Abstract
Gas chromatography-mass spectrometry and nuclear magnetic resonance spectroscopy were used to study the metabolism of deuterated n-alkanes (C6 to C12) and 1-13C-labeled n-hexane by a highly enriched sulfate-reducing bacterial culture. All substrates were activated via fumarate addition to form the corresponding alkylsuccinic acid derivatives as transient metabolites. Formation of d14-hexylsuccinic acid in cell extracts from exogenously added, fully deuterated n-hexane confirmed that this reaction was the initial step in anaerobic alkane metabolism. Analysis of resting cell suspensions amended with 1-13C-labeled n-hexane confirmed that addition of the fumarate occurred at the C-2 carbon of the parent substrate. Subsequent metabolism of hexylsuccinic acid resulted in the formation of 4-methyloctanoic acid, and 3-hydroxy-4-methyloctanoic acid was tentatively identified. We also found that 13C nuclei from 1-13C-labeled n-hexane became incorporated into the succinyl portion of the initial metabolite in a manner that indicated that 13C-labeled fumarate was formed and recycled during alkane metabolism. Collectively, the findings obtained with a sulfate-reducing culture using isotopically labeled alkanes augment and support the previously proposed pathway (H. Wilkes, R. Rabus, T. Fischer, A. Armstroff, A. Behrends, and F. Widdel, Arch. Microbiol. 177:235-243, 2002) for metabolism of deuterated n-hexane by a denitrifying bacterium.
The anaerobic biodegradation of n-alkanes is now a well-documented process that has been demonstrated to occur under nitrate-reducing, sulfate-reducing, and methanogenic conditions. Several sulfate-reducing and denitrifying bacterial strains and enrichment cultures capable of complete n-alkane mineralization have been described (1, 7, 8, 13, 16, 17, 19, 20). Anaerobic metabolism of n-alkanes has been shown to occur by at least two mechanisms, both of which include addition of carbon to the parent substrate. An activation mechanism involves an initial carboxylation reaction. Using the sulfate-reducing isolate strain Hxd3, So et al. (21) showed that 13C-bicarbonate was added to the C-3 position of the parent alkane, while subsequent reactions involved elimination of the two adjacent terminal carbon atoms, yielding a fatty acid with one less carbon atom than the original hydrocarbon. Alternately, the primary attack on an alkane can be initiated by addition to the double bond of fumarate with the formation of alkylsuccinic acid derivatives (13, 18). This mechanism seems to be more widespread as it has now been found in three separate anaerobic cultures (6, 7, 13, 18, 24). This reaction resembles the anaerobic biodegradation of toluene and xylene, in which the aryl methyl carbon of the substrate is added across the double bond of fumarate to form benzylsuccinate or methylated derivatives (2, 3). However, alkanes are not activated at the terminal methyl group. The succinyl moiety is attached to the parent alkane at the C-2 or C-3 position (7, 13, 18). The biochemistry of this conversion is incomplete, but electron paramagnetic resonance spectroscopy has suggested a possible radical mechanism since an organic radical was detected in n-hexane-grown cells of the denitrifying bacterium strain HxN1 (18). Similarly, the benzylsuccinate synthase catalyzing toluene activation was shown to be a glycyl radical enzyme (12, 14).
Subsequent steps in anaerobic alkane metabolism are beginning to be elucidated. Studies of strain HxN1 grown with deuterated n-hexane or fumarate revealed the formation of a suite of fatty acid metabolites (23). The labeling patterns associated with identification of 4-methyloctanoic acid suggested that hexylsuccinate [(1-methylpentyl)succinate] underwent a carbon skeleton rearrangement and decarboxylation step prior to further oxidation. The identification of other transient metabolites, such as 4-methyloct-2-enoic acid and 3-hydroxy-4-methyloctanoic acid, allowed Wilkes et al. (23) to propose an anaerobic alkane pathway that included the regeneration of fumarate from propionate in a reaction sequence involving yet another carbon skeleton rearrangement similar to the known methylmalonyl-coenzyme A (methylmalonyl-CoA) pathway (11).
We examined the metabolism of isotopically labeled n-alkanes by a highly enriched sulfate-reducing bacterial culture. We focused on the metabolic fate of 1-13C-labeled n-hexane rather than on deuterium-labeled parent alkanes. Our findings largely agree with the pathway for alkane degradation by a denitrifying bacterium initially proposed by Wilkes et al. (23). However, we could neither confirm nor rule out the possibility that a carbon skeleton rearrangement metabolite was produced during alkane metabolism.
MATERIALS AND METHODS
Sulfate-reducing enrichment culture.
An alkane-degrading culture (ALDC) was enriched from oily sludge collected from a U.S. Navy wastewater storage facility. The enrichment was cultivated under strictly anaerobic conditions (sulfate reducing) in a brackish mineral medium (22) as previously described (13). After repeated transfers (20%) and preparation of dilution series in the mineral medium with hexane or decane as a growth substrate, the culture consisted of morphologically similar short oval rods and less than 1% other bacteria. The culture was routinely grown in 160-ml serum bottles containing 25 ml of the mineral medium (22) supplemented with 15 to 25 μl of sterile anoxic n-C6, n-C8, n-C10, or n-C12. Rates of alkane metabolism were determined in exponentially growing cultures before the optical density at 600 nm reached 0.2. All preparations were sealed with Teflon-lined stoppers and incubated inverted at 31°C.
