environments in which 2,4-DNT accumulates are toxic for plant development (8, 9), we evaluated whether inoculation of contaminated media with P. fluorescens RE alters the 2,4-DNT toxicity for plant growth. We first monitored the effects on early seedling development by analyzing A. thaliana and N. tabacum plants cultured in vitro. Seed germination and cotyledon expansion were drastically inhibited by 200 μM 2,4-DNT (Fig. 4A). On the other hand, these processes were not altered by inoculation of uncontaminated media with P. fluorescens strains (Fig. 4A). P. fluorescens RE, but not the parental P. fluorescens WT strain, was able to counteract 2,4-DNT toxicity, rescuing normal early plant development (Fig. 4A). As expected, Burkholderia sp. strain DNT also counteracted 2,4-DNT toxicity. However, this effect was observed only for N. tabacum since the bacterium drastically inhibited A.thaliana seed germination (Fig. 4A) and caused rapid chlorosis and plant mortality upon inoculation of A. thaliana plant roots that were comparable to the chlorosis and plant mortality caused by the pathogenic organism P. syringae pv. tomato (1) (Fig. 4B).
FIG. 4.
Relief of 2,4-DNT toxicity for A. thaliana and N. tabacum development in vitro by P. fluorescens RE. (A) Early stages of seedling growth in Gamborg’s medium supplemented (+2,4-DNT) or not supplemented (−2,4-DNT) with 200 μM 2,4-DNT. Media were either not treated with bacteria (−bacteria) or supplemented with the following strains (2 × 108 CFU/ml): P. fluorescens WT, P. fluorescens RE, and Burkholderia sp. strain DNT. Images were taken 10 days after seeds were plated. (Insets) Enlargements of representative seeds or seedlings. Bars = 1 mm. (B) Effects of inoculation of Burkholderia sp. strain DNT onto A. thaliana plants. Fifteen-day-old plants grown in solid medium were incubated with 2.5 × 107 CFU/ml of Burkholderia sp. strain DNT, P. syringae pv. tomato (a pathovar that causes disease and mortality of A. thaliana plants in vitro), and P. fluorescens RE. Images were taken 2 and 12 days after bacterial inoculation (dpi).
We next assessed whether P. fluorescens RE ameliorates 2,4-DNT toxicity in soil. Nonsterile soils were either treated or not treated with 2,4-DNT (500 μM) and then maintained without additional treatment or inoculated with P. fluorescens RE, P. fluorescens WT, or Burkholderia sp. strain DNT. Vernalized A. thaliana seeds (45 to 50 seeds per assay) were sown on these soils, and plant growth was recorded for 30 days. The toxicity of 2,4-DNT was shown by reductions in the level of seed germination at 5 days after sowing (Table 2). Once again, inoculation of P. fluorescens RE into the contaminated soil counteracted 2,4-DNT toxicity, rescuing normal germination and seedling development (Table 2 and Fig. 5). Burkholderia sp. strain DNT had a similar protective effect (Table 2 and Fig. 5),and in this natural environment it neither hindered A.thaliana seed germination nor inhibited plant development, as observed in synthetic media (Fig. 4). Although inoculation of P. fluorescens WT, which lacked the 2,4-DNT-detoxifying genes, increased the percentage of surviving seedlings by 14 days after sowing (Table 2), confirming that it is a plant growth-promoting bacterium, the seedlings displayed reduced growth and slight chlorosis and they did not develop further for the following 30 days (Fig. 5). Together, these results indicated that P. fluorescens RE, like Burkholderia sp. strain DNT, had long-lasting protective effects on plant growth in 2,4-DNT-contaminated soil.
TABLE 2.
2,4-DNT toxicity for A. thaliana seedlings
| 2,4-DNT | Strain | Seedling survival (%)c
|
|||
|---|---|---|---|---|---|
| Inoculated soila
|
Inoculated seedsb
|
||||
| 5 days after sowing | 14 days after sowing | 5 days after sowing | 14 days after sowing | ||
| − | 52.0 ± 4.0 | 50.0 ± 6.0 | 65.0 ± 17.0 | 65.0 ± 11.0 | |
| + | 31.0 ± 1.0 | 8.0 ± 2.0 | 35.0 ± 3.0 | 11.0 ± 5.0 | |
| + | P. fluorescens WT | 38.0 ± 8.0 | 47.0 ± 13.0 | 26.0 ± 6.0 | 4.0 ± 2.0 |
| + | P. fluorescens RE | 56.0 ± 8.0 | 60.0 ± 6.0 | 20.0 ± 2.0 | 42.0 ± 10.0 |
| + | Burkholderia sp. strain DNT | 59.0 ± 3.0 | 61.0 ± 1.0 | 36.0 ± 12.0 | 50.0 ± 10.0 |
Forty-five to 50 A. thaliana seeds were sown on planting mixture containing or not containing 2,4-DNT (500 μM) and P. fluorescens WT, P. fluorescens RE, or Burkholderia sp. strain DNT.
