Abstract
Ant lions are insect larvae that feed on the liquefied internal components of insect prey. Prey capture is assisted by the injection of toxins that are reportedly derived from both the insect and bacterial symbionts. These larvae display interesting gut physiology where the midgut is not connected to the hindgut, preventing elimination of solid waste until adulthood. The presence of a discontinuous gut and the potential involvement of bacteria in prey paralyzation suggest an interesting microbial role in ant lion biology; however, the ant lion microbiota has not been described in detail. We therefore performed culture-independent 16S rRNA gene sequence analysis of the bacteria associated with tissues of an ant lion, Myrmeleon mobilis. All 222 sequences were identified as Proteobacteria and could be subdivided into two main groups, the α-Proteobacteria with similarity to Wolbachia spp. (75 clones) and the γ-Proteobacteria with similarity to the family Enterobacteriaceae (144 clones). The Enterobacteriaceae-like 16S rRNA gene sequences were most commonly isolated from gut tissue, and Wolbachia-like sequences were predominant in the head and body tissue. Fluorescence in situ hybridization analyses supported the localization of enterics to gut tissue and Wolbachia to nongut tissue. The diversity of sequences isolated from freshly caught, laboratory-fed, and laboratory-starved ant lions were qualitatively similar, although the libraries from each treatment were significantly different (P = 0.05). These results represent the first culture-independent analysis of the microbiota associated with a discontinuous insect gut and suggest that the ant lion microbial community is relatively simple, which may be a reflection of the diet and gut physiology of these insects.
Insects are one of the most diverse groups of organisms on the planet, with widely varied behaviors and ecological niches. This diversity will likely be reflected in an abundance of intriguing host-bacterium interactions, and accumulating evidence indicates that bacteria play important roles in the nutrition (1, 5, 8, 10, 18, 40), reproduction (1, 4, 10, 58, 68), development (69), and even behavior (60) of their insect hosts. However, the microbiota of many biologically diverse insect taxa remain uncharacterized. One such unexplored group is the genus Myrmeleon.
Species of Myrmeleon are an example of a widely distributed predatory insect with interesting pit-building behavior (Fig. 1A) during the larval stage (Fig. 1B). The larvae, called ant lions, use the pits to capture insect prey, which are rapidly paralyzed and killed through the injection of toxins and digestive enzymes. The larvae then feed on the liquefied contents of the prey and discard the carcass. Ant lions are semisedentary with a variable and intermittent diet and are capable of withstanding starvation for up to 3 months (32).
FIG. 1.
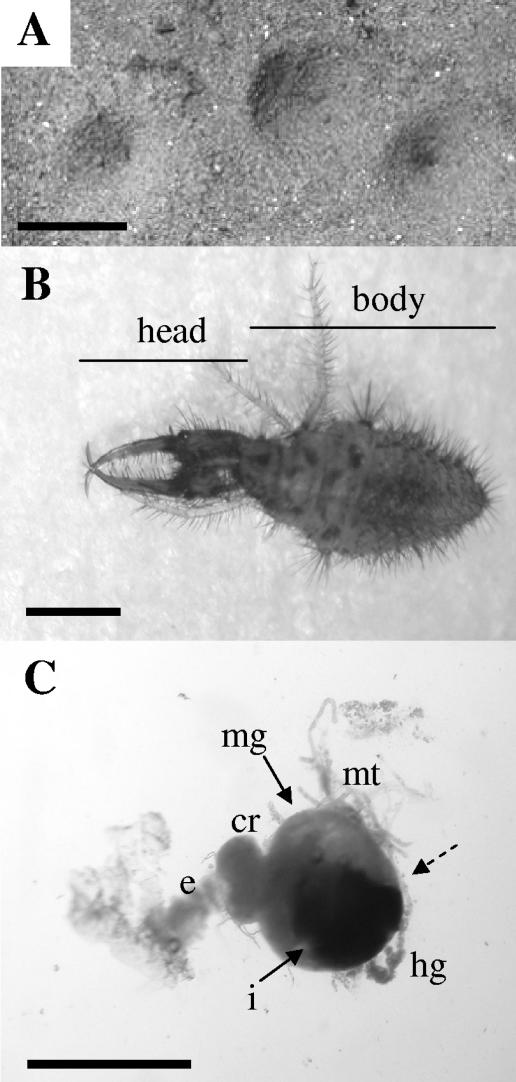
Ant lion habitat and morphology. A. Conical pits constructed by ant lion larvae in sandy soil. The scale bar represents 2 cm. B. First-instar M. mobilis. Lines depict regions sampled as head and body tissue in experiments. The scale bar represents 0.1 cm. C. Representative gastrointestinal tract dissected from the animal shown in panel B. The scale bar equals 0.1 cm. The dashed arrow points to the constriction between the midgut and hindgut which results in a discontinuous gut. cr, crop; e, esophagus; hg, hindgut; i, dark pellet of indigestible material in midgut; mg, midgut; mt, Malpighian tubules.
Myrmeleon larvae have a discontinuous gut (Fig. 1C), in which the midgut ends as a blind sac rather than being connected to the hindgut (24, 28, 65). During the three larval instars, which last 1 to 2 years, the hindgut is considered nonfunctional (65), and any indigestible material consumed by the ant lion is retained as a pellet in the midgut until the adult stage. In adults, the gut becomes continuous and the pellet is voided immediately after emergence (64). This physiological adaptation of the gut during the larval stages creates an interesting habitat where microbes would be confined and subjected to the alternating secretory and absorptive phases of the midgut (64), unlike in a continuous gut, where secretion and absorption can be performed in separate gut regions. In addition, the intermittent feeding of the ant lion and its inability to void solid waste could restrict the size or composition of the microbial community, although this has not been investigated.
Recently, there has also been interest in the bacterial associates of ant lions, based on their potential role in prey paralyzation. Although some toxins are apparently insect derived (43, 67), compounds produced by bacterial isolates cultured from the Japanese ant lion, Myrmeleon bore, have been implicated as contributing to prey paralyzation and/or death and include GroEL from Enterobacter aerogenes and sphingomyelinase C from Bacillus cereus (47, 66).
The presence of a discontinuous gut and the potential involvement of bacteria in the feeding process of ant lions suggest an interesting microbial role in ant lion biology. Little is known, however, about the ant lion microbial community and its distribution within host tissue. To begin to address these issues, we chose to focus our studies on the larvae of Myrmeleon mobilis, an ant lion species routinely found in the Southeastern United States. Our goals were to characterize the M. mobilis microbial community using 16S rRNA gene sequence analysis and to use fluorescence in situ hybridization (FISH) to identify tissue-specific interactions between ant lions and their microbiota. Here, we report the first culture-independent characterization of the ant lion microbiota and, to our knowledge, of the bacteria associated with a discontinuous insect gut.
MATERIALS AND METHODS
Insect collection and culturing.
Ant lions were collected from the State Botanical Garden of Georgia at the University of Georgia in Athens, Ga. Representative adults were identified as M. mobilis by John D. Oswald, Department of Entomology, Texas A&M University. Insects used in all experiments were approximately 0.5 cm in length (first instar) and of similar body shape and pigmentation. Any ant lions not sampled immediately were placed in autoclaved sandy soil and maintained at room temperature (23 to 25°C). Laboratory-fed ant lions were offered mealworms twice weekly and were kept in the laboratory for 1 to 2 weeks. Laboratory-starved ant lions were not fed mealworms and were kept in the laboratory for 21 to 28 days before sampling.
