Abstract
A small-oligonucleotide microarray prototype was designed with probes specific for the universal 16S rRNA and cpn60 genes of several pathogens that are usually encountered in wastewaters. In addition to these two targets, wecE-specific oligonucleotide probes were included in the microarray to enhance its discriminating power within the Enterobacteriaceae family. Universal PCR primers were used to amplify variable regions of 16S rRNA, cpn60, and wecE genes directly in Escherichia coli and Salmonella enterica serovar Typhimurium genomic DNA mixtures (binary); E. coli, S. enterica serovar Typhimurium, and Yersinia enterocolitica genomic DNA mixtures (ternary); or wastewater total DNA. Amplified products were fluorescently labeled and hybridized on the prototype chip. The detection sensitivity for S. enterica serovar Typhimurium was estimated to be on the order of 0.1% (104 S. enterica genomes) of the total DNA for the combination of PCR followed by microarray hybridization. The sensitivity of the prototype could be increased by hybridizing amplicons generated by PCR targeting genes specific for a bacterial subgroup, such as wecE genes, instead of universal taxonomic amplicons. However, there was evidence of PCR bias affecting the detection limits of a given pathogen as increasing amounts of a different pathogen were spiked into the test samples. These results demonstrate the feasibility of using DNA microarrays in the detection of waterborne pathogens within mixed populations but also raise the problem of PCR bias in such experiments.
Microbial water quality assessment and the rapid detection of pathogenic microorganisms remain great challenges in ecotoxicology, public health maintenance, and, more recently, bioterrorism prevention (25). The main origin of human waterborne pathogens is the guts of humans and animals. Water contamination events often result from discharges from wastewater treatment facilities, overflowing sanitary sewer systems, waste materials that find their way into domestic and industrial sewage, and runoff of animal fecal matters during storm events (21). Bacteria within contaminated water represent a highly diversified group. Numerous Salmonella spp., Escherichia spp., Shigella spp., Yersinia spp., and Klebsiella spp. within the Enterobacteriaceae are human waterborne pathogens found in domestic wastewater. In particular, Escherichia coli strains constitute an important set of waterborne pathogens composed of numerous Shiga toxin-producing strains and enterohemorrhagic (e.g., O157: H7) strains associated with potable (14, 32) and recreational (7, 22) water.
Several nucleic acid-based methods have been developed for the rapid detection of pathogens in food, soil, and water with high degrees of sensitivity and specificity and without the need for complex cultivation (3, 4, 9, 24, 40). In general, these methods allow detection within hours, rather than days as is normally required by culture techniques. Due to its high sensitivity and specificity, PCR is the most commonly employed molecular tool (16). A major limitation to this approach is the utilization of one specific primer pair per gene detection reaction. Although multiple primer sets may be successfully combined in one reaction, they rarely exceed more than six primer sets due to the generation of nonspecific products or false negatives. Another difficulty with multiplex PCR is that it requires additional postamplification analysis to discriminate the products. Size separation by electrophoresis is frequently used to discriminate multiplex PCR products, but this requires additional labor and that the amplicons of each reaction be significantly different in size, which can limit the primer pairs that can potentially be multiplexed. Consequently, general pathogen detection by PCR can be both labor-intensive and costly.
Microarrays represent an important advance in molecular detection technology, allowing the simultaneous detection of specifically labeled DNAs from many different pathogenic organisms on a small glass slide containing thousands of surface-immobilized DNA probes. Both basic types of microarrays, i.e., immobilized oligonucleotide probes and PCR amplicons, have been used successfully to detect (40) and/or characterize (5) pathogens. As the sensitivity of microarrays hybridized with total genomic DNA from complex mixtures is usually inadequate to provide detection of low pathogen concentrations (30), the hybridized DNA (target) usually consists of PCR amplicons (10, 40). This mode of pathogen detection necessitates the combination of many PCRs prior to their hybridization on microarrays. Wilson et al. (40) used 140 amplicons to characterize 18 pathogenic species, thus constraining the use of microarrays for routine detection of pathogens in wastewater. Target DNA amplification with universal primers to ubiquitous genes prior to microarray hybridization can circumvent this limitation (12, 23, 28, 36). The cpn60 gene codes for GroEL, an essential, highly conserved chaperonin protein which displays moderate DNA sequence diversity, making this gene useful in bacterial taxonomy applications (8, 17, 20). However, within the Enterobacteriaceae, 16S rRNA and cpn60 sequences may share sufficient similarity to generate cross-hybridization reactions, even when short oligonucleotides are used as probes. As the majority of water pathogens belong to this family, discrimination on the basis of 16S rRNA and cpn60 sequences is challenging. However, sequence diversity within the wecE gene, an Enterobacteriaceae-specific gene which forms part of the wec gene cluster involved in enterobacterial common antigen biosynthesis, has been shown to discriminate among the Enterobacteriaceae most frequently found in water (2).
The aim of this study was to assess the detection efficacy of an oligonucleotide-based microarray designed with probes specific for the universal targets of 16S rRNA and cpn60 genes in addition to the Enterobacteriaceae-specific wecE genes of several pathogens usually encountered in wastewater.
MATERIALS AND METHODS
Environmental wastewater sampling and concentration.
Raw wastewater samples (20 liters) were collected from the Fabreville wastewater treatment plant influent (Laval, Quebec, Canada) and stored at 4°C in sterile plastic containers until centrifugation (approximately 1 to 2 h). To concentrate raw wastewater solids, 900-ml volumes of raw wastewater were centrifuged in a swinging-bucket rotor (Beckman JS-4.2) at 3,000 × g for 15 min at room temperature. Each wastewater pellet was resuspended in 10 ml of conserved supernatant. After this concentration step, the pellet was stored at −20°C until DNA extraction. Bacterial cell concentrations in raw water were determined by culturing on plate count agar for heterotrophic counts and on mTEC agar for E. coli counts (34,35). All cell counts were calculated by averaging results from three separate plating experiments.
Genomic DNA preparation.
Genomic DNA was extracted using a boiling method (13) from pure cultures of E. coli K-12, Salmonella enterica serovar Typhimurium (ATCC 14028), and Yersinia enterocolitica (ATCC 23715). A bead beating technique was used to extract genomic DNA from wastewater samples, followed by ammonium acetate purification (41). A final polyvinylpolypyrrolidone column purification step, modified from the procedure of Berthelet et al. (6), was performed on the precipitated wastewater DNA. Briefly, 1 ml of acid-washed polyvinylpolypyrrolidone in 20 mM potassium phosphate (pH 7.0) was added to a microspin column (Amersham Biosciences Inc., Québec, Canada), placed inside a 2-ml collection tube, and centrifuged for 3 min at 700 × g at room temperature, and the column was placed into a new sterile collection tube. The wastewater DNA extract was warmed for 10 min at 37°C and loaded onto the column. The column was centrifuged for 3 min at 700 × g at room temperature, and the purity of the collected DNA was verified by spectrophotometry using the A260/A280 ratio.