Forty-five to 50 A. thaliana seeds were coated or not coated with one of the strains and sown on bacterium-free planting mixture containing or not containing 2,4-DNT (500 μM).
The number of surviving plants was determined 5 or 14 days after sowing. The values are percentages based on the number of seeds sown.
FIG. 5.
Relief of 2,4-DNT toxicity for A. thaliana growth in soil by P. fluorescens RE. Soils were either supplemented (+2,4-DNT) or not supplemented (−2,4-DNT) with 500 μM 2,4-DNT and either not treated with bacteria (−bacteria) or supplemented with P. fluorescens WT, P.fluorescens RE, or Burkholderia sp. strain DNT (2 × 108 CFU/ml). A. thaliana vernalized seeds were transferred to soil, and plant growth was recorded for 1 month. Images were taken 30 days after sowing. Several plants were randomly removed to facilitate evaluation of the remaining plants.
Interestingly, similar protective results were obtained when seeds were just coated with P. fluorescens RE or Burkholderia sp. strain DNT and then sown on soils that were not amended with bacteria and treated with 500 μM 2,4-DNT (Table 2). In this case, the plant size was slightly reduced compared to the size in a previous assay (not shown). However, this experiment clearly indicated that P. fluorescens RE effectively counteracted 2,4-DNT plant toxicity in such conditions.
DISCUSSION
In the present investigation we analyzed the potential use of the P. fluorescens ATCC 17400 plant growth-promoting rhizobacterial strain to degrade 2,4-DNT by transferring the dnt genes. By conjugative transfer of pJS1 we confirmed that all factors required for complete degradation of 2,4-DNT and growth on 2,4-DNT as the sole source of carbon, nitrogen, and energy reside on the megaplasmid. However, the acquired 2,4-DNT degradative phenotype of pJS1-borne P. fluorescens MP was clearly lost in the absence of 2,4-DNT. Then, by utilizing a suicide chromosomal integration system, we transferred the dntAbAcAd, dntB, and dnt lower operon genes to create P. fluorescens RE, which showed long-term stability of the 2,4-DNT degradative phenotype in 2,4-DNT-free media. This achievement established for the first time that there can be intergeneric transfer and stable inheritance of the 2,4-DNT-degrading capability by chromosomal integration of the genes encoding the 2,4-DNT catabolic pathway, overcoming the limitation associated with degradation ineffectiveness due to plasmid instability (29). Furthermore, it is important to point out that the use of mini-Tn5 transposon vectors minimizes the possibility of horizontal genetic transfer to other indigenous bacteria found in the environment and therefore significantly reduces the ecological risk (7).
P. fluorescens RE has several advantages over other bacterial systems. First, it maintains the psychrotolerant property of P.fluorescens WT since it can grow at temperatures as low as 5°C. Furthermore, P. fluorescens RE was capable of efficiently degrading 2,4-DNT at 10°C, indicating that the 2,4-DNT-degrading enzymes remain active at this temperature. In contrast, Burkholderia sp. strain DNT exhibited cold sensitivity, and it was unable to sustain a complete degradation process at low temperatures. In this bacterium there was accumulation of toxic 2,4-DNT intermediates that probably had a poisonous effect. In this sense, the advantageous cold resistance of P.fluorescens RE reflects the environmental robustness of this strain, indicating its potential for use as a vehicle for in situ bioremediation processes.
Second, we describe here the capacity of the P. fluorescens RE strain to overcome 2,4-DNT toxicity for plant growth. This protective effect was shown in synthetic medium in which P.fluorescens RE was the only microorganism present, indicating that this strain biodegrades 2,4-DNT in such conditions. In addition, a single inoculation with P. fluorescens RE in nonsterile soils was sufficient to rescue normal plant growth for at least 1 month. A similar effect was observed for the genetically unmodified organism Burkholderia sp. strain DNT. These results led us to assume that in this environment the bacteria survive, that sufficient numbers are maintained, and that in situ expression of dnt genes takes place in both bacterium-plant-soil microcosms. It is important to point out that addition of cosubstrates was not required for 2,4-DNT detoxification in soil, as this process occurs in vitro. However, it is expected that plant exudates could provide a carbon source for the bacteria to grow, allowing proliferation and stimulating the metabolic activity (20). In addition, P. fluorescens RE might compete successfully with other microorganisms for the exudate nutrients and efficiently colonize the plant root. Interestingly, soaking seeds with P. fluorescens RE resulted in relief of 2,4-DNT plant toxicity. This implies that such a seed treatment may allow delivery of the bacterium to the seedling rhizosphere, which in turn could be a successful strategy in a potential rhizoremediation approach. Although further experiments are necessary to analyze these potential properties of the recombinant strain, it is known that Pseudomonas spp. are the most abundant rhizosphere species (20), and several successful rhizoremediation experiments have been carried out with P. fluorescens strains to clean up soils polluted with trichloroethylene and polychlorinated biphenyls (2, 34).
