Abstract
A thermophilic syntrophic bacterium, Pelotomaculum thermopropionicum strain SI, was grown in a monoculture or coculture with a hydrogenotrophic methanogen, Methanothermobacter thermautotrophicus strain ΔH. Microscopic observation revealed that cells of each organism were dispersed in a monoculture independent of the growth substrate. In a coculture, however, these organisms coaggregated to different degrees depending on the substrate; namely, a large fraction of the cells coaggregated when they were grown on propionate, but relatively few cells coaggregated when they were grown on ethanol or 1-propanol. Field emission-scanning electron microscopy revealed that flagellum-like filaments of SI cells played a role in making contact with ΔH cells. Microscopic observation of aggregates also showed that extracellular polymeric substance-like structures were present in intercellular spaces. In order to evaluate the importance of coaggregation for syntrophic propionate oxidation, allowable average distances between SI and ΔH cells for accomplishing efficient interspecies hydrogen transfer were calculated by using Fick's diffusion law. The allowable distance for syntrophic propionate oxidation was estimated to be approximately 2 μm, while the allowable distances for ethanol and propanol oxidation were 16 μm and 32 μm, respectively. Considering that the mean cell-to-cell distance in the randomly dispersed culture was approximately 30 μm (at a concentration in the mid-exponential growth phase of the coculture of 5 × 107 cells ml−1), it is obvious that close physical contact of these organisms by coaggregation is indispensable for efficient syntrophic propionate oxidation.
In anaerobic digestors, organic matter is converted to methane and CO2 via various intermediates (1, 27). Among the most important intermediate metabolites are volatile fatty acids (VFAs), such as acetate, propionate, and butyrate, and it has been reported that accumulation of VFAs results in a significant decrease in the methane production efficiency in such digestors (16, 17, 37, 38). VFAs are, however, unfavorable substrates for anaerobes, since oxidation of these substrates to H2 and CO2 (or formate) is endoergonic under standard conditions (i.e., the changes in the Gibbs free energy are positive [ΔG°′ > 0]) (Table 1) and is thermodynamically feasible only when the H2 partial pressure (or formate concentration) is kept low (3, 11, 29, 34). For instance, thermodynamic estimation has predicted that H2 partial pressures as low as 10 Pa and 100 Pa are necessary for the oxidation of propionate and butyrate, respectively (29, 34). Since H2 and formate are scavenged mainly by the carbonate respiration of methanogenic archaea, syntrophic association of VFA-oxidizing bacteria (called syntrophs) and methanogenic archaea is considered indispensable for efficient VFA oxidation (27, 36).
TABLE 1.
Standard Gibbs free energy changes (ΔG°′) for anaerobic reactions (pH 7)a
| Compound | Reaction | ΔG°′ (kJ mol−1) |
|---|---|---|
| Hydrogen-releasing reactions | ||
| Propionate | CH3CH2COO− + 3H2O → CH3COO− + HCO3− + 3H2 + H+ | 76.1 |
| Ethanol | CH3CH2OH + H2O → CH3COO− + 2H2 + H+ | 9.6 |
| 1-Propanol | CH3CH2CH2OH + H2O → CH3CH2COO− + 2H2 + H+ | 3.0 |
| Hydrogen-consuming reaction | ||
| Hydrogen | H2 + 1/4HCO3− + 1/4H+ → 1/4CH4 + 3/4H2O | −33.9 |
| Syntrophic methanogenesis | ||
| Propionate | CH3CH2COO− + 3/4H2O → 3/4CH4 + CH3COO− + 1/4HCO3− + 1/4H+ | −25.6 |
| Ethanol | CH3CH2OH + 1/2HCO3− → 1/2CH4 + CH3COO− + 1/2H2O + 1/2H+ | −58.2 |
| 1-Propanol | CH3CH2CH2OH + 1/2HCO3− → 1/2CH4 + CH3CH2COO− + 1/2H2O + 1/2H+ | −64.8 |
As described previously, syntrophic VFA oxidation depends on interspecies electron (as H2 and formate) transfer. Researchers have suggested that close physical contact between syntrophs and methanogens is important for efficient interspecies electron transfer (5, 28, 34). Using a diffusion theory, they have elegantly simulated the inverse relationship between the average distance from syntrophs to methanogens and the hydrogen flux between the organisms (5, 28). Fluorescence in situ hybridization (FISH) has shown that syntrophic bacteria occurred close to methanogenic archaea in granular sludge taken from highly efficient upflow anaerobic sludge blanket (UASB) reactors (5, 10, 13), which also suggests that close physical contact is important.
