Abstract
Only one isolate each of the class “Spartobacteria” (subdivision 2 of the phylum Verrucomicrobia) and of subdivision 3 of Verrucomicrobia have previously been reported to grow in laboratory culture. Using media that had been used successfully in other studies to isolate members of diverse groups of soil bacteria, we generated a collection of over 1,200 isolates from soil from a pasture. An oligonucleotide probe that targets the 16S rRNA genes of verrucomicrobia was used to screen this collection, and 14 new verrucomicrobia were identified. Nine of these belonged to the class “Spartobacteria” and were related to “Chthoniobacter flavus.” Five further isolates were members of subdivision 3 and were related to the only known isolate of this subdivision. The differences in the 16S rRNA gene sequences of the new isolates and previously described isolates, of up to 10%, indicated that the new isolates represent new species and genera. All but two of the verrucomicrobial isolates were from colonies that first became visible one or more months after inoculation of plates with soil, but subcultures grew more rapidly. Analysis of PCR-amplified 16S rRNA genes in the pasture soil showed that members of the class “Spartobacteria” were more numerous than members of subdivision 3. Isolates of subdivision 3 were only found on plates receiving an inoculum that yielded a mean of 29 colonies per plate, while members of the class “Spartobacteria” were only found on plates receiving a more dilute inoculum that resulted in a mean of five colonies per plate. This suggested that colony development by members of the class “Spartobacteria” was inhibited by other culturable bacteria.
Soils are known to be populated by genetically diverse communities of bacteria, with estimates of many thousands of species per gram (9, 43). Surveys of the phylogenetic diversity, based on collections of cloned, PCR-amplified 16S rRNA gene sequences, have revealed the presence of many species, genera, and families, and even orders, classes and phyla of bacteria for which there are no known cultured representatives (5, 20, 31). One of the major phylogenetic groupings of globally distributed soil bacteria that is represented by few cultivated isolates is the phylum Verrucomicrobia (18-20).
Hugenholtz et al. (20) divided the phylum Verrucomicrobia into five subdivisions on the basis of 16S rRNA gene sequence divergence. There are also some lineages that are only poorly represented by 16S rRNA gene sequences and have been given no uniform designations. Only very few cultured isolates and recognized species of the phylum Verrucomicrobia are known (7, 8, 12, 19, 21, 23, 34, 36, 38, 41, 45). Based on their detection in libraries of PCR-amplified 16S rRNA genes, members of the phylum Verrucomicrobia are very widely distributed and found in many different habitats (20, 31), including occurrences as symbionts (30, 45). 16S rRNA genes originating from members of the phylum Verrucomicrobia make up to 12% of all of the bacterial 16S rRNA genes detected in PCR-based surveys of soil bacterial communities (5). Most of the sequences originate from members of the class “Spartobacteria.” One such 16S rRNA gene, designated EA25, was estimated to originate from a species with a population size of up to 2 × 108 cells per gram of soil (27) and therefore could represent 1 to 10% of all of the bacteria in that soil.
Members of the phylum Verrucomicrobia have been shown to make up 1 to 10% of the bacterial 16S rRNA in soils (4, 6). The member of the class “Spartobacteria” from which one 16S rRNA gene, designated DA101, originated, made up a significant part of bacterial 16S rRNA in another soil system (15, 16). It has been suggested that the amount of 16S rRNA, the product of the 16S rRNA gene, is indicative of bacterial activity (25). From estimates of the contribution of their 16S rRNA to the total 16S rRNA pool, it can be concluded that members of the phylum Verrucomicrobia are active members of the soil microbial community.
The evidence available therefore indicates that members of the phylum Verrucomicrobia are a globally distributed, abundant, and active group of soil bacteria. However, in the absence of cultivated isolates from soil, very little is known about the biology of members of this phylum. Although these bacteria are postulated to be important members of the soil microbial community (5), information on their roles and ecology is based only on correlations of their abundance, based on 16S rRNA, with environmental parameters (4). The complexity of soil microbial communities (9, 43) means that metagenomic approaches to studying soil bacteria and assembling genomes of uncultured bacteria to understand their physiologies seems impractical at present (44). To enable easier assessment of their roles, we have been attempting to isolate members of this phylum from soil. Here we report on a simple approach that generated a collection of new strains of the phylum Verrucomicrobia.
MATERIALS AND METHODS
Soil collection.
The soil used in the present study was collected from a rotationally grazed perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) pasture at the Dairy Research Institute, Ellinbank, Victoria, Australia. The sample site is located at 38°14.55′S, 145°56.11′E. Characteristics of the soil have been published elsewhere (37, 39). Soil cores were collected, transported, and sieved as described by Davis et al. (11). Dry weights and total cell counts were determined as described by Sait et al. (32).
DNA extraction and PCR.
Soil cores were divided into 2-cm sections by depth (32). DNA was extracted from 1-g aliquots of sieved and mixed soil from each section as described by O'Farrell and Janssen (28) and 4 to 9 ng used per PCR. 16S rRNA genes of verrucomicrobia were amplified from this DNA by PCR using the primer pair VMB537f and VMB1295r (Table 1) as described by O'Farrell and Janssen (28). 16S rRNA genes from most bacteria were amplified by using the primer pair BAC27f and BAC1492r (Table 1). For this amplification, the PCR mixture contained PCR buffer (QIAGEN Pty., Ltd., Clifton Hill, Victoria, Australia), 1 μl of the template DNA, 1.25 mM MgCl2, 100 pmol of each primer, 1 M betaine, and 6.25% (vol/vol) dimethyl sulfoxide in a total volume of 40 μl overlaid with 2 drops of mineral oil (Promega Corp., Annandale, New South Wales, Australia). After heating at 94°C for 2 min, 2.5 U of Taq DNA polymerase (QIAGEN) and 100 nmol of each deoxyribonucleoside phosphate were added to each reaction in a volume of 10 μl. Amplification occurred during 42 cycles consisting of annealing at 48°C for 15 s, extension at 72°C for 60 s, and denaturation at 94°C for 15 s. After 42 cycles, there was a final annealing step at 48°C for 90 s and a extension step at 72°C for 6 min.
TABLE 1.