When isotopically labeled substrates were employed, hexane-, octane-, or decane-grown ALDC cells were incubated with 20 μl of the corresponding protonated or fully deuterated (CDN Isotopes, Pointe-Claire, Quebec, Canada) alkane. Cultures used for 13C nuclear magnetic resonance (NMR) analysis were grown under sulfate-limiting conditions with 15 μl of 1-13C-labeled n-hexane (Isotec, Miamisburg, OH) and incubated for 6 weeks. The alkane degradation results were in good stoichiometric agreement with the theoretically expected amount of sulfate reduction (94% ± 5%), so the progress of various experiments was monitored by electron acceptor consumption by ion chromatography (5) or by protein accumulation (BCA protein assay reagent kit; Pierce, Rockford, IL). Alkane losses were monitored by gas chromatography (GC) as previously described (13).
Preparation of cell extracts and suspensions.
The cells in the ALDC (1 liter) were anaerobically collected in the late exponential phase by centrifugation (16,000 × g, 15 min, 4°C) in sealed bottles and washed once in anoxic 20 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.2) containing 5 mM MgCl2 and 1 mM dithiothreitol. The washed cells were resuspended in 10 ml of the same buffer and divided into two aliquots. Each aliquot was centrifuged again and suspended in MOPS buffer (pH 7.2) containing TiCl3 (1 mM) or Tris-HCl buffer (100 mM; pH 7.8) containing 5 mM MgCl2 and 1 mM TiCl3. Two milliliters of each cell suspension was disrupted with a French press (69 mPa), and the resulting crude extracts were used for in vitro assays. Unbroken cells were diluted 2.5-fold in the appropriate buffers and used for comparative purposes. All incubations were conducted inside sealed test tubes in a well-working anaerobic glove box containing an N2-H2 (95:5) atmosphere. The in vitro assay mixtures (total volume, 1.0 ml) contained 0.9 ml of crude extract, 1.5 μmol [1,2,3,4-13C]fumaric acid (CIL, Andover, MA), and 10 μl fully deuterated d14-n-hexane (CDN Isotopes, Pointe-Claire, PQ, Canada). The protein concentration in crude extracts was 2.8 mg/ml. The reaction was stopped after 16 or 26 h of incubation by addition of 50 μl of 6 N HCl, and the assay mixtures were extracted and analyzed by gas chromatography coupled with mass spectrometry (MS) (see below). Concentrated cell suspensions were incubated identically and used for comparative purposes. However, some preparations were also supplemented with 0.14 mmol sulfate. The final volume was adjusted to 1.0 ml, and the cell suspensions were incubated for 3 days prior to extraction and metabolite analysis.
Extraction and metabolite analysis.
All preparations were acidified to pH <2 with H2SO4 prior to extraction with ethyl acetate. In some cases, cultures were initially treated with NaOH (pH >12) for 30 min to cleave presumed CoA thioester derivatives. Organic extracts were concentrated by rotary evaporation and then reacted with N,O-bis(trimethylsilyl)trifluoroacetamide (Pierce Chemical Co., Rockford, IL) to obtain trimethylsilyl (TMS) derivatives. Derivatized metabolites were separated using an HP 5890 GC equipped with a DB-5 capillary column (30 m by 0.25 mm [inside diameter]; J&W Scientific, Folsom, CA). The temperature was held at 40°C for 2 min, raised at a rate of 4°C per min to 240°C, and then held at 240°C for 8 min. The temperature of the injector and the transfer line from the GC to the MS was kept at 250°C. An HP 5970 MS detector was used in the scan mode from 50 to 600 mass units. Metabolites were identified based on matching the GC retention times and mass spectral profiles with those of authentic standards or by comparison with previously published mass spectra. 4-Methyloctanoic acid was acquired from Sigma-Aldrich (St. Louis, MO). n-Octylsuccinic acid was prepared by base hydrolysis from n-octylsuccinic anhydride (TCI America, Portland, OR) as previously described (9).
13C nuclear magnetic resonance spectroscopic analysis.
Underivatized ethyl acetate extracts of 13C-amended cultures were concentrated by rotary evaporation and transferred into 5-mm NMR tubes. Extracts were dried under N2 and redissolved in 1,4-dioxane-d8 (CIL, Andover, MA). An additional culture was amended with unlabeled hexane to ensure that the observed NMR signals did not result from 13C impurities present in the solvents used. The NMR spectra of the organic solvent-extracted samples were obtained with a Unity INOVA 400-MHz NMR (Varian) with a 13C resonance frequency of 100.567 MHz. The proton-decoupled 13C spectra were obtained at 30°C using a standard inverse-gated pulse sequence. The following experimental parameters were employed: sweep width, 25,000 Hz; acquisition time, 1.199 s; recycle time, 2 s; and number of acquisitions, 35,000. The data were processed with 1-Hz line broadening. Chemical shifts were referenced relative to the center peak of the 1,4-dioxane pentet centered at 66.0 ppm. Metabolite chemical shift values were predicted using ChemNMR 5.0 (CambridgeSoft, Cambridge, MA). Predicted chemical shifts were allowed a tolerance of ±2 ppm. Pure (1-phenylethyl)succinic acid (ethylbenzylsuccinic acid) used for comparative NMR experiments was synthesized and purified as described by Bickford et al. (4). Additional NMR experiments included distortionless enhancement by polarization transfer (number of protons attached to each carbon), heteronuclear single quantum coherence (1H-13C coupling), and heteronuclear multiple-bond correlation (long-range 1H-13C coupling).