Despite the recognition of the importance of physical contact between syntrophs and methanogens in aggregated biomass (e.g., UASB granules) (5, 28), it is not yet clear if these organisms also make physical contact in suspended cultures. In addition, the effects of growth substrates on the physical contact have not been examined yet. We also considered the possibility that defined coculture experiments would provide valuable information about how the organisms establish close physical contact. In the present study, therefore, we investigated coaggregation of a syntrophic propionate-oxidizing bacterium, Pelotomaculum thermopropionicum strain SI (13, 14), and an H2-consuming methanogen, Methanothermobacter thermautotrophicus strain ΔH (33), in cocultures. These organisms were chosen because previous studies have shown that they represent important populations in high-temperature anaerobic digestors (1, 13, 19, 31). Emphasis was placed on the conditions and processes for coaggregation and the theoretical background.
MATERIALS AND METHODS
Strains and culture conditions.
P. thermopropionicum SI (=DSM 13744) was grown in a monoculture or coculture with M. thermautotrophicus ΔH (=DSM 1053) in a 100-ml serum vial containing 50 ml of a culture medium described elsewhere (32). Strain SI was kindly provided by Hiroyuki Imachi (Nagaoka University of Technology, Nagaoka, Japan), while strain ΔH was obtained fromDeutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). The medium was supplemented with 0.1% Bacto yeast extract (Difco) and a growth substrate at a concentration of 17 mM. Cultivation was conducted at 55°C under an atmosphere consisting of N2 plus CO2 (80:20, vol/vol) without shaking. A monoculture of strain ΔH was grown as described above except that the gas phase was replaced with H2-CO2 (80:20, vol/vol). In order to examine microbial growth and hydrogen release, cultivation was initiated by inoculating the medium with 5 ml of a preculture in the same medium. To inhibit the H2 consumption by ΔH cells, 2-bromoethanesulfonate (2-BES) was added at a concentration of 6 mM immediately after cultivation was begun(9).
Microscopic methods.
Phase-contrast micrographs were taken using an E600 optical microscopy (Nikon). Cells in a culture were counted using a BX60 fluorescence microscope (Olympus), after the cells were stained with 2 mg liter−1 of 4′,6-diamidino-2-phenylindole (DAPI) for 2 min and collected on an Isopore membrane (0.22 μm; Millipore). SI cells could be distinguished from ΔH cells by color, shape, and fluorescence intensity. SI cells were elliptical and white and exhibited relatively strong fluorescence due to their high permeability to DAPI. By contrast, ΔH cells were rod shaped and green and exhibited relatively weak fluorescence due to their stiff cell wall made of pseudomurein. ΔH cells could also be identified by F420 autofluorescence (13).
In order to determine the ratio of the number of SI cells adhering to ΔH cells (RSI-ΔH) to the total number of SI cells (single cells plus adhering cells), more than 1,000 DAPI-stained cells from more than six different samples were analyzed with a fluorescence microscope, and the ratio was calculated with the following equation:
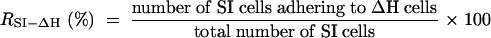 |
(1) |
Cells in aggregates were not considered for this calculation.
Electron microscopy.
Field emission-scanning electron microscopy (FE-SEM) was performed as described previously (15). Cells were grown in the presence of glass plates, each glass plate was carefully removed from the culture, and cells on the plate were fixed with 1.25% glutaraldehyde and 1.3% osmium tetraoxide. After cells were dehydrated using a graded series of ethanol solutions, they were dried using an HCP-2 drier (Hitachi). The resulting specimen was coated with osmium using a CVD coating device (Hitachi). The specimen was observed with an S4500 FE-SEM (Hitachi) at 5 kV.
Transmission electron microscopy (TEM) was also performed as described previously (15). Cells were negatively stained with phosphotungstic acid and observed with an H7000 TEM (Hitachi) at 75 kV.
AI.
The aggregation index (AI) was determined by methods described previously (6, 20). After the optical density at 600 nm (OD600) of a culture was measured using a DU640 spectrophotometer (Beckman), aggregated cells were dispersed by gentle homogenization using a tissue grinder (PTFE type; Asahi Techno Glass), until the OD600 no longer increased. The AI was calculated using the following equation:
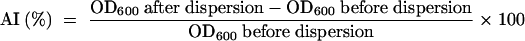 |
(2) |
Estimation of interspecies distance.
A theoretical average interspecies distance between SI and ΔH cells was estimated by using Fick's diffusion law (5, 28), which is expressed by the following equation:
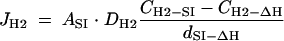 |
(3) |
where JH2 is an H2 flux between SI and ΔH cells, ASI is the surface area of an SI cell, DH2 is the H2 diffusion constant in water, CH2-SI is the H2 concentration immediately outside an SI cell, CH2-ΔH is the H2 concentration immediately outside a ΔH cell, and dSI-ΔH is the average distance between SI and ΔH cells.
Gas chromatography.