Oligonucleotides used as probes for DNA-DNA hybridization or as primers for PCR-mediated amplification of 16S rRNA genes
| Oligonucleotide | Use | Sequence (5′ to 3′)a | Target
|
Source or referenced | |
|---|---|---|---|---|---|
| Bacterial group(s) | 16S rRNA regionb | ||||
| EUB338 | Hybridization | GCTGCCTCCCGTAGGAGT | Most Bacteria | 338-355 | 11 |
| EUB338 suitec | Hybridization | GCTGCCTCCCGTAGGAGT | Most Bacteria | 338-355 | 11 |
| GCAGCCACCCGTAGGTGT | Planctomycetes, Chloroflexi, OP11 | 338-355 | 11 | ||
| GCTGCCACCCGTAGGTGT | Verrucomicrobia | 338-355 | 11 | ||
| VER47 | Hybridization | GACTTGCATGTCTTAWC | Verrucomicrobia | 47-64 | 4 |
| VER727 | Hybridization | TCCAGGGACTCGCCTTC | Subdivision 3 Verrucomicrobia | 727-743 | - |
| VER1112 | Hybridization | GGCAACAGTTTCACAGGGG | “Spartobacteria” | 1112-1129 | - |
| BAC27f | PCR | GAGTTTGATCMTGGCTCAG | Most Bacteria | 9-27 | 23 |
| BAC1492r | PCR | GGYTACCTTGTTACGACTT | Most cellular organisms | 1492-1510 | 27 |
| VMB537f | PCR | GCCAGCAGCCGCGGTAATACA | Most Verrucomicrobia | 516-537 | 30 |
| VMB1295r | PCR | GCAGMCTICAATCTGAACTGRGC | Most Verrucomicrobia | 1295-1317 | 30 |
Nonstandard symbols: I, inosine; M, equimolar A or C; R, equimolar A or G; Y, equimolar C or T.
Target in 16S rRNA, E. coli numbering (2).
Equimolar mix of all three oligonucleotides used.
-, Philip Hugenholtz, personal communication.
Clone library preparation.
PCR products were purified, A-tailed, and cloned in Escherichia coli as described elsewhere (28). The cloned 16S rRNA gene fragments were reamplified and screened (28) by digestion of 1 μg of DNA with 3 U of the restriction endonuclease HhaI (New England Biolabs, Beverly, Mass.) at 37°C for 3 h. This enzyme was selected because of its potential to distinguish different groups of verrucomicrobia based on predicted fragment patterns from known 16S rRNA gene sequences. Multiple clones yielding each digestion pattern were sequenced (28). Only 1 of the 18 patterns yielded sequences that were affiliated with different classes of bacteria, and so all 10 of the inserts corresponding to that restriction fragment pattern were sequenced.
Cultivation experiments.
Plate count experiments were prepared as described by Davis et al. (11). Four or six replicate experiments were set up with each medium, and each experiment consisted of three different inoculum levels, each with three or five replicate plates (11). The plates were incubated in sealed polyethylene bags at 25°C in the dark for up to 6 months. Some experiments were incubated at 25°C under an air atmosphere enriched with 5% (vol/vol) CO2 in a model 3157 CO2 incubator (Forma Scientific, Marietta, Ohio) that had been calibrated at 25°C by using a Fyrite CO2 analyzer (Bacharach, Pittsburgh, Pa.).
At 1-month intervals, the plates were viewed on a light box under a magnifying lens (up to ×1.5 magnification), and visible colonies were counted. The significance of differences in viable counts was tested by using analysis of variance and two-tailed Student t tests.
Where required, colonies were subcultured onto the medium on which they first appeared but solidified with agar (11). Cultures were grown in liquid media as described by Sangwan et al. (36). Cell sizes were determined from phase-contrast photomicrographs (36).
Media.
Media were prepared as described by Joseph et al. (23). The media used were as follows: VL55 plus 0.05% (wt/vol) xylan; VL55 plus amino acid mix (23); VL55 plus 2 mM N-acetylglucosamine; VL55 plus a mix of d-glucose, d-galactose, d-xylose, and l-arabinose (0.5 mM [each]) (GGXA); and dilute nutrient broth (DNB). Media were solidified with 0.8% (wt/vol) gellan when used in plates (23). Cyclic AMP (Sigma, Castle Hill, New South Wales, Australia) was added as required to the cooled medium from a filter-sterilized (0.2-μm pore size) stock solution just prior to dispensing the medium into plates. “C. flavus” was grown to late exponential phase in liquid culture by using medium VL55 with 4 mM glucose (36). Culture supernatant from these liquid cultures was prepared by centrifugation to remove cells, followed by filter sterilization (0.2-μm pore size). The supernatant was added as required to the cooled medium just prior to dispensing the completed medium into plates. Medium stocks were adjusted so that the final concentrations of VL55 medium base, gellan, and substrate (xylan or amino acids) were the same as in parallel control experiments without the addition of culture supernatant.
Controls for hybridization.
To prepare some of the plasmid controls to act as hybridization controls, almost-complete 16S rRNA genes were PCR amplified (32) from pure cultures, A-tailed, ligated into the vector pGEM T-Easy (Promega, Annandale, New South Wales, Australia), and transformed into chemically competent E. coli JM109 (Promega) as recommended by the supplier. The pure cultures used were Verrucomicrobium spinosum DSM 4136, Planctomyces limnophilus DSM 3776, Haloferax volcanii NCIMB 2012, Opitutus terrae DSM 11246, Bradyrhizobium sp. strain Ellin153, Arthrobacter sp. strain Ellin159, and isolate Ellin202, which is a member of the phylum Bacteroidetes. The identity of the inserts was confirmed after amplification with the primers GEM189r (5′-AGCGGATAACAATTTCACACAGG-3′) and GEM2987f (5′-CCCAGTCACGACGTTGTAAAACG-3′), which target regions of the vector flanking the cloning site. These products were then purified by using the UltraClean PCR Clean-up DNA Purification kit (MoBio Laboratories, Solana Beach, Calif.) according to the manufacturer's instructions and sequenced (32). Other controls were almost-complete 16S rRNA genes in the pGEM T-Easy vector (Table 2). These genes had been amplified by PCR from a sample of DNA extracted from soil from the Ellinbank sample site (L. Schoenborn and P. H. Janssen, unpublished data).
TABLE 2.