Tissue dissection and DNA isolation.
Ant lions were anesthetized by placing them at −20°C for 2 min and were surface disinfested by rinsing them five times in sterile distilled water with vortexing. Removal of soil particles and microorganisms was monitored microscopically and by dilution plating of the final-wash liquid on 1/10-strength tryptic soy agar (Becton Dickinson and Company, Sparks, Md.). The fore-, mid-, and hindgut tissues (gastrointestinal [GI] tract) were isolated by ventral incision and removal using forceps (Fig. 1C). Head tissue was isolated by removing the head from the body using a scalpel blade, and body tissue was defined as the remaining tissue after GI tract and head removal (Fig. 1B). Tissue samples from each ant lion were processed individually. Each tissue sample was immediately placed into a 1.5-ml microcentrifuge tube on ice in 200 μl of 1× phosphate buffered saline (PBS) (260 mM NaCl, 10 mM Na2HPO4, 10 mM NaH2PO4, pH 7.2). Tissue was homogenized using a pestle (BIO PLAS, Inc., San Rafael, Calif.) and sonicated for 30 s in a Bransonic 1510 bath sonicator (Branson Ultrasonics Corp., Danbury, Conn.). DNA was isolated either using the EasyDNA kit (Invitrogen, Carlsbad, Calif.) or through bead beating with a Mini-Beadbeater (Biospec Products Inc., Bartlesville, Okla.) and a method based on that described previously by Furlong et al. (23). Briefly, 400 μl of bead-beating buffer (0.1 M NaCl, 0.5 M Tris HCl, pH 8.0, and 10% sodium dodecyl sulfate) was added to a 2-ml screw-cap vial containing the homogenized tissue and 0.1 g of 0.1-mm glass beads (Biospec Products, Inc.). Samples were run for 3 min at 4,600 rpm. The supernatant was extracted sequentially with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) and chloroform and precipitated with ethanol.
16S rRNA gene sequence analysis.
The purified DNA was used as a template for PCR amplification of community 16S rRNA gene partial sequences using the universal bacterial primers 27F and 1492R (39) and KOD HiFi DNA polymerase (Novagen, Madison, Wis.), according to the manufacturer's protocols for amplification. PCR primers were synthesized by Integrated DNA Technologies (Coralville, Iowa). PCR products were cloned using the Zero Blunt TOPO PCR cloning kit (Invitrogen) and sequenced at the University of Michigan DNA Sequencing Core Facility. A total of 35 clone libraries representing each tissue type from individual ant lions were constructed. Restriction digestion of plasmids isolated from individual clones identified those containing DNA inserts of approximately 1,500 bp, of which 3 to 22 were randomly chosen for sequencing, depending on the tissue type sampled (Table 1). Sequence length ranged from approximately 1,466 bp (Wolbachia-like sequences) to 1,506 bp. Sequences were checked for chimeras using Chimera Check from the Ribosomal Database Project-II (15, 16) and Bellerophon (34). Sequences were assigned to phylogenetic classes and checked for closely related database sequences using the Classifier and Seqmatch tools, respectively, from the Ribosomal Database Project-II (15). Sequence alignments were performed using ClustalX (59) under the default settings with a gap-opening penalty of 10.0 and a gap extension penalty of 0.1 or 0.2 for pairwise and multiple alignments, respectively. Distance matrices were constructed using the DNADIST program within the PHYLIP version 3.6 software package (21, 22) using the Jukes-Cantor correction for multiple substitutions. Operational taxonomic unit (OTU) groupings were determined using DOTUR with the furthest-neighbor clustering algorithm (54) and were reported at a 2% distance level. Total species richness using Chao1 as a nonparametric richness estimator was calculated using DOTUR. Phylogenetic trees were constructed using PAUP 4.0b (Sinauer Associates, Inc., Sunderland, Mass.). Trees were illustrated using TreeView (49). Statistical comparisons of library diversity were performed using ∫-LIBSHUFF (55).
TABLE 1.
Numbers of ant lions sampled and 16S rRNA gene sequences analyzed per tissue type and feeding treatment
| Feeding treatment | Tissue type
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Head
|
GI tract
|
Body
|
Whole ant lion
|
|||||
| No. of ant lions | No. of sequences | No. of ant lions | No. of sequences | No. of ant lions | No. of sequences | No. of ant lions | No. of sequences | |
| Freshly caught | 4 | 24 | 7 | 50 | 1 | 5 | 1 | 12 |
| Laboratory fed | 6 | 31 | 4 | 27 | 0 | |||
| Laboratory starved | 5 | 25 | 5 | 23 | 1 | 3 | 1 | 22 |
| Total | 15 | 80 | 16 | 100 | 2 | 8 | 2 | 34 |
Cryosectioning.
Whole ant lions were fixed in 4% paraformaldehyde at 4°C for 4 h under vacuum, sucrose infused at 4°C for 12 to 16 h under vacuum, frozen in tissue-mounting medium, and sectioned (18 μm thick) using a cryostat (3). Sections were mounted onto Superfrost Plus slides (Fisher Scientific, Hampton, N.H.) and fixed a second time using 4% paraformaldehyde for 5 min at room temperature. Slides were washed with 1× PBS for 2 min and dehydrated through an ethanol series (50%, 80%, and 100% ethanol for 2 min each). Slides were stored with dessicant at −80°C.
Fluorescence in situ hybridization.
The oligonucleotide probes used for FISH were synthesized by Integrated DNA Technologies and included probe D that is specific for Enterobacteriaceae (48) labeled with Cy5, probe W2 that is specific for the genus Wolbachia (31) labeled with Alexa Fluor 546, and the universal bacterial probe EUB338 labeled with Oregon green 488 (2). The nonsense probe NON338, which is the reverse complement of EUB338 (Oregon green 488 labeled), or RNase A treatment (50 ng μl−1; Calbiochem, San Diego, Calif.) was used as a control for nonspecific hybridization. Forty microliters of prehybridization buffer (0.9 M NaCl, 20 mM Tris HCl, pH 7.4, 20% formamide, 0.01% sodium dodecyl sulfate) was added to each slide, and the samples were covered with a Hybrislip (RPI Corp., Mt. Prospect, Ill.) and incubated in a humid chamber at 50°C. After 30 min, 0.5 μl of each probe (50 mM stock) was added beneath each Hybrislip, and samples were incubated at 50°C for 2 h. Slides were placed in washing buffer (20 mM Tris HCl, pH 7.4, 180 mM NaCl, 0.01% sodium dodecyl sulfate), incubated at 50°C for 15 min, rinsed with ice-cold sterile distilled water, and air dried. Slides were mounted with Fluoroguard antifading solution (Bio-Rad, Hercules, Calif.) and visualized using a Leica TCS SP2 spectral confocal microscope (Leica Microsystems Inc., Exon, Pa.) equipped with a 63.0× HCX PL APO water immersion objective using the excitation wavelengths of 488 nm, 543 nm, and 633 nm. Fluorescence images were pseudocolored and reconstructed from visual sections using Leica Confocal software and were labeled using Adobe Photoshop version 7.0. Transmitted-light images were captured using a Nikon Coolpix 5000 camera (Nikon, Inc., Melville, N.Y.).