After a liquid sample was passed through a 0.22-μm membrane (type GV; Millipore) and acidified with concentrated HCl, volatile fatty acids and short-chain alcohols were analyzed using a gas chromatograph (GC-2010; Shimadzu) with a flame ionization detector and a DB-FFAP column (Shimadzu). The operation temperatures were as follows: injection temperature, 250°C; column temperature, 40 to 240°C (increased at a rate of 10°C min−1); and detector temperature, 300°C. The methane, hydrogen, nitrogen, and carbon dioxide in the headspace of a serum vial were measured using a gas chromatograph (GC-14A; Shimadzu) equipped with a thermal conductivity detector and a molecular sieve 5A 60-80/Porapack Q 80-100 column (Shimadzu). The column, injection, and detector temperatures were 50°C, 100°C, and 80°C, respectively.
RESULTS
Influence of culture conditions on aggregation.
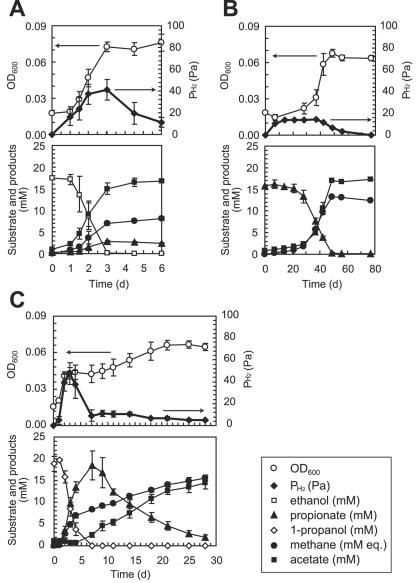
P. thermopropionicum SI was grown in cocultures with M. thermautotrophicus ΔH on propionate, ethanol, or 1-propanol (Fig. 1) andalso in monocultures on fumarate or pyruvate (data not shown). It has been reported that strain SI produces acetate, H2, and CO2 from ethanol and propionate, while formate did not contribute significantly to the syntrophic growth with the methanogen (14). Figure 1 also shows the production of relevant products and intermediate metabolites. The data show that growth on propionate was much slower than growth on ethanol or 1-propanol. The amounts of methane produced in these cultures were in good agreement with the amounts estimated according to the equations in Table 1. As shown in Fig. 1C, the propanol culture exhibited two phases of growth, a first phase in which 1-propanol was transformed to propionate and a second phase in which propionate was oxidized. The amount of propionate that accumulated after the first phase was equivalent to the amount of 1-propanol added when cultivation wasbegun.
FIG. 1.
Syntrophic oxidation of ethanol (A), propionate (B), and 1-propanol (C) by P. thermopropionicum SI in coculture with M. thermautotrophicus ΔH. The methane concentration was expressed as “mM equivalents” (mM eq.) by assuming that all methane was present in the aqueous phase. The data are means ± standard deviations obtained from three independent cultures.
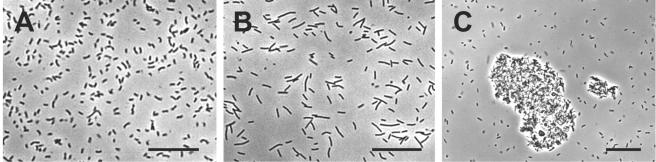
When strain SI was grown alone on fumarate or pyruvate, the cells were fully dispersed, and no aggregates were observed even in the stationary phase (Fig. 2A). Strain ΔH was also completely dispersed in a monoculture (Fig. 2B). By contrast, when the organisms were grown in a coculture, large cell aggregates were observed in the stationary phase (Fig. 2C). Aggregates were observed in all cocultures, albeit to different degrees. AI values were determined using equation 1 to more quantitatively compare aggregation trends under different culture conditions (Table 2). It was found that aggregation was most substantial when organisms were grown on propionate and less substantial when organisms were grown on ethanol. The aggregation trend in the first phase of the propanol culture was similar to that in the ethanol culture, while the second phase and the propionate culture exhibited similar trends (Table2).
FIG. 2.
Phase-contrast micrographs of an SI monoculture grown on fumarate (A), a ΔH monoculture grown on H2 plus CO2 (B), and a coculture of strains SI and ΔH grown on propionate (C). All micrographs were taken in the stationary phase (a phase in which the OD600 was no longer elevated). Bars, 25 μm.
TABLE 2.
AI and ratio of the number of SI-ΔH pairs to the total number of dispersed SI cells under different culture conditionsa
| Cells | Substrate | AI (%)b | RSI-ΔH (%)c |
|---|---|---|---|
| SI | Fumarate | 0.2 ± 0.3 | NAd |
| ΔH | H2-CO2 | 0.4 ± 0.3 | NA |
| SI + ΔH | Ethanol | 5.6 ± 0.5 | 27.4 ± 7.4 |
| Propionate | 19.1 ± 0.4 | 49.6 ± 7.5 | |
| 1-Propanol | 6.3 ± 1.4 | 30.4 ± 6.3 | |
| Propionatee | 11.2 ± 2.8 | 50.4 ± 8.3 |
The data are means ± standard deviations (n > 3).