RESULTS
Growth and metabolite formation with H- and d-alkanes.
The ALDC grew with n-alkanes ranging from C6 to C12. The biodegradation rate with individual alkanes decreased as the alkane chain length increased, presumably reflecting differences in the relative aqueous solubilities of the substrates. Hexane, decane, and dodecane were degraded at rates of 2.45, 1.76, and 0.95 μmol alkane/day (±5%), respectively. The fumarate addition metabolites associated with protonated or deuterated hexane, octane, and decane showed the characteristic mass spectral features associated with anaerobic alkane degradation (9, 13; data not shown). All six protonated succinyl metabolites showed abundant MS fragments at m/z 73 and m/z 147, attesting to their diderivatization with N,O-bis(trimethylsilyl)trifluoroacetamide (16). The McLafferty rearrangement ion associated with the GC-MS analysis of these metabolites was also observed (13, 15). This included major ions at m/z 262 and m/z 264 for the protonated and deuterated fumarate addition metabolites, respectively (9, 13). Fragmentation of the McLafferty rearrangement signal [HOSi(CH3)3 and DOSi(CH3)3] that resulted in ions at m/z 172 and 173, respectively, was also noted. Losses of CO2H or CO2D (or both) from the McLafferty rearrangement signal accounted for the m/z 217 ion in the spectra of the fumarate addition metabolites obtained from protonated alkanes or the m/z 218 and 219 ions in the spectra of the corresponding deuterated alkane metabolites. The ion with the largest mass in the spectra of these metabolites occurred at m/z 331, 359, 387, or 415 with protonated hexane, octane, decane, or dodecane as the parent substrate, while the corresponding fragments for the deuterated alkanes were at m/z 345, 377, 409, and 441. The latter ions were 14, 18, 22, and 26 atomic mass units larger than the corresponding protonated derivatives and accounted for all of the deuterium atoms present in the isotopically labeled parent alkane. However, these masses are not the masses for the molecular ions (M+) of the metabolites but rather are the masses for the M+-15 ions, as is typical of TMS esters (16). Thus, all of the alkanes studied were metabolized by the ALDC in an analogous fashion.
Multiple GC peaks that had the same MS features were typically observed for the succinyl metabolites produced during anaerobic alkane biodegradation. This finding presumably reflected the formation of diasteriomers, as previously described (13, 18). A synthesized authentic standard of n-octylsuccinic acid analyzed in the same fashion resolved as a single GC peak with a different retention time but the same MS fragmentation pattern as the fumarate addition metabolite of octane. Therefore, the alkane metabolites detected also likely bore the succinyl moiety at a subterminal position in a manner similar to that reported previously (13, 18).
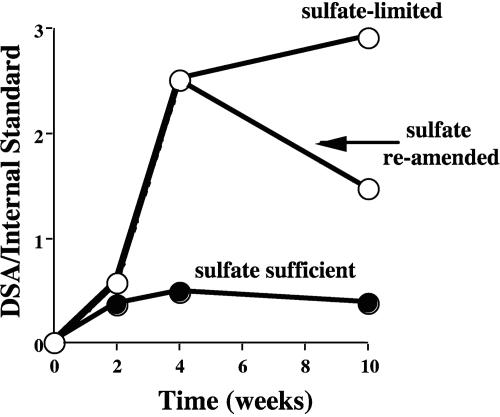
The pattern of alkylsuccinic acid accumulation was monitored as the ALDC was grown on fully deuterated decane (C10D22) under sulfate-sufficient and sulfate-limited conditions. Use of the deuterated substrate allowed us to track the formation of the addition product de novo and to distinguish it from the nonisotopically labeled metabolite pool. Under sulfate-sufficient conditions, complete mineralization of decane according to the theoretically expected stoichiometry was observed (25 mM Na2SO4 for 3 mM decane), while only about one-half of the initial decane added could be mineralized with 10 mM Na2SO4. Metabolites were typically found only at trace levels but accumulated when sulfate was limiting (Fig. 1). Subsequent reamendment with sulfate caused the level of decylsuccinic acid to decrease, attesting to the transient nature of the metabolite (Fig. 1).
FIG. 1.
Formation of d-decylsuccinic acid (DSA) from deuterated decane by the ALDC with different amounts of sulfate. The amount of decylsuccinic acid is expressed as the GC-MS response of the metabolite peak relative to a hexadecane internal standard (0.033 μl) added to each extract prior to analysis.
Formation of hexylsuccinic acid in vitro and in cell suspension experiments.
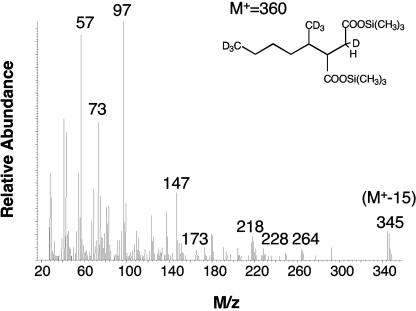
In experiments with crude cell extracts and nongrowing cell suspensions, d14-n-hexane and [1,2,3,4-13C]fumarate were used to distinguish reaction products from preexisting metabolites. The reactions were performed at two different pH values (pH 7.2 and 7.8) to determine the optimal conditions. Hexylsuccinic acid was formed both in the crude extracts and in the whole-cell suspensions. Crude extracts catalyzed the formation of hexylsuccinic acid only at pH 7.2; no metabolite was formed at pH 7.8. However, in the cell suspensions, hexylsuccinate was formed at both pHs. The presence of sulfate in the cell suspensions did not influence the pattern of metabolite accumulation as the fumarate addition product was detected both in its presence and in its absence. The hexylsuccinic acid metabolite contained all of the deuterium atoms from d14-n-hexane, but it did not incorporate any 13C from [1,2,3,4-13C]fumarate (Fig. 2).
