Abstract
Thirteen Escherichia coli strains harboring stx2e were isolated from 11,056 human stools. This frequency corresponded to the presence of the stx2e allele in 1.7% of all Shiga toxin-producing E. coli (STEC) strains. The strains harboring stx2e were associated with mild diarrhea (n = 9) or asymptomatic infections (n = 4). Because STEC isolates possessing stx2e are porcine pathogens, we compared the human STEC isolates with stx2e-harboring E. coli isolated from piglets with edema disease and postweaning diarrhea. All pig isolates possessed the gene encoding the F18 adhesin, and the majority possessed adhesin involved in diffuse adherence; these adhesins were absent from all the human STEC isolates. In contrast, the high-pathogenicity island encoding an iron uptake system was found only in human isolates. Host-specific patterns of interaction with intestinal epithelial cells were observed. All human isolates adhered to human intestinal epithelial cell lines T84 and HCT-8 but not to pig intestinal epithelial cell line IPEC-J2. In contrast, the pig isolates completely lysed human epithelial cells but not IPEC-J2 cells, to which most of them adhered. Our data demonstrate that E. coli isolates producing Shiga toxin 2e have imported specific virulence and fitness determinants which allow them to adapt to the specific hosts in which they cause various forms of disease.
Shiga toxin (Stx)-producing Escherichia coli (STEC) isolates, which cause diarrhea and hemolytic-uremic syndrome (HUS) in humans (19, 26, 50), generally cause minimal or no injury in their animal host reservoirs (26). The only naturally occurring diseases in animals caused by STEC are swollen head syndrome in chickens (44) and edema disease in piglets (20). Edema disease is characterized by vascular necrosis, edema, and neurological signs and can be fatal (20). Although the exact mechanisms that lead to edema disease are unknown, Stx2e and adherence-mediating virulence factors such as the F18 adhesin, F4 fimbriae, and adhesin involved in diffuse adherence (AIDA) seem to be common among strains isolated from diseased pigs (21, 35). In one study, the presence of Stx2e in the erythrocyte fraction was strongly associated with clinical disease (30). stx2e is the most frequent stx2 variant found in fecal samples from pigs (14), and it was the second most common stx2 variant in environmental STEC isolates (54). In the latter study, the stx2e variant was found not only in STEC strains isolated from pig samples but also in isolates from a dairy cattle herd, suggesting that such strains spread from pigs to cattle (54).
Stx2e-producing E. coli strains have also occasionally been isolated from humans (5, 15, 40, 52). The majority of the patients had uncomplicated diarrhea (5, 15, 40), and some had HUS (52). However, the frequency with which Stx2e-producing STEC strains occur in humans, their virulence factors, their mechanisms of interaction with the human host, their reservoir(s), and their mode(s) of transmission are poorly understood.
Here, we compared the putative virulence genes in Stx2e-producing E. coli strains isolated from humans and diseased pigs in order to assess the extent to which they are related. We also analyzed the interactions of the two groups of organisms with homologous and heterologous intestinal epithelial cells in vitro in a search for characteristics that might be related to adaptation in the host.
MATERIALS AND METHODS
Bacterial strains.
After screening 11,056 stools (9,206 from patients with diarrhea or HUS and 1,850 from asymptomatic individuals), we isolated 13 E. coli strains containing the stx2e gene. These isolates were obtained from patients with uncomplicated diarrhea (n = 9) or from asymptomatic carriers (n = 4) and were recovered at the Institute of Hygiene and Microbiology, University of Würzburg, Würzburg, Germany, and the Institute of Hygiene, University Hospital Münster, Münster, Germany, during routine diagnostic examinations and epidemiological investigations between January 1997 and December 2003. The procedures used for STEC isolation from stools have been described previously (15). Briefly, enriched primary stool cultures were screened using PCRs for stx and eae genes, and STEC strains were isolated from PCR-positive stools using colony blot hybridization with digoxigenin-labeled stx probes (15). The 13 human isolates showed no geographical or temporal linkage. A subset of these strains was investigated for stx2e transcription in a previous study (57). Twelve porcine STEC strains harboring stx2e were isolated from German piglets with edema disease or postweaning diarrhea (35), while one strain (strain E57) was isolated from a pig with diarrhea in Canada (27).
stx subtyping.
The isolated strains were tested for stx1 and stx2 using the primer pairs KS7-KS8 (stxB1 and stxB1c) and LP43-LP44 (stxA2 and stxA2 variants) (Table 1). stx1 and stx1c were distinguished by HhaI restriction of the KS7-KS8 PCR products (16, 56). The strategy used to distinguish stx2 from its variants has been described previously (15). Briefly, STEC strains positive in the PCR with primers LP43 and LP44 were subjected to PCR with primers GK3 and GK4 (Table 1), and the amplification products were digested with HaeIII (New England Biolabs, Frankfurt, Germany) to differentiate between stx2 and stx2c (15). Isolates in which amplification products could not be elicited with primers GK3 and GK4 were tested further for the presence of the stx2d (41) and stx2e (55) genes using primers VT2-cm and VT2-f and primers FK1 and FK2, respectively (Table 1). Strains positive in the PCR with primers FK1 and FK2, which target the stxB2e subunit gene (15), were confirmed to contain the stxA2e subunit gene using the PCR with primers FK9 and FK10 (13) (Table 1). The presence of stx2e in PCR-positive isolates was confirmed by Southern blot hybridization with digoxigenin-labeled stxA2e and stxB2e probes derived from stx2e-harboring human isolate VUB-EH60 (40) by PCRs with primer pairs FK9-FK10 and FK1-FK2 (Table 1), respectively. Moreover, the identity of stx2e genes was verified by nucleotide sequence analysis performed as described previously (57).
TABLE 1.