The values were estimated by using equation 1 for stationary-phase cultures.
The values were estimated by using equation 2 for exponential-phase cultures.
NA, not analyzed.
Data for cells at the propionate oxidation phase of the 1-propanol culture (Fig. 1C).
In the mid-exponential growth phase (OD600, approximately 0.03), cell aggregates were also observed in cocultures, although the sizes were relatively small compared to the aggregates observed in the stationary phase (data not shown). In these aggregates, ΔH cells were shown to be the major components, while many SI cells were also incorporated into the aggregates, indicating that SI and ΔH cells coaggregated. When dispersed cells in coculture were observed with a microscope, many SI cells were found to adhere to ΔH cells to form SI-ΔH pairs in the mid-exponential growth phase; in contrast, pairs of SI cells or pairs of ΔH cells were not frequent (data not shown). Pairs of SI-ΔH cells were, however, rarely observed in the stationary-phase cultures. We estimated RSI-ΔH values for dispersed SI cells in the mid-exponential growth phase under different growth conditions using equation 2 (Table 2). The RSI-ΔH values show that pairs of SI-ΔH cells were most abundant in the propionate coculture.
FE-SEM observation of coculture.
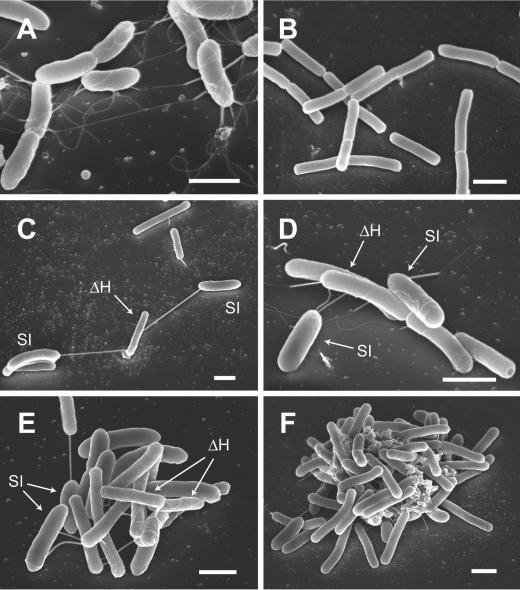
FE-SEM was used to gain insight into mechanisms underlying the connection and coaggregation of SI and ΔH cells. Compared to conventional scanning electron microscopy, FE-SEM improves the spatial resolution by using a field emission electron source, minimizes sample charge-up and damage by operating at lower electron-accelerating voltages, and gives better images for immediate biological surfaces by reducing electron penetration (25). For FE-SEM, cells were grown in the presence of glass plates at the bottom of a culture vial, which resulted in many cells being deposited (loosely attached) onto each glass plate.
Electron micrographs showed clear cell morphology, cell-cell contact, and the formation of large cell aggregates (Fig. 3). In these micrographs, SI cells are relatively thick and sausage shaped, while ΔH cells are relatively thin, straight, and rod shaped. It was found that SI cells produced long flagellum-like filaments (length, ∼10 μm) in a monoculture (Fig. 3A). These filaments were also observed by TEM (data not shown), although a previous report indicated that strain SI was nonmotile (14). In contrast, strain ΔH produced no filaments (Fig. 3B). When a coculture of SI and ΔH was observed by FE-SEM, the cells were found to be connected with the flagellum-like filaments of SI cells (Fig. 3C); it is likely that this is the initial step for SI cells to make contact with ΔH cells. When SI cells approached ΔH cells, flagellum-like filaments coiled around ΔH cells (Fig. 3D and E). In relatively small aggregates, the filaments were observed to intertwine with each other (Fig. 3E).In large aggregates, extracellular polymer substance (EPS)-like structures were seen in intercellular spaces (Fig. 3F). These results suggest that flagellum-like filaments of strain SI play roles in coaggregation of SI and ΔH cells. In addition, we need to consider the possibility that EPS-like material is involved in coaggregation.
FIG. 3.
Representative FE-SEM images of a monoculture of strain SI grown on fumarate (A), a monoculture of strain ΔH grown on H2 plus CO2 (B), and a coculture of SI and ΔH cells grown on propionate (C to F). Bars, 1 μm.
Estimation of interspecies distances.