PCR amplification conditions of DNA mixtures.
The specific compositions of the different complex genomic DNA mixtures used as PCR templates are presented in Table 1. To generate 16S rRNA gene, cpn60, or wecE amplicons from these mixtures, different amounts S. enterica serovar Typhimurium, E. coli, and Y. enterocolitica genomic DNA, ranging from 50 ng to 50 fg, were added in the master mix as templates for the PCRs. Published universal primers (15) were used for amplification of 16S rRNA (F1, 5′-GAGTTTGATCCTGGCTCAG-3′; R2, 5′-GWATTACCGCGGCKGCTG-3′). For cpn60 amplicons, the primers wdF (5′-GAIIIIGCIGGIGAYGGNCANCANAC-3′) and wdR (5′-KIYKITCICCRAANCCNGGNGCYTT-3′) were used. These are based on published primers H279 and H280 (20), with the modification that inosines near the 3′ ends of the primers were replaced by mixed base positions to decrease nonspecific priming. The wecE gene primers (wecE1, 5′-AGGGCGTGATGTCCACTTAC-3′; wecE2, 5′-GAAGAACTGGCTGCGGTTAG-3′) were newly designed.
TABLE 1.
Binary and ternary mixture compositions
| Mixture | DNA (ng)
|
%
|
||||
|---|---|---|---|---|---|---|
| Y. enterocolitica | S. enterica serovar Typhimurium | E. coli | Extracted wastewater | Salmonella | Yersinia | |
| Binary | 0 | 0.50 | 49.5 | 0 | 1 | 0 |
| 0 | 5 × 10−2 | 50.0 | 0 | 0.1 | 0 | |
| 0 | 5 × 10−3 | 50.0 | 0 | 0.01 | 0 | |
| 0 | 5 × 10−4 | 50.0 | 0 | 0.001 | 0 | |
| Ternary | 0.50 | 5 × 10−2 | 49.5 | 0 | 0.1 | 1 |
| 5 × 10−2 | 5 × 10−2 | 50.0 | 0 | 0.1 | 0.1 | |
| Complex environmental DNA | 0 | 2.50 | 0 | 47.5 | 5.0 | 0 |
| 0 | 0.50 | 0 | 49.5 | 1.0 | 0 | |
| 0 | 5 × 10−2 | 0 | 50.0 | 0.1 | 0 | |
The PCR mixture included 5 μl of 10 × PCR buffer (100 mM Tris-HCl, pH 9.0, 15 mM MgCl2, and 500 mM KCl), 0.5 μl of 20 mM deoxynucleoside triphosphates, 1 μl of each of the forward and reverse primers (stock concentration, 25 μM), 0.5 μl (2.5 units) of Taq DNA polymerase (Amersham Biosciences), 50 ng of genomic DNA extracted from pure culture or environmental wastewater samples, and sterile distilled water added to give a 50-μl final volume. For cpn60 amplification, 1 μl of 100 mM MgCl2 solution was also added to the master mixture.
The annealing temperatures used during amplification were 52°C for 16S rRNA gene, 60°C for cpn60, and 55°C for wecE primers. Amplifications were performed in a GeneAmp PCR system 9700 (Perkin-Elmer) according to the following scheme: a hot start for 3 min at 94°C; 40 amplification cycles of 1 min at 94°C, 1 min at the annealing temperature described above, and 30 s (wecE amplification) or 1 min (16S rRNA and cpn60 amplifications) at 72°C; and a final extension for 7 min at 72°C. The lengths of the amplicons generated were approximately 528 bp for the 16S rRNA gene, 555 bp for cpn60, and 188 bp for wecE. An aliquot (5 μl) of each amplification reaction product was electrophoresed in a 1.5% (wt/vol) agarose gel containing ethidium bromide and 1× Tris-acetate buffer (pH 8). DNA bands were visualized under UV light. Amplicons were purified with the QIAquick PCR purification kit (QIAGEN Inc., Ontario, Canada) according to the manufacturer's instructions before being labeled.
Oligonucleotide probe design.
16S rRNA and cpn60 sequences of different pathogenic strains were compiled from GenBank, the ARB database (27), the Ribosomal Database Project (11), and the cpn60 database (19). Specific 16S rRNA and cpn60 oligonucleotides were designed using OligoPicker software (39). Initial design criteria were as follows: length between 18 and 26 bases, a maximum of no more than 11 continuous matches between a probe and nontarget species, no more than six repetitive bases, and a minimum melting temperature of 55°C. The wecE probes were chosen from an earlier publication (2). Specificity of candidate probes was verified by BLAST searches against GenBank. Multiple DNA alignments used to design generic probes were performed by using the CLUSTALW program (33). Oligonucleotide probes were purchased and synthesized by Integrated DNA Technologies (Coralville, IA). The probe sequences, their sizes, the references used, and the corresponding bacterial indicators are listed in Table 2. For a positive control, the general 16S rRNA probe S-D-Bact-0338-a-A-18 was used to detect the presence of bacteria. Negative controls were composed of two plant-specific Arabidopsis oligonucleotide sequences in addition to three printing buffer spots.
TABLE 2.