Estimation of bacterial numbers in the GI tract.
The GI tracts of freshly caught first-instar ant lions were each placed in 50 μl of filter-sterilized 1× PBS, homogenized with a pestle, and sonicated for 30 s. One microliter of each sample was dilution plated onto 1/10-strength tryptic soy agar, plates were incubated at 28°C for 24 h, and colonies were counted to determine the average CFU per GI tract. Incubation beyond 24 h and up to 3 days did not yield additional colonies. The remaining 49 μl of gut homogenate was stained with 4′,6′-diamidino-2-phenlindole (DAPI) (Molecular Probes, Eugene, Oreg.) using a variation of a previously described method (51). Briefly, 1 ml of filter-sterilized 1× PBS and 20 μM DAPI was added to each homogenate. Samples were incubated for 10 min in the dark at room temperature and filtered onto a prewetted 0.22-μm black polycarbonate filter (GE Osmonics, Inc., Minnetonka, Minn.). Filters were rinsed with 1 ml of 1× PBS and mounted onto microscope slides using Fluoroguard antifading solution (Bio-Rad). Filters were visualized using a Nikon Eclipse E600 epifluorescence microscope equipped with a Nikon 96165 tricolor filter cube and a 100× Plan Fluor oil immersion objective. The area of the field of view was calculated using a micrometer, and the number of bacterium-like objects were counted in randomly chosen fields of view until 20 fields or 400 bacteria were counted. These numbers were used to estimate the number of bacteria per GI tract. The volume of the GI tract of first-instar ant lions was estimated by measuring the dimensions of dissected GI tracts and calculating volumes.
Nucleotide sequence accession numbers.
All 156 unique 16S rRNA gene sequences are available in the GenBank database under the accession numbers DQ068777 to DQ068932. The nine 16S rRNA gene sequences from cultured ant lion isolates and seven 16S rRNA gene sequences from laboratory-cultured mealworms (Tenebrio molitor) are available under accession numbers DQ163936 to DQ163944 and DQ163945 to DQ163951, respectively.
RESULTS
Our goals were to characterize the M. mobilis microbial community using 16S rRNA gene sequence analysis and to identify tissue-specific associations between ant lions and their microbiota using FISH. For consistency, we focused on late-first-instar ant lions, which were approximately 0.5 cm in length. These animals fed on insect prey since hatching, yet they were small enough to be cryosectioned.
We constructed a total of 35 16S rRNA gene sequence libraries from various tissue types of individual ant lions, including the head, GI tract, remaining body (body minus the head and GI tract), and whole bodies. In addition, animals were subjected to three different feeding treatments, including being freshly caught from the field, caught from the field and maintained in the laboratory on a diet of mealworms, or caught from the field and maintained in the laboratory without feeding (Table 1). Out of 251 sequences, 29 were discarded as chimeras (approximately 12%).
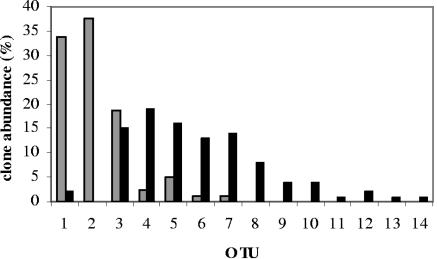
The remaining 222 sequences were grouped into 14 OTUs based on a 2% distance level (Table 2) using DOTUR (54). Of the 222 sequences, 156 were unique, with identical sequences found within eight OTUs (OTUs 1 to 5 and 7 to 9) and with identical sequences found in more than one ant lion for five OTUs (OTUs 1 to 3, 5, and 7). The libraries were dominated by two main groups of sequences, those with similarity to Wolbachia (75 sequences; OTUs 1 and 2) and those with similarity to the Enterobacteriaceae (144 sequences; OTUs 3 to 10, 12, and 13) (Fig. 2). The Wolbachia-like sequences were most commonly amplified from the head samples, whereas the Enterobacteriaceae-like sequences were amplified most commonly from the GI tract tissue samples (Fig. 3). ∫-LIBSHUFF analysis (55) of the head and GI tract libraries determined that they are significantly different (P = 0.05).
TABLE 2.
Phylogenetic groupings for all 222 16S rRNA gene sequences from Myrmeleon mobilis larvae and distribution of clones within tissue types and OTUs among ant lions
| OTUa | Representative clone | Class | Sequence database matchb (organism; % identity; GenBank accession no.) | No. of clones | No. of unique clones | No. of clones from each tissue type
|
No. of ant lions harboring each OTU
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head | GI tract | Body | Whole ant lion | Freshly caught (n = 8) | Lab fed (n = 6) | Lab starved (n = 6) | ||||||
| 1 | s1h19 | α-Proteobacteria | Wolbachia pipientis wRi; 98; AY833061 | 39 | 13c,d | 27 | 2 | 1 | 9 | 6 | 3 | 4 |
| 2 | f3h11 | α-Proteobacteria | Wolbachia pipientis wRi; 97; AY833061 | 36 | 13c,d | 30 | 0 | 6 | 0 | 5 | 4 | 2 |
| 3 | s2s6 | γ-Proteobacteria | Serratia marcescens KRED; 99; AB061685 | 31 | 28c,d | 15 | 15 | 0 | 1 | 2 | 4 | 3 |
| 4 | f5s7 | γ-Proteobacteria | Citrobacter murliniae; 99; AF025369 | 28 | 27 | 2 | 19 | 0 | 7 | 0 | 4 | 2 |
| 5 | f1s3 | γ-Proteobacteria | Raoultella ornithinolytica; 99; U78182 | 23 | 16c,d | 4 | 16 | 0 | 3 | 3 | 4 | 3 |
| 6 | s6s1 | γ-Proteobacteria | Enterobacter asburiae; 99; AB004744 | 20 | 20 | 1 | 13 | 0 | 6 | 4 | 1 | 2 |
| 7 | 3s2 | γ-Proteobacteria | Arsenophonus endosymbiont of Aleuroplatus gelatinosus; 98; AY264665 | 16 | 12c | 1 | 14 | 1 | 0 | 1 | 0 | 3 |
| 8 | 4s31 | γ-Proteobacteria | Brenneria quercina DSM4561; 99; AJ233416 | 8 | 7 | 0 | 8 | 0 | 0 | 1 | 0 | 0 |
| 9 | 6s25 | γ-Proteobacteria | Buttiauxella izardii; 98; AJ233404 | 8 | 7 | 0 | 4 | 0 | 4 | 1 | 0 | 1 |
| 10 | 7s2 | γ-Proteobacteria | Enterobacter sp. strain NAB3b; 98; AY395009 | 7 | 7 | 0 | 4 | 0 | 3 | 2 | 0 | 0 |
| 11 | 1s5 | β-Proteobacteria | Aquabacterium parvum; 96; AF035052 | 2 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| 12 | bb2s3 | γ-Proteobacteria | Enterobacter sp. clone o16; 98; AY376693 | 2 | 2 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| 13 | s2s7 | γ-Proteobacteria | Citrobacter diversus; 97; AF025372 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 14 | s1s9a | γ-Proteobacteria | Acinetobacter junii S33; 99; AB101444 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Total | 222 | 156 | 80 | 100 | 8 | 34 | ||||||
Determined using DOTUR (54), with a 2% distance level.