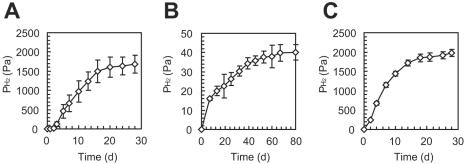
We were interested in examining how coaggregation contributed to interspecies electron transfer between SI and ΔH cells. In this case, H2 was the only compound that contributed to the interspecies electron transfer (14). A relationship between the H2 flux between SI and ΔH and the average distance between the cells was described by using Fick's diffusion law (equation 3). In the present study, in order to estimate “dSI-ΔH” values for syntrophic oxidation of propionate, ethanol, and 1-propanol, the other parameters were determined as follows. The JH2 value was calculated from a substrate oxidation rate in the coculture experiment (Fig. 1) by using the chemical equation in Table 1, and the value was equivalent to that calculated from the methane production rate. The ASI value was estimated by assuming that an SI cell was cylindrical with a diameter of 0.4 μm and a length of 0.9 μm. The DH2 value at 55°C was obtained from reference 40. For CH2-SI, we used the maximum H2 concentration below which substrate oxidation could proceed; this value was determined by experiments in which H2 consumption and methane production by ΔH cells were completely inhibited by adding 2-BES (Fig. 4). A partial pressure (in Pa) was converted to an aqueous concentration (in μM) by using Henry's constant for H2 at 55°C (7,722 Pa) (41). The CH2-SI values determined (i.e., the maximum H2 concentrations) were equivalent to the values predicted thermodynamically. For CH2-ΔH, we used the theoretical minimum H2 concentration above which an H2-consuming methanogen can gain energy by carbonate respiration (11). Since the maximum and minimum H2 concentrations were used as CH2-SI and CH2-ΔH, respectively, the “dSI-ΔH” value was defined as the allowable average interspecies distance for accomplishing syntrophic oxidation at an observed substrate oxidation rate. The values determined are summarized in Table 3.
FIG. 4.
Determination of the maximum H2 partial pressures for ethanol (A), propionate (B), and 1-propanol (C) oxidation. Hydrogen consumption by ΔH cells was inhibited by adding 2-BES. The data are means ± standard deviations obtained from three independent cultures.
TABLE 3.
Parameters in Fick's equation and allowable interspecies distances
Table 3 shows that the dSI-ΔH value for propionate is much less than the dSI-ΔH values for ethanol and 1-propanol. The OD600 in the mid-exponential growth phase of an SI-ΔH coculture was approximately 0.04 (Fig. 1), which corresponded to approximately 5 × 107 cells ml−1. In this case, the theoretical mean distance of randomly dispersed cells was calculated to be 30.4 μm. Clearly, in order to accomplish syntrophic propionate oxidation at the rate observed, the average interspecies distance should be much less than the distance between randomly dispersed cells (2 μm versus 30 μm). The allowable average interspecies distances for ethanol and propanol oxidation were, however, much greater than the distance for propionate oxidation. These estimates clearly show that close physical contact was particularly important for syntrophic propionate oxidation.
DISCUSSION
In the present study we found that P. thermopropionicum SI and M. thermautotrophicus ΔH coaggregated when they were grown in a syntrophic coculture (Fig. 2 and 3). This coaggregation was dependent on the growth substrate; thus, coaggregation was more significant when cells were grown on propionate than when cells were grown on ethanol or 1-propanol (Table 2). We considered the possibility that this coaggregation trend was correlated with the energetics of the oxidation reaction; i.e., coaggregation was particularly important for energetically unfavorable syntrophic propionate oxidation. Thermodynamic analyses have estimated that syntrophic propionate oxidation is energetically feasible only when the circumstantial H2 partial pressure is very low (27, 28, 34); in the present study, propionate oxidation by SI cells stopped when the H2 partial pressure was approximately 40 Pa (Fig. 4). The diffusion theory has suggested that at such a low H2 concentration, the distance between cells of an H2 producer and cells of an H2 consumer should be very small to obtain a sufficient rate of H2 flux between them (34); actually, we estimated that the interspecies distance should be less than 2 μm for propionate oxidation at the rate observed (Table 3). We concluded that strains SI and ΔH coaggregated in order to achieve efficient interspecies H2 flux.
The FE-SEM data suggest that flagellum-like filaments produced by strain SI are involved in coaggregation of SI and ΔH cells. Flagella have been known to play important roles in adhesion of bacteria to a variety of materials (23). O'Toole and Kolter showed that flagella were essential for the initial attachment of bacteria to abiotic surfaces (24). Tasteyre et al. have shown that flagellum proteins play a role in adherence of Clostridium difficile to mouse cecal mucus (35). They demonstrated that the flagellum cap protein FliD was highly adhesive to mouse cells (35). It has also been suggested that the flagellum cap protein is important for adhesion of Pseudomonas aeruginosa to mucin (2). Although involvement of flagella in prokaryotic interspecies attachment has not been well described, in the present study we found that many SI and ΔH cells were connected with flagellum-like filaments of SI cells (Fig. 3C, D, and E). In particular, the frequent long-distance connection of these cells with the filaments suggests that a tip structure of the filaments may play a role in initiating the contact. In addition to flagella, some bacterial strains (e.g., clostridia) (18, 21) have been known to produce unidentified thick filaments that connect cells of these strains. In order to identify the filament of strain SI, molecular characterization of component proteins is necessary.