Plasmids bearing 16S rRNA genes used as positive and negative controls for testing the specificity of oligonucleotide probe hybridization, with the phylogenetic affiliations of the source organism of each gene, and whether it contains a target site for probes used in this studya
| Plasmid | Source of 16S rRNA gene | GenBank accession no. | Match to oligonucleotide probe
|
||||
|---|---|---|---|---|---|---|---|
| EUB suite | EUB338 | VER47 | VER727 | VER1112 | |||
| pVS | Verrucomicrobium spinosum, Verrucomicrobiae | X90515 | + | − | + | − | − |
| pEB1006 | Clone EB1006, “Spartobacteria” | AY395325 | + | − | + | − | + |
| pEB1007 | Clone EB1007, “Spartobacteria” | AY395326 | + | − | + | − | + |
| pEB1116 | Clone EB1116, “Spartobacteria” | AY395435 | + | − | + | − | + |
| pEB1106 | Clone EB1106, subdivision 3 of Verrucomicrobia | AY395425 | + | − | + | + | − |
| pPB90-1 | Opitutus terrae DSM 11246, subdivision 4 of Verrucomicrobia | AJ229235 | + | − | + | − | − |
| pEB1005 | Clone EB1005, subdivision 1 of Acidobacteria | AY395324 | + | + | − | − | − |
| pEB1063 | Clone EB1063, subdivision 7 of Acidobacteria | AY395382 | + | + | − | − | − |
| pEB1034 | Clone EB1034, genus Methylobacterium of Alphaproteobacteria | AY395353 | + | + | − | − | − |
| pEB1113 | Clone EB1113, Alphaproteobacteria | AY395432 | + | + | − | − | − |
| pEllin153 | Bradyrhizobium sp. strain Ellin153, Alphaproteobacteria | AF408995 | + | + | − | − | − |
| pEB1079 | Clone EB1079, Betaproteobacteria | AY395398 | + | + | − | − | − |
| pEB1030 | Clone EB1030, Betaproteobacteria | AY395349 | + | + | − | − | − |
| pEB1054 | Clone EB1054, Gammaproteobacteria | AY395373 | + | + | − | − | − |
| pEB1076 | Clone EB1076, Deltaproteobacteria | AY395395 | + | + | − | − | − |
| pEB1047 | Clone EB1047, Planctomycetes | AY395366 | − | − | − | − | − |
| p290 | Planctomyces limnophilus DSM 3776, Planctomycetes | X62911 | + | − | − | − | − |
| pEllin159 | Arthrobacter sp. strain Ellin159, subclass Actinobacteridae of Actinobacteria | AF409001 | + | + | − | − | − |
| pEB1016 | Clone EB1016, subclass Actinobacteridae of Actinobacteria | AY395335 | + | + | − | − | − |
| pEB1133 | Clone EB1133, subclass Acidimicrobidae of Actinobacteria | AY395452 | + | + | − | − | − |
| pEB1101 | Clone EB1101, subclass Rubrobacteridae of Actinobacteria | AY395420 | + | + | − | − | − |
| pEB1065 | Clone EB1065, Chloroflexi | AY395384 | + | + | − | − | − |
| pEB1010 | Clone EB1010, genus Bacillus of Firmicutes | AY395329 | + | + | − | − | − |
| pXG002 | Isolate Ellin202, Bacteroidetes | AF432101 | + | + | − | − | − |
| pEB1002 | Clone EB1002, Gemmatimonadetes | AY395321 | + | + | − | − | − |
| pHfv | Haloferax volcanii NCIMB 2012, Archaea | AY425724 | − | − | − | − | − |
Some of these were also used as standards for quantitative hybridizations.
E. coli JM109 containing the pGEM T-Easy vector with the appropriate 16S rRNA gene insert was grown in Luria-Bertani broth (35) supplemented with ampicillin (100 μg/ml) and 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The cells were harvested from 30 ml of an overnight culture by centrifugation at 2,000 × g for 20 min at room temperature, and the plasmid was extracted from the cell pellet by using a Plasmid Midi Kit (QIAGEN, Clifton Hill, Victoria, Australia) according to the manufacturer's instructions. Plasmids were suspended in water, and the amounts of DNA in each preparation were quantified by UV spectrophotometry (35).
Oligonucleotide probe preparation.
Probes for DNA-DNA hybridization (Table 1) were purchased as high-pressure liquid chromatography-purified oligonucleotides from GeneWorks (Thebarton, South Australia, Australia) and labeled with a single digoxigenin molecule at the 3′ terminus by using the DIG Oligonucleotide 3′-End Labeling Kit (Roche Diagnostics Australia, Castle Hill, New South Wales, Australia) according to the manufacturer's instructions.
Hybridization methods to screen isolates.
Almost-complete 16S rRNA genes were amplified from isolates by PCR (32). Plasmids (ca. 3 ng) and PCR products (1 μl) were applied to 63-by-228-mm positively charged nylon membranes with a 0.45-μm pore size (Roche) and immobilized by cross-linking three times at the “optimal cross-link” setting in a XL-1000 Spectrolinker (Spectronics Corp., Westbury, N.Y.). Membranes were treated prior to hybridization in 40 ml of hybridization buffer (0.1% [wt/vol] N-lauroylsarcosine, 0.02% [wt/vol] sodium dodecyl sulfate, 1% [wt/vol] Blocking Reagent [Roche], 750 mM NaCl, and 75 mM citric acid [pH 7.0] with NaOH) at the hybridization temperature in a glass tube in a Shake'n'Stack hybridization oven (Hybaid). After at least 1 h, the hybridization buffer was replaced with fresh hybridization buffer containing the appropriate labeled oligonucleotide probe or probe suite at a total concentration of 5 pmol/ml, which was boiled for 10 min and then placed on ice for 5 min just before use. After overnight hybridization, the membrane was washed twice with concentrated wash solution (0.1% [wt/vol] sodium dodecyl sulfate, 300 mM NaCl, and 30 mM citric acid [pH 7.0] with NaOH) for 15 min each time on a rocking platform at room temperature. The membrane was then washed twice for 15 min each at the hybridization temperature in dilute wash solution (0.1% [wt/vol] sodium dodecyl sulfate, 75 mM NaCl, and 7.5 mM citric acid [pH 7.0] with NaOH) in a glass tube in a hybridization oven before being washed in blocking solution (1% [wt/vol] skim milk powder, 150 mM NaCl, and 100 mM maleic acid [pH 7.5] with NaOH) on a rocking platform for at least 1 h at room temperature. The blocking solution was replaced by fresh blocking solution containing 0.075 U of anti-digoxigenin-alkaline phosphatase Fab fragments (Roche) per milliliter and then incubated on a rocking platform for 30 min at room temperature. The membrane was then washed twice for 15 min each at room temperature in predetection washing buffer (0.3% [wt/vol] Tween 20, 150 mM NaCl, and 100 mM maleic acid [pH 7.5] with NaOH) before being incubated for 2 min at room temperature in detection buffer (100 mM NaCl and 100 mM Tris [pH 9.5] with HCl) and then placed between two sheets of polyethylene film. Chemiluminescent substrate solution, consisting of 250 μM disodium 3-(4- methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)phenyl phosphate (CSPD; Roche) in detection buffer or 250 μM disodium 2-chloro-5-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)-1-phenyl phosphate (CDP-Star; Roche) in detection buffer was added at 0.5 ml per 100 cm2 of membrane. The membrane was first incubated at room temperature for 5 min, after which it was placed between two fresh sheets of polyethylene film and, if CSPD was being used, incubated at 37°C for 15 min. The membrane was then exposed to Lumi-Film Chemiluminescent Detection Film (Roche) at room temperature overnight for CSPD or 20 to 40 min for CDP-Star.
Quantitative hybridization.