FIG. 2.
Mass spectrum of TMS-derivatized d14-hexylsuccinic acid formed during incubation of cell-free ALDC extracts incubated with d14-n-hexane.
GC-MS results with 13C-labeled hexane.
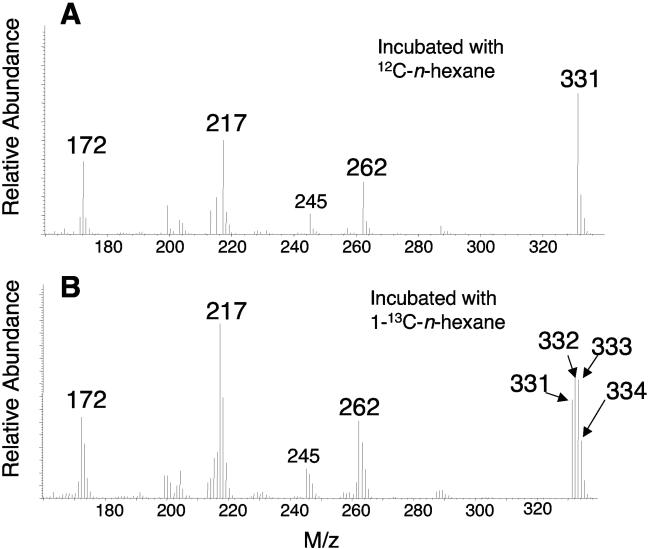
In cultures incubated with 1-13C-labeled n-hexane, hexylsuccinic acid was also detected (as the di-TMS ester). The MS profile of this metabolite revealed M+-15 ions at m/z 331, 332, 333, and 334, suggesting that the extracted molecule incorporated up to three 13C atoms (Fig. 3). Such a molecule could have resulted if the 13C label of the n-hexane substrate ultimately produced fumarate that was then repeatedly recycled back into the metabolic scheme. The relative abundance of the M+-15 fragment at m/z 334 was consistently one-half that of the fragment at m/z 332 and 333; this value is much greater than would be expected based upon the natural abundance alone.
FIG. 3.
Portion of the total mass spectrum of the di-TMS-derivatized hexylsuccinic acid formed by the ALDC during n-hexane (A) or 1-13C-labeled n-hexane (B) metabolism.
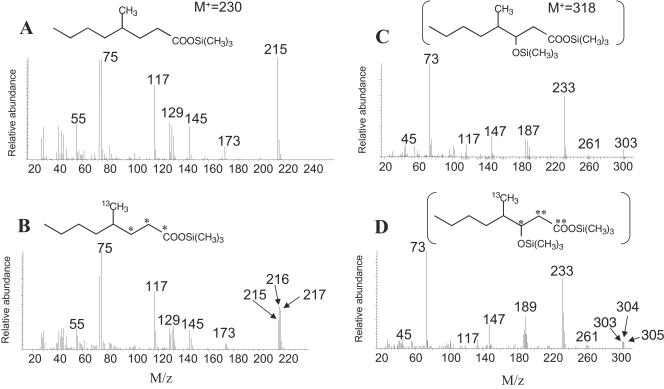
In addition, 4-methyloctanoic acid was positively identified in culture supernatants by GC-MS (Fig. 4A and B). In the organic extracts of 12C-hexane-amended culture supernatants, the GC retention time and the MS profile of this metabolite were identical to those of an authentic standard (not shown). This metabolite was previously shown to be produced during hexane degradation by a nitrate-reducing isolate (23). For the 13C-amended culture extracts, there was a peak whose GC retention time also matched the GC retention time of authentic 4-methyloctanoic acid, but the MS profile differed slightly in that fragment ions were shifted up to 2 mass units higher than those of the standard (Fig. 4B). A third metabolite (Fig. 4C and D) was tentatively identified as TMS-derivatized 3-hydroxy-4-methyloctanoic acid based on fragmentation comparisons with the same methyl-esterified metabolite proposed previously (23). In the MS profile reported by Wilkes et al. (23), this metabolite had a mass of 188 and a base peak at m/z 103, a difference of 85 mass units. Similarly, in the MS profile of the peak detected in our unlabeled culture extracts, a presumptive M+-15 of m/z 303 was seen, resulting in a molecular mass of 318 (Fig. 4C). With this metabolite, a base peak was observed at m/z 233, also a difference of 85 mass units, like the difference observed by Wilkes et al. (23). Other key ions were at m/z 73, 147 (indicating that two functional groups were derivatized with TMS), and 187 [loss of —CH2-COO-Si(CH3)3]. In the MS profile presented by Wilkes et al. (23), a comparable fragment was also seen at m/z 115 (loss of —CH2-COO-CH3). In the [13C]hexane-amended cultures, a metabolite with similar MS features was also detected and eluted at the same GC retention time, but it had fragments up to 2 mass units higher (Fig. 4D). Based on these comparisons we tentatively identified this metabolite, but confirmation awaits the availability of an authentic standard.
FIG. 4.