Microorganisms utilize EPS materials for creating networks of cells in biofilms (7). In addition, EPS materials have also been found in dense granular assemblages of microorganisms in aerobic and anaerobic wastewater treatment facilities (4, 8, 22, 30). McSwain et al. have suggested that the formation and stability of granules are dependent on noncellular protein cores (22). In the present study, we found that EPS-like structures were present in intercellular spaces of SI-ΔH aggregates (Fig. 3F). We stained coaggregates of SI and ΔH cells with calcofluor (a dye for staining polysaccharides) and found that intercellular EPS-like structures contained polysaccharides (unpublished data). Since a genome analysis of strain ΔH revealed that ΔH possesses genes for polysaccharide metabolism (33), it is possible that strain ΔH was responsible for the production of EPS; however, there has been no report describing production of polysaccharides by strain ΔH. It would be interesting if ΔH produces polysaccharides only in the presence of a syntrophic partner. We also need to examine whether EPS-like structures really contribute to coaggregation.
The diffusion theory (i.e., Fick's law) has been applied to simulation of the importance of close physical contact of syntrophs and methanogens for syntrophic VFA oxidation (5, 34), although to our knowledge there has been no example that practically applied this theory to analysis of syntrophic VFA-oxidizing consortia. In the present study we introduced the available experimental data into Fick's equation to estimate the distances between SI and ΔH cells allowable for accomplishing substrate oxidation at the rates observed (Table 3). As a result, the estimated values reasonably explained why SI and ΔH cells should coaggregate, demonstrating the utility of this theory. An interesting feature of this theory is the relationship among an H2 flux (corresponding to substrate oxidation kinetics), an H2 concentration gradient (reflecting the thermodynamics of substrate oxidation), and an average distance between an H2 producer and a consumer. Accordingly, we are able to correlate thermodynamics with kinetics for syntrophic substrate oxidation. In order to further improve the applicability of the diffusion theory for analysis of syntrophic consortia, a radial diffusion model (3) may be useful. In addition, for fine-scale analyses, functional heterogeneity within a culture needs to be considered.
P. thermopropionicum strain SI was isolated from a UASB reactor as a representative propionate-oxidizing bacterium (13, 14), and FISH has shown that Pelotomaculum group bacteria are abundant populations in UASB granules (14). For M. thermautotrophicus, abundant closely related organisms have been detected in high-temperature anaerobic digestors by 16S rRNA gene analyses (19, 31) and immunological detection (1). The results of these studies have suggested that strains SI and ΔH represent important organisms in high-temperature methanogenic digestors. We therefore believe that the findings obtained in the present study have profound implications for understanding the formation of UASB granules. Several different mechanisms have been proposed so far for the granulation of anaerobic organisms (12). Weigant has proposed the “spaghetti theory” (39), in which Methanosaeta (formerly Methanothrix) filaments play primary roles in the granulation. Another idea is the “Cape Town hypothesis,” which proposes involvement of extracellular polypeptides from Methanobacterium (26). Based on the results of the present study, we propose an alternative possibility, that coaggregation of VFA-oxidizing syntrophs and H2-consuming methanogens facilitates initiation of granulation. To address this possibility, coaggregation of these organisms will be analyzed in the initial phase of granulation in an UASB reactor.
In conclusion, the present study provides data that can bridge the gap between the ecological process for granulation and the molecular mechanisms for cell adhesion. In order to better understand the granulation process, studies should move toward molecular and genomic analyses of the relevant organisms. We are currently conducting genome sequencing of P. thermopropionicum strain SI with the hope of gaining insight into mechanisms that underlie the syntrophism of this organism with its methanogenic partner.
Acknowledgments
We thank Hiroyuki Imachi (Nagaoka University of Technology) for providing P. thermopropionicum strain SI and Yasuo Igarashi (University of Tokyo), Hideki Harada (Nagaoka University of Technology), and Yoichi Kamagata (Agency of Industrial Science and Technology) for valuable suggestions. We also thank Hiroshi Ikenaga for continuous encouragement and Reiko Hirano for technical assistance.
This work was supported by New Energy and Industrial Technology Development Organization (NEDO).