Probes
| Probe name according to reference 1 | Sequence | Target species | Accession no. of reference file | Position of probe in reference file |
|---|---|---|---|---|
| S-G-Salm-0455-a-A-24 | TTG CTG CGG TTA TTA ACC ACA ACA | Salmonella spp. | Z47544 | 471-448 |
| S-S-E.coli-0462-a-A-23 | CGT CAA TGA GCA AAG GTA TTA AC | E. coli, Shigella spp. | J01859 | 483-461 |
| S-S-E.coli-0453-a-A-25 | GAG CAA AGG TAT TAA CTT TAC TCC C | E. coli, Shigella spp. | J01859 | 476-452 |
| S-S-Y.ente-0452-a-A-25 | CAC AAA GGT TAT TAA CCT TTA TGC C | Y. enterocolitica | Z75316 | 450-426 |
| S-G-Yers-0079-a-A-22 | CGC CGG CAA AGT AGT AAA CTA C | Yersinia spp. | Z75316 | 73-52 |
| S-G-Vibr-0154-a-A-22 | GTA TTA GCC ATC GTT TCC AAT G | Vibrio spp. | X76337 | 168-147 |
| S-F-Ente-0383-a-S-22 | AGC CTG ATG CAG CCG TAT GCA G | Enterobacteriaceae | U92194 | 382-403 |
| S-G-Salm-0467-a-S-22 | TAC CGC AGC TAA TTG ACG TTA C | Salmonella spp. | U92195 | 466-487 |
| S-Ss-S.parA-0222-a-S-22 | ATC ACA TGT GCC CAG ATG CCA T | Salmonella serovar Paratyphi A | U88546 | 222-243 |
| S-Ss-S.parC-1006-a-S-22 | CTT TCC AGA GAT GAG TTT GTG C | Salmonella serovar Paratyphi C | U88548 | 1005-1026 |
| S-Ss-S.entH-0994-a-S-22 | ACA TCC ACG GGA AGT TTT CAG A | Salmonella serovar Heidelberg | AF276989 | 966-987 |
| S-Ss-S.entA-0076-a-S-22 | GAA GCA GCT TGC ACG TAG CTG A | Salmonella serovar Agona | U92197 | 76-97 |
| S-Ss-S.entE-1015-a-S-22 | GAT CCA TTT GTG CCT TAG GGA A | Salmonella serovar Enteritidis | U90318 | 1014-1035 |
| S-Ss-S.entT-1088-a-S-22 | GGT TAA GTC CCG CTA CGA TCG C | Salmonella serovar Thompson C1 | AF057363 | 1092-1113 |
| S-D-Bact-0338-a-A-18 | GCT GCC TCC CGT AGG AGT | Domain Bacteria | ||
| C-S-A.thal-0896-a-A-25a | AAC AAC AAC ATC TGG CGT AAG AGT G | Arabidopsis thaliana | AY042821 | 920-896 |
| C-S-A.thal-0871-a-A-25a | CCA AAC AAT GCT TGG CCA GCC CAC C | Arabidopsis thaliana | AY042821 | 895-871 |
| C-S-E.coli-0369-a-A-22 | TTC TTC AAC TGC AGC GGT AAC A | E. coli | X07850 | 860-840 |
| C-S-Y.ente-0470-a-A-21 | GTG CAA TCA GCT CAC CCA CAG | Y. enterocolitica | X68526 | 490-470 |
| C-S-Y.ente-0623-a-A-23 | AAG TTC AAT AGA ACC GGT TTC TG | Y. enterocolitica | X82212 | 645-623 |
| C-S-V.chol-0447-a-A-21 | GAC GAG AGG CTT TCG CTA CGC | Vibrio cholerae | AF230940 | 467-447 |
| C-S-V.chol-0464-a-A-24 | GCC CAC GCT AGA GTC TGA GTT AGC | V. cholerae, V. mimicus | AF230940 | 205-182 |
| C-Ss-S.Typh-0366-a-A-22 | TTC AAC CGC CGC AGC AAC CGC T | Salmonella serovar Typhimurium | AB033231 | 886-865 |
| W-Ss-Salm-0588-a-S-22 | TCG TAC GCT AAT TGA ACG CGC G | Salmonella serovar Typhimurium | AE008883 | 8741-8762 |
| W-Ss-Salm-0497-a-S-22 | TAG GGA CGA TCG GTC ACA TCG G | Salmonella serovar Typhimurium | AE008883 | 8650-8671 |
| W-S-E.coli-0497-a-S-22 | TGG GAA CCA TTG GTC ATA TTG G | E. coli, Shigella spp. | M87049 | 28762-28783 |
| W-G-Kleb-unk.-a-S-20 | ATC GCA CGC TGG TGG AAC GC | Klebsiella spp. | Unpublished | |
| W-G-Serr-unk.-a-S-20 | ATC CGG CCC TGA TCG ATC GG | Serratia spp. | Unpublished | |
| W-G-Yers-unk.-a-S-25 | CAC TAT CGG CCA TAT TGG TTG CTT T | Yersinia spp. | Unpublished | |
| W-G-Yers-unk.-a-S-23 | GCT TAT CAA TGA CCC GTC ACT GA | Yersinia spp. | Unpublished | |
| W-S-Y.pest-0517-a-S-23 | TCT GGG TAC CAT TGG CCA TAT TG | Yersinia pestis | AJ414159.1 | 81913-81891 |
| W-G-Yers-unk.-b-S-23 | CAC TAT CGG CCA TAT TGG TTG CT | Yersinia spp. | Unpublished | |
| W-G-Entb-unk.-a-S-21 | TCG TGC GCT GGT TGA GCG TGC | Enterobacter spp. | Unpublished | |
| W-F-Ente-0523-a-S-21 | TTT AGC TTC CAT GAA ACC AAA | Enterobacteriaceae | AL627279 | 22787-22767 |
| W-F-Ente-0526-a-S-20 | AGC TTC CAT GAA ACC AAA AA | Enterobacteriaceae | AL627279 | 22784-22765 |
| W-G-Prot-unk.-a-S-26 | CAT CTA TTT TTA CCC TTA TGA AGG GC | Proteus spp. | Unpublished | |
| W-G-Prov-unk.-a-S-24 | CGC TTT ATA GGA CGC GAA TTG TAC | Providencia spp. | Unpublished | |
| W-G-Citr-unk.-a-S-24 | AAA ATT AGT CGA ACG TGC AGA GAT | Citrobacter spp. | Unpublished |
Negative controls.
Microarray prototype and printing.
Our water pathogen microarray prototype contains a combination of short, 18- to 26-mer oligonucleotide probes specific for 16S rRNA (15 probes) and cpn60 (six probes). Fifteen short wecE oligonucleotide probes (∼20 bases) were also printed on the prototype to enhance its discriminating power. The sequences of all printed 18- to 26-mer oligonucleotides are listed in Table 2.
In preparation for printing, lyophilized oligonucleotides were suspended in water to obtain a stock concentration of 100 pmol/μl. These solutions were diluted in dimethyl sulfoxide (50%, vol/vol) to a final concentration of 50 pmol/μl. Ten microliters of each probe was transferred into a 384-well microplate and stored at −20°C until printing onto Corning GAPS II slides (Corning Co., Corning, N.Y.) with a Virtek ChipWriter, using Telechem SMP3 microspotting pins. Each probe was printed in triplicate. Slides were processed through UV cross-linking (600 mJ) followed by heat treatment (80°C overnight) and stored in the dark at room temperature until use. Quality control of the printing was assessed by terminal transferase labeling of the printed material (38). Printed slides showed uniform spot intensity and morphology, with an average spot fluorescence of 54,000 pixels.
Amplicon labeling and hybridization.
Two micrograms of purified amplicons was chemically labeled with a Mirus Cy5 Label IT nucleic acid labeling kit (Mirus, Madison, Wis.) according to the manufacturer's instructions. As the labeling reaction was performed in a small volume (30 μl), a quick spin was performed after 30min of incubation to minimize evaporation loss. The tubes were incubated for 4 h at 37°C in the dark, after which unreacted reagents were removed using a QIAquick PCR purification kit (QIAGEN Inc., Ontario, Canada).