PCR primers and conditions used in this study
| Primer | Sequence (5′-3′) | Target(s) | PCR conditionsa
|
Size of PCR product (bp) | Reference | Positive controlb | ||
|---|---|---|---|---|---|---|---|---|
| Denaturation | Annealing | Extension | ||||||
| KS7 | CCCGGATCCATGAAAAAAACATTATTAATAGC | stxB1 | 94°C, 30 s | 52°C, 60 s | 72°C, 40 s | 285 | 15 | EDL933 |
| KS8 | CCCGAATTCAGCTATTCTGAGTCAACG | stxB1c | ||||||
| LP43 | ATCCTATTCCCGGGAGTTTACG | stxA2 and variants | 94°C, 30 s | 57°C, 60 s | 72°C, 60 s | 584 | 15 | EDL933 |
| LP44 | GCGTCATCGTATACACAGGAGC | |||||||
| GK3 | ATGAAGAAGATGTTTATG | stxB2, stxB2c | 94°C, 30 s | 52°C, 60 s | 72°C, 40 s | 260 | 15 | EDL933 |
| GK4 | TCAGTCATTATTAAACTG | |||||||
| VT2-cm | AAGAAGATATTTGTAGCGG | stxB2d | 94°C, 30 s | 55°C, 60 s | 72°C, 60 s | 256 | 41 | EH250 |
| VT2-f | TAAACTGCACTTCAGCAAAT | |||||||
| FK1 | CCCGGATCCAAGAAGATGTTTATAG | stxB2e | 94°C, 30 s | 55°C, 60 s | 72°C, 40 s | 280 | 15 | VUB-EH60 |
| FK2 | CCCGAATTCTCAGTTAAACTTCACC | |||||||
| FK9 | CCCGGATCCATGAAGTGTATATTGTTA | stxA2e | 94°C, 30 s | 52°C, 60 s | 72°C, 60 s | 260 | 13 | VUB-EH60 |
| FK10 | CCCGAATTCAGCACAATCCGCCGCCAT | |||||||
| SK1 | CCCGAATTCGGCACAAGCATAAGC | eae | 94°C, 30 s | 52°C, 60 s | 72°C, 60 s | 863 | 15 | EDL933 |
| SK2 | CCCGGATCCGTCTCGCCAGTATTCG | Conserved | ||||||
| Iha-I | CAGTTCAGTTTCGCATTCACC | iha | 94°C, 30 s | 56°C, 60 s | 72°C, 90 s | 1,305 | 46 | 4797/97 |
| Iha-II | GTATGGCTCTGATGCGATG | |||||||
| SAADF | CGTGATGAACAGGCTATTGC | saa | 94°C, 30 s | 52°C, 60 s | 72°C, 40 s | 119 | 16 | 3937/97c |
| SAADR | ATGGACATGCCTGTGGCAAC | |||||||
| E643f | TATCAGGCCAATCAAAACAG | efa-1d | 94°C, 30 s | 50°C, 60 s | 72°C, 60 s | 974 | 22 | 493/89 |
| E1598r | AGACACTGGTAAATTTCGC | |||||||
| E5242f | TAAGCGAGCCCTGATAAGCA | efa-2d | 94°C, 30 s | 55°C, 60 s | 72°C, 60 s | 630 | 22 | 493/89 |
| E5854r | CGTGTTGCTTGCCTTTGC | |||||||
| E7044f | TGTCTAACTGGATTGTATGGC | efa-3d | 94°C, 30 s | 56°C, 60 s | 72°C, 60 s | 685 | 22 | 493/89 |
| E7710r | ATGTTGTTCCCGGCCCAGT | |||||||
| sfpA-U | AGCCAAGGCCAAGGGATTATTA | sfpA | 94°C, 30 s | 59°C, 60 s | 72°C, 60 s | 440 | 17 | 493/89 |
| sfpA-L | TTAGCAACAGCAGTGAAGTCTC | |||||||
| HlyA1 | GGTGCAGCAGAAAAAGTTGTAG | EHEC hlyA | 94°C, 30 s | 57°C, 60 s | 72°C, 90 s | 1,550 | 45 | EDL933 |
| HlyA4 | TCTCGCCTGATAGTGTTTGGTA | |||||||
| esp-A | AAACAGCAGGCACTTGAACG | espP | 94°C, 30 s | 56°C, 60 s | 72°C, 150 s | 1,830 | 56 | EDL933 |
| esp-B | GGAGTCGTCAGTCAGTAGAT | |||||||
| D1 | CGTCAGGAGGATGTTCAG | etpD | 94°C, 30 s | 56°C, 60 s | 72°C, 70 s | 1,062 | 56 | EDL933 |
| D13R | CGACTGCACCTGTTCCTGATTA | |||||||
| wkat-B | CTTCCTGTTCTGATTCTTCTGG | katP | 94°C, 30 s | 56°C, 60 s | 72°C, 150 s | 2,125 | 56 | EDL933 |
| wkat-F | AACTTATTTCTCGCATCATCC | |||||||
| Cdt I-f | TGGTGAGAATCGGAACTG | cdt-IA | 94°C, 30 s | 51°C, 60 s | 72°C, 60 s | 418 | 6 | 6468/62 |
| Cdt I-r | CATTCCATCAGGTTTGTC | |||||||
| Cdt II-f | AATCCCTATCCCTGAACC | cdt-IIA | 94°C, 30 s | 52°C, 60 s | 72°C, 60 s | 542 | 6 | 9142/88 |
| Cdt II-r | GTTCTATTGGCTGTGGTG | |||||||
| Cdt III-f | AAACAGGACGGTAATAATGACTAATA | cdt-III | 94°C, 30 s | 54°C, 60 s | 72°C, 180 s | 2,230 | 6 | 1404 |
| Cdt III-r | GTGATCTCCTTCCATGAAAATATAGT | Complete | ||||||
| c338f | AGCATTAAATAAAAGCACGA | cdt-VAe | 94°C, 30 s | 52°C, 60 s | 72°C, 60 s | 1,329 | 23 | 493/89 |
| c2135r | TACTTGCTGTGGTCTGCTAT | |||||||
| c1309f | AGCACCCGCAGTATCTTTGA | cdt-VBe | 94°C, 30 s | 52°C, 60 s | 72°C, 60 s | 1,363 | 23 | 493/89 |
| c2166r | AGCCTCTTTTATCGTCTGGA | |||||||
| P105 | GTCAACGAACATTAGATTAT | cdt-VCe | 94°C, 30 s | 49°C, 60 s | 72°C, 60 s | 748 | 23 | 493/89 |
| c2767r | ATGGTCATGCTTTGTTATAT | |||||||
| RTsubAF | CGAATGTTTTTCTTGCTCCAG | subA | 94°C, 30 s | 53°C, 60 s | 72°C, 60 s | 220 | 38 | 3706/02f |
| RTsubAR | ACACTGCTGACAGGATGATAAG | |||||||
| espI-I | ATGGACAGAGTGGAGACAG | espI | 94°C, 30 s | 52°C, 60 s | 72°C, 60 s | 560 | 46 | 4797/97 |
| espI-II | GCCACCTTTATTCTCACCA | |||||||
| Irp2 FP | AAGGATTCGCTGTTACCGGAC | irp2g | 94°C, 30 s | 60°C, 60 s | 72°C, 60 s | 280 | 25 | 5720/96 |
| Irp2 RP | TCGTCGGGCAGCGTTTCTTCT | |||||||
| FyuA f | TGATTAACCCCGCGACGGGAA | fyuAg | 94°C, 30 s | 63°C, 60 s | 72°C, 90 s | 880 | 25 | 5720/96 |
| FyuA r | CGCAGTAGGCACGATGTTGTA | |||||||
| UN19 | CTGGGTGACATTATTGCTTGG | orfAh | 94°C, 60 s | 64°C, 60 s | 72°C, 90 s | 370 | 35 | 2787 |
| UN20 | TTTGCTTGTGCGGTAGACTG | |||||||
| UN21 | TGAAAACATTAAGGGCTCG | orfBi | 94°C, 60 s | 64°C, 60 s | 72°C, 90 s | 450 | 35 | 2787 |
| UN22 | CCGGAAACATTGACCATACC | |||||||
| UN23 | CAGTTTATCAATCAGCTCGGG | orfBj | 94°C, 60 s | 64°C, 60 s | 72°C, 90 s | 543 | 35 | 2787 |
| UN24 | CCACCGTTCCGTTATCCTC | |||||||
| fedA1 | GTGAAAAGACTAGTGTTTATTTC | fedA | 94°C, 60 s | 56°C, 60 s | 72°C, 60 s | 230 | 35 | 2787 |
| fedA2 | CTTGTAAGTAACCGCGTAAGC | |||||||
| TerF1 | TTACAATCCGGACAAAACA | terF | 94°C, 30 s | 55°C, 60 s | 72°C, 60 s | 244 | 51 | EDL933 |
| TerF2 | CAATGACAACGGTGATCG | |||||||
| UreC-f | TCTAACGCCACAACCTGTAC | ureC | 94°C, 60 s | 60°C, 60 s | 72°C, 60 s | 398 | 32 | EDL933 |
| UreC-r | GAGGAAGGCAGAATATTGGG | |||||||
All PCRs included 30 cycles, followed by a final extension of 5 min at 72°C.
The PCR positive control strains were the strains described in the references unless indicated otherwise.
saa+ E. coli O91:NM (16).
Three different regions of efa1 were targeted to detect the whole gene (9,996 bp) (22).
The presence of three open reading frames encoding cytolethal distending toxin V was investigated.
subA+ E. coli O113:H21 from our collection (H. Karch, unpublished).