Finally, P. fluorescens RE was found to be harmless for the plant species tested here, A. thaliana and N. tabacum, whereas Burkholderia sp. strain DNT was toxic for A. thaliana development in vitro. In the case of Burkholderia sp. strain DNT and A. thaliana, the bacterium eliminated seed germination and was lethal to the plants when it was inoculated into roots of young plants, and it was more aggressive than the virulent pathogen P. syringae pv. tomato DC3000. The basis of Burkholderia sp. strain DNT toxicity for in vitro A. thaliana development remains to be determined, as do the reasons that this bacterium does not affect plant growth in soil. However, it has been proposed that B. cepacia is a phytopathogen that causes bacterial rot of onions (4). In addition, it is important to be cautious with Burkholderia species since several studies have indicated that virtually all of them can be opportunistic human pathogens in immunocompromised patients and can occasionally infect healthy individuals (5, 21, 30). One important advantage is the fact that P. fluorescens RE growth is drastically reduced at 37°C, which reduces the possibility that it can infect humans or warm-blooded animals.
In conclusion, our results provide evidence that the use of a harmless and psychrotolerant rhizobacterial host for the transfer of 2,4-DNT degradative ability is an interesting approach for generation of biological vehicles for cleaning environments contaminated with this priority pollutant.
Supplementary Material
Acknowledgments
We are grateful to Jim Spain, Glenn R. Johnson, and Shirley Nishino for providing Burkholderia sp. strain DNT, 4M5NC, 2,4,5-THT, and the plasmids containing the dnt genes used for P. fluorescens recombinant strain construction. We thank Bernardo González for technical assistance with the megaplasmid transfer and Raquel Kremer for allowing us to use the high-performance liquid chromatography equipment. We thank Susana Genti for valuable discussions and comments on the manuscript.
This work was supported in part by grants from the Secretaría de Ciencia y Técnica (Universidad Nacional de Córdoba), the Agencia Córdoba Ciencia, the Agencia Nacional de Promoción Científica y Técnica, and Fundación Antorchas.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bais, H. P., B. Prithiviraj, A. K. Jha, F. M. Ausubel, and J. M. Vivanco. 2005. Mediation of pathogen resistance by exudation of antimicrobials from roots. Nature 434:217-2121. [DOI] [PubMed] [Google Scholar]
- 2.Brazil, G. M., L. Kenefick, M. Callanan, A. Haro, V. de Lorenzo, D. N. Dowling, and F. O'Gara. 1995. Construction of a rhizosphere pseudomonad with potential to degrade polychlorinated biphenyls and detection of bph gene expression in the rhizosphere. Appl. Environ. Microbiol. 61:1946-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruhn, C., H. Lenke, and H.-J. Knackmuss. 1987. Nitrosubstituted aromatic compounds as nitrogen sources for bacteria. Appl. Environ. Microbiol. 53:208-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkholder, W. 1950. Sour skin, a bacterial rot of onions bulbs. Phytopathology 40:115-118. [Google Scholar]
- 5.Coenye, T., and P. Vandamme. 2003. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 5:719-729. [DOI] [PubMed] [Google Scholar]
- 6.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived mini-transposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 7.de Lorenzo, V., M. Herrero, J. M. Sánchez, and K. N. Timmis. 1998. Mini-transposons in microbial ecology and environmental biotechnology. FEMS Microbiol. Ecol. 27:211-224. [Google Scholar]
- 8.Dodard, S. G., A. Y. Renoux, J. Hawari, G. Ampleman, S. Thiboutot, and G. I. Sunahara. 1999. Ecotoxicity characterization of dinitrotoluenes and some of their reduced metabolites. Chemosphere 38:2071-2079. [DOI] [PubMed] [Google Scholar]
- 9.Dutta, S. K., G. P. Hollowell, F. M. Hashem, and L. D. Kuykendall. 2003. Enhanced bioremediation of soil containing 2,4-dinitrotoluene by a genetically modified Sinorhizobium meliloti. Soil Biol. Biochem. 35:667-675. [Google Scholar]
- 10.Gaballa, A., P. D. Abeysinghe, G. Urich, S. Matthijs, H. De Greve, P. Cornelis, and N. Koedam. 1997. Trehalose induces antagonism towards Pythium debaryanum in Pseudomonas fluorescens ATCC 17400. Appl. Environ. Microbiol. 63:4340-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong, P., R. G. Kuperman, and G. I. Sunahara. 2003. Genotoxicity of 2,4- and 2,6-dinitrotoluene as measured by the Tradescantia micronucleus (Trad-MCN) bioassay. Mutat. Res. 538:13-18. [DOI] [PubMed] [Google Scholar]