Microarrays were prehybridized at 37°C for 1 hour with 14 μl of prewarmed (37°C) DIG Easy Hyb buffer (Hoffmann-La Roche Limited, Ontario, Canada) containing 10 μg of denatured salmon sperm DNA (Invitrogen Life Technologies, Ontario, Canada) under 22- by 22-mm coverslips (Sigma-Aldrich Canada Ltd., Ontario, Canada). Afterwards, the coverslips were removed by dipping the slides into 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and the slides dried by centrifugation at room temperature in a 50-ml conical tube for 5 min at 150 × g.
For detection threshold determination, 1 μg of labeled amplicons obtained after the amplification of binary or ternary DNA mixtures or total environmental genomic DNA was dried in a Speedvac (Savant model no. SVC200H). For specificity experiments, 25 ng of amplicons obtained from pure genomic DNA was dried in a Speedvac. Dried DNA was resuspended into 10.5 μl of prewarmed (37°C) DIG Easy Hyb buffer and 1 μl of salmon sperm DNA. DNA was then chemically denatured and neutralized for 5 min at room temperature as described by the manufacturer (Mirus Cy5 Label IT nucleic acid labeling kit). Microarrays were hybridized with the labeled DNA mix under a coverslip for 4hours at 37°C. After hybridization, coverslips were removed in 0.1× SSC, the microarrays were washed three times in prewarmed (37°C) 0.1× SSC-0.1% (vol/vol) sodium dodecyl sulfate for 5 min and one time in 1× SSC for 10 min, and the slide was dried by centrifugation (150 × g, 5 min, room temperature). All hybridizations were done in triplicate.
Data acquisition and analysis.
Hybridized arrays were imaged using a fluorescence scanner (ScanArray; Canberra-Packard, Mississauga, Ontario) and ScanArray software version 2.1. Three complete arrays were printed on each slide and could be hybridized simultaneously yet independently using separate coverslips. This approach minimizes array variation resulting from minor fluctuations in different external parameters such as temperature. Fluorescent spot intensities were quantified using QuantArray software version 3.0 (Canberra-Packard) after normalizing the data by subtracting local background from the recorded spot intensities from arrays on the same slide. The median value for each set of triplicate spotted probes was compared to the median for the buffer spots, and probes that had a signal-to-noise fluorescence ratio of greater than 2.0 (i.e., log2 of ratios >1) on replicate arrays were considered positive (26).
RESULTS
PCR amplification sensitivity on pure cultures.
The PCR amplification sensitivities for three different taxonomic genes (16S rRNA gene, cpn60, and wecE) were estimated using different S. enterica serovar Typhimurium genomic DNA amounts ranging from 50 ng to 50 fg as PCR templates. The detection thresholds, assessed by visualization of the appropriately sized fragment after electrophoresis in agarose gels, were similar to those reported by other groups (40). Under our standard PCR conditions, the 16S rRNA and wecE amplifications gave similar thresholds, where 500 fg was the minimum amount of S. enterica serovar Typhimurium DNA template required for detection (data not shown). As 5 fg of genomic DNA represents approximately one S. enterica serovar Typhimurium genome, the PCR detection limit for these two targets corresponds to approximately 102 bacterial genomes. For the cpn60 amplification reactions, the detection limit was higher than the previous estimate and corresponded to 5 pg or 103 genomes (data not shown).
Microarray validation.
To evaluate the specificities of the printed oligonucleotide probes, 25 ng of Cy5-labeled 16S rRNA gene, cpn60, or wecE amplicons, produced from either E. coli, S. enterica serovar Typhimurium, or Y. enterocolitica genomic DNA, was individually hybridized to the water pathogen microarray. Hybridization with 16S rRNA E. coli or Y.enterocolitica amplicons resulted in positive signals detected with their specific probes as well as the general bacterial S-D-Bact-0338-a-A-18 probe (Fig. 1). Hybridization of the 16S rRNA amplicons from S. enterica serovar Typhimurium resulted in positive signals for the S-G-Salm-0455-a-A-24 and S-G-Salm-0467-a-S-22 oligonucleotides, which are homologous to S. enterica serovar Typhimurium as well as to a number of other Salmonella species (Fig. 1). As expected, a positive signal was again detected with the S-D-Bact-0338-a-A-18 probe. All three 16S rRNA amplicons (E. coli, S. enterica serovar Typhimurium, and Y. enterocolitica) hybridized with the general S-F-Ente-0383-a-S-22 probe. One cross-hybridization was observed with the 16S rRNA probe for Vibrio spp. when Salmonella amplicons were hybridized (Fig. 1). By contrast, testing the microarrays with cpn60 amplicons gave only the expected signals at a ratio of intensities of >2, while results for wecE also showed the expected signals, with weak cross-hybridization only to heterologous probes (C-Ss-S.Typh-0366-a-A-22 and S-G-Salm-0467-a-S-22) (data not shown).
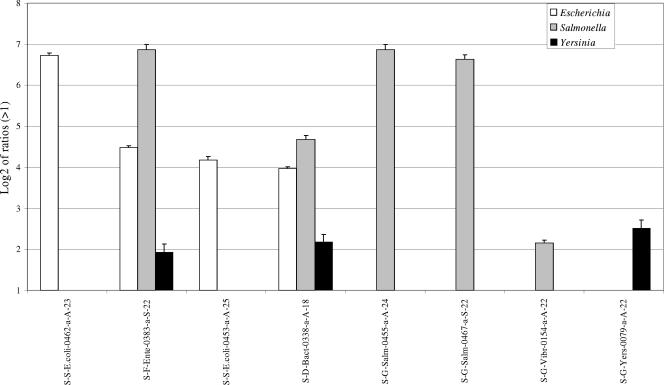
FIG. 1.
Hybridization of 16S rRNA fragments amplified from purified genomic S. enterica serovar Typhimurium, E. coli, and Y. enterocolitica DNAs on the prototype microarray. Hybridization of 25 ng of 16S rRNA amplicons from genomic DNAs of E. coli, S. enterica serovar Typhimurium, and Y. enterocolitica is shown. Results are shown as the logarithm (base 2) ratio of the probe's fluorescence intensity relative to control (buffer) spots, after normalization. The error bars represent standard errors, and each result represents the average of six spot intensities derived from two different microarray hybridizations.
Detection thresholds in mixed genomic DNA backgrounds.
To evaluate the microarray prototype detection limits in a more complex environment, different amounts of pure culture genomic DNA were added to create either binary (E. coli and S. enterica serovar Typhimurium) or ternary (E. coli, S. enterica serovar Typhimurium, and Y. enterocolitica) genomic DNA mixtures or were added to total DNA extracted from wastewater (Table 1). In wastewater environments, E. coli strains are generally predominant in comparison to Salmonella spp. or Yersinia spp., and heterotrophic plate counts of our samples showed that 1.2 (±0.1) × 106 CFU/ml were present in the raw wastewater. In the same samples, the E. coli concentration was determined to be 1.0 (±0.1) × 104 CFU/ml when plated on mTEC medium. In order to mimic these conditions, the binary and ternary DNA samples had a large excess of purified E. coli genomic DNA. To obtain well-defined hybridization signals, 1μg of total labeled taxonomic amplicons, generated from each type of DNA mixture, was hybridized on the microarray.