Markers for the HPI. The presence of additional HPI genes, their links, and the insertion site of HPI were determined previously (25).
orfA encodes a 45-kDa protein which is required to modify AIDA-I to adhere to target cells (3).
The primer amplifies a fragment from the coding region for AIDA-I (35).
The primer amplifies a fragment from the coding region for AIDAC (35).
PCR.
PCRs were performed with a Biometra TGradient 96 cycler (Biometra GmbH, Göttingen, Germany) (16, 48). The PCR primers, target sequences, conditions, and positive controls are shown in Table 1. E. coli C600 was used as a negative control. The specificity of PCR products was confirmed by analyzing the sequences of representative amplicons (6, 48).
Southern blot hybridization.
Southern blot hybridization of plasmid DNA with digoxigenin-labeled enterohemorrhagic E. coli (EHEC) hlyA, katP, espP, and etpD probes was performed as described previously (58).
Phenotypic methods.
Isolates were serotyped using antisera against E. coli O antigens 1 to 181 and H antigens 1 to 56 (42). Stx production was tested using a commercial latex agglutination assay (verotoxin-producing E. coli reverse passive latex agglutination; Denka Seiken Co., Ltd., Tokyo, Japan). Fermentation of sorbitol was detected on sorbitol MacConkey (SMAC) agar plates after overnight incubation. The enterohemolytic phenotype was investigated on enterohemolysin agar containing 5% defibrinated and washed sheep erythrocytes and 10 mM CaCl2 (45). Resistance to tellurite was determined from the ability of isolates to grow on cefixime-tellurite (CT)-SMAC agar (Oxoid, Hampshire, United Kingdom) (7). Urease activity was examined in urea degradation broth (Heipha) after 24 h of incubation at 37°C (9, 17).
Cell cultures.
The T84 cell line (human colonic carcinoma epithelial cells; ATCC CCL-248) and the HCT-8 cell line (human ileocecal adenocarcinoma cells; ATCC CCL-244) were used. The culture medium for T84 cells contained a 1:1 mixture of Dulbecco's modified Eagle medium and Ham's F-12 medium (Cambrex Bioscience, Verviers, Belgium) supplemented with 10% (vol/vol) fetal calf serum (FCS) (Cambrex). HCT-8 cells were grown in RPMI 1640 (Cambrex) supplemented with 10% FCS, 2 mM l-glutamine, and 1 mM sodium pyruvate (Cambrex). The IPEC-J2 cell line (4) from jejunal epithelial cells of a neonatal piglet was maintained in a 1:1 mixture of Dulbecco's modified Eagle medium and Ham's F-12 medium (Cambrex) supplemented with 5% FCS. All cell cultures were grown at 37°C in 5% CO2 until they reached confluence, and then they were subcultured using a 0.1% trypsin-EDTA solution (Cambrex).
Interaction of stx2e-harboring E. coli with intestinal epithelial cells.
For the adherence assay, cells were grown on coverslips in six-well plates (Corning Inc., Corning, N.Y.) which were seeded with 1 × 106 T84 or HCT-8 cells/well or 2.5 × 105 IPEC-J2 cells/well; the plates were incubated at 37°C with 5% CO2 until the cultures were semiconfluent. One hundred fifty microliters of a bacterial overnight culture in Luria-Bertani broth (8 × 107 to 1 × 108 CFU) was added to the cells and allowed to attach for 5 h. The cells were then thoroughly washed with phosphate-buffered saline (Cambrex), fixed with 70% ethanol, and stained with 10% Giemsa stain (Merck). The adherence assay was performed in parallel in the absence and presence of 0.5% (wt/vol) d-mannose (Roth, Karlsruhe, Germany) in the growth medium. For quantitative analysis, the numbers of bacteria attached to one cell were determined, and the results were scored as follows: ++++, >100 bacteria attached; +++, 50 to 100 bacteria attached; ++, 10 to 50 bacteria attached; +, 1 to 10 bacteria attached; −, no bacteria attached. Enteropathogenic E. coli strain 2348/69 (O127:H6) (33) and E. coli K-12 strain C600 were used as positive and negative controls, respectively. To ensure that the bacteria interacted specifically with the intestinal epithelial cells, the assays with all strains were also performed in wells without cells. To test the effects of culture supernatants on the intestinal epithelial cells, strains were grown with aeration (180 rpm) in Luria-Bertani broth overnight, the bacterial cells were removed by centrifugation (8,000 rpm, 15 min), and the supernatants were filter sterilized (pore size, 0.22 μm; Schleicher & Schuell GmbH, Dassel, Germany). The presence of Stx2e was verified by the latex agglutination assay as described above.
RESULTS
Frequency of stx2e-containing E. coli in human stools.
A total of 747 STEC strains were isolated from 11,056 stools from patients with HUS or diarrhea or asymptomatic individuals. The 13 stx2e-harboring STEC strains isolated during the period studied thus accounted for 1.7% of all STEC isolates and were in 0.12% of all stool samples investigated. All STEC strains harboring stx2e were isolated from patients with mild diarrhea (n = 9) or from asymptomatic carriers (n = 4). None was associated with HUS.
Diagnostic characteristics of stx2e-harboring strains.
Table 2 compares the serotypes of human STEC isolates containing stx2e with those of stx2e-positive STEC isolates from pigs. The human isolates belonged to none of the serotypes associated with edema disease in piglets, and more than one-half were nontypeable with antisera against currently known E. coli O antigens, suggesting that they might represent new serotypes. All but one human strain and all but three porcine strains produced Stx2e, as demonstrated by a commercial latex agglutination assay. All isolates fermented sorbitol on SMAC agar within 24 h, and none grew on CT-SMAC agar. This is consistent with the absence in all of the strains of terF (Table 2), which is used as a marker for the ter cluster that encodes tellurite resistance (7, 51). Similarly, in accordance with the absence of the EHEC hlyA gene in all 26 strains (Table 2), none displayed an enterohemolytic phenotype on enterohemolysin agar. None of the strains investigated possessed the ureC gene (Table 2), a marker for the ure gene cluster (18, 32), and accordingly none of them produced urease.
TABLE 2.
Distribution of putative virulence genes and other genes investigated among stx2e-harboring E. coli strains isolated from humans and pigs
| Gene | Predicted product or markera | Human isolates (n = 13)b
|
Porcine isolates (n = 13)c
|
||
|---|---|---|---|---|---|
| No. positive | No. negative | No. positive | No. negative | ||
| stx2e | Stx2e | 13 | 0 | 13 | 0 |
| eae | Intimin | 0 | 13 | 0 | 13 |
| iha | Iha | 0 | 13 | 0 | 13 |
| efa1 | Efa1 | 0 | 13 | 0 | 13 |
| saa | Saa | 0 | 13 | 0 | 13 |
| sfpA | SfpA | 0 | 13 | 0 | 13 |
| fedA | Major subunit of F18 | 0 | 13 | 13 | 0 |
| orfA | AIDA-MP | 0 | 13 | 13 | 0 |
| orfB | AIDA | 0 | 13 | 11 | 2 |
| espI | Serine protease EspI | 0 | 13 | 10 | 3 |
| espP | Serine protease EspP | 0 | 13 | 0 | 13 |
| EHEC hlyA | EHEC hemolysin | 0 | 13 | 0 | 13 |
| etpD | Type II secretion system | 0 | 13 | 0 | 13 |
| katP | Catalase peroxidase | 0 | 13 | 0 | 13 |
| cdtd | CDT | 0 | 13 | 0 | 13 |
| subA | Subtilase cytotoxin A subunit | 0 | 13 | 0 | 13 |
| irp2 | HPI | 5 | 8 | 0 | 13 |
| fyu A | HPI | 5 | 8 | 0 | 13 |
| terF | Tellurite resistance | 0 | 13 | 0 | 13 |
| ureC | Urease | 0 | 13 | 0 | 13 |
Iha, iron-regulated gene A homologue adhesin; Efa1, EHEC factor for adherence; Saa, Shiga toxin-producing E. coli autoagglutinating adhesin; SfpA, major pillin subunit of sorbitol-fermenting STEC O157 plasmid-encoded fimbriae (Sfp); AIDA-MP, AIDA modifying protein (3); F18, fimbrial adhesin; CDT, cytolethal distending toxin.