Plasmid controls were quantified by using the PicoGreen dsDNA Quantitation Kit (Molecular Probes, Eugene, Oreg.). From the known plasmid and insert sizes, the number of targets in the standards was calculated. A series of four standards of between 108 and 109 target copies was prepared by dilution in water, and each was blotted in triplicate on each membrane. PCR products in which target copy number was to be estimated were purified by using the Ultraclean PCR Clean-up DNA Purification Kit (MoBio Laboratories, Inc., Solana Beach, Calif.) and diluted in water to yield four different dilutions that would yield hybridization signals comparable to those produced by the standards, and each was blotted onto the membrane in triplicate. Hybridization was carried out as described above.
Each PCR product was quantified by using the EUB338 probe, the VER727 probe, and the VER1112 probe, with a different membrane for each probe. The standards (Table 2) were pEllin153 for EUB338, pEB1106 for VER727, and pEB1116 for VER1112. The signal intensities were measured from scanned images of the exposed film by using NIH Image (National Institutes of Health, Bethesda, Md.), and the number of copies in the PCR product calculated from the standards. The number of targets detected using each of the latter two probes was expressed as a proportion of the target number estimated by using the EUB338 probe.
Phylogenetic analysis.
The primary sequences of 16S rRNA genes of cultured bacteria were determined as described by Sait et al. (32). The phylogenetic affiliations of these bacteria and of cloned 16S rRNA genes were deduced by using BLAST in GenBank (1). The 16S rRNA gene sequences obtained in the present study have been deposited in the GenBank databases under accession numbers AY960764 to AY960781 and DQ087988 to DQ088032. For more detailed phylogenetic analyses, 16S rRNA gene sequences were aligned against selected sequences extracted from GenBank, using the program ClustalX version 1.81 (42). This alignment was then manually checked and corrected, and regions of uncertain alignment eliminated, using the software SeAl version 1.d1 (A. Rimbaut, Department of Zoology, University of Oxford, Oxford, United Kingdom). Analyses of the isolates were restricted to the unambiguously aligned regions totaling 1,158 positions of the 16S rRNA gene. Evolutionary analyses were carried out with the PHYLIP package version 3.573c (J. Felsenstein, Department of Genome Sciences, University of Washington, Seattle [14]). Evolutionary distances between pairs of microorganisms were determined by the Jukes and Cantor method (24) and the maximum-likelihood method implemented in the Dnadist program, and dendrograms were derived with the Fitch and Neighbor programs, using the Fitch-Margoliash method (17) and a neighbor-joining algorithm (33), respectively. The significance of the nodes was tested by bootstrap analysis generating Jukes-Cantor evolutionary distances, using the least-squares and neighbor-joining algorithms to produce 1,000 dendrograms, and then compiling consensus dendrograms, using the programs Seqboot, Dnadist, Fitch, Neighbor, and Consense. Maximum-likelihood and maximum-parsimony analyses of the sequence data used the programs Dnaml and Dnapars, respectively. Dendrograms were represented graphically with the software TreeViewPPCversion 1.4 (R. D. M. Page, Division of Environmental and Evolutionary Biology, University of Glasgow, Glasgow, United Kingdom). The topologies and bootstrap values of dendrograms produced by using different methods were not significantly different.
Identities between 16S rRNA genes were calculated from uncorrected distances by using ClustalX, after alignment of smaller sets of unedited sequences.
RESULTS AND DISCUSSION
Optimization of probe specificity.
Only two cultures of aerobic soil verrucomicrobia, belonging to the class “Spartobacteria” and to subdivision 3, have been reported (22, 23). Because verrucomicrobia seem to form colonies only rarely, we used an oligonucleotide probe to detect colonies of these bacteria that developed on solid media. The probe VER47 (4), which is complementary to a region of the 16S rRNA genes of most members of the phylum Verrucomicrobia, bound only to 16S rRNA genes from members of the phylum Verrucomicrobia (Table 2) at hybridization temperatures of 40 to 45°C. A standard hybridization temperature of 44.5°C was therefore selected for further work. The EUB338 suite (10), which contains oligonucleotides that are complementary to a region of the 16S rRNA gene of nearly all members of the domain Bacteria, bound only to 16S rRNA genes from bacteria with a suitable probe binding site (Table 2) at hybridization temperatures of 55 to 57°C. A standard hybridization temperature of 55°C was selected for further work. Similar optimization studies identified standard hybridization temperatures of 46 and 52°C for the probes VER727 and VER1112, respectively.
The single probe EUB338, which targets most bacteria (10), bound specifically to DNA containing an appropriate target at a hybridization temperature of 55°C, which was used for quantitative work. Attempts to quantify bacterial 16S rRNA genes using the EUB338 suite of three probes (10) that target a wider range of bacteria proved to be problematic (data not shown). The three probes target the same region of the gene (10) (Table 1), and we suspect that imperfectly matching probes bound in the initial hybridization and were subsequently lost in the wash steps, reducing signal intensity relative to the intensity using EUB338 alone.
Abundance of verrucomicrobia in soil.
The abundance of members of the different classes (subdivisions) of the phylum Verrucomicrobia in the Ellinbank soil was assessed by analyzing libraries of PCR-amplified and cloned 16S rRNA genes prepared by using a primer pair that amplifies these genes from members of the phylum Verrucomicrobia (38). Members of the class “Spartobacteria” were the dominant verrucomicrobia (Table 3), as judged by the relative abundance of genes in these libraries. Members of the class Verrucomicrobiae (subdivision 1) and of subdivisions 3 and 4 and of a new lineage within the phylum Verrucomicrobia were all rarer (Table 3). The sequence that represented the new lineage, EVS233 (GenBank accession DQ088008) did not group with any of the recognized subdivisions (20, 36) of the phylum in phylogenetic analyses of the 16S rRNA gene sequences (not shown). Some 16S rRNA genes from members of the phylum Planctomycetes were also amplified, but it is not known whether this is because they are more abundant in absolute or in relative terms in the upper sections of the soil. The primer pair used therefore did not exclusively amplify verrucomicrobial DNA but did indicate that members of the class “Spartobacteria” were the most numerous verrucomicrobia.
TABLE 3.
Cloned 16S rRNA genes amplified from soil DNA by using the VMB537f-VMB1295r primer pair targeting verrucomicrobia
| Soil depth (cm) | Total no. of clones screeneda | No. of clones affiliated with phylum Planctomycetes | No. affiliated with phylum Verrucomicrobia
|
GenBank accession no. of sequenced clones | ||||
|---|---|---|---|---|---|---|---|---|
| Verrucomicrobiae | “Spartobacteria” | Subdivision
|
New lineage | |||||
| 3 | 4 | |||||||
| 0-2 | 45 | 14 | 1 | 25 | 3 | 2 | 0 | DQ087988 to DQ088002 |
| 2-4 | 39 | 6 | 0 | 31 | 1 | 0 | 1 | DQ088003 to DQ088010 |
| 4-6 | 33 | 0 | 3 | 29 | 1 | 0 | 0 | DQ088011 to DQ088018 |
| 6-8 | 31 | 1 | 1 | 28 | 1 | 0 | 0 | DQ088019 to DQ088022 |
| 8-10 | 31 | 1 | 0 | 28 | 1 | 1 | 0 | DQ088023 to DQ088032 |
| Total | 179 | 22 | 5 | 141 | 7 | 3 | 1 | |
Screening by analyzing restriction fragment patterns.