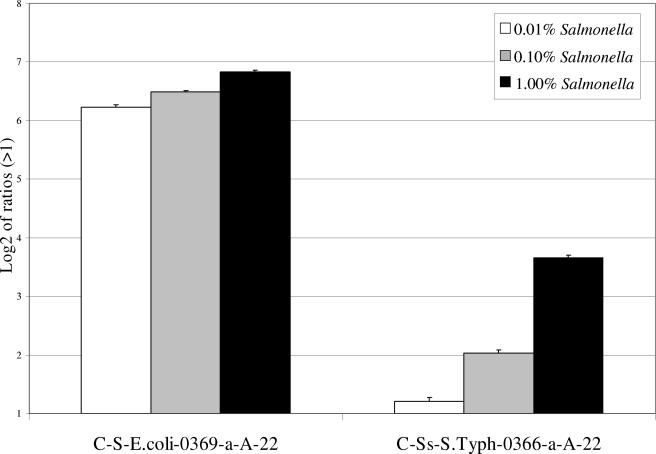
When decreasing amounts of S. enterica serovar Typhimurium genomic DNA were added to E. coli DNA, amplification and hybridization of total cpn60 amplicons showed that S. enterica serovar Typhimurium was clearly detectable in the 0.1% (50-pg) or 1% (500-pg) Salmonella binary mixtures (Fig. 2). A weak signal was obtained with the 0.01% (5-pg) mixture but was <2 in intensity ratio. On the basis that 50 ng of genomic DNA is equivalent to 1 × 107 S. enterica serovar Typhimurium cells, the threshold of the method corresponds to the presence of 103 to 104 specific genomes. The presence of S. enterica serovar Typhimurium DNA at levels lower than 0.01% gave negative results. Similar detection limits were obtained for both the 16S rRNA and wecE sets of amplicons from the binary mixtures (data not shown).
FIG. 2.
Hybridization of cpn60 fragments amplified from different binary (E. coli-Salmonella) mixtures on the prototype microarray. Hybridization of 1 μg of cpn60 fragments amplified from mixtures containing 0.01%, 0.10%, and 1.00% S. enterica serovar Typhimurium is shown. Results are shown as the logarithm (base 2) ratio of the probe's fluorescence intensity relative to control (buffer) spots, after normalization. Only two array probes specific for the cpn60 gene of either E. coli or Salmonella showed a positive hybridization signal. The error bars represent standard errors, and each result represents the average of six spot intensities derived from two different microarray hybridizations.
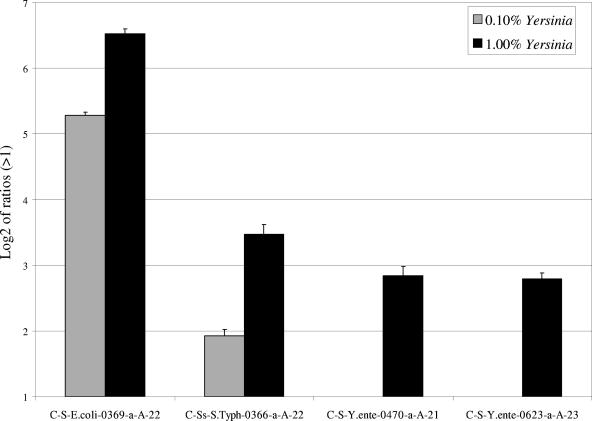
In the ternary DNA mixtures where Yersinia genomic DNA was added at 1.0% to fixed concentrations of S. enterica serovar Typhimurium and E. coli DNA (Table 1), universal amplification and hybridization of cpn60 amplicons showed that this gene was specifically detected for all three organisms (Fig. 3). The strongest signals were observed for E. coli and Salmonella probes that are specific for 99% and 0.1% of the total DNA, respectively. Yersinia, present at an equivalent concentration as S. enterica serovar Typhimurium (0.1%), failed to produce a signal (Fig. 3). If Yersinia DNA was added at a 10-fold-higher concentration than Salmonella, the signal increased to a level approximating that of Salmonella (Fig. 3). In parallel experiments, a similar pattern was observed for Yersinia 16S rRNA amplicons but not wecE, which had similar intensities as Salmonella at similar concentrations (data not shown).
FIG. 3.
Hybridizations of cpn60 fragments amplified from different ternary (E. coli-Salmonella-Yersinia) mixtures on the prototype microarray. Hybridization of 1 μg of cpn60 fragments amplified from mixtures of 0.1% S. enterica serovar Typhimurium and either 0.1% or 1% Yersinia enterocolitica is shown. Results are shown as the logarithm (base 2) ratio of the probe's fluorescence intensity relative to control (buffer) spots, after normalization. The error bars represent standard errors, and each result represents the average of six spot intensities derived from two different microarray hybridizations.