The serotypes were O8:H10 (one strain), O8:H19 (one strain), O8:H- (one strain), O8:HNT (two strains), ONT:H10 (one strain), ONT:H19 (two strains), and ONT:H- (five strains).
The serotypes were O138:K81 (one strain), O139:K12 (one strain), O139:K82 (eight strains), O141:K45 (one strain), O141:K85 (one strain), and O149:K91 (one strain).
The presence of cdt-I, cdt-II, cdt-III, and cdt-V alleles (6) was investigated.
Distribution of virulence genes.
Genes encoding various adhesins, such as intimin, the iron-regulated gene A homologue adhesin (Iha) (49), EHEC factor for adherence (Efa1) (22, 34), STEC autoagglutinating adhesin (Saa) (37), and Sfp fimbriae (17), which are frequently found in STEC strains harboring stx2 and the variants stx2c and stx2d (15, 16, 17, 22), were not found in any of the 26 E. coli strains harboring the stx2e allele (Table 2). All of the porcine isolates, but none of the human isolates, possessed fedA, which encodes the major subunit of F18 fimbrial adhesin (35). Most of the porcine isolates (11 of 13) contained the orfB gene, which encodes AIDA (2) (Table 1). The orfA gene encoding a 45-kDa protein, which is required to modify AIDA so that it adheres to target cells (3), was present in all porcine isolates (Table 2). Ten of the 13 porcine isolates but none of the human isolates contained espI (Table 2), which is located on a pathogenicity island termed the locus of proteolysis activity which is inserted into selC of locus of enterocyte effacement-negative stx2d-harboring STEC strains (46). espI encodes a novel serine protease (EspI) which cleaves swine pepsin A and human apolipoprotein A-I (46). In contrast, the espP gene encoding plasmid-encoded serine protease (EspP) in E. coli O157:H7, as well as the other plasmid-borne genes of STEC strains, such as EHEC hlyA, katP, and etpD (48, 58), were absent from all 26 strains investigated (Table 2). Similarly, various alleles encoding cytolethal distending toxin (cdt-I, cdt-II, cdt-III, and cdt-V), some of which were previously identified in a subset of eae-negative STEC strains from patients (6), were absent from all human and porcine stx2e-harboring E. coli isolates (Table 2). Also, none of the strains investigated possessed the subA gene encoding the A subunit of the subtilase cytotoxin (38).
HPI is present in human STEC isolates that harbor stx2e.
Five of the 13 human E. coli isolates that possessed stx2e, but none of the corresponding porcine isolates, contained irp2 and fyuA (Table 2), which are components of an iron uptake-mediating gene cluster located on the high-pathogenicity island (HPI) (25). This prompted us to investigate whether a complete HPI is present in these five human isolates. Moreover, we compared HPIs of stx2e-harboring E. coli strains with previously characterized HPIs in STEC isolates belonging to serogroups O26 and O128 and Yersinia pestis (25). To do this, all five stx2e-harboring E. coli strains were subjected to 14 additional PCRs which target the other HPI genes or link consecutive genes (25). The results of the HPI analysis of these strains and a comparison of the HPIs of stx2e-harboring STEC isolates with the HPIs of other STEC strains and Y. pestis are summarized in Table 3. Each of the five stx2e-containing STEC isolates yielded amplicons that were of the same size as those detectable in the positive control STEC and Y. pestis strains in each of the PCRs targeting single HPI genes or links of the genes that constitute the siderophore yersiniabactin biosynthetic cluster (ybtS, ybtQ, ybtA, irp2, irp1, ybtU, ybtT, and ybtE) and the fyuA gene encoding the yersiniabactin receptor (Table 3) (PCRs IV to X and XII to VIII). Moreoever, similar to HPI of Y. pestis, but unlike HPIs of the STEC strains belonging to serogroups O26 and O128, the HPI in each of the five stx2e-harboring E. coli strains contained the insertion element IS100 (PCR XI) (Table 3). The sizes of the amplicons elicited from the integrase gene (int) (PCR III) (Table 3) in four of the five stx2e-containing STEC strains were identical to the size of the amplicon elicited from STEC O26 strain 5720/96 (Table 3), which was previously shown to possess a truncated int gene (25). In contrast, one remaining strain possessing stx2e (24059/97) yielded an int amplicon that was the same size as the amplicons of STEC O128 strain 3172/87 and Y. pestis strain (Table 3), both of which contain an intact int gene (25).
TABLE 3.
PCR analysis of HPIs in stx2e-harboring E. coli strains of human origin and comparison with HPIs of other STEC strains and Y. pestis
| Strain or speciesa | Serogroup | Results of PCRs targeting HPI genes or their linksb
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR I, asnT-int(1) | PCR II, asnT-int(2) | PCR III, int | PCR IV, ybtS | PCR V, ybtQ | PCR VI, ybtA | PCR VII, irp2 | PCR VIII, irp1 | PCR IX, ybtE-fyuA | PCR X, fyuA | PCR XI, IS100 | PCR XII, int-ybtS | PCR XIII, ybtS-ybtQ | PCR IV, ybtQ-ybtA | PCR XV, ybtA-irp2 | PCR XVI, irp2-irp1 | PCR XVII, irp1-ybtT | PCR XVIII, ybtT-fyuA | ||
| 5720/96c | O26 | 900 | 1,100 | 900 | 160 | 800 | 230 | 280 | 240 | 360 | 780 | − | 800 | 2,800 | 2,800 | 1,300 | 300 | 1,700d | 2,500 |
| 3172/97c | O128 | 1,200 | 1,400 | 1,200 | 160 | 800 | 230 | 280 | 240 | 360 | 780 | − | 800 | 2,800 | 2,800 | 1,300 | 300 | 1,700d | 2,500 |
| Y. pestisc | NAg | 1,255 | 1,500 | 1,203 | 160 | 797 | 233 | 286 | 237 | 359 | 780 | 100 | 830 | 2,797 | 2,805 | 1,340 | 300 | 1,762 | 2,518 |
| 24059/97 | ONT | +e | +e | +e | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 3357/98 | ONT | +f | +f | +f | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 665/00 | O8 | +f | +f | +f | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| E01/233 | ONT | +f | +f | +f | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| E02/25 | ONT | +f | +f | +f | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| E. coli C600 | NAg | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
E. coli strains 5720/96 and 3172/97 and Y. pestis strain KIM6 were analyzed previously to determine their HPI structures (25) and were used as positive controls in this study; E. coli C600 was a negative control. The other five strains are human STEC isolates containing stx2e.
The numbering of the PCRs and the sequences targeted are the same as described by Karch et al. (25). int is the integrase gene; ybtS, ybtQ, ybtA, irp2, irp1, ybtE, and ybtT are components of the siderophore yersiniabactin biosynthetic gene cluster; fyuA encodes yersiniabactin receptor; and IS100 is an insertion element. PCR results: +, amplicon of the same size as that elicited from the positive control strains was obtained, unless indicated otherwise; −, no PCR product was obtained.
The values for PCRs I to XVIII indicate the sizes (in bp) of amplicons obtained in the PCRs with the positive control strains. −, no PCR product was obtained. The sizes of the Y. pestis PCR products are the sizes in the previously published HPI sequence (25).