(A and B) Mass spectra of 4-methyloctanoic acid (as the TMS derivative) produced during n-hexane (A) or 1-13C-labeled n-hexane (B) metabolism by the ALDC. (C and D) Mass spectra of tentatively identified 3-hydroxy-4-methylocanoic acid (as the TMS derivative) produced during n-hexane (C) or 13C-labeled n-hexane (D) degradation. The asterisks in panels B and D indicate possible sites of 13C incorporation from labeled regenerated fumarate molecules.
13C nuclear magnetic resonance spectroscopic analysis.
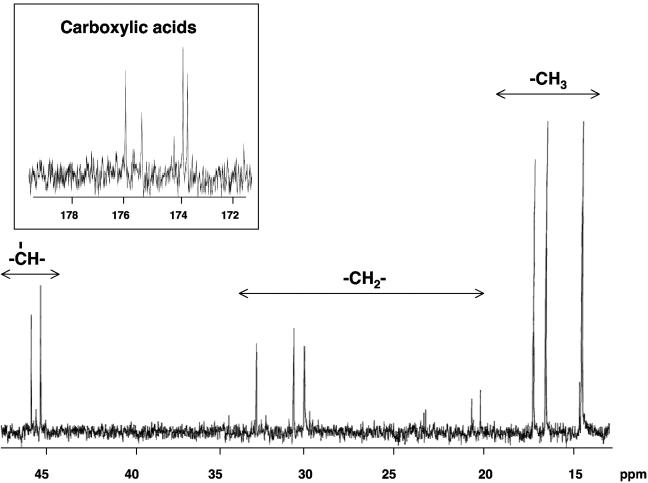
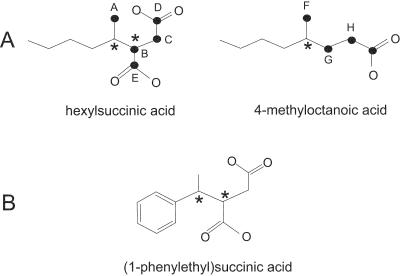
The 13C NMR spectrum of the unlabeled control sample did not display any signals other than that of the solvent 1,4 dioxane-d8 (data not shown), demonstrating that all the signals present in the 13C-labeled sample spectrum resulted from 1-13C-labeled n-hexane and metabolites. Figure 5 shows the 13C NMR spectrum of the 13C-labeled sample after 6 weeks of incubation. 13C NMR signals were detected only in the alkyl (0 to 50 ppm) and carboxylic acid (170 to 180 ppm) regions. Table 1 shows the chemical shift of each signal and indicates its functional group. The presence of a limited number of distinct 13C NMR signals indicates that there was formation of discrete 13C-labeled metabolites rather than uniform incorporation of the 13C label into microbial lipid molecules, which, if it had occurred, would have given rise to a great array of fatty acid methylene signals. The comparison shown in Table 2 of 13C NMR spectral chemical shifts with predicted 13C chemical shifts for carbons expected to be 13C labeled according to the pathway outlined by Wilkes et al. (23) and compounds observed by GC-MS supports the presence of hexylsuccinic acid and 4-methyloctanoic acid. Figure 6A shows the carbon atoms expected to be 13C labeled based on the proposed pathway of Wilkes et al. (23) and demonstrates that hexylsuccinic acid is unique in that it contains two chiral centers, one at the C-2 carbon of hexane and the other at the methine carbon of the succinic acid moiety (carbon B in Fig. 6A). Depending on the isomeric configuration of these sites, it is possible that the 13C-labeled carbons A, B, C, D, and E are in different chemical environments. If these chemical environments are unique, each 13C-labeled carbon should produce two 13C NMR signals, one for each of the enantiomeric configurations. As noted in Table 1, this effect was observed for all the 13C-labeled carbons in hexylsuccinic acid. Likewise, 4-methyloctanoic acid has a chiral center at the C-2 carbon of the hexane backbone, resulting in two 13C NMR signals for the methylene carbon labeled G in Fig. 6A. This NMR chiral effect was observed and confirmed using an authentic (1-phenylethyl)succinic acid standard, which also has two chiral carbons (Fig. 6B). The 13C NMR spectrum of the latter compound had “doublets” for the carbons present in the 1-phenylethyl and succinic acid moieties. Other NMR experiments (distortionless enhancement by polarization transfer, heteronuclear single quantum coherence, and heteronuclear multiple-bond correlation) confirmed that these “doublets” resulted from different enantiomeric configurations of (1-phenylethyl)succinic acid (data not shown). Finally, the 13C NMR results also strongly indicate that the C-2 carbon of hexane is the prominent site for addition to fumarate, rather than the C-1 or C-3 carbon. Fumarate addition to the C-1 and C-3 carbons of hexane results in predicted chemical shifts of 26.7 and 11.8 ppm, respectively, neither of which was detected in the 13C NMR spectrum. Moreover, the presence of carboxylic acid signals between 171 and 176 ppm illustrated that there was incorporation of 13C label into the carboxylic acid functional groups of hexylsuccinic acid and 4-methyloctanoic acid.
FIG. 5.
13C NMR spectrum of the 13C-labeled metabolites produced by the ALDC after 6 weeks of incubation.
TABLE 1.
NMR data for signals detected in cultures grown on [1-13C]-labeled n-hexane
| 13C (ppm) | Functional group |
|---|---|
| 14.34 | Methyl (C-1 hexane) |
| 14.80 | Methyl |
| 16.41 | Methyl |
| 17.12 | Methyl |
| 20.16 | Methyl |
| 20.62 | Methyl |
| 30.38 | Methylene |
| 30.98 | Methylene |
| 33.18 | Methylene |
| 45.70 | Methine |
| 46.25 | Methine |
| 173.88 | Carboxylic acid |
| 174.02 | Carboxylic acid |
| 175.32 | Carboxylic acid |
| 175.84 | Carboxylic acid |
TABLE 2.