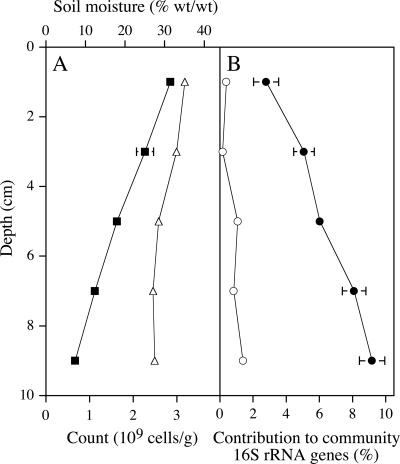
The number of 16S rRNA genes from members of the class “Spartobacteria” and members of subdivision 3 was estimated in PCR products generated by using a different primer pair that amplifies these genes from nearly all bacteria. This estimation was made by using the group-specific oligonucleotide probes VER1112 and VER727 (Table 1). Genes from members of the class “Spartobacteria” were found to be more numerous than genes from members of subdivision 3 (Fig. 1), supporting the results obtained in the clone library analysis. The total number of cells in the soil, determined by microscopy, decreased with depth (Fig. 1), whereas the contribution of members of the class “Spartobacteria” increased, so that the absolute number of spartobacteria did not change greatly with depth. We concluded that sufficiently large populations of spartobacteria were present in the Ellinbank soil to use this as an inoculum for isolating members of this class.
FIG. 1.
Distribution of bacteria in the Ellinbank soil. Each point represents the results for a pooled 2-cm section (0 to 2, 2 to 4, 4 to 6, 6 to 8, and 8 to 10 cm). (A) The total cell count (▪), determined by microscopy, decreased with depth, whereas soil moisture (▵) was relatively constant. (B) The oligonucleotide probe VER1112 that targets 16S rRNA gene sequences of members of the class “Spartobacteria” (•) indicated that 16S rRNA genes from members of this class were more numerous than homologs from subdivision 3 (○) in PCR products generated by using a bacterium-specific primer pair. The errors bars represent SDs when they are larger than the symbol.
Selection of media.
We tested five different media for their ability to support colony formation by these soil bacteria: VL55 plus xylan, VL55 plus amino acids, VL55 plus GGXA, VL55 plus N-acetylglucosamine, and DNB. The number of CFU on these media were not significantly different (P = 0.17 to 0.97 for the different comparisons), with a mean count of 3.4 × 108 (standard deviation [SD] = 1.3 × 108, n = 20). This is 22% of the mean microscopic cell count (Fig. 1A). Two of these media, VL55 plus xylan and VL55 plus amino acids, were also used with three different supplements: 10 μM cyclic AMP (3), 2% (vol/vol) supernatant of a culture of “Chthoniobacter flavus,” and 20% (vol/vol) “C. flavus” culture supernatant. The addition of these supplements did not significantly change the viable counts obtained (P = 0.24 to 0.95 for the different comparisons). We also tested the effect of incubating the plates under air enriched with 5% (vol/vol) CO2 to mimic the higher CO2 partial pressures found in soil air (29). There was no significant difference in the viable count obtained compared to a control experiment incubated under normal air (P = 0.64). Isolates from all of these experiments were selected for screening to find new isolates of the phylum Verrucomicrobia.
Screening of isolates.
A total of 1,208 isolates yielded PCR products that bound probes in the EUB338 suite (Table 4), indicating the presence of 16S rRNA genes of members of the domain Bacteria. These isolates were then screened in a separate hybridization experiment for the ability to bind the probe VER47, complementary to a region of the 16S rRNA gene of most members of the phylum Verrucomicrobia (4). Of the 1,208 products 19 bound the VER47 probe, and the 16S rRNA genes amplified from these cultures were sequenced. The PCR product from one of these appeared to have resulted from a mixed culture, based on the multiple base identifications in the sequencing chromatographs. This culture was not able to be subcultured further and was not analyzed in any more detail. Between 1,327 and 1,480 nucleotides of the sequences of the 16S rRNA genes of the remaining 18 cultures were determined (Table 5).
TABLE 4.
Screening of isolates to identify members of the phylum Verrucomicrobia
| Medium | No. of EUB338 suite-positive cultures | No. of VER47- positive cultures | No. of confirmed members of the phylum Verrucomicrobia |
|---|---|---|---|
| DNB | 99 | 2 | 2 |
| VL55 + GGXA | 47 | 0 | 0 |
| VL55 + N-acetylglucosamine | 52 | 0 | 0 |
| VL55 + xylan | 348 | 8 | 7 |
| VL55 + amino acids | 116 | 4 | 2 |
| VL55 + xylan + cAMPa | 51 | 1 | 0 |
| VL55 + amino acids + cAMP | 35 | 0 | 0 |
| VL55 + xylan + 2% (vol/vol) supernatant | 52 | 1 | 1 |
| VL55 + amino acids + 2% (vol/vol) supernatant | 43 | 0 | 0 |
| VL55 + xylan + 20% (vol/vol) supernatant | 93 | 2 | 1 |
| VL55 + amino acids + 20% (vol/vol) supernatant | 112 | 0 | 0 |
| VL55 + xylan + CO2 | 160 | 1 | 1 |
| Total | 1,208 | 19 | 14 |
cAMP, cyclic AMP.
TABLE 5.