The 1,700-bp amplicon indicates that the ybtU gene located between irp1 and ybtT is present and intact.
+, amplicons that were the same size as those from Y. pestis and E. coli strain 3172/97 were obtained.
+, amplicons that were the same size as those from E. coli strain 5720/96 were obtained.
NA, not available.
The integration site of HPI in stx2e-harboring STEC strains was investigated using PCRs (25) linking the int gene of HPI with three different tRNA loci (asnT, asnU, and asnV). These sites are used by HPI to integrate into the chromosomes of pathogenic yersiniae (10). Amplicons that were 900 and 1,100 bp long, similar to those in STEC O26 strain 5720/96, were obtained from four strains in two different PCRs connecting asnT with the int gene (PCRs I and II) (Table 3). In these two PCRs, the remaining stx2e-positive strain yielded amplicons that were 1,200 and 1,500 bp long and were similar to those in Y. pestis and STEC strain 3172/97 (Table 3). asnU-int and asnV-int PCRs were negative for all strains investigated (data not shown). These findings demonstrate that in STEC strains harboring stx2e HPI is located near asnT, similar to the location in STEC O26 and O128 and Y. pestis (25). These PCR analyses also confirmed that four of the five strains harboring stx2e (3357/98, 665/00, E01/233, and E02/25), like STEC O26 strain 5720/96, possess an HPI with a truncated int gene. In contrast, the remaining stx2e-positive strain, 24059/97, like STEC O128 strain 3172/97 and Y. pestis, contains an HPI with an intact int gene (Table 3). Together, these data demonstrate that each of the five STEC human isolates harboring stx2e possesses a complete HPI whose structure is closer to that of Y. pestis than to that of STEC strains belonging to serogroups O26 and O128.
Interaction of stx2e-containing STEC with cultured intestinal epithelial cells.
The known STEC adhesins are absent from E. coli strains containing stx2e (Table 2). Therefore, we investigated whether stx2e-containing STEC can adhere to intestinal epithelial cells in vitro. As shown in Table 4, all human strains adhered with various intensities to human cell lines T84 and HCT-8. The presence of d-mannose in the culture medium did not inhibit the adherence of most of these strains; the only exception was strain 3096/00 (Table 4), which adhered more strongly to T84 cells in the absence than in the presence of 0.5% d-mannose. This suggests that an as-yet-unidentified adhesin(s), different from type 1 pili, plays a role in the adherence of human STEC strains harboring stx2e to human intestinal epithelial cells. However, none of the human stx2e-containing STEC strains adhered to pig intestinal epithelial cell line IPEC-J2 (Table 4).
TABLE 4.
Interaction of stx2e-harboring STEC strains from humans and pigs with intestinal epithelial cells from homologous and heterologous hosts
| Strain | Serotype | Source | Adherence to cell linea:
|
||
|---|---|---|---|---|---|
| T84 | HCT-8 | IPEC-J2 | |||
| 2771/97 | ONT:H- | Human | +++ | ++ | − |
| 3054/97 | O8:HNT | Human | ++ | +++ | − |
| 3583/97 | ONT:H- | Human | + | +++ | − |
| 24059/97 | ONT:H10 | Human | ++ | +++ | − |
| 24066/97 | ONT:H- | Human | + | + | − |
| 26725/97 | ONT:H- | Human | + | +++ | − |
| 3229/98 | O8:H- | Human | + | ++ | − |
| 3357/98 | ONT:H19 | Human | + | ++ | − |
| 3615/99 | O8:H10 | Human | ++ | +++ | − |
| 665/00 | O8:H19 | Human | +++ | + | − |
| 3096/00 | O8:HNT | Human | ++ | ++ | − |
| E01/233 | ONT:H- | Human | + | ++ | − |
| E02/25 | ONT:H19 | Human | ++ | +++ | − |
| S103G | O141:K45 | Pig | CLb | CL | + |
| S105G | O139:K12 | Pig | 80%c | CL | + |
| S115G | O139:K82 | Pig | CL | CL | ++ |
| S116G | O139:K82 | Pig | CL | CL | ++ |
| S123G | O139:K82 | Pig | CL | CL | ++ |
| S125G | O139:K82 | Pig | CL | CL | ++ |
| S126G | O139:K82 | Pig | CL | CL | − |
| S128G | O139:K82 | Pig | 90%c | CL | ++++ |
| S130G | O149:K91 | Pig | 90%c | CL | + |
| S131G | O139:K82 | Pig | CL | CL | + |
| S132G | O139:K82 | Pig | CL | CL | +++ |
| S138G | O139:K82 | Pig | CL | CL | +++ |
| E57 | O138 | Pig | CL | CL | ++ |
| 2348/69 | O127:H6 | Human EPEC (positive control)d | ++ | ++++ | − |
| C600 | Not available | Laboratory strain (negative control) | − | + | − |
After 5 h of incubation of bacteria with cells in the presence of 0.5% d-mannose, the adherence was quantified based on the number of bacteria attached to one cell, as follows: ++++, >100 bacteria; +++, 50 to 100 bacteria; ++, 10 to 50 bacteria; +, 1 to 10 bacteria; −, no bacteria attached.
CL, complete lysis of cells after 5 h of incubation with bacteria.
Percentage of cells which underwent lysis after 5 h of incubation with bacteria.
EPEC, enteropathogenic E. coli.
In contrast to human strains, which lysed none of the intestinal epithelial cell lines investigated, most pig isolates completely lysed human intestinal epithelial cells during a 5-h incubation (Table 4). However, with an equally long incubation period, the pig isolates did not lyse IPEC-J2 cells (Table 4), but 12 of the 13 strains adhered (Table 4). Representative patterns of the interaction of human and pig STEC strains with intestinal epithelial cells from homologous and heterologous hosts are shown in Fig. 1. No lysis of human intestinal epithelial cells was observed with sterile-filtered culture supernatants of the 26 strains, 22 of which contained Stx2e as determined by the latex agglutination assay.
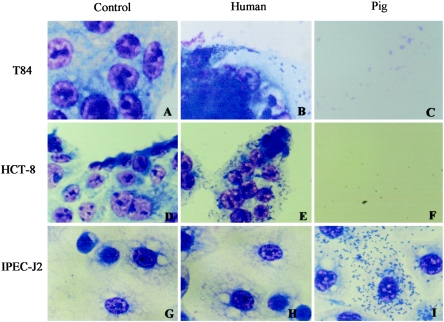
FIG. 1.
Interaction of human and pig stx2e-containing STEC isolates with intestinal epithelial cells from homologous and heterologous hosts. (A, D, and G) Control (untreated) cells of cell lines T84 (human), HCT-8 (human), and IPEC-J2 (pig), respectively. (B) Adherence of human strain 24059/97 to T84 cells. (E) Adherence of human strain 3583/97 to HCT-8 cells. (H) Lack of adherence of human strain E02/25 to IPEC-J2 cells. (C and F) Lysis of T84 and HCT-8 cells by pig strains S103G and S115G, respectively. (I) Adherence of pig strain S128G to IPEC-J2 cells. No adherence was observed with any of the strains tested in wells without intestinal epithelial cells, indicating that the adherence is cell dependent.
DISCUSSION
STEC ecology is complex and only partially understood. This is due to the marked heterogeneity of STEC strains. Epidemiological studies and molecular profiling indicate that most STEC infections in humans are food-borne and that the source of the pathogen is a nonhuman reservoir (26). Recent studies indicate that besides ruminants, swine also harbor STEC capable of causing human illness (11, 14, 43). A study conducted in the United States showed that 13% and 6% of STEC strains isolated from swine feces during a farm survey possessed the stx1 and stx2 genes, respectively (14). These genes are typically found in human STEC strains; however, the majority (80%) of these strains harbored the stx2e variant (14) known to cause edema disease in weaned pigs (20). Although strains causing pig edema disease have been extensively characterized (1, 21, 35), to our knowledge this is the first detailed analysis of phenotypic and molecular characteristics of human stx2e-containing STEC isolates. Both serotyping and molecular profiling demonstrated that Stx2e-producing STEC strains that cause human diseases are different from the strains that cause edema disease in pigs. Moreover, the two groups vary in their interactions with intestinal epithelial cells.