REFERENCES
- 1.Ahring, B. K. 1995. Methanogenesis in thermophilic biogas reactors. Antonie Leeuwenhoek 67:91-102. [DOI] [PubMed] [Google Scholar]
- 2.Arora, S. K., B. W. Ritchings, E. C. Almira, S. Lory, and R. Ramphal. 1998. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect. Immun. 66:1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boone, D. R., R. L. Johnson, and Y. Liu. 1989. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Appl. Environ. Microbiol. 55:1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Beer, D., V. O'Flaharty, J. Thaveesri, P. Lens, and W. Verstraete. 1996. Distribution of extracellular polysaccharides and flotation of anaerobic sludge. Appl. Microbiol. Biotechnol. 46:197-201. [Google Scholar]
- 5.De Bok, F. A. M., C. M. Plugge, and A. J. M. Stams. 2004. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 38:1368-1375. [DOI] [PubMed] [Google Scholar]
- 6.Fine, D. H., D. Furgang, J. Kaplan, J. Charlesworth, and D. H. Figurski. 1999. Tenacious adhesion of Actinobacillus actinomycetemcomitans strain CU1000 to salivary-coated hydroxyapatite. Arch. Oral Biol. 44:1063-1076. [DOI] [PubMed] [Google Scholar]
- 7.Geesey, G. G. 1982. Microbial exopolymers: ecological and economic considerations. ASM News 48:9-14. [Google Scholar]
- 8.Grotenhuis, J. T. C., M. Smit, A. A. M. Van Lammeren, A. J. M. Stams, and A. J. B. Zehnder. 1991. Localization and quantification of extracellular polymers in methanogenic granular sludge. Appl. Microbiol. Biotechnol. 36:115-119. [Google Scholar]
- 9.Gunsalus, R. P., J. A. Romesser, and R. S. Wolfe. 1978. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry 17:2374-2377. [DOI] [PubMed] [Google Scholar]
- 10.Harmsen, H. J. M., H. M. P. Kengen, A. D. L. Akkermans, A. J. M. Stams, and W. M. de Vos. 1996. Detection and localization of syntrophic propionate-oxidizing bacteria in granular sludge by in situ hybridization using 16S rRNA-based oligonucleotide probes. Appl. Environ. Microbiol. 62:1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper, S. R., and F. G. Pohland. 1986. Recent developments in hydrogen management during anaerobic biological wastewater treatment. Biotechnol. Bioeng. 28:585-602. [DOI] [PubMed] [Google Scholar]
- 12.Hulshoff-Pol, L. W., S. I. de Castro Lopes, G. Lettinga, and P. N. L. Lens. 2004. Anaerobic sludge granulation. Water Res. 38:1376-1389. [DOI] [PubMed] [Google Scholar]
- 13.Imachi, H., Y. Sekiguchi, Y. Kamagata, A. Ohashi, and H. Harada. 2000. Cultivation and in situ detection of a thermophilic bacterium capable of oxidizing propionate in syntrophic association with hydrogenotrophic methanogens in a thermophilic methanogenic granular sludge. Appl. Environ. Microbiol. 66:3608-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imachi, H., Y. Sekiguchi, Y. Kamagata, S. Hanada, A. Ohashi, and H. Harada. 2002. Pelotomaculum thermopropionicum gen. nov., sp. nov., an anaerobic, thermophilic, syntrophic propionate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 52:1729-1735. [DOI] [PubMed] [Google Scholar]
- 15.Ishii, S., J. Koki, H. Unno, and K. Hori. 2004. Two morphological types of cell appendages on a strongly adhesive bacterium, Acinetobacter sp. strain Tol 5. Appl. Environ. Microbiol. 70:5026-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaspar, H. F., and K. Wuhrmann. 1978. Product inhibition in sludge digestion. Microb. Ecol. 4:241-248. [DOI] [PubMed] [Google Scholar]
- 17.Kaspar, H. F., and K. Wuhrmann. 1978. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl. Environ. Microbiol. 36:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhner, C. H., C. Matthies, G. Acker, M. Schmittroth, A. S. Gößner, and H. L. Drake. 2000. Clostridium akagii sp. nov. and Clostridium acidisoli sp. nov.: acid-tolerant, N2-fixing clostridia isolated from acidic forest soil and litter. Int. J. Syst. Evol. Microbiol. 50:873-881. [DOI] [PubMed] [Google Scholar]
- 19.Leclerc, M., J.-P. Delgènes, and J.-J. Godon. 2004. Diversity of the archaeal community in 44 anaerobic digesters as determined by single strand conformation polymorphism analysis and 16S rDNA sequencing. Environ. Microbiol. 6:809-819. [DOI] [PubMed] [Google Scholar]
- 20.Malik, A., and K. Kakii. 2003. Intergeneric coaggregations among Oligotropha carboxidovorans and Acinetobacter species present in activated sludge. FEMS Microbiol. Lett. 224:23-28. [DOI] [PubMed] [Google Scholar]
- 21.Matthies, C., C. H. Kuhner, G. Acker, and H. L. Drake. 2001. Clostridium uliginosum sp. nov., a novel acid-tolerant, anaerobic bacterium with connecting filaments. Int. J. Syst. Evol. Microbiol. 51:1119-1125. [DOI] [PubMed] [Google Scholar]
- 22.McSwain, B. S., R. L. Irvine, M. Hausner, and P. A. Wilderer. 2005. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge. Appl. Environ Microbiol. 71:1051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moens, S., and J. Vanderleyden. 1996. Functions of bacterial flagella. Crit. Rev. Microbiol. 22:67-100. [DOI] [PubMed] [Google Scholar]
- 24.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 25.Pawley, J. 1997. The development of field-emission scanning electron microscopy for imaging biological surfaces. Scanning 19:324-336. [PubMed] [Google Scholar]
- 26.Sam-Soon, P., R. E. Loewenthal, P. L. Dold, and G. R. Marais. 1987. Hypothesis for pelletisation in the upflow anaerobic sludge bed reactor. Water SA (Pretoria) 13:69-80. [Google Scholar]
- 27.Schink, B. 1997. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61:262-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schink, B., and R. K. Thauer. 1988. Energetics of syntrophic methane formation and the influence of aggregation, p. 5-17. In G. Lettinga, A. J. B. Zehnder, J. T. C. Grotenhuis, and L. W. Hulshoff-Pol (ed.), Granular anaerobic sludge; microbiology and technology. Pudoc, Wageningen, TheNetherlands.