- 12.Haigler, B. E., W.-C. Suen, and J. C. Spain. 1996. Purification and sequence analysis of 4-methyl-5-nitrocatechol oxygenase from Burkholderia sp. strain DNT. J. Bacteriol. 178:6019-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haigler, B. E., G. R. Johnson, W.-C. Suen, and J. C. Spain. 1999. Biochemical and genetic evidence for meta-ring cleavage of 2,4,5-trihydroxytoluene in Burkholderia sp. strain DNT. J. Bacteriol. 181:965-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Agency for Research on Cancer. 1996. 2,4-Dinitrotoluene, 2,6-dinitrotoluene and 3,5-dinitrotoluene. IARC (Int. Agency Res. Cancer) Monogr. Eval. Carcinog. Risks Hum. 65:309-368. [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, G. R., R. K. Jain, and J. C. Spain. 2000. Properties of the trihydroxytoluene oxygenase from Burkholderia cepacia R34: an extradiol dioxygenase from the 2,4-dinitrotoluene pathway. Arch. Microbiol. 173:86-90. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, G. R., R. K. Jain, and J. C. Spain. 2002. Origins of the 2,4-dinitrotoluene pathway. J. Bacteriol. 184:4219-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keith, L. H., and W. A. Telliard. 1979. Priority pollutants. I. A perspective view. Environ. Sci. Technol. 13:416-423. [Google Scholar]
- 18.Klemba, M., B. Jackobs, R.-M. Wittich, and D. Pieper. 2000. Chromosomal integration of tcb chlorocatechol degradation pathway genes as a means of expanding the growth substrate range of bacteria to include haloaromatics. Appl. Environ. Microbiol. 66:3255-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kröckel, L., and D. D. Focht. 1987. Construction of chlorobenzene-utilizing recombinants by progenitive manifestation of a rare event. Appl. Environ. Microbiol. 53:2470-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuiper, I., E. L. Lagendijk, G. V. Bloemberg, and B. J. J. Lugtenberg. 2004. Rhizoremediation: a beneficial plant-microbe interaction. Mol. Plant-Microbe Interact. 17:6-15. [DOI] [PubMed] [Google Scholar]
- 21.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144-156. [DOI] [PubMed] [Google Scholar]
- 22.Nishino, S. F., G. C. Paoli, and J. C. Spain. 2000. Aerobic degradation of dinitrotoluenes and pathway for bacterial degradation of 2,6-dinitrotoluene. Appl. Environ. Microbiol. 66:2139-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Sousa, C., V. de Lorenzo, and A. Cebolla. 1997. Modulation of gene expression through chromosomal positioning in Escherichia coli. Microbiology 143:2071-2078. [DOI] [PubMed] [Google Scholar]
- 25.Spanggord, R. J., J. C. Spain, S. F. Nishino, and K. E. Mortelmans. 1991. Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl. Environ. Microbiol. 57:3200-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanier, R. Y., N. J. Palleroni, and M. Doudoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 27.Suen, W.-C., and J. C. Spain. 1993. Cloning and characterization of Pseudomonas sp. strain DNT genes for 2,4-dinitrotoluene degradation. J. Bacteriol. 175:1831-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suen, W.-C., B. E. Haigler, and J. C. Spain. 1996. 2,4-Dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J. Bacteriol. 178:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmis, K. N., and D. H. Pieper. 1999. Bacteria designed for bioremediation. Trends Biotechnol. 17:201-204. [DOI] [PubMed] [Google Scholar]
- 30.Valvano, M. A., K. E. Keith, and S. T. Cardona. 2005. Survival and persistence of opportunistic Burkholderia species in host cells. Curr. Opin. Microbiol. 8:99-105. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, U. F., J. P. Morrissey, and F. O'Gara. 2001. Pseudomonas for biocontrol of phytopathogens: from functional genomics to commercial exploitation. Curr. Opin. Biotechnol. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 32.Whiteley, M., M. R. Parsek, and E. P. Greenberg. 2000. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 182:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiegel, D., and J. Glazebrook. 2002. Arabidopsis: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Yee, D. C., J. A. Maynard, and T. K. Wood. 1998. Rhizoremediation of trichloroethylene by a recombinant, root-colonizing Pseudomonas fluorescens strain expressing toluene ortho-monooxygenase constitutively. Appl. Environ. Microbiol. 64:112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.