Most laboratories do not routinely screen for Stx2e-producing STEC in the way that they screen for E. coli O157:H7. The former strains would be overlooked on sorbitol MacConkey agar because all isolates investigated ferment sorbitol. They also do not grow on CT-SMAC agar, a medium routinely used to isolate E. coli O157:H7 (26, 50), and this is due to their tellurite sensitivity, as demonstrated in this study. Furthermore, EHEC hlyA, the structural gene encoding EHEC hemolysin (45), was not present in any of the stx2e-harboring strains investigated. As a result, none of the strains showed the characteristic enterohemolytic phenotype. Although EHEC hemolysin production is a useful marker for the detection of STEC (9, 48, 53), it cannot be used to detect strains producing Stx2e. In addition, all Stx2e-producing STEC investigated lacked ureC, which we used as a marker for the ure operon (18, 24). It has recently been reported (32) that the presence of ureC distinguishes STEC strains belonging to the major serogroups associated with human diseases (O157, O26, O103, O111, and O145) from diarrheagenic E. coli belonging to other pathogroups (32). On the basis of this finding, ureC has been recommended as a target in the screening for such STEC strains (32). Our data, on the other hand, demonstrate that STEC strains harboring stx2e would be missed by this screening procedure because of the absence of ureC. Thus, because of the poor repertoire of diagnostically useful phenotypic markers in stx2e-positive STEC, the detection of the stx2e gene with PCR, as used in this study, followed by colony blot hybridization, should be superior to the culture methods for screening primary stool cultures. The PCR approach enabled us to show that the frequency with which strains possessing the stx2e allele occur in human stools is very low (0.12%) and that such strains can be present in stools of asymptomatic subjects. This raises a question about the etiological role of these strains in human diseases. In this study, we were unable to identify stx2e-positive STEC strains in association with bloody diarrhea or HUS, although such strains have been isolated from an HUS patient by other workers (52). The absence of data on the anti-O157 lipopolysaccharide antibody response in the HUS patient of Thomas et al. (52), however, does not allow exclusion of a coinfection with E. coli O157:H7, which might have caused the HUS.
It is well established that STEC strains produce factors other than Stx that are potentially injurious to the human host (6, 8, 22, 23, 28, 34, 37, 38, 49). Intimin, the best-characterized STEC adhesin (33, 59), mediates attaching and effacing lesions in vitro and in animal models (33), but there are several other putative adherence factors, including Iha (49), Saa (37), and Efa1 (34), which also mediate adherence in vitro. However, all of these factors are absent from the stx2e-containing strains investigated here. Notably, we observed different patterns when the strains interacted with intestinal epithelial cells. Whereas stx2e-containing STEC strains from humans adhered to human epithelial cells, they did not adhere to pig intestinal epithelial cells. Although the molecular basis of this phenomenon is not known, differences in receptor-binding capacities of intestinal epithelial cells from different hosts are likely to be involved. Moreover, porcine strains were able to lyse human, but not porcine, intestinal epithelial cells. The cell lysis is not attributable to Stx2e because of its rapid occurrence (after 5 h) and because T84 cells do not express Gb3 and Gb4, the receptors for Stx2e (29, 53, 55). Moreover, sterile culture supernatants containing Stx2e displayed no visible lysis of the intestinal epithelial cells used in this study. The factors determining the interaction of Stx2e-producing STEC strains with intestinal epithelial cells from the homologous and heterologous species are not known, but it is likely that the differences in the interaction are due to differences in the molecular mechanisms involved. We have initiated transposon mutagenesis experiments to determine which genes are involved in the ability of porcine strains to cause the cell lysis.
It is noteworthy that the plasmid-encoded cytolysin EHEC hemolysin and cytolethal distending toxin, a potent toxin produced by a subset of STEC strains associated with human disease (6, 23, 39) and by Stx2f-producing STEC strains found in pigeons (31), are absent from STEC strains producing Stx2e. Furthermore, in addition to having no EHEC hlyA, all Stx2e-producing strains also lack other plasmid-borne genes, such as katP, espP, and etpD, which are usually present in STEC strains harboring stx1 and stx2 (9, 48, 58) and their variants (stx1c, stx2c, or stx2d) (16, 48). Our finding that known putative virulence determinants of STEC strains pathogenic for humans are absent from Stx2e-producing human isolates extends a previous observation by our group (13). Although we found a close relatedness between one human and four porcine E. coli O101 strains by DNA fingerprinting, the virulence factors typically found in porcine STEC (i.e., heat-stable and heat-labile enterotoxins and F107 fimbriae) were absent from the human isolate (13). Moreover, this isolate also lacked virulence factors (eae and EHEC hemolysin) typical of STEC pathogenic for humans (13). This indicated that the pathogenicity of the human Stx2e-producing E. coli O101 strain might involve different mechanisms. Taken together, these data demonstrate that the mechanisms of pathogenicity of Stx2e-producing STEC strains associated with human diseases warrant further investigation.
Although swine are a potential reservoir of STEC strains that cause human illness (11, 14, 43), in an analysis of 11,056 stool samples from humans we were unable to detect the Stx2e-producing strains belonging to serogroups O138, O139, and O141 which are associated with edema disease in piglets (1, 35). Furthermore, a detailed characterization of the Stx2e-producing strains isolated from humans showed that they lack virulence factors, such as AIDA and F18 adhesins, that are frequently found in Stx2e-producing strains associated with pig edema disease (1, 21, 35). Therefore, it is unlikely that the Stx2e-producing STEC strains that cause pig edema disease are human pathogens. Moreover, only some of the serotypes identified among the Stx2e-producing STEC strains from humans in this study and in a study by Beutin et al. (O43:H30, O60:H4, O91:H21, Ont:H10, and Ont:H19) (5) have been found among the stx2e-harboring STEC strains isolated from healthy pigs (14). These data indicate that there may be additional, as-yet-unknown reservoirs of STEC strains harboring stx2e and that the extent to which these porcine strains play a role in the epidemiology of human infections needs further investigation. The majority of Stx2e-producing STEC strains isolated from humans in this study failed to agglutinate with O antisera currently available for serotyping of E. coli. Therefore, the development of diagnostic sera against such strains, which might represent novel O serogroups, would improve laboratory diagnosis of them and thus increase our understanding of their epidemiology.
The presence of the HPI of pathogenic yersiniae in a subset of the human stx2e-containing STEC strains is noteworthy. This island confers virulence in highly pathogenic Yersinia species. HPI is also widely distributed among other Enterobacteriaceae (36), especially extraintestinal pathogenic E. coli strains that cause bacteremia and urosepsis in humans (47) and septicemia in poultry (12), and it contributes to the virulence of such strains (47). Recently, HPI was also found in certain serotypes of STEC pathogenic to humans, and it has been hypothesized that HPI can contribute to the fitness of such strains in diverse environments under iron limitation conditions (25). HPI contains a P4-like integrase (int) gene at the 5′ end and the fyuA gene encoding the receptor for yersiniabactin and pesticin at the 3′ end of the HPI core. A cluster of genes encoding the siderophore yersiniabactin is located between int and fyuA (25). Moreover, HPI in Y. pestis contains the insertion element IS100 upstream of fyuA (25). Our examination of the structure of HPI, identified in the five human stx2e-containing STEC strains, showed that each of these strains contained a complete HPI structurally similar to HPI in Y. pestis. However, three of the five HPI-positive STEC strains in this study were isolated from patients with diarrhea, and two were isolated from asymptomatic carriers, making it impossible to speculate on the putative contribution of HPI to the pathogenicity of such strains.