Actual and predicted 13C NMR signals for proposed metabolitesa
| Predicted results (ppm) | NMR results (ppm) | Functional group |
|---|---|---|
| Hexylsuccinic acid | ||
| 14.0 | 14.34 | Methyl (if addition to fumarate occurred at C-5 rather than at C-2) |
| 17.0 | 16.41 | Methyl closest to succinic acid |
| 32.7 | 30.98 | Methylene in succinic acid |
| 44.0 | 45.70, 46.25 | Methine in succinic acid |
| 4-Methyloctanoic acid | ||
| 14.0 | 14.34 | Methyl (if addition to fumarate occurred at C-5 rather than at C-2) |
| 19.7 | 17.12 | Methyl closest to succinic acid |
| 32.0 | 30.38 | Methylene “alpha” to carboxylic |
| 33.2 | 33.58 | Methylene “beta” to carboxylic |
For hexane the 14.34 ppm signal is also from unreacted 13C-labeled hexane. This signal also appears for metabolites in which the fumarate is added to the unlabeled end of hexane.
FIG. 6.
(A) Molecular structure of hexylsuccinic acid and 4-methyloctanoic acid showing 13C-labeled carbons (solid circles), as predicted by the cycling of fumarate in the proposed pathway (23), and chiral carbons (asterisks). (B) Molecular structure of (1-phenylethyl)succinic acid showing chiral carbons (asterisks) used for comparison in NMR experiments.
DISCUSSION
Our study confirmed that fumarate addition reactions represent a common mechanism for anaerobic alkane oxidation independent of alkyl chain length. All alkanes tested, ranging from C6 to C12, formed corresponding alkylsuccinic acid derivatives. The transient nature of alkylsuccinate formation confirmed that the addition product is the first step leading to complete oxidation, rather than a dead-end product. The in vitro experiment showing the formation of hexylsuccinic acid from fully deuterated hexane with retention of all deuterium atoms in crude cell extracts confirmed that this is indeed an initial step in the anaerobic alkane degradation pathway. Subsequent reactions following alkylsuccinate formation are not well documented. The pathway proposed by Wilkes et al. (23) was based on metabolic studies of denitrifying alkane-degrading strain HxN1 cultures growing with unlabeled and deuterated hexane and amended with 2,3-d2-fumarate. The detection of 4-methyloctanoic acid with deuterium in the C-3 position and also 4-methyloct-2-enoic acid, 3-hydroxy-4-methyloctanoic acid, and 2-methylhexanoic acid led to a hypothetical cycle in which alkane oxidation might be coupled to fumarate regeneration. The identification of 4-methyloctanoic acid with deuterium in the C-3 position was crucial evidence that supported the proposed model. A deuterium atom in the C-3 position suggested that the product of fumarate addition, hexylsuccinic acid, could possibly undergo carbon rearrangement of the carbon skeleton prior to decarboxylation. If such a rearrangement occurred, a deuterium atom from the C-2 position was moved toward the methyl group with opposite migration of a carboxyl group (or its thioester), yielding (2-methylhexyl)malonyl-CoA. After decarboxylation the product, 4-methyloctanoyl-CoA, would go through β-oxidation, yielding 2-methylhexanoyl-CoA, as well as the intermediate metabolites 4-methyloct-2-enoic acid and 3-hydroxy-4-methyloctanoic acid, all of which were identified as methyl esters in strain HxN1 culture extracts. Another cycle of β-oxidation would produce propionyl-CoA and butyryl-CoA from 2-methylhexanoyl-CoA. According to the model, butyryl-CoA would be oxidized further to acetyl-CoA and CO2, and propionyl-CoA would be transformed to fumarate via transcarboxylation with (2-methylhexyl)malonyl-CoA as the carboxyl donor, thus regenerating a cosubstrate and closing the cycle. (2-Methylhexyl)malonyl-CoA was not detected in the study. However, while the detection of 3-d1-4-methyloctanoic acid as a product strongly argued in favor of simultaneous rearrangement and decarboxylation, proof was lacking.
Our studies on [13C]hexane metabolism produced more data that support the proposed model. The GC-MS and 13C NMR results indicate that fumarate addition indeed occurs at the C-2 carbon of n-hexane. Moreover, our results illustrate that multiple 13C nuclei from 13C-labeled hexane molecules are incorporated into the metabolites, demonstrating that 13C-labeled fumarate is produced and recycled in this process. In extracts of cultures grown with 13C-labeled n-hexane, GC-MS detection of M+-15 ions at m/z 331, 332, 333, and 334 (Fig. 3B) illustrated that the extracted hexylsuccinic acid molecule contained up to three 13C atoms, showing that the 13C label at the C-1 carbon of hexane had been recycled multiple times through the degradation pathway. Likewise, the lack of 13C incorporation from labeled [1,2,3,4-13C]fumarate into hexylsuccinic acid in the in vitro and the cell suspension experiments suggested that preformed fumarate from internal cellular pools was used preferentially, thereby indirectly supporting the model for cosubstrate regeneration. Such selectivity in the formation of a fumarate addition product could also be explained by the fact that substitution of heavy isotopes into molecules produces a stronger bond (10), and therefore more energy may be required for formation of a new 12C-13C bond in the alkyl addition reaction, thereby possibly placing thermodynamic constraints on the involvement of exogenously added [1,2,3,4-13C]fumarate in cell metabolism.