Identity of VER47-positive isolates and their phylogenetic affiliations as determined by BLAST analysis in GenBank databases (1)a
| Isolate | 16S rRNA gene sequence
|
Medium (month of colony appearance) | Affiliation(s) | |
|---|---|---|---|---|
| Length (bp) | GenBank accession no. | |||
| Ellin501 | 1,348 | AY960764 | VL55 + amino acids (2) | Verrucomicrobia, “Spartobacteria” |
| Ellin502 | 1,371 | AY960765 | VL55 + xylan (2) | Verrucomicrobia, “Spartobacteria” |
| Ellin503 | 1,327 | AY960766 | VL55 + amino acids (2) | Verrucomicrobia, “Spartobacteria” |
| Ellin504 | 1,473 | AY960767 | VL55 + amino acids (3) | Rubrobacteridae |
| Ellin505 | 1,438 | AY960768 | VL55 + xylan (3) | Bacillus |
| Ellin506 | 1,468 | AY960769 | DNB (3) | Verrucomicrobia, “Spartobacteria” |
| Ellin507 | 1,448 | AY960770 | DNB (3) | Verrucomicrobia, “Spartobacteria” |
| Ellin508 | 1,480 | AY960771 | VL55 + xylan (3) | Verrucomicrobia, “Spartobacteria” |
| Ellin509 | 1,480 | AY960772 | VL55 + xylan (3) | Verrucomicrobia, “Spartobacteria” |
| Ellin510 | 1,424 | AY960773 | VL55 + xylan (3) | “Deinococcus-Thermus” |
| Ellin511 | 1,362 | AY960774 | VL55 + xylan + 2% (vol/vol) supernatant (3) | Verrucomicrobia, “Spartobacteria” |
| Ellin512 | 1,428 | AY960775 | VL55 + xylan + cAMP (3) | “Deinococcus-Thermus” |
| Ellin513 | 1,361 | AY960776 | VL55 + xylan + 20% (vol/vol) supernatant (3) | Verrucomicrobia, “Spartobacteria” |
| Ellin514 | 1,443 | AY960777 | VL55 + xylan + CO2 (1) | Verrucomicrobia, subdivision 3 |
| Ellin515 | 1,481 | AY960778 | VL55 + xylan (1) | Verrucomicrobia, subdivision 3 |
| Ellin516 | 1,357 | AY960779 | VL55 + xylan (2) | Verrucomicrobia, subdivision 3 |
| Ellin517 | 1,429 | AY960780 | VL55 + xylan (>3) | Verrucomicrobia, subdivision 3 |
| Ellin518 | 1,479 | AY960781 | VL55 + xylan (>3) | Verrucomicrobia, subdivision 3 |
Details of the medium on which each was isolated and the month in which the colony that the isolate originated from first became visible are also given. cAMP, cyclic AMP.
Of these 18 isolates, 14 were members of the phylum Verrucomicrobia (Table 5). The 16S rRNA genes of the 14 confirmed verrucomicrobia all contained an exact match to the VER47 probe binding site. The other four products were derived from bacteria that were clearly not members of the phylum Verrucomicrobia. One, Ellin505, was from a member of the genus Bacillus in the phylum Firmicutes and had a 16S rRNA gene sequence most similar (99% nucleotide identity) to those of a group of Bacillus species including Bacillus arbutinivorans, B. drentensis, B. senegalensis, B. djibelorensis, and B. bataviensis. A second product, from culture Ellin504, was from a member of the subclass Rubrobacteridae of the phylum Actinobacteria and most closely related (97 to 98% nucleotide identity) toisolates Ellin301 and Ellin325, recently isolated from the Ellinbank site (32), and also to Conexibacter woesei (40). Members of the subclass Rubrobacteridae are only rarely isolated from soils, and the presence of this isolate in the collection of cultured soil bacteria is therefore noteworthy. Neither of these two false-positive sequences contained a perfect VER47 probe-binding site. The remaining two products were from members of the “Thermus-Deinococcus” phylum (Table 5). Both of these isolates had 16S rRNA genes that had a region that matched all but the 3′-nucleotide of the VER47 probe. This probe-binding site was in the same position as that of the fully complementary binding site found in verrucomicrobia. We conclude that the VER47 probe designed by Buckley and Schmidt (4) was an accurate tool for detecting colonies of verrucomicrobia, with an acceptable incidence of false positives.
Characteristics of new isolates.
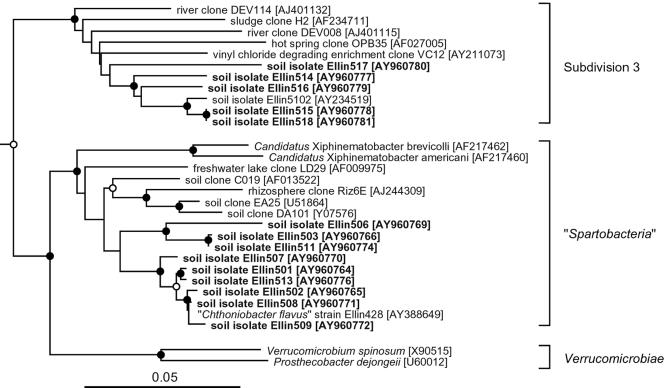
Nine of the new isolates clearly grouped within the newly proposed class “Spartobacteria” (36), equivalent to subdivision 2 (20), of the phylum Verrucomicrobia (Fig. 2). They clustered into two lineages that were supported by bootstrap values of >97%. One group, consisting of six of the isolates, grouped with the cultured species “Chthoniobacter flavus” (36), and had 16S rRNA gene sequences that were 96.9 to 99.9% identical to that of “C. flavus.” Three other isolates were more distantly related to “C. flavus” and had 16S rRNA genes that were only 89.9 to 93.2% identical to that of “C. flavus” and 88.5 to 93.2% identical to those of the other six isolates. The 16S rRNA gene sequences of “C. flavus” and the six related isolates were 96.4 to 99.9% identical to each other, while the other three isolates had gene sequences that were 91.2 to 99.4% identical to each other. Sequence identities of <96% have been used to define the genus level separation of bacteria (13). On this basis, the degree of sequence identity between the different isolates of “Spartobacteria” obtained in the present study suggests that they belong to least two genera, one of which is “Chthoniobacter.” The other is probably a new genus.
FIG. 2.
Evolutionary-distance dendrogram showing the relationships of newly isolated bacteria (in boldface) and other members of the class “Spartobacteria” and of subdivision 3 within the phylum Verrucomicrobia, based on comparisons of their 16S rRNA gene sequences. Also shown are two cultured members of the class Verrucomicrobiae. The dendrogram was constructed by using the Fitch-Margoliash (17) method from Jukes-Cantor (24) distances. The sequences of the 16S rRNA genes of Opitutus terrae (GenBank accession no. AJ229235) and Victivallis vadensis (GenBank accession AY049713) were used as outgroups and to root the tree; they are not shown in the figure. The GenBank accession numbers for the 16S rRNA gene sequences are given after each bacterium or clone name. Analyses using different algorithms resulted in very similar dendrograms. The number of times each branch point was recovered in dendrograms constructed from 1,000 bootstrapped datasets is indicated by symbols: •, recovered in >90%; ○, recovered in 75 to 89%. Nodes with no symbol were recovered in <75% of the bootstrapped datasets. The scale bar indicates 0.05 changes per nucleotide position.