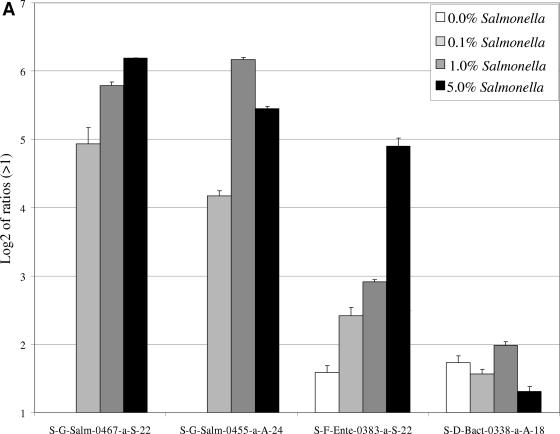
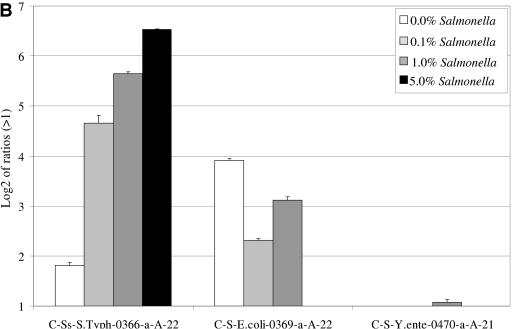
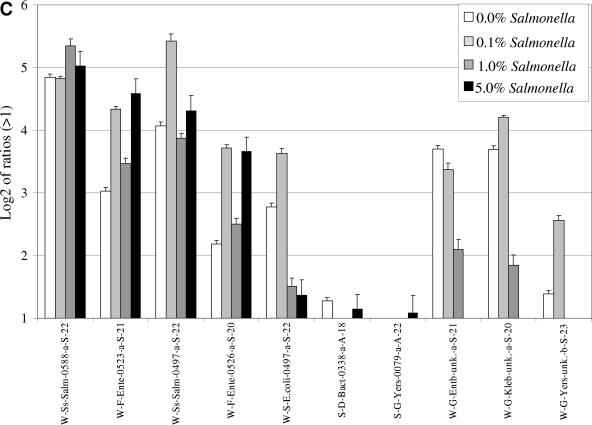
When variable amounts of S. enterica serovar Typhimurium DNA were added to a highly complex DNA sample (wastewater-extracted total DNA), hybridizations with either 16S rRNA (Fig. 4A) or cpn60 (Fig. 4B) amplicons generated from the wastewater mixture produced specific fluorescent signals for the Salmonella-specific probes at as low as 50 pg of DNA (0.1% of total added S. enterica serovar Typhimurium DNA). No signals were observed for the Salmonella-specific probes when hybridizing Cy5-labeled 16S rRNA amplicons generated from the wastewater DNA in non-Salmonella-supplemented wastewater DNA. In contrast to the absence of signals observed with S. enterica serovar Typhimurium- or E. coli-specific probes for the 16S rRNA gene, positive signals for the Salmonella-specific cpn60 and wecE probes were observed after hybridization with either total cpn60 (Fig. 4B) or wecE (Fig. 4C) amplicons produced from non-Salmonella-supplemented wastewater DNA. In addition to the Salmonella results, it was also found that the presence of E. coli in wastewater samples was detected by the cpn60 and wecE probes in non-Salmonella-supplemented DNA. The signal for Salmonella-specific cpn60 was relatively weak compared to the E. coli signal (Fig. 4B), whereas the Salmonella wecE signal was stronger than the E. coli wecE signal (Fig. 4C).
FIG.4.
Hybridization of 16S rRNA, cpn60, or wecE amplicons generated from different ratios of S. enterica serovar Typhimurium DNA in wastewater DNA. Variable amounts of S. enterica serovar Typhimurium DNA were added to wastewater DNA (0%, 0.1%, 1.0%, and 5.0% final concentrations). The mixtures were subjected to PCR using universal 16S rRNA (A), cpn60 (B), or wecE (C) primers. One microgram of each amplicon was labeled and separately hybridized to the prototype microarray. Results are shown as the logarithm (base 2) ratio of the probe's fluorescence intensity relative to control (buffer) spots, after normalization. The error bars represent standard errors, and each result represents the average of six spot intensities derived from two different microarray hybridizations.
Hybridization of total wecE amplicons derived from non-Salmonella-supplemented wastewater DNA produced signals with general Salmonella species-specific probes (W-Ss-Salm-0588-a-S-22 and W-Ss-Salm-0497-a-S-22) as well as with the E.coli, Enterobacter, Yersinia, and Klebsiella wecE probes (Fig. 4C). For the most part, we observed that the signals for Salmonella and Enterobacteriaceae probes increased with the addition of S.enterica serovar Typhimurium DNA. While the signals for Enterobacter, Yersinia, and Klebsiella decreased and disappeared completely at the highest concentration of S. enterica serovar Typhimurium DNA, the signal for E. coli decreased but never disappeared. A small amount of cross-hybridization, just above the ratio of 2, was also observed with two 16S probes, S-D-Bact-0338-a-A-18 and S-G-Yers-0079-a-A-22.
DISCUSSION
Discrimination between closely related bacterial species or genera is relatively difficult when using a single specific taxonomic gene in PCR-based assays, especially within complex environmental samples (29). Although incorporating more taxonomic identifiers would circumvent this problem, the increase in cost and labor rapidly becomes unrealistic. In this feasibility study aimed at detecting pathogens in wastewater samples, both points were addressed, first by employing three different taxonomic genes and second by harnessing the parallel processing power of DNA microarrays. With regard to the taxonomic genes, two correspond to universal target sequences (16S rRNA and cpn60 genes), and the third (wecE) belongs to the wec cluster specific to the Enterobacteriaceae family (2). Inclusion of a nonuniversal target, such as the wecE gene, is advantageous when higher sensitivity is desired. Another important factor behind the choice of genes capable of discriminating among different bacterial species is that they can be amplified from a complex genomic DNA mixture by using a single set of universal primers. Hybridization of the individually amplified 16S rRNA gene, cpn60, and wecE fragments from E. coli, S. enterica serovar Typhimurium, or Y. enterocolitica strains showed that all the cpn60 and wecE oligonucleotide probes printed on the microarray can specifically discriminate between the three bacterial species. The S. enterica serovar Typhimurium 16S cross-hybridization that occurred with the S-G-Vibr-0154-a-A-22 probe can be explained by a high percent similarity (87%) between them.
Although PCR amplification remains an important tool for bacterial detection, precise identification of PCR products requires additional measures such as the use of specific beacons. However, in addition to their cost, the design of beacons for real-time PCR can face difficult design issues with complex samples such as feces (31). Microarrays coupled with PCR can serve as a set of parallel dot blots to enhance microbial detection and identification. Under our PCR conditions, the detection limits of the 16S rRNA and wecE PCRs using genomic DNA from a single strain are in the same range (approximately 100 bacterial genomes). Therefore, the sensitivity of the these two reactions is sufficiently high to be applicable for the detection of low levels of bacteria in complex mixtures. For the cpn60 PCR, the detection limit was unexpectedly higher (10-fold) than those of the two other reactions but remains at acceptable levels.
When complex samples are used as templates for PCR amplification, PCR bias may occur and manifest itself by a nonproportional amplification of the less abundant species (37). To address this concern, hybridization thresholds for a specific species were assessed using variable amounts of a specific genomic DNA in binary, ternary, and complex DNA mixtures. The binary and ternary mixtures were composed mainly of genomic DNA from E. coli to mimic conditions encountered in wastewater, where E. coli is predominant. By using amplicons produced from binary or ternary genomic DNA mixtures (S.enterica serovar Typhimurium and E. coli genomic DNAs), we demonstrated that our 16S rRNA gene, cpn60, and wecE microarray oligonucleotide probes can detect between 103 and 104 S. enterica serovar Typhimurium genomes in a 50-ng DNA sample. The addition of genomic DNA from Y. enterocolitica to the mixture to a ratio of 1% does not affect the detection limit of the microarray for S. enterica serovar Typhimurium; therefore, detection sensitivity was not affected by the addition of a third DNA component in the mixture. Since the observed thresholds were identical when binary and ternary mixtures were tested, it is reasonable to conclude that little or no PCR bias had occurred under our conditions. However, the sensitivity of the microarray appears to vary slightly depending on the microorganisms tested. The intensities of the 16S rRNA and cpn60 signals for Yersinia DNA were lower than those obtained with Salmonella DNA when both bacterial DNAs were added at a ratio of 0.1% in the ternary mixture. The reason for this variation is unknown, since the 16S rRNA and cpn60 genetic sequences of Salmonella and Yersinia share similar G/C ratios. Moreover, hybridization conditions favoring Salmonella over Yersinia amplicons can be eliminated, since the amplicon sizes and the annealing temperatures of the printed probes specific for both bacteria are identical. Using amplicons generated from Salmonella-wastewater DNA mixtures, a detection threshold of >104 S. enterica serovar Typhimurium genomes was obtained. This environmental detection limit constitutes only an estimate, since the initial quantity of S.enterica serovar Typhimurium present in the original wastewater sample was unknown. Previous culture studies on domestic wastewater treatment have shown that the amount of S. enterica serovar Typhimurium cells is generally two orders of magnitude lower than that of E. coli (18).