Determined using Seqmatch (15) based on highest similarity score for both type and nontype strains with sequences >1,450 nucleotides.
Identical sequences found in >1 ant lion with the same feeding treatment.
Identical sequences found in ant lions with different feeding treatments.
FIG. 2.
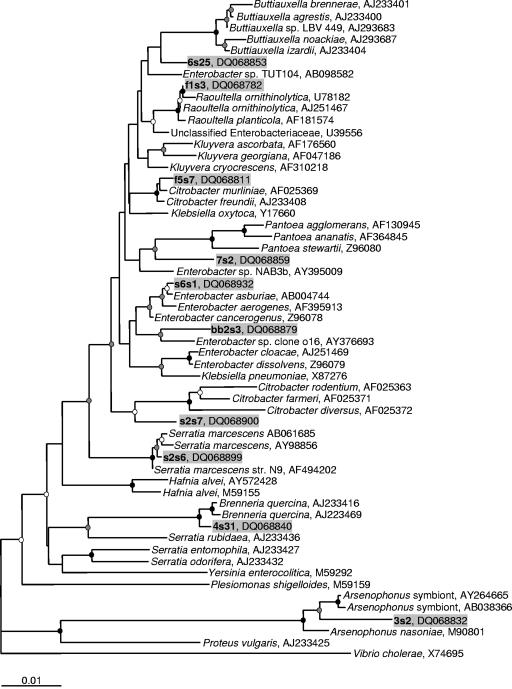
Inferred phylogenetic tree based on 16S rRNA gene sequences of representative clones belonging to the Enterobacteriaceae (OTUs 3 to 10, 12, and 13), including selected database sequences. The tree was generated using the neighbor-joining method with 2,000 bootstrap replicates using 1,416 nucleotides. Maximum-likelihood analysis resulted in similar tree topology (data not shown). Scientific names represent Bergey's classification from the Ribosomal Database Project-II website (15) and are followed by the GenBank accession number. Representative clones are listed in boldface type followed by GenBank accession numbers and include background shading. Branch points supported by bootstrap values of >90% are represented by black circles, >70% are represented by gray circles, and >50% are represented by white circles. Branches without circles are unresolved (bootstrap values of <50%). Database sequences included were chosen using the SeqMatch tool from the Ribosomal Database Project-II (15) with the addition of other sequences representing genera belonging to the Enterobacteriaceae. The tree was rooted with a 16S rRNA gene sequence from Vibrio cholerae, and the use of multiple different outgroup sequences from other Proteobacteria did not affect tree topology. The bar represents 0.01 substitutions per site.
FIG. 3.
The percentage of 16S rRNA gene sequences isolated from the head (gray bars; n = 80) or GI tract (black bars; n = 100) of ant lions that belong to each of the 14 OTUs (Table 2).
To determine if feeding history affected the microbial community associated with the ant lions, we compared the distribution of the16S rRNA gene sequences generated from the freshly caught, laboratory-fed, and laboratory-starved animals among the 14 OTUs. Our analysis indicates that the microbiota is significantly different between feeding treatments (P = 0.05), although there are several OTUs in common between all treatments (Table 2). To determine whether enough sequences had been analyzed to represent the diversity of each community, the total richness of the communities associated with each feeding treatment was calculated using DOTUR with the Chao1 richness estimator (12) at a 2% distance level. In this way, we calculated theoretical totals of 9 OTUs in the freshly caught ant lions, 5 OTUs in the laboratory-fed ant lions, and 10 OTUs in the laboratory-starved ant lions, which corresponds closely to the 9, 5, and 9 experimentally observed OTUs, respectively. This suggests that we sequenced enough clones to detect the diversity of OTUs present in each feeding treatment. Rarefaction curve analysis supports this conclusion (data not shown).
We were concerned that the EasyDNA kit used to isolate DNA from ant lion tissue might selectively lyse the bacteria, leading to a misrepresentation of the microbial community present in these insects. Therefore, we also prepared DNA from two freshly caught ant lions using a bead-beating method and amplified 16S rRNA gene sequences from these samples. All 28 sequences analyzed could be grouped within OTU 1, 2, 3, 6, 10, or 12 (based on a 2% distance level estimated by DOTUR), suggesting that we had not missed a major community member using the EasyDNA method.
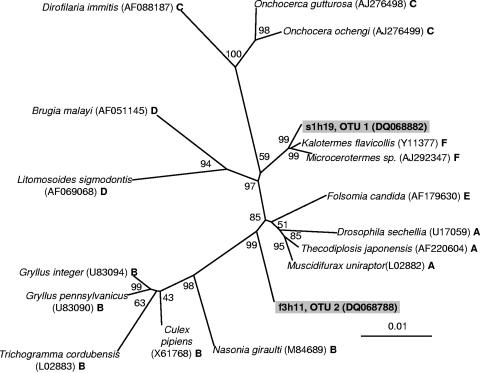
Interestingly, the DOTUR analysis placed the Wolbachia-like sequences into two OTUs at a 2% distance level (Table 2). Wolbachia species are widespread insect-associated bacteria that are known to manipulate the sexual reproduction of their hosts (68) and have been arranged into six supergroups (A to F) based on ftsZ and 16S rRNA gene sequence analysis (41). To investigate whether the two Wolbachia-like OTUs were of the same or different supergroups, we used 16S rRNA gene sequences from Wolbachia belonging to each supergroup and a representative sequence from each OTU to construct an unrooted phylogenetic tree (Fig. 4). This analysis suggests that OTU 1 and OTU 2 belong to different supergroups, although sequence analysis using other gene markers will be necessary for precise supergroup assignment. Sequences belonging to both OTU 1 and OTU 2 were isolated from 8 out of 20 ant lions sampled. Although sequences belonging to OTU 1 and OTU 2 were not isolated from each ant lion sampled, the number of sequences analyzed from each ant lion was modest (Table 1), and it appears possible that larvae of M. mobilis harbor two distinct types of Wolbachia.
FIG. 4.
Unrooted phylogenetic tree of Wolbachia-like sequences based on 16S rRNA genes showing positions of members of the six Wolbachia supergroups A to F (41) and a representative clone from OTUs 1 and 2. The tree was generated using the neighbor-joining method with 10,000 bootstrap replicates using 1,290 nucleotides. Maximum-likelihood analysis resulted in the same tree topology (data not shown). Scientific names represent insect host species and are followed by GenBank accession numbers and the Wolbachia supergroup designations. The bar represents 0.01 substitutions per site. Using other clones to represent OTU 1 or OTU 2 did not affect tree topology.