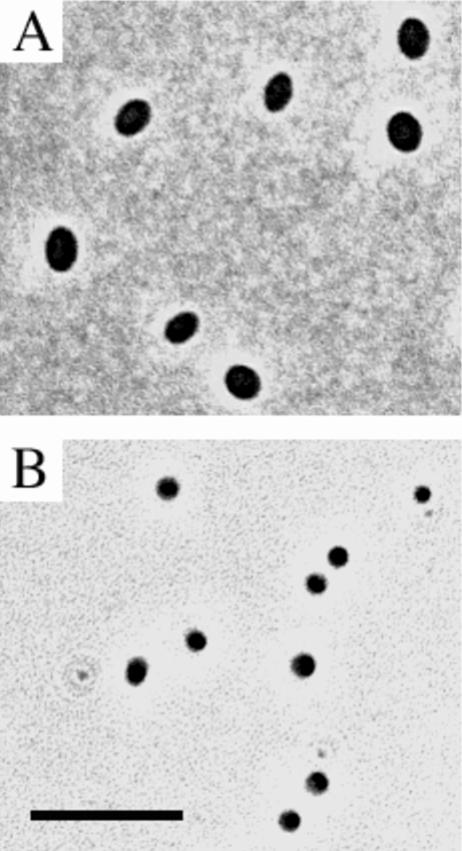
The isolates belonging to “Spartobacteria” formed yellow, low, convex colonies with entire edges, a smooth surface and an amorphous structure when grown on plates. The colonies of isolate Ellin503 were distinctly paler than those of the other six isolates. The cells of all of these isolates were ovoid (Fig. 3A), 1.2 μm long, and 0.8 to 1.0 μm in diameter; stained gram negative; and were very similar to those of “C. flavus.” In liquid culture, visible turbidity was observable after an incubation period of 3 to 4 days at 25°C. Colonies appeared on solid media after 5 to 7 days of incubation at 25°C, except for culture Ellin501, which took 12 to 14 days to grow up into visible colonies. Growth upon subcultivation therefore did not take as long as the time required for colony formation in the initial isolation experiments (Table 4). The isolates cultured on media supplemented with supernatant from “C. flavus” (Tables 4 and 5) did not require the addition of supernatant for subculture. A more detailed characterization will be required to confirm and delineate potential new species and genera.
FIG. 3.
Phase contrast photomicrographs, showing cells of isolate Ellin502, a member of the class “Spartobacteria” (A), and isolate Ellin514, a member of subdivision 3 (B). The scale bar represents 5 μm for both panels.
The remaining five isolates were members of subdivision 3 (20) of the phylum Verrucomicrobia (Fig. 2). They grouped together with an earlier isolate from the same site, Ellin5102 (23). The 16S rRNA gene sequences of Ellin5102 and the five new isolates were 89.4 to 99.8% identical to each other. On this basis, the degree of sequence identity between the different isolates obtained in the present study suggest the presence of different species among the isolates. These isolates formed cream-colored, flat, smooth, and entire colonies when grown on plates. The cells were cocci (Fig. 3B), 0.6 to 0.8 μm in diameter, and stained gram negative. In liquid subcultures, visible turbidity was observable after an incubation period of 3 weeks at 30°C. When subcultured onto solid media, colonies appeared after 9 to 12 days of incubation at 30°C. Growth was slower at 25°C. As with members of the “Spartobacteria,” colonies formed more rapidly than in the initial isolation experiment (Table 5). One of the isolates was from the experiment incubated under CO2 (Tables 4 and 5), but it did not require elevated CO2 concentrations for successful subculture.
Evaluation of approach.
All but two of the verrucomicrobial isolates initially formed colonies that first became visible more than 1 month after inoculation of the plates with diluted soil. Extended incubation time has been shown to be a significant factor in the isolation of members of many rarely isolated but abundant groups of soil bacteria (11), and this also seems to be true for members of “Spartobacteria” and subdivision 3 of Verrucomicrobia.
All of the isolates affiliated with the class “Spartobacteria” originated from plates inoculated with the equivalent of 2 × 10−7 g of dry soil, which yielded a mean of 4.8 colonies per plate (SD = 2.5). Only 12% of the colonies screened originated from such plates. No members of the class “Spartobacteria” were found among the 88% of colonies that were found on plates that received a 10-fold-denser inoculum. In contrast, all of the isolates belonging to subdivision 3 originated from plates inoculated with the equivalent of 2 × 10−6 g of dry soil, which yielded a mean of 28.8 colonies per plate (SD = 5.6), and none were detected among the colonies that were formed on plates with a less-dense inoculum. Members of subdivision 3 of Verrucomicrobia were estimated to be rarer in the soil under investigation, making up <1% of the bacterial community. It is likely that this is why they were only detected on plates with more concentrated inocula. Members of “Spartobacteria” were more common in this soil, making up ca. 4 to 9% of the bacterial community. This is why they appeared on plates with more dilute inocula. They were only detectable on plates with lower total colony numbers (<12 per plate), and may be inhibited by other bacteria on plates with denser inocula.
Acknowledgments
We thank Philip Hugenholtz for designing the probes VER727 and VER1112 and for helpful comments on the manuscript. We thank Cameron Gourley and Sharon Aarons (Dairy Research Institute, Ellinbank, Victoria, Australia) for help with access to the sampling site.
This study was supported by a grant from the Australian Research Council.
REFERENCES
- 1.Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, and D. L. Wheeler. 2005. GenBank. Nucleic Acids Res. 33:D34-D38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brosius, J., M. L. Palmer, P. J. Kennedy, and H. F. Noller. 1978. Complete nucleotide sequence of the 16S rRNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4801-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruns, A., H. Cypionka, and J. Overmann. 2002. Cyclic AMP and acyl homoserine lactones increase the cultivation efficiency of heterotrophic bacteria from the central Baltic Sea. Appl. Environ. Microbiol. 68:3978-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley, D. H., and T. M. Schmidt. 2001. Environmental factors influencing the distribution of rRNA from verrucomicrobia in soil. FEMS Microbiol. Ecol. 35:105-112. [DOI] [PubMed] [Google Scholar]
- 5.Buckley, D. H., and T. M. Schmidt. 2002. Exploring the biodiversity of soil: a microbial rainforest, p. 183-208. In J. T. Staley and A.-L. Reysenbach (ed.), Biodiversity of microbial life. Wiley-Liss, Inc., New York, N.Y.