In conclusion, Stx2e-producing E. coli strains, although having stx2e in common, appear to have independently imported and exchanged virulence determinants. This has led to differences in the pathogenicity profiles and forms of disease, suggesting that there has been specific host adaptation.
Acknowledgments
This study was supported by a grant from the Interdisciplinary Center of Clinical Research (IZKF) Münster (project Ka2/061/04), by grants from the Bundesministerium für Bildung und Forschung (BMBF) Project Network of Competence Pathogenomics Alliance (“Functional Genomic Research on Enterohaemorrhagic, Enteropathogenic and Enteroaggregative Escherichia coli”; grants 119523 and 207800), and by a grant from the Deutsche Forschungsgemeinschaft (DFG) (grant Wi-1436/4-3).
We are very grateful to Angelika Fruth and Helmut Tschäpe (Robert Koch Institute, Wernigerode, Germany) for serotyping the human STEC isolates and to Philip I. Tarr (Washington University School of Medicine, St. Louis, Mo.) for fruitful and extensive discussions concerning the manuscript. We thank M. Hülsmann and N. Brandt for excellent technical assistance.
REFERENCES
- 1.Aarestrup, F. M., S. E. Jorsal, P. Ahrens, N. E. Jensen, and A. Meyling. 1997. Molecular characterization of Escherichia coli strains isolated from pigs with edema disease. J. Clin. Microbiol. 35:20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz, I., and M. A. Schmidt. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benz, I., and M. A. Schmidt. 1992. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27). Infect. Immun. 60:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berschneider, H. M. 1989. Development of normal cultured small intestinal epithelial cell line which transports Na and Cl. Gastroenterology 96:A41. [Google Scholar]
- 5.Beutin, L., G. Krause, S. Zimmermann, S. Kaulfuss, and K. Gleier. 2004. Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J. Clin. Microbiol. 42:1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielaszewska, M., M. Fell, L. Greune, R. Prager, A. Fruth, H. Tschäpe, M. A. Schmidt, and H. Karch. 2004. Characterization of cytolethal distending toxin genes and expression in Shiga toxin-producing Escherichia coli strains of non-O157 serogroups. Infect. Immun. 72:1812-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielaszewska, M., P. I. Tarr, H. Karch, W. Zhang, and W. Mathys. 2005. Phenotypic and molecular analysis of tellurite resistance among enterohemorrhagic Escherichia coli O157:H7 and sorbitol-fermenting O157:NM clinical isolates. J. Clin. Microbiol. 43:452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielaszewska, M., B. Sinha, T. Kuczius, and H. Karch. 2005. Cytolethal distending toxin from Shiga toxin-producing Escherichia coli O157 causes irreversible G2/M arrest, inhibition of proliferation and death of human endothelial cells. Infect. Immun. 73:552-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielaszewska, M., W. Zhang, P. I. Tarr, A.-K. Sonntag, and H. Karch. 2005. Molecular profiling and phenotype analysis of Escherichia coli O26:H11 and O26:NM: secular and geographic consistency of enterohemorrhagic and enteropathogenic isolates. J. Clin. Microbiol. 43:4225-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchrieser, C., R. Brosch, S. Bach, A. Guiyoule, and E. Carniel. 1998. The high pathogenicity island of Yersinia pseudotuberculosis can be inserted into any of the three chromosomal Asn tRNA genes. Mol. Microbiol. 30:965-978. [DOI] [PubMed] [Google Scholar]
- 11.Cornick, N. A., and A. F. Helgerson. 2004. Transmission and infectious dose of Escherichia coli O157:H7 in swine. Appl. Environ. Microbiol. 70:5331-5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewers, C., T. Janssen, S. Kiessling, H. C. Philipp, and L. H. Wieler. 2004. Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Vet. Microbiol. 104:91-101. [DOI] [PubMed] [Google Scholar]
- 13.Franke, S., D. Harmsen, A. Caprioli, D. Pierard, L. H. Wieler, and H. Karch. 1995. Clonal relatedness of Shiga-like toxin-producing Escherichia coli O101 strains of human and porcine origin. J. Clin. Microbiol. 33:3174-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratamico, P. M., L. K. Bagi, E. J. Bush, and B. T. Solow. 2004. Prevalence and characterization of Shiga toxin-producing Escherichia coli in swine feces recovered in the National Animal Health Monitoring System's Swine 2000 study. Appl. Environ. Microbiol. 70:7173-7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich, A. W., M. Bielaszewska, W.-L. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich, A. W., J. Borell, M. Bielaszewska, A. Fruth, H. Tschäpe, and H. Karch. 2003. Shiga toxin 1c-producing Escherichia coli strains: phenotypic and genetic characterization and association with human disease. J. Clin. Microbiol. 41:2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrich, A. W., K. V. Nierhoff, M. Bielaszewska, A. Mellmann, and H. Karch. 2004. Phylogeny, clinical associations, and diagnostic utility of the pilin subunit gene (sfpA) of sorbitol-fermenting, enterohemorrhagic Escherichia coli O157:H−. J. Clin. Microbiol. 42:4697-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrich, A. W., R. Köck, M. Bielaszewska, W. Zhang, H. Karch, and W. Mathys. 2005. Distribution of the urease gene cluster among and urease activities of enterohemorrhagic Escherichia coli O157 isolates from humans. J. Clin. Microbiol. 43:546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber, A., H. Karch, F. Allerberger, H. M. Verweyen, and L. B. Zimmerhackl. 2002. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997-2000, in Germany and Austria: a prospective study. J. Infect. Dis. 186:493-500. [DOI] [PubMed] [Google Scholar]
- 20.Gyles, C. L. 1993. Escherichia coli, p. 164-187. In C. L. Gyles and C. O. Thoen (ed.), Pathogenesis of bacterial infections in animals. Iowa State University Press, Ames.