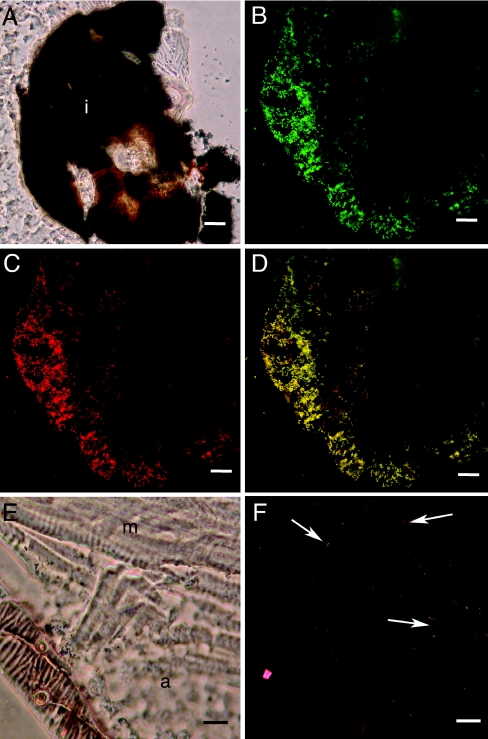
To identify where the bacteria were associating with ant lion tissue, we used FISH with cryosections of ant lion tissue, from the head through the lower abdomen, using the general eubacterial probe EUB338 (2) and the group-specific probes D for the Enterobacteriaceae (48) and W2 for Wolbachia (31). Bacterium-like objects that hybridized with EUB338 also hybridized with either W2 or probe D (Fig. 5), and no bacterium-like objects were observed when sections were hybridized with the nonsense probe or treated with RNase (data not shown). Bacterium-like objects hybridizing with probe D were only found associated with the indigestible food material located at the region of the GI tract where the midgut is discontinuous with the hindgut (Fig. 5A to D). No bacterium-like objects were observed in the hindgut. In contrast, bacterium-like objects hybridizing with the Wolbachia-specific W2 probe were found scattered among various tissue types, including adipose and muscle (Fig. 5E and F), but were not associated with the GI tract tissue or the GI tract contents (data not shown).
FIG. 5.
Fluorescence in situ hybridization of ant lion cryosections. A. Transmitted-light micrograph of a cryosection through the abdomen of an ant lion showing the dark pellet of indigestible material at the barrier between the midgut and the hindgut (i). B to D. Corresponding confocal micrographs of FISH using EUB338 (green, panel B) and probe D (red, panel C) and overlay showing EUB338 and probe D (panel D). Yellow objects associated with the undigested material are hybridizing with EUB338 and probe D (panel D). E. Transmitted-light micrograph of a cryosection through the abdomen of the ant lion showing adipose (a) and muscle (m) tissue near the body wall. D. Corresponding confocal micrograph of FISH using EUB338 (green) and W2 (red). Yellow-orange objects scattered in tissue (arrows indicate examples) are hybridizing with EUB338 and W2. For A to D, scale bars represent 20 μm; for E and F, scale bars represent 10 μm.
Our FISH results suggested that large numbers of bacteria are present in the GI tract of ant lions (Fig. 5A to D). To approximate the number of bacteria in the GI tract, we dilution plated GI tract homogenates from three freshly caught ant lions. After overnight incubation, there were an average of 2.8 × 106 CFU per GI tract (standard error, ±8.5 × 105; range, 1.8 × 106 to 4.1 × 106). Direct counts of DAPI-stained bacterium-like cells in the gut homogenates did not reveal greater numbers of bacteria present in GI tracts relative to plating (average, 1.7 × 106; standard error, ±8.2 × 104; range, 1.6 × 106 to 1.8 × 106), suggesting that many or most of the bacteria present are easily culturable. Direct counting of additional gut homogenates with and without DAPI staining and/or fixation yielded similar results (data not shown). Sequence analysis of 16S rRNA genes from nine streak-purified isolates identified all isolates as members of the Enterobacteriaceae and six isolates as belonging to either OTU 3, OTU 9, or OTU 10 (data not shown). It has been reported previously that the numbers of culturable bacteria present can approach direct counts of DAPI-stained bacteria in human fecal samples (29), sea ice bacteria (38), and the rainbow trout intestine (33). Our results indicate that a large proportion of the ant lion GI tract microbiota is culturable and that some of these culturable bacteria are similar to previously described enteric bacteria. These results agree with our culture-independent sequence analysis of the GI tract, which identified a large proportion of the GI tract microbiota as belonging to the Enterobacteriaceae (Table 2 and Fig. 2), although we cannot explain the consistently larger population size estimates using plate counts compared to those with direct counts.
By measuring the dimensions of dissected GI tract tissue from first-instar larvae (Fig. 1C), the approximate gut volume was estimated to be 1 μl. This volume will vary with the feeding history of the animal, and in particular, large meals will greatly distend the gut. As the ant lion processes the liquid and voids the liquid waste into the environment, the gut volume decreases. In addition, depending on the frequency with which the animal has been feeding, the size of the pellet of undigested material in the midgut will vary, effectively changing the volume of this gut section. Using our estimation of an average gut volume of 1 μl and the presence of approximately 2 × 106 bacteria per GI tract, the density is nearly 2 × 109 bacteria per ml.
DISCUSSION
Ant lions are insects with fascinating predatory behavior and gut physiology that live under dry conditions with a variable and intermittent food source. The combination of a discontinuous gut and an inconsistent food source creates an interesting gut microbial habitat. In addition, it has previously been reported that bacteria may be involved in the ability of ant lions to paralyze their prey (47, 66). These observations suggest a novel role(s) for bacteria in the biology of these insects. To our knowledge, a comprehensive description of the microbiota associated with the discontinuous gut of any insect has not been performed, although it has been reported that unidentified bacteria can be found in the midgut of insects with this physiological adaptation (27, 44).
The presence of a discontinuous gut during larval stages is not unique to ant lions. This modification has also previously been described in other fluid-feeding insects such as the nymphs of the milkweed bug (44), larvae of the cotton stainer (6), cattle grubs (7), stinkbugs (27), and some hymenopterans, which include bees, ants, and wasps (13). Although it would appear to be detrimental, the advantages of a discontinuous gut may include avoidance of soiling the larval habitat, improved regulation of osmotic balance, efficient extraction of nutrients from food, and maintenance of dietary balance (27). Considering that the ant lions commonly live in dry soil and have a complex pitfall trap, it is possible that a discontinuous gut might be important for maintaining osmotic balance and preventing the disruption and soiling of the prey-capturing pit.
The ingestion of a relatively nutrient-rich fluid diet and the variable and intermittent food source along with the inability to void solid waste could influence the diversity of the microbiota associated with ant lion gut tissue. The results of our culture-independent study of the bacteria found in the ant lion GI tract suggest that community diversity is limited, with the libraries dominated by Enterobacteriaceae-like 16S rRNA gene sequences. Our libraries contained sequences with similarity to Enterobacter aerogenes (OTUs 6 and 12) but not Bacillus cereus (Table 2 and Fig. 2), bacteria that were isolated from the Japanese ant lion, M. bore (47, 66), suggesting that the microbial communities may vary between Myrmeleon species. Further research is needed to determine the presence and/or extent of microbial community variation between ant lion species.
Our study focused on the microbiota associated with first-instar larvae, but limited 16S rRNA gene sequence analysis of second- and third-instar larvae suggests that the microbiota is qualitatively similar throughout the three larval stages (data not shown). Although these insects maintain a similar lifestyle and feeding habit during the three larval instars, their size does dramatically increase (up to 1.5 cm), possibly affecting the population size and diversity of the microbiota. Similar in-depth characterization of later instars will be necessary to determine whether microbial distribution and diversity change between the larval stages and, in addition, between pupation and adulthood, where the insects have dramatically different morphologies and lifestyles.