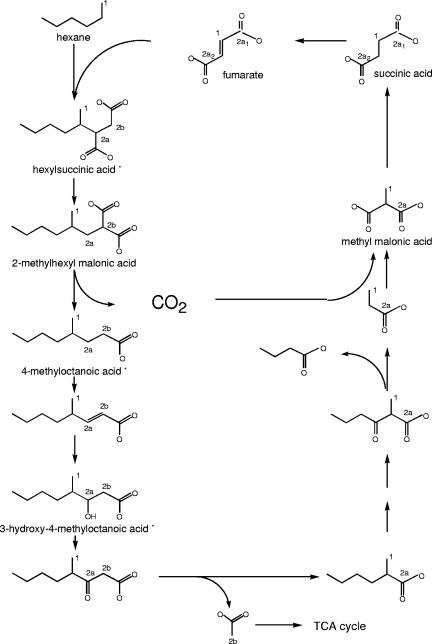
Figure 7 shows the pathway for anaerobic alkane metabolism that was initially proposed by Wilkes et al. (23) and that is supported by the results of the present study. If fumarate adds to the C-2 carbon adjacent to the 13C-labeled C-1 carbon of a molecule of n-hexane, the 13C-labeled C-1 carbon is incorporated as the C-2 carbon of fumarate upon completion of one metabolic cycle (carbon 1 of fumarate in Fig. 7). Addition of this 13C-labeled fumarate to another molecule of 1-13C-labeled n-hexane, either to the 13C-labeled C-2 carbon or to the unlabeled C-3 carbon of fumarate, results in a covalent bond with the C-2 carbon of the hexane. If the former occurs, then a 13C-labeled methine site (carbon 2a of hexylsuccinic acid in Fig. 7) is created with a predicted chemical shift of 44.0 ppm. If the latter occurs, a 13C-labeled methylene site (carbon 2b of hexylsuccinic acid in Fig. 7) is produced with a predicted chemical shift of 32.7 ppm. Signals with very similar chemical shifts (i.e., 45.70 and 46.25, and 30.98 and 33.18 ppm) were detected in our 13C NMR spectrum. The presence of two methine signals, as well as the two methylene signals, results from the fact that hexylsuccinic acid contains two chiral centers; therefore, methine and methylene carbons 2a and 2b reside in slightly different chemical environments formed by the enantiomers of hexylsuccinic acid, thereby giving rise to two signals for each carbon atom. The nearly equal peak areas (Fig. 5) illustrate that there is minimal stereochemical specificity upon addition of hexane to fumarate. This chiral “doublet” effect is also observed with the 13C NMR signals of the 13C-labeled methyl group of the hexane backbone and both of the 13C-labeled carboxylic acids (Fig. 5, inset). The fact that the carboxylic acid functional groups in the succinic acid moiety of hexylsuccinic acid are 13C labeled strongly indicates that multiple cycling of fumarate occurs. The fact that the experimentally observed 13C NMR chemical shifts closely match those predicted based on the original proposed pathway (23) further supports the validity of the pathway. Moreover, the presence of multiple 13C nuclei in metabolites supports the idea of a cyclical pathway, with 13C becoming incorporated into fumarate.
FIG. 7.
Pathway of alkane metabolism under sulfate-reducing conditions based on the pathway proposed by Wilkes et al. (23). The numbered carbons indicate the positions of isotopically heavy carbon atoms (13C). The number indicates the metabolic cycle number, and the lowercase letter and subscript indicate the sites of additional label incorporation as a result of pathway cycling. Metabolites that were experimentally detected using 13C NMR and/or GC-MS are indicated by asterisks.
The detection of 4-methyloctanoic acid with a deuterium in the C-3 position laid the foundation for the proposed alkane degradation pathway (23). Our studies with [13C]hexane strongly suggest that this metabolite belongs in the pathway as it was identified by GC-MS during alkane metabolism, its presence was supported by 13C NMR analysis, and cultivation experiments showed that the ALDC could use it as a growth substrate (data not shown). The presence of this metabolite indicated that decarboxylation of hexylsuccinic acid had occurred and apparently requires carbon skeleton rearrangement in a manner similar to that proposed by Wilkes et al. (23). However, the presence of (2-methylhexyl)malonyl-CoA to prove this contention directly was not detected. Nevertheless, the existence of this molecule cannot be ruled out, as this compound could conceivably be destroyed under the acidic conditions used for metabolite extraction. Alternately, the relative rates of the reactions leading from hexylsuccinic acid to 4-methyloctanoic acid could preclude accumulation of the rearrangement intermediate at a detectable level.
Common metabolites formed during the course of alkane biodegradation, as well as the presence of multiple 13C atoms in the metabolites at the positions predicted by the pathway of Wilkes et al. (23), strongly suggest that the sulfate-reducing culture, ALDC, and a denitrifying strain, HxN1, not only both initiate alkane degradation by fumarate addition but most probably share the entire pathway.