An unexpected result occurred in our wecE amplicon hybridizations in that the intensities of some non-Salmonella hybridization signals (W-G-Kleb-unk.-a-S-20 and W-G-Entb-unk.-a-S-21 probes) decreased when the ratio of S. enterica serovar Typhimurium DNA in the mixture was increased (Fig. 4C). This observation might be explained by PCR amplification bias, where the PCR seems to favor amplification of the most abundant bacterial species to the detriment of the less abundant ones. This observation can be problematic if a bacterial species is predominant in an environmental mixture in comparison to others. A weaker or absent PCR amplification might be obtained for the less abundant bacteria, and therefore, the detection threshold of the microarray for these bacteria might be affected.
Of the three taxonomic genes used in this study, only wecE hybridization signals were observed for Salmonella when the genes were amplified from wastewater DNA not spiked with S.enterica serovar Typhimurium DNA. Thus, the detection sensitivity for the wecE amplicons is higher than that for either the 16S rRNA or cpn60 gene. This increased sensitivity could be due to at least three factors. First, since the size of the wecE amplicons (188 bp) is 2.8-fold lower than that of either the 16S rRNA (528 bp) or cpn60 (555 bp) amplicons, using 1 μg of labeled wecE amplicons would represent a higher number of molecules hybridized on a molar basis. Second, the lower detection limit of the wecE amplicons can also be explained by the fact that wecE amplification is specific for one eubacterial family (Enterobacteriaceae), in contrast to the ubiquitous 16S rRNA or cpn60 gene. Therefore in community DNA, the diversity of wecE would be lower than that of either 16S rRNA or cpn60, resulting in a higher relative abundance. Finally, the wecE probe (Table 2) was designed to detect several species of Salmonella other than S. enterica serovar Typhimurium. Since the wecE primers amplified the wecE genes for only a limited diversity of microorganisms, they might favor the detection of more specific bacteria than with the 16S rRNA or cpn60 primers, which would amplify the appropriate gene from all bacteria present in the wastewater sample. Thus, to increase the sensitivity of the prototype for wastewater pathogens, it may be advantageous to target amplicons generated from PCRs targeting a limited group of bacteria instead of universal PCRs specific for all bacteria.
In summary, we have designed a specific and sensitive microarray that can be utilized for the detection of several bacterial species in wastewater samples. Amplification and fluorescent labeling of the 16S rRNA, cpn60, and wecE genes from extracted community DNA show specific detection of each of the microorganisms studied when hybridized to oligonucleotide probes printed on the wastewater prototype microarray. Although sensitivity may vary depending on the microorganisms tested, detection sensitivity can be increased by targeting amplicons specific for a limited group of bacteria instead of universal taxonomic amplicons from a broad spectrum of bacteria.
Acknowledgments
This work was supported by contract 01-HHE-1 from the Water Environment Research Foundation of the United States (under U.S. Environmental Protection Agency Cooperative Agreement no. CR-827345-01) and by funds from the Canadian Water Network.
REFERENCES
- 1.Alm, E. W., D. B. Oerther, N. Larsen, D. A. Stahl, and L. Raskin. 1996. The oligonucleotide probe database. Appl. Environ. Microbiol. 62:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayardelle, P., and M. Zafarullah. 2002. Development of oligonucleotide primers for the specific PCR-based detection of the most frequent Enterobacteriaceae species DNA using wec gene templates. Can. J. Microbiol. 48:113-122. [DOI] [PubMed] [Google Scholar]
- 3.Bej, A. K. 2003. Molecular based methods for the detection of microbial pathogens in the environment. J. Microbiol. Methods 53:139-140. [DOI] [PubMed] [Google Scholar]
- 4.Bej, A. K., J. L. DiCesare, L. Haff, and R. M. Atlas. 1991. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl. Environ. Microbiol. 57:1013-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekal, S., R. Brousseau, L. Masson, G. Prefontaine, J. Fairbrother, and J. Harel. 2003. Rapid identification of Escherichia coli pathotypes by virulence gene detection with DNA microarrays. J. Clin. Microbiol. 41:2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthelet, M., L. G. Whyte, and C. W. Greer. 1996. Rapid, direct extraction of DNA from soils for PCR analysis using polyvinylpolypyrrolidone spin columns. FEMS Microbiol. Lett. 138:17-22. [DOI] [PubMed] [Google Scholar]
- 7.Brewster, D. H., M. I. Brown, D. Robertson, G. L. Houghton, J. Bimson, and J. C. Sharp. 1994. An outbreak of Escherichia coli O157 associated with a children's paddling pool. Epidemiol. Infect. 112:441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brousseau, R., J. E. Hill, G. Prefontaine, S. H. Goh, J. Harel, and S. M. Hemmingsen. 2001. Streptococcus suis serotypes characterized by analysis of chaperonin 60 gene sequences. Appl. Environ. Microbiol. 67:4828-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Call, D. R., M. K. Borucki, and F. J. Loge. 2003. Detection of bacterial pathogens in environmental samples using DNA microarrays. J. Microbiol. Methods 53:235-243. [DOI] [PubMed] [Google Scholar]
- 10.Call, D. R., F. J. Brockman, and D. P. Chandler. 2001. Detecting and genotyping Escherichia coli O157: H7 using multiplexed PCR and nucleic acid microarrays. Int. J. Food Microbiol. 67:71-80. [DOI] [PubMed] [Google Scholar]
- 11.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook, K. L., A. C. Layton, H. M. Dionisi, J. T. Fleming, and G. S. Sayler. 2004. Evaluation of a plasmid-based 16S-23S rDNA intergenic spacer region array for analysis of microbial diversity in industrial wastewater. J. Microbiol. Methods 57:79-93. [DOI] [PubMed] [Google Scholar]
- 13.Daigle, F., J. Harel, J. M. Fairbrother, and P. Lebel. 1994. Expression and detection of pap-, sfa-, and afa-encoded fimbrial adhesin systems among uropathogenic Escherichia coli. Can. J. Microbiol. 40:286-291. [DOI] [PubMed] [Google Scholar]