- 29.Schmidt, J. E., and B. K. Ahring. 1993. Effects of hydrogen and formate on the degradation of propionate and butyrate in thermophilic granules from an upflow anaerobic sludge blanket reactor. Appl. Environ. Microbiol. 59:2546-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt, J. E., and B. K. Ahrin. 1994. Extracellular polymers in granular sludge from different upflow anaerobic sludge blanket (UASB) reactors. Appl. Microbiol. Biotechnol. 42:457-462. [Google Scholar]
- 31.Sekiguchi, Y., Y. Kamagata, K. Syutsubo, A. Ohashi, H. Harada, and K. Nakamura. 1998. Phylogenetic diversity of mesophilic and thermophilic granular sludges determined by 16S rRNA gene analysis. Microbiology 144:2655-2665. [DOI] [PubMed] [Google Scholar]
- 32.Sekiguchi, Y., Y. Kamagata, K. Nakamura, A. Ohashi, and H. Harada. 2000. Syntrophothermus lipocalidus gen. nov., sp. nov., a novel thermophilic, syntrophic, fatty-acid-oxidizing anaerobe which utilizes isobutyrate. Int. J. Syst. Evol. Microbiol. 50:771-779. [DOI] [PubMed] [Google Scholar]
- 33.Smith, D. R., L. A. Doucette-Stamm, C. Deloughery, H. Lee, J. Dubois, T. Aldredge, R. Bashirzadeh, D. Blakely, R. Cook, K. Gilbert, D. Harrison, L. Hoang, P. Keagle, W. Lumm, B. Pothier, D. Qiu, R. Spadafora, R. Vicaire, Y. Wang, J. Wierzbowski, R. Gibson, N. Jiwani, A. Caruso, D. Bush, H. Safer,D. Patwell, S. Prabhakar, S. McDougall, G. Shimer, A. Goyal, S. Pietrokovski, G. M. Church, C. J. Daniels, J.-I. Mao, P. Rice, J. Nölling, and J. N. Reeve. 1997. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J. Bacteriol. 179:7135-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stams, A. J. M. 1994. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Leeuwenhoek 66:271-294. [DOI] [PubMed] [Google Scholar]
- 35.Tasteyre, A., M.-C. Barc, A. Collignon, H. Boureau, and T. Karjalainen. 2001. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 69:7937-7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thauer, R. K., K. Jungermann, and K. Decker. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41:100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueno, Y., S. Haruta, M. Ishii, and Y. Igarashi. 2001. Changes in product formation and bacterial community by dilution rate on carbohydrate fermentation by methanogenic microflora in continuous flow stirred tank reactor. Appl. Microbiol. Biotechnol. 57:65-73. [DOI] [PubMed] [Google Scholar]
- 38.Van Lier, J. B., J. L. S. Martin, and G. Lettinga. 1996. Effect of temperature on the anaerobic thermophilic conversion of volatile fatty acids by dispersed and granular sludge. Water Res. 30:199-207. [Google Scholar]
- 39.Wiegant, W. W. 1987. The “spaghetti theory” on anaerobic granular sludge formation, or the inevitability of granulation, p. 146-152. In G. Lettinga, A. J. B. Zehnder, J. T. C. Grotenhuis, and L. W. Hulshoff Pol (ed.), Granular anaerobic sludge; microbiology and technology. Centre for Agricultural Publishing and Documentation, Wageningen, The Netherlands.
- 40.Wise, D. L., and G. Houghton. 1966. The diffusion coefficients of ten slightly soluble-gases in water at 10-60°C. Chem. Eng. Sci. 21:999-1010. [Google Scholar]
- 41.Young, C. L. 1981. Hydrogen and deuterium. IUPAC solubility data series, vol. 5/6. Pergamon Press, New York, N.Y.