Our analysis indicates that the ant lion microbiota is relatively simple, a characteristic identified in the GI tract of other insects using culture-independent methods. In the midgut of the gypsy moth, between 7 and 15 phylotypes were described, with the number depending on feeding treatment (9). Although the gypsy moth and ant lion microbial communities share similar members (bacteria of the genera Enterobacter, Serratia, and Pantoea), the M. mobilis community is dominated by α- and γ-Proteobacteria, whereas the gypsy moth microbiota consists of a slightly more diverse bacterial community, including the Actinobacteria, the Cytophaga-Flexibacter-Bacteroides group, low-G+C gram-positive bacteria, and the α- and γ-Proteobacteria. Although the analysis was not confined to the GI tract, 16S rRNA gene sequence analysis of aphids also identified a simple microbial community consisting of the primary aphid symbiont Buchnera and up to three other taxa of accessory bacteria with similarity to α- and γ-Proteobacteria and the Firmicutes (30). Alternatively, a rich diversity of microorganisms was found in culture-independent analyses of the GI tract in scarab beetle larvae (19), termites (56), and biting midges (11). With the limited number of insect types whose gut microbial communities have been characterized using culture-independent methods, it is difficult to draw many conclusions as to how diet or gut physiology affects microbial community structure, but it is possible that in ant lions, a diet of nutrient-rich fluid and a discontinuous gut may be correlated with a less diverse microbiota than those of insects with a continuous gut whose diet consists of soil (scarab beetle larvae and certain termites) or blood meal (biting midges).
Considering that ant lions consume the liquefied contents of other insects, it would not be unreasonable to assume that the diversity of 16S rRNA gene sequences from ant lion tissues could be influenced by transient bacteria ingested during feeding. To address this concern, we compared 16S rRNA gene clone libraries from ant lions from three feeding treatments. We predicted that if transient bacteria ingested from prey were influencing the bacterial community, ant lions that have been feeding on different types of insect prey in the environment would contain a more diverse community than laboratory-confined ant lions that were fed laboratory-raised mealworms or ant lions that were confined in the laboratory without food. Statistical analysis indicated that the communities differed between the three treatments and that the freshly caught and laboratory-starved ant lions had the most diverse microbial communities. However, there is a numerically dominant core group of bacteria consisting of Wolbachia (OTUs 1 and 2) and a subset of the Enterobacteriaceae (OTUs 3, 5, and 6) present within all treatments. These core groups might play a more important role in the biology of the ant lion, whereas other groups could be “contaminants” from insect prey.
It also does not appear that the microbiota of ant lions was obtained from the mealworms they were fed in the laboratory. Analysis of seven 16S rRNA gene sequences obtained from mealworms using the Classifier tool from the Ribosomal Database Project-II (15) revealed α-Proteobacteria with similarity to the genus Caulobacter and bacteria belonging to the phylum Firmicutes (data not shown), groups that were not identified in our culture-independent analysis of ant lions that were fed mealworms. This is not a comprehensive identification of the mealworm microbiota, but it raises questions as to whether and/or to what extent the bacteria present in insect prey are incorporated into or influence the ant lion microbial community. Further experiments using FISH with probes specific to the OTUs that are dominant in ant lions combined with attempts to clear these bacteria using antibiotic treatments may shed more light on the importance of these bacterial groups to the genus Myrmeleon.
Whereas a majority of the 16S rRNA gene sequences associated with the GI tract tissue were Enterobacteriaceae like, Wolbachia-like sequences were more commonly found in the non-GI tract tissue (Fig. 3), and the FISH analysis identified bacterium-like objects hybridizing with the Wolbachia-specific probe in many different tissue types, including muscle and adipose tissue (Fig. 5E and F). These results indicate that the population of M. mobilis ant lions we sampled contains Wolbachia, possibly from two distinct supergroups (Fig. 4). Although we identified clones belonging to both OTU 1 and OTU 2 from 40% of the ant lions sampled, because ant lions can live in mixed species populations (20, 42), and larvae are difficult to type morphologically (J. D. Oswald, personal communication), it is possible that some sampled ant lion larvae were not M. mobilis and contain only one type of Wolbachia. Alternatively, only a small number of clones were sequenced from each ant lion and tissue sample (Table 1), and the possibility exists that additional sequencing would reveal that all ant lions containing Wolbachia belong to OTU 1 and OTU 2. In any case, 80% of ant lions sampled contained either OTU 1 or OTU2, suggesting that Wolbachia is present in this type of insect. Infections with multiple Wolbachia types has been observed in other insect species (37, 46, 50, 62) and, if fully verified, would not be unique to M. mobilis.
Because Wolbachia can influence insect reproduction, most studies on the interactions between Wolbachia species and their hosts have focused on reproductive tissues. However, Wolbachia species are also routinely found in insect somatic tissues (14, 17, 45), so this characteristic of ant lions is not unusual. Similarly to our FISH results (Fig. 5E and F), it has been reported in weevils (31) and aphids (26) that FISH analysis using the Wolbachia-specific probe W2 identifies low densities of Wolbachia-like cells scattered throughout somatic tissue as opposed to the dense aggregations present in germ cells. In our study, we did not detect any regions of ant lion tissue harboring high densities of Wolbachia, such as those in the reproductive tissue of other insects. The reproductive organs of ant lions appear towards the end of the third and final larval instar (65); therefore, we would not expect to detect any reproductive tissue in the first-instar insects used in these experiments. Further study will be necessary to determine whether the Wolbachia species associated with M. mobilis display differential tissue tropism throughout the life cycle of the insect or influence reproduction, and it will be interesting to determine whether Wolbachia associates with other ant lion species. Ant lions can be found in the environment as populations of mixed species (20, 42), and Wolbachia may be involved in the generation and maintenance of reproductive isolation in these populations.
Interestingly, this is the first report of the identification of Wolbachia in the insect order Neuroptera. Systematic surveys of Wolbachia distributions among insect populations in Panama (62), Britain (63), and North America (61) determined that 16 to 22% of insects sampled contain Wolbachia, suggesting that one to five million insect species are infected (62). Unfortunately, no insects from the Neuroptera were sampled in these studies. It is possible that an even greater percentage of insects harbor Wolbachia than previously thought.
Our study also identified 16S rRNA gene sequences associated with the GI tract tissue with similarity to that of Arsenophonus nasoniae (OTU 7), which has been implicated as the causative agent of the son killer trait in the parasitic wasp Nasonia vitripennis (25, 35). In N. vitripennis, infections are passed from mother to offspring through the hemolymph of the parasitized host, with infections originating in the midgut of larvae and spreading to other tissues when the insects near the adult stages (35). A related bacterium, Arsenophonus triatominarum, is found intracellularly dispersed throughout numerous tissue types in the adult triatomine bug, Triatoma infestans. A. triatominarum is found in the gut lumen of T. infestans embryos and only begins to spread to individual organs in the second-instar larval stage (36). Organisms related to A. nasoniae have also been identified in whiteflies (57), psyllids (57), and aphids (52, 53). Arsenophonus-like sequences were only identified in four ant lions (Table 2), but further analysis may identify these bacteria as commonly being present in M. mobilis, and if so, it will be fascinating to investigate the role that these bacteria play in the biology of their host.