- 14.Dev, V. J., M. Main, and I. Gould. 1991. Waterborne outbreak of Escherichia coli O157. Lancet 337:1412. [DOI] [PubMed] [Google Scholar]
- 15.Dorsch, M., and E. Stachebrandt. 1992. Some modifications in the procedure of direct sequencing of PCR amplified 16S rDNA. J. Microbiol. Methods 16:271-279. [Google Scholar]
- 16.Foy, C. A., and H. C. Parkes. 2001. Emerging homogeneous DNA-based technologies in the clinical laboratory. Clin. Chem. 47:990-1000. [PubMed] [Google Scholar]
- 17.Goh, S. H., S. Potter, J. O. Wood, S. M. Hemmingsen, R. P. Reynolds, and A. W. Chow. 1996. HSP60 gene sequences as universal targets for microbial species identification: studies with coagulase-negative staphylococci. J. Clin. Microbiol. 34:818-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hench, K. R., G. K. Bissonnette, A. J. Sexstone, J. G. Coleman, K. Garbutt, and J. G. Skousen. 2003. Fate of physical, chemical, and microbial contaminants in domestic wastewater following treatment by small constructed wetlands. Water Res. 37:921-927. [DOI] [PubMed] [Google Scholar]
- 19.Hill, J. E., S. L. Penny, K. G. Crowell, S. H. Goh, and S. M. Hemmingsen. 2004. cpnDB: a chaperonin sequence database. Genome Res. 14:1669-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, J. E., R. P. Seipp, M. Betts, L. Hawkins, A. G. Van Kessel, W. L. Crosby, and S. M. Hemmingsen. 2002. Extensive profiling of a complex microbial community by high-throughput sequencing. Appl. Environ. Microbiol. 68:3055-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurst, C. J. 1997. Overview of water microbiology as it relates to public health, p. 133-242. In C. J. Hurst (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 22.Keene, W. E., J. M. McAnulty, F. C. Hoesly, L. P. Williams, Jr., K. Hedberg, G. L. Oxman, T. J. Barrett, M. A. Pfaller, and D. W. Fleming. 1994. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7 and Shigella sonnei. N. Engl. J. Med. 331:579-584. [DOI] [PubMed] [Google Scholar]
- 23.Keramas, G., D. D. Bang, M. Lund, M. Madsen, S. E. Rasmussen, H. Bunkenborg, P. Telleman, and C. B. Christensen. 2003. Development of a sensitive DNA microarray suitable for rapid detection of Campylobacter spp. Mol. Cell. Probes 17:187-196. [DOI] [PubMed] [Google Scholar]
- 24.Lee, C. Y., G. Panicker, and A. K. Bej. 2003. Detection of pathogenic bacteria in shellfish using multiplex PCR followed by CovaLink NH microwell plate sandwich hybridization. J. Microbiol. Methods 53:199-209. [DOI] [PubMed] [Google Scholar]
- 25.Lemarchand, K., L. Masson, and R. Brousseau. 2004. Molecular biology and DNA microarray technology for microbial quality monitoring of water. Crit. Rev. Microbiol. 30:145-172. [DOI] [PubMed] [Google Scholar]
- 26.Loy, A., K. Kusel, A. Lehner, H. L. Drake, and M. Wagner. 2004. Microarray and functional gene analyses of sulfate-reducing prokaryotes in low-sulfate, acidic fens reveal concurrence of recognized genera and novel lineages. Appl. Environ. Microbiol. 70:6998-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCabe, K. M., Y. H. Zhang, B. L. Huang, E. A. Wagar, and E. R. McCabe. 1999. Bacterial species identification after DNA amplification with a universal primer pair. Mol. Genet. Metab. 66:205-211. [DOI] [PubMed] [Google Scholar]
- 29.Pillai, S. D. 1997. Rapid molecular detection of microbial pathogens: breakthroughs and challenges. Arch. Virol. Suppl. 13:67-82. [DOI] [PubMed] [Google Scholar]
- 30.Rhee, S. K., X. Liu, L. Wu, S. C. Chong, X. Wan, and J. Zhou. 2004. Detection of genes involved in biodegradation and biotransformation in microbial communities by using 50-mer oligonucleotide microarrays. Appl. Environ. Microbiol. 70:4303-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinttila, T., A. Kassinen, E. Malinen, L. Krogius, and A. Palva. 2004. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 97:1166-1177. [DOI] [PubMed] [Google Scholar]
- 32.Swerdlow, D. L., B. A. Woodruff, R. C. Brady, P. M. Griffin, S. Tippen, H. D. Donnell, Jr., E. Geldreich, B. J. Payne, A. Meyer, Jr., J. G. Wells, et al. 1992. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann. Intern. Med. 117:812-819. [DOI] [PubMed] [Google Scholar]
- 33.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ultee, A., N. Souvatzi, K. Maniadi, and H. Konig. 2004. Identification of the culturable and nonculturable bacterial population in ground water of a municipal water supply in Germany. J. Appl. Microbiol. 96:560-568. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Environmental Protection Agency. 2000. Improved enumeration methods for recreational water quality indicators: enterococci and Escherichia coli. EPA/821/R-97/004. Office of Science and Technology, U.S. Environmental Protection Agency, Washington, D.C.
- 36.van der Giessen, J. W., A. Eger, J. Haagsma, R. M. Haring, W. Gaastra, and B. A. van der Zeijst. 1992. Amplification of 16S rRNA sequences to detect Mycobacterium paratuberculosis. J. Med. Microbiol. 36:255-263. [DOI] [PubMed] [Google Scholar]
- 37.Vora, G. J., C. E. Meador, D. A. Stenger, and J. D. Andreadis. 2004. Nucleic acid amplification strategies for DNA microarray-based pathogen detection. Appl. Environ. Microbiol. 70:3047-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, J. 2002. Terminal deoxynucleotidyl transferase quality control protocol. https://dnacore.mgh.harvard.edu/microarray/protocol-tdt.shtml.
- 39.Wang, X., and B. Seed. 2003. Selection of oligonucleotide probes for protein coding sequences. Bioinformatics 19:796-802. [DOI] [PubMed] [Google Scholar]
- 40.Wilson, W. J., C. L. Strout, T. Z. DeSantis, J. L. Stilwell, A. V. Carrano, and G. L. Andersen. 2002. Sequence-specific identification of 18 pathogenic microorganisms using microarray technology. Mol. Cell. Probes 16:119-127. [DOI] [PubMed] [Google Scholar]
- 41.Yu, Z., and W. W. Mohn. 1999. Killing two birds with one stone: simultaneous extraction of DNA and RNA from activated sludge biomass. Can. J. Microbiol. 45:269-272. [Google Scholar]