- 6.Buckley, D. H., and T. M. Schmidt. 2003. Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ. Microbiol. 5:441-452. [DOI] [PubMed] [Google Scholar]
- 7.Chin, K.-J., D. Hahn, U. Hengstmann, W. Liesack, and P. H. Janssen. 1999. Characterization and identification of numerically abundant culturable bacteria from the anoxic bulk soil of rice paddy microcosms. Appl. Environ. Microbiol. 65:5042-5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin, K.-J., W. Liesack, and P. H. Janssen. 2001. Opitutus terrae gen. nov., sp. nov., to accommodate novel strains of the division “Verrucomicrobia” isolated from rice paddy soil. Int. J. Syst. Evol. Microbiol. 51:1965-1968. [DOI] [PubMed] [Google Scholar]
- 9.Curtis, T. P., W. T. Sloan, and J. C. Scannell. 2002. Estimating prokaryotic diversity and its limits. Proc. Natl. Acad. Sci. USA 99:10494-10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daims, H., A. Bruhl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 11.Davis, K. E. R., S. J. Joseph, and P. H. Janssen. 2005. Effects of growth medium, inoculum size, and incubation time on the culturability and isolation of soil bacteria. Appl. Environ. Microbiol. 71:826-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derrien, M., E. E. Vaughan, C. M. Plugge, and W. M. de Vos. 2004. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54:1469-1476. [DOI] [PubMed] [Google Scholar]
- 13.Everett, K. D., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 14.Felsenstein, J. 1989. PHYLIP: phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 15.Felske, A., and A. D. L. Akkermans. 1998. Prominent occurrence of ribosomes from an uncultured bacterium of the Verrucomicrobiales cluster in grassland soils. Lett. Appl. Microbiol. 26:219-223. [DOI] [PubMed] [Google Scholar]
- 16.Felske, A., A. Wolterink, R. van Lis, and A. D. L. Akkermans. 1998. Phylogeny of the main bacterial 16S rRNA sequences in Drentse A grassland soils (The Netherlands). Appl. Environ. Microbiol. 64:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitch, W. M., and E. Margoliash. 1967. Construction of phylogenetic trees. Science 155:279-284. [DOI] [PubMed] [Google Scholar]
- 18.Floyd, M. M., J. Tang, M. Kane, and D. Emerson. 2005. Captured diversity in a culture collection: case study of the geographic and habitat distributions of environmental isolates held at the American Type Culture Collection. Appl. Environ. Microbiol. 71:2813-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedlund, B. P., J. J. Gosink, and J. T. Staley. 1997. Verrucomicrobia div. nov., a new division of the Bacteria containing three new species of Prosthecobacter. Antonie Leeuwenhoek 72:29-38. [DOI] [PubMed] [Google Scholar]
- 20.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen, P. H., A. Schuhmann, E. Mörschel, and F. A. Rainey. 1997. Novel anaerobic ultramicrobacteria belonging to the Verrucomicrobiales lineage of bacterial descent isolated by dilution culture from anoxic rice paddy soil. Appl. Environ. Microbiol. 63:1382-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen, P. H., P. S. Yates, B. E. Grinton, P. M. Taylor, and M. Sait. 2002. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl. Environ. Microbiol. 68:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph, S. J., P. Hugenholtz, P. Sangwan, C. A. Osborne, and P. H. Janssen. 2003. Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl. Environ. Microbiol. 69:7210-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, Inc., New York, N.Y.
- 25.Kemp, P. F. 1995. Can we estimate bacterial growth rates from rRNA content?, p. 279-302. In I. Joint (ed.), Molecular ecology of aquatic microbes. Springer-Verlag, Berlin, Germany.
- 26.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom.
- 27.Lee, S.-Y., J. Bollinger, D. Bezdicek, and A. Ogram. 1996. Estimation of the abundance of an uncultured soil bacterial strain by a competitive quantitative PCR method. Appl. Environ. Microbiol. 62:3787-3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Farrell, K. A., and P. H. Janssen. 1999. Detection of verrucomicrobia in a pasture soil by PCR-mediated amplification of 16S rRNA genes. Appl. Environ. Microbiol. 65:4280-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul, E. A., and F. E. Clark. 1996. Soil microbiology and biochemistry, 2nd ed. Academic Press, Inc., San Diego, Calif.
- 30.Petroni, G., S. Spring, K.-H. Schleifer, F. Verni, and G. Rosati. 2000. Defensive extrusive ectosymbionts of Euplotidium (Ciliophora) that contain microtubule-like structures are bacteria related to Verrucomicrobia. Proc. Natl. Acad. Sci. USA 97:1813-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rappé, M. S., and S. J. Giovannoni. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57:369-394. [DOI] [PubMed] [Google Scholar]
- 32.Sait, M., P. Hugenholtz, and P. H. Janssen. 2002. Cultivation of globally distributed soil bacteria from phylogenetic lineages previously only detected in cultivation-independent surveys. Environ. Microbiol. 4:654-666. [DOI] [PubMed] [Google Scholar]
- 33.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 34.Sakai, T., K. Ishizuka, and I. Kato. 2003. Isolation and characterization of a fuciodan-degrading marine bacterium. Mar. Biotechnol. 5:409-416. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Sangwan, P., X. Chen, P. Hugenholtz, and P. H. Janssen. 2004. Chthoniobacter flavus gen. nov., sp. nov., the first pure-culture representative of subdivision two, Spartobacteria classis nov., of the phylum Verrucomicrobia. Appl. Environ. Microbiol. 70:5875-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sargeant, I. J., and J. K. M. Skene. 1970. Some properties of the krasnozems of southern Victoria, Australia. Aust. J. Soil Res. 8:281-295. [Google Scholar]
- 38.Shieh, W. Y., and W. D. Jean. 1998. Alterococcus agarolyticus, gen. nov., sp. nov., a halophilic thermophilic bacterium capable of agar degradation. Can. J. Microbiol. 44:637-645. [DOI] [PubMed] [Google Scholar]
- 39.Singh, D. K., P. W. G. Sale, C. J. P. Gourley, and C. Hasthorpe. 1999. High phosphorus supply increases persistence and growth of white clover in grazed dairy pastures during dry summer conditions. Aust. J. Exp. Agric. 39:579-585. [Google Scholar]
- 40.Singleton, D. R., M. A. Furlong, A. D. Peacock, D. C. White, D. C. Coleman, and W. B. Whitman. 2003. Solirubrobacter pauli gen. nov., sp. nov., a mesophilic bacterium within the Rubrobacteridae related to common soil clones. Int. J. Syst. Evol. Microbiol. 53:485-490. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson, B. S., S. A. Eichorst, J. T. Wertz, T. M. Schmidt, and J. A. Breznak. 2004. New strategies for cultivation and detection of previously uncultured microbes. Appl. Environ. Microbiol. 70:4748-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torsvik, V., J. Goksøyr, and F. L. Daae. 1990. High diversity in DNA of soil bacteria. Appl. Environ. Microbiol. 56:782-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tringe, S. G., C. von Mering, A. Kobayashi, A. A. Salamov, K. Chen, H. W. Chang, M. Podar, J. M. Short, E. J. Mathur, J. C. Detter, P. Bork, P. Hugenholtz, and E. M. Rubin. 2005. Comparative metagenomics of microbial communities. Science 308:554-557. [DOI] [PubMed] [Google Scholar]
- 45.Vandekerkhove, T. T. M., A. Willems, M. Gillis, and A. Coomans. 2000. Occurrence of novel verrucomicrobial species, endosymbiotic and associated with parthenogenesis in Xiphinema americanum-group species (Nematoda, Longidoridae). Int. J. Syst. Evol. Microbiol. 50:2197-2205. [DOI] [PubMed] [Google Scholar]