Culture-independent analysis of the microbiota of ant lions has revealed a relatively simple community, a trait that may be related to the food source and gut physiology of these insects. These data will be useful for comparative analysis of the microbiota of diverse ant lion species of various geographic regions to determine the extent to which the microbial communities vary within this particular type of insect. As more culture-independent analyses of the microbiota associated with the GI tracts of diverse insect types emerge, it will be interesting to more thoroughly compare community diversity as related to food type and physiological adaptations of the gut to gain a better understanding of how these characteristics are related to the types of microorganisms associated with insects. In addition, further analysis of the interactions between ant lions and their microbial community through targeting of specific bacterial types will help to decipher the role(s) these bacteria play in the biology of these interesting insects.
Acknowledgments
We thank Beth Richardson for instruction on cryosectioning methods and William Whitman for the use of his Mini-Beadbeater.
This research was supported by a National Science Foundation postdoctoral fellowship in microbial biology to A.K.D. (DBI-0301367) and a University of Georgia Research Council junior faculty research grant to E.V.S.
REFERENCES
- 1.Aksoy, S. 2000. Tsetse—a haven for microorganisms. Parasitol. Today 16:114-118. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., B. Roger, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 4.Bandi, C., A. M. Dunn, G. D. Hurst, and T. Rigaud. 2001. Inherited microorganisms, sex-specific virulence and reproductive parasitism. Trends Parasitol. 17:88-94. [DOI] [PubMed] [Google Scholar]
- 5.Baumann, P., L. Baumann, C. Y. Lai, D. Rouhbakhsh, N. A. Moran, and M. A. Clark. 1995. Genetics, physiology, and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu. Rev. Microbiol. 49:55-94. [DOI] [PubMed] [Google Scholar]
- 6.Berridge, M. J. 1965. The physiology of excretion in the cotton stainer, Dysdercus fasciatus signoret. I. Anatomy, water excretion and osmoregulation. J. Exp. Biol. 43:511-521. [DOI] [PubMed] [Google Scholar]
- 7.Boulard, C. 1969. Anatomie et histologie du tube digestif de la larve d'Hypoderma bovis (Diptere Oestriforme). Ann. Soc. Ent. Fr. 5:371-387. [Google Scholar]
- 8.Breznak, J. A. 1982. Intestinal microbiota of termites and other xylophagous insects. Annu. Rev. Microbiol. 36:323-343. [DOI] [PubMed] [Google Scholar]
- 9.Broderick, N. A., K. F. Raffa, R. M. Goodman, and J. Handelsman. 2004. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl. Environ. Microbiol. 70:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. John Wiley & Sons, New York, N.Y.
- 11.Campbell, C. L., D. L. Mummey, E. T. Schmidtmann, and W. C. Wilson. 2004. Culture-independent analysis of midgut microbiota in the arbovirus vector Culicoides sonorensis (Diptera: Ceratopogonidae). J. Med. Entomol. 41:340-348. [DOI] [PubMed] [Google Scholar]
- 12.Chao, A. 1984. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 11:265-270. [Google Scholar]
- 13.Chapman, R. F. 1985. Structure of the digestive system, p. 165-211. In G. A. Kerkut and L. I. Gilbert (ed.), Comprehensive insect physiology biochemistry and pharmacology. Pergamon Press, New York, N.Y.
- 14.Cheng, Q., T. D. Ruel, W. Zhou, S. K. Moloo, P. Majiwa, S. L. O'Neill, and S. Aksoy. 2000. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med. Vet. Entomol. 14:44-50. [DOI] [PubMed] [Google Scholar]
- 15.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou, F. Rousset, and S. L. O'Neill. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29:153-160. [DOI] [PubMed] [Google Scholar]
- 18.Douglas, A. E. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43:17-37. [DOI] [PubMed] [Google Scholar]
- 19.Egert, M., B. Wagner, T. Lemke, A. Brune, and M. W. Friedrich. 2003. Microbial community structure in midgut and hindgut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 69:6659-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisner, T., I. T. Baldwin, and J. Conner. 1993. Circumvention of prey defense by a predator: ant lion vs. ant. Proc. Natl. Acad. Sci. USA 90:6716-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felsenstein, J. 1989. PHYLIP-Phylogeny Inference Package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 22.Felsenstein, J. 2004. PHYLIP (Phylogeny Inference Package) version 3.6. Department of Genome Sciences, University of Washington, Seattle, Wash.
- 23.Furlong, M. A., D. R. Singleton, D. C. Coleman, and W. B. Whitman. 2002. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 68:1265-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaumont, J. 1976. L'appareil digestif des larves de planipennes. Ann. Sci. Nat. Zool. Biol. Anim. 12:145-250. [Google Scholar]
- 25.Gherna, R. L., J. H. Werren, W. Weisburg, R. Cote, C. R. Woese, L. Mandelco, and D. Brenner. 1991. Arsenophonus nasoniae gen. nov., sp. nov., the causative agent of the son-killer trait in the parasitic wasp Nasonia vitripennis. Int. J. Syst. Bacteriol. 41:563-565. [Google Scholar]
- 26.Gomez-Valero, L., M. Soriano-Navarro, V. Perez-Brocal, A. Heddi, A. Moya, J. M. Garcia-Verdugo, and A. Latorre. 2004. Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid Cinara cedri. J. Bacteriol. 186:6626-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodchild, A. J. P. 1963. Studies on the functional anatomy of the intestines of Heteroptera. Proc. Zool. Soc. Lond. 141:851-910. [Google Scholar]
- 28.Hagen, H. A. 1872. On the larvae of the Hemerobina. Proc. Bost. Soc. Nat. Hist. 15:243-248. [Google Scholar]
- 29.Harmsen, H. J., G. R. Gibson, P. Elfferich, G. C. Raangs, A. C. Wildeboer-Veloo, A. Argaiz, M. B. Roberfroid, and G. W. Welling. 1999. Comparison of viable cell counts and fluorescence in situ hybridization using specific rRNA-based probes for the quantification of human fecal bacteria. FEMS Microbiol. Lett. 183:125-129. [DOI] [PubMed] [Google Scholar]
- 30.Haynes, S., A. C. Darby, T. J. Daniell, G. Webster, F. J. Van Veen, H. C. Godfray, J. I. Prosser, and A. E. Douglas. 2003. Diversity of bacteria associated with natural aphid populations. Appl. Environ. Microbiol. 69:7216-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heddi, A., A. M. Grenier, C. Khatchadourian, H. Charles, and P. Nardon. 1999. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc. Natl. Acad. Sci. USA 96:6814-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrich, B., and M. J. E. Henrich. 1984. The pit-trapping foraging strategy of the antlion, Myrmeleon immaculatus DeGeer (Neuroptera: Myrmeleontidae). Behav. Ecol. Sociobiol. 14:151-160. [Google Scholar]
- 33.Huber, I., B. Spanggaard, K. F. Appel, L. Rossen, T. Nielsen, and L. Gram. 2004. Phylogenetic analysis and in situ identification of the intestinal microbial community of rainbow trout (Oncorhynchus mykiss, Walbaum). J. Appl. Microbiol. 96:117-132. [DOI] [PubMed] [Google Scholar]
- 34.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 35.Huger, A. M., S. W. Skinner, and J. H. Werren. 1985. Bacterial infections associated with the son-killer trait in the parasitoid wasp Nasonia (= Mormoniella) vitripennis (Hymenoptera: Pteromalidae). J. Invertebr. Pathol. 46:272-280. [DOI] [PubMed] [Google Scholar]
- 36.Hypsa, V., and C. Dale. 1997. In vitro culture and phylogenetic analysis of “Candidatus Arsenophonus triatominarum,” an intracellular bacterium from the triatomine bug, Triatoma infestans. Int. J. Syst. Bacteriol. 47:1140-1144. [DOI] [PubMed] [Google Scholar]
- 37.Jamnongluk, W., P. Kittayapong, V. Baimai, and S. L. O'Neill. 2002. Wolbachia infections of tephritid fruit flies: molecular evidence for five distinct strains in a single host species. Curr. Microbiol. 45:255-260. [DOI] [PubMed] [Google Scholar]
- 38.Junge, K., F. Imhoff, T. Staley, and J. W. Deming. 2002. Phylogenetic diversity of numerically important Arctic sea-ice bacteria cultured at subzero temperature. Microb. Ecol. 43:315-328. [DOI] [PubMed] [Google Scholar]
- 39.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Inc., New York, N.Y.