REFERENCES
- 1.Aeckersberg, F., F. Bak, and F. Widdel. 1991. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 156:5-14. [Google Scholar]
- 2.Beller, H. R., and A. M. Spormann. 1997. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain PRTOL1. Appl. Environ. Microbiol. 63:3729-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beller, H. R., and A. M. Spormann. 1997. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J. Bacteriol. 179:670-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickford, W. G., G. S. Fisher, F. G. Dollear, and C. E. Swift. 1948. Autoxidation of fats. I. Preparation and oxidation of alkylbenzene-maleic anhydride adducts. J. Am. Oil Chem. Soc. July:251-254. [Google Scholar]
- 5.Caldwell, M. E., R. M. Garrett, R. C. Prince, and J. M. Suflita. 1998. Anaerobic biodegradation of long-chain n-alkanes under sulfate-reducing conditions. Environ. Sci. Technol. 32:2191-2195. [Google Scholar]
- 6.Callaghan, A. V., L. M. Gieg, K. G. Kropp, J. M. Suflita, and L. Y. Young. 2003. Fumarate addition during hexadecane degradation by the sulfate-reducer AK-01. Abstr. Am. Soc. Microbiol. 103rd Gen. Meet., abstr. Q-038, p. 521.
- 7.Cravo-Laureau, C., V. Grossi, D. Raphel, R. Matheron, and A. Hirschler-Réa. 2005. Anaerobic n-alkane metabolism by a sulfate-reducing bacterium, Desulfatibacillum aliphaticivorans strain CV2803T. Appl. Environ. Microbiol. 71:3458-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrenreich, P., A. Behrends, J. Harder, and F. Widdel. 2000. Anaerobic oxidation of alkanes by newly isolated denitrifying bacteria. Arch. Microbiol. 173:58-64. [DOI] [PubMed] [Google Scholar]
- 9.Gieg, L. M., and J. M. Suflita. 2002. Detection of anaerobic metabolites of saturated and aromatic hydrocarbons in petroleum-contaminated aquifers. Environ. Sci. Technol. 36:3755-3762. [DOI] [PubMed] [Google Scholar]
- 10.Grossman, E. L. 2002. Stable carbon isotopes as indicators of microbial activity in aquifers, p. 728-742. In C. J. Hurst, R. L. Crawford, G. R. Knudsen, M. J. McInerney, and L. D. Stetzenbach (ed.), Manual of environmental microbiology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 11.Kremer, D. R., and T. A. Hansen. 1988. Pathway of propionate degradation in Desulfobulbus propionicus. FEMS Microbiol. Lett. 49:273-277. [Google Scholar]
- 12.Krieger, C. J., W. Roseboom, S. P. J. Albracht, and A. M. Spormann. 2001. A stable organic free radical in anaerobic benzylsuccinate synthase of Azoarcus sp. strain T. J. Biol. Chem. 276:12924-12927. [DOI] [PubMed] [Google Scholar]
- 13.Kropp, K. G., I. A. Davidova, and J. M. Suflita. 2000. Anaerobic oxidation of n-dodecane by an addition reaction in a sulfate-reducing bacterial enrichment culture. Appl. Environ. Microbiol. 66:5393-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuthner, B., C. Leutwein, H. Schulz, P. Hörth, W. Haehnel, E. Schiltz, H. Schägger, and J. Heider. 1998. Biochemical and genetical characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalyzing the first step in anaerobic toluene metabolism. Mol. Microbiol. 28:615-628. [DOI] [PubMed] [Google Scholar]
- 15.McLafferty, F. W. 1980. Interpretation of mass spectra, 3rd ed. University Science Books, Mill Valley, Calif.
- 16.Pierce, A. E. 1968. Silylation of organic compounds. Pierce Chemical Co., Rockford, Ill.
- 17.Rabus, R., H. Wilkes, A. Schramm, G. Harms, A. Behrends, R. Amann, and F. Widdel. 1999. Anaerobic utilization of alkylbenzenes and n-alkanes from crude oil in an enrichment culture of denitrifying bacteria affiliating with the β-subclass of Proteobacteria. Environ. Microbiol. 1:145-157. [DOI] [PubMed] [Google Scholar]
- 18.Rabus, R., H. Wilkes, A. Behrends, A. Armstroff, T. Fischer, and F. Widdel. 2001. Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl)succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J. Bacteriol. 183:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rueter, P., R. Rabus, H. Wilkes, F. Aeckersberg, F. A. Rainey, H. W. Jannasch, and F. Widdel. 1994. Anaerobic oxidation of hydrocarbons in crude oil by new types of sulphate-reducing bacteria. Nature 372:455-458. [DOI] [PubMed] [Google Scholar]
- 20.So, C. M., and L. Y. Young. 1999. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes. Appl. Environ. Microbiol. 65:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.So, C. M., C. D. Phelps, and L. Y. Young. 2003. Anaerobic transformation of alkanes to fatty acids by a sulfate-reducing bacterium, strain Hxd3. Appl. Environ. Microbiol. 69:3892-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, New York, N.Y.
- 23.Wilkes, H., R. Rabus, T. Fischer, A. Armstroff, A. Behrends, and F. Widdel. 2002. Anaerobic degradation of n-hexane in a denitrifying bacterium: further degradation of the initial intermediate (1-methylpentyl)succinate via C-skeleton rearrangement. Arch. Microbiol. 177:235-243. [DOI] [PubMed] [Google Scholar]
- 24.Wilkes, H., S. Kühner, C. Bolm, T. Fischer, A. Classen, F. Widdel, and R. Rabus. 2003. Formation of n-alkane- and cycloalkane-derived organic acids during anaerobic growth of a denitrifying bacterium with crude oil. Org. Geochem. 34:1313-1323. [Google Scholar]