- 21.Ha, S. K., C. Choi, and C. Chae. 2003. Prevalence of a gene encoding adhesin involved in diffuse adherence among Escherichia coli isolates in pigs with postweaning diarrhea or edema disease. J. Vet. Diagn. Investig. 15:378-381. [DOI] [PubMed] [Google Scholar]
- 22.Janka, A., M. Bielaszewska, U. Dobrindt, and H. Karch. 2002. Identification and distribution of the enterohemorrhagic Escherichia coli factor for adherence (efa1) gene in sorbitol-fermenting Escherichia coli O157:H−. Int. J. Med. Microbiol. 292:207-214. [DOI] [PubMed] [Google Scholar]
- 23.Janka, A., M. Bielaszewska, U. Dobrindt, L. Greune, M. A. Schmidt, and H. Karch. 2003. The cytolethal distending toxin (cdt) gene cluster in enterohemorrhagic Escherichia coli O157:H− and O157:H7: characterization and evolutionary considerations. Infect. Immun. 71:3634-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janka, A., G. Becker, A.-K. Sonntag, M. Bielaszewska, U. Dobrindt, and H. Karch. 2005. Presence and characterization of a mosaic genomic island which distinguishes sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H− from E. coli O157:H7. Appl. Environ. Microbiol. 71:4875-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karch, H., S. Schubert, D. Zhang, W. Zhang, H. Schmidt, T. Ölschläger, and J. Hacker. 1999. A genomic island, termed high pathogenicity island, is present in certain non-O157 Shiga toxin-producing Escherichia coli clonal lineages. Infect. Immun. 67:5994-6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karch, H., M. Bielaszewska, M. Bitzan, and H. Schmidt. 1999. Epidemiology and diagnosis of Shiga toxin-producing Escherichia coli infections. Diagn. Microbiol. Infect. Dis. 34:229-243. [DOI] [PubMed] [Google Scholar]
- 27.Konowalchuk, J., J. I. Speirs, and S. Stavric. 1977. Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lathem, W. W., T. E. Grys, S. E. Witowski, A. G. Torres, J. B. Kaper, P. I. Tarr, and R. A. Welch. 2002. StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol. Microbiol. 45:277-288. [DOI] [PubMed] [Google Scholar]
- 29.Lingwood, C. A. 1996. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 4:147-153. [DOI] [PubMed] [Google Scholar]
- 30.Matise, I., N. A. Cornick, J. E. Samuel, and H. W. Moon. 2003. Binding of Shiga toxin 2e to porcine erythrocytes in vivo and in vitro. Infect. Immun. 71:5194-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morabito, S., G. Dell'Omo, U. Agrimi, H. Schmidt, H. Karch, T. Cheasty, and A. Caprioli. 2001. Detection and characterization of Shiga toxin-producing Escherichia coli in feral pigeons. Vet. Microbiol. 82:275-283. [DOI] [PubMed] [Google Scholar]
- 32.Nakano, M., T. Iida, M. Ohnishi, K. Kurokawa, A. Takahashi, T. Tsukamoto, T. Yasunaga, T. Hayashi, and T. Honda. 2001. Association of the urease gene with enterohemorrhagic Escherichia coli strains irrespective of their serogroups. J. Clin. Microbiol. 39:4541-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 35.Niewerth, U., A. Frey, T. Voss, C. Le Bouguenec, G. Baljer, S. Franke, and M. A. Schmidt. 2001. The AIDA autotransporter system is associated with F18 and stx2e in Escherichia coli isolates from pigs diagnosed with edema disease and postweaning diarrhea. Clin. Diagn. Lab. Immunol. 8:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oelschlaeger, T. A., D. Zhang, S. Schubert, E. Carniel, W. Rabsch, H. Karch, and J. Hacker. 2003. The high-pathogenicity island is absent in human pathogens of Salmonella enterica subspecies I but present in isolates of subspecies III and VI. J. Bacteriol. 185:1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paton, A. W., P. Srimanote, M. C. Woodrow, and J. C. Paton. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 69:6999-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paton, A. W., P. Srimanote, U. M. Talbot, H. Wang, and J. C. Paton. 2004. A new family of potent AB5 cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 200:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickett, C. L., R. B. Lee, A. Eyigor, B. Elitzur, E. M. Fox, and N. A. Strockbine. 2004. Patterns of variations in Escherichia coli strains that produce cytolethal distending toxin. Infect. Immun. 72:684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierard, D., L. Huyghens, S. Lauwers, and H. Lior. 1991. Diarrhoea associated with Escherichia coli producing porcine oedema disease verotoxin. Lancet 338:762. [DOI] [PubMed] [Google Scholar]
- 41.Pierard, D., G. Muyldermas, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prager, R., A. Fruth, and H. Tschäpe. 2003. Subtyping of pathogenic E. coli strains using flagellar (H) antigens: serotyping vs. fliC-polymorphism. Int. J. Med. Microbiol. 292:477-486. [DOI] [PubMed] [Google Scholar]
- 43.Rios, M., V. Prado, M. Trucksis, C. Arellano, C. Borie, M. Alexandre, A. Fica, and M. M. Levine. 1999. Clonal diversity of Chilean isolates of enterohemorrhagic Escherichia coli from patients with hemolytic-uremic syndrome, asymptomatic subjects, animal reservoirs, and food products. J. Clin. Microbiol. 37:778-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salvadori, M. R., A. T. Yamada, and T. Yano. 2001. Morphological and intracellular alterations induced by cytotoxin VT2y produced by Escherichia coli isolated from chickens with swollen head syndrome. FEMS Microbiol. Lett. 197:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt, H., W.-L. Zhang, U. Hemmrich, S. Jelacic, W. Brunder, P. I. Tarr, U. Dobrindt, J. Hacker, and H. Karch. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schubert, S., B. Picard, S. Gouriou, J. Heesemann, and E. Denamur. 2002. Yersinia high-pathogenicity island contributes to virulence in Escherichia coli causing extraintestinal infections. Infect. Immun. 70:5335-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonntag, A., R. Prager, M. Bielaszewska, W. Zhang, A. Fruth, H. Tschape, and H. Karch. 2004. Phenotypic and genotypic analyses of enterohemorrhagic Escherichia coli O145 strains from patients in Germany. J. Clin. Microbiol. 42:954-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarr, P. I., S. S. Bilge, J. C. Vary, S. Jelacic, R. L. Habeeb, T. R. Ward, M. R. Baylor, and T. E. Besser. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 68:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga toxin-producing Escherichia coli and the haemolytic uraemic syndrome. Lancet 365:1073-1086. [DOI] [PubMed] [Google Scholar]
- 51.Taylor, D., M. Rooker, M. Keelen, L.-K. Ng, I. Martin, N. T. Perna, N. T. V. Burland, and F. R. Blattner. 2002. Genome variability of O islands encoding tellurite resistance in enterohemorrhagic Escherichia coli O157:H7 isolates. J. Bacteriol. 184:4690-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas, A., T. Cheasty, H. Chart, and B. Rowe. 1994. Isolation of Vero cytotoxin-producing Escherichia coli serotypes O9ab:H- and O101:H-carrying VT2 variant gene sequences from a patient with haemolytic uraemic syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 13:1074-1076. [DOI] [PubMed] [Google Scholar]
- 53.Valdivieso-Garcia, A., D. L. MacLeod, R. C. Clarke, C. L. Gyles, C. Lingwood, B. Boyd, and A. Durette. 1996. Comparative cytotoxicity of purified Shiga-like toxin-IIe on porcine and bovine aortic endothelial and human colonic adenocarcinoma cells. J. Med. Microbiol. 45:331-337. [DOI] [PubMed] [Google Scholar]
- 54.Vernozy-Rozand, C., M. P. Montet, Y. Bertin, F. Trably, J. P. Girardeau, C. Martin, V. Livrelli, and L. Beutin. 2004. Serotyping, stx2 subtyping, and characterization of the locus of enterocyte effacement island of Shiga toxin-producing Escherichia coli and E. coli O157:H7 strains isolated from the environment in France. Appl. Environ. Microbiol. 70:2556-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstein, D. L., M. P. Jackson, J. E. Samuel, R. K. Holmes, and A. D. O'Brien. 1988. Cloning and sequencing of a Shiga-like toxin type II variant from an Escherichia coli strain responsible for edema disease of swine. J. Bacteriol. 170:4223-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, W., M. Bielaszewska, T. Kuczius, and H. Karch. 2002. Identification, characterization and distribution of a Shiga toxin 1 gene variant (stx1c) in Escherichia coli isolated from humans. J. Clin. Microbiol. 40:1441-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, W., M. Bielaszewska, A. W. Friedrich, T. Kuczius, and H. Karch. 2005. Transcriptional analysis of genes encoding Shiga toxin 2 and its variants in Escherichia coli. Appl. Environ. Microbiol. 71:558-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, W.-L., M. Bielaszewska, A. Liesegang, H. Tschäpe, H. Schmidt, M. Bitzan, and H. Karch.. 2000. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26. J. Clin. Microbiol. 38:2134-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, W.-L., B. Kohler, E. Oswald, L. Beutin, H. Karch, S. Morabito, A. Caprioli, S. Suerbaum, and H. Schmidt. 2002. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J. Clin. Microbiol. 40:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]