- 40.Lilburn, T. G., K. S. Kim, N. E. Ostrom, K. R. Byzek, J. R. Leadbetter, and J. A. Breznak. 2001. Nitrogen fixation by symbiotic and free-living spirochetes. Science 292:2495-2498. [DOI] [PubMed] [Google Scholar]
- 41.Lo, N., M. Casiraghi, E. Salati, C. Bazzocchi, and C. Bandi. 2002. How many Wolbachia supergroups exist? Mol. Biol. Evol. 19:341-346. [DOI] [PubMed] [Google Scholar]
- 42.Lucas, J. R., and L. Stange. 1981. Key and descriptions to the Myrmeleon larvae of Florida. Fla. Entomol. 64:207-216. [Google Scholar]
- 43.Matsuda, K., H. Suzuki, F. Nakanishi, K. Shio, K. Komai, and K. Nishimura. 1995. Purification and characterization of a paralytic polypeptide from larvae of Myrmeleon bore. Biochem. Biophys. Res. Commun. 215:167-171. [DOI] [PubMed] [Google Scholar]
- 44.Miles, P. W. 1958. Retention of food residues in the midgut by nymphs of the milkweed bug, Oncopeltus fasciatus Dall. Nature 182:959.13590198 [Google Scholar]
- 45.Min, K. T., and S. Benzer. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 94:10792-10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mouton, L., H. Henri, M. Bouletreau, and F. Vavre. 2003. Strain-specific regulation of intracellular Wolbachia density in multiply infected insects. Mol. Ecol. 12:3459-3465. [DOI] [PubMed] [Google Scholar]
- 47.Nishiwaki, H., K. Ito, K. Otsuki, H. Yamamoto, K. Komai, and K. Matsuda. 2004. Purification and functional characterization of insecticidal sphingomyelinase C produced by Bacillus cereus. Eur. J. Biochem. 271:601-606. [DOI] [PubMed] [Google Scholar]
- 48.Ootsubo, M., T. Shimizu, R. Tanaka, T. Sawabe, K. Tajima, M. Yoshimizu, Y. Ezura, T. Ezaki, and H. Oyaizu. 2002. Oligonucleotide probe for detecting Enterobacteriaceae by in situ hybridization. J. Appl. Microbiol. 93:60-68. [DOI] [PubMed] [Google Scholar]
- 49.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 50.Perrot-Minnot, M. J., L. R. Guo, and J. H. Werren. 1996. Single and double infections with Wolbachia in the parasitic wasp Nasonia vitripennis: effects on compatibility. Genetics 143:961-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 52.Russell, J. A., A. Latorre, B. Sabater-Munoz, A. Moya, and N. A. Moran. 2003. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 12:1061-1075. [DOI] [PubMed] [Google Scholar]
- 53.Sandstrom, J. P., J. A. Russell, J. P. White, and N. A. Moran. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217-228. [DOI] [PubMed] [Google Scholar]
- 54.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schloss, P. D., B. R. Larget, and J. Handelsman. 2004. Integration of microbial ecology and statistics: a test to compare gene libraries. Appl. Environ. Microbiol. 70:5485-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt-Wagner, D., M. W. Friedrich, B. Wagner, and A. Brune. 2003. Phylogenetic diversity, abundance, and axial distribution of bacteria in the intestinal tract of two soil-feeding termites (Cubitermes spp.). Appl. Environ. Microbiol. 69:6007-6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spaulding, A. W., and C. D. von Dohlen. 2001. Psyllid endosymbionts exhibit patterns of co-speciation with hosts and destabilizing substitutions in ribosomal RNA. Insect Mol. Biol. 10:57-67. [DOI] [PubMed] [Google Scholar]
- 58.Stouthamer, R., J. A. Breeuwer, and G. D. Hurst. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71-102. [DOI] [PubMed] [Google Scholar]
- 59.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trexler, J. D., C. S. Apperson, L. Zurek, C. Gemeno, C. Schal, M. Kaufman, E. Walker, D. W. Watson, and L. Wallace. 2003. Role of bacteria in mediating the oviposition responses of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 40:841-848. [DOI] [PubMed] [Google Scholar]
- 61.Werren, J. H., and D. M. Windsor. 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. R. Soc. Lond. B 267:1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Werren, J. H., W. Zhang, and L. R. Guo. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. B 261:55-63. [DOI] [PubMed] [Google Scholar]
- 63.West, S. A., J. M. Cook, J. H. Werren, and H. C. Godfray. 1998. Wolbachia in two insect host-parasitoid communities. Mol. Ecol. 7:1457-1465. [DOI] [PubMed] [Google Scholar]
- 64.Wheeler, W. M. 1930. Demons of the dust. W.W. Norton & Company, Inc., New York, N.Y.
- 65.Withycombe, C. L. 1924. Some aspects of the biology and morphology of the Neuroptera, with special reference to the immature stages and their possible phylogenetic significance. Trans. Ent. Soc. Lond. 72:303-411. [Google Scholar]
- 66.Yoshida, N., K. Oeda, E. Watanabe, T. Mikami, Y. Fukita, K. Nishimura, K. Komai, and K. Matsuda. 2001. Protein function. Chaperonin turned insect toxin. Nature 411:44. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida, N., H. Sugama, S. Gotoh, K. Matsuda, K. Nishimura, and K. Komai. 1999. Detection of ALMB-toxin in the larval body of Myrmeleon bore by anti-N-terminus peptide antibodies. Biosci. Biotechnol. Biochem. 63:232-234. [DOI] [PubMed] [Google Scholar]
- 68.Zimmer, C. 2001. Wolbachia. A tale of sex and survival. Science 292:1093-1095. [DOI] [PubMed] [Google Scholar]
- 69.Zurek, L., C. Schal, and D. W. Watson. 2000. Diversity and contribution of the intestinal bacterial community to the development of Musca domestica (Diptera: Muscidae) larvae. J. Med. Entomol. 37:924-928. [DOI] [PubMed] [Google Scholar]