Abstract
A unique lineage of bacteria belonging to the order Bacteroidales was identified as an intracellular endosymbiont of the protist Pseudotrichonympha grassii (Parabasalia, Hypermastigea) in the gut of the termite Coptotermes formosanus. We identified the 16S rRNA, gyrB, elongation factor Tu, and groEL gene sequences in the endosymbiont and detected a very low level of sequence divergence (<0.9% of the nucleotides) in the endosymbiont population within and among protist cells. The Bacteroidales endosymbiont sequence was affiliated with a cluster comprising only sequences from termite gut bacteria and was not closely related to sequences identified for members of the Bacteroidales attached to the cell surfaces of other gut protists. Transmission electron microscopy showed that there were numerous rod-shaped bacteria in the cytoplasm of the host protist, and we detected the endosymbiont by fluorescence in situ hybridization (FISH) with an oligonucleotide probe specific for the 16S rRNA gene identified. Quantification of the abundance of the Bacteroidales endosymbiont by sequence-specific cleavage of rRNA with RNase H and FISH cell counting revealed, surprisingly, that the endosymbiont accounted for 82% of the total bacterial rRNA and 71% of the total bacterial cells in the gut community. The genetically nearly homogeneous endosymbionts of Pseudotrichonympha were very abundant in the gut symbiotic community of the termite.
Symbiotic microorganisms that inhabit the guts of termites enable the termites to feed on lignocelluloses (4, 13, 21). The gut microbiota forms a dense and complex symbiotic community consisting of both flagellated protists (single-cell eukaryotes) and prokaryotes. Recent culture-independent studies based on molecular sequences have enabled us to classify the gut symbionts phylogenetically as both protists (22, 26) and prokaryotes (9, 16-18, 23-25, 28, 29). These studies revealed that a great majority of the prokaryotes in the gut are novel organisms that have not been cultivated, which has limited our knowledge of termite gut symbionts.
Associations of prokaryotes with gut protists are frequently observed, and gut protists themselves are the hosts of prokaryotic symbionts (21). In fact, the protist-associated prokaryotes comprise a significant portion of the gut microbial community (3). Previous observations revealed the presence of methanogens within cells (as endosymbionts) and spirochetes attached to cell surfaces (as ectosymbionts) of the gut protists, and these groups of prokaryotes have been identified in situ and classified phylogenetically by using molecular sequences (references 8, 12, 20, 33, and 35 and references therein). Recently, bacteria belonging to the order Bacteroidales were identified as ectosymbionts of a number of protist species in termite guts (18, 30, 35), but they belong to at least three polyphyletic lineages in this order (18). These studies and other studies have gradually disclosed bacterial species associated with gut protists of termites and the spatial distribution of the members of the bacterial community. However, there are numerous types of associations with diverse protists in termite guts, and very few studies have investigated the associated bacteria from the point of view of phylogenetic classification, the extent of genetic heterogeneity, and the abundance of the whole community in the gut.
In this study, we investigated the endosymbiotic bacteria of the protist genus Pseudotrichonympha (Parabasalia, Hypermastigea). The Pseudotrichonympha protists are some of the common protists in subterranean termites (family Rhinotermitidae) (14), which include economically important pests of buildings, such as Coptotermes formosanus, which is distributed worldwide. We found that the endosymbiont of Pseudotrichonympha protists was affiliated with a unique phylogenetic lineage in the order Bacteroidales and comprised the predominant population in the gut symbiotic community of C. formosanus.
MATERIALS AND METHODS
Termites and protist.
The termites C. formosanus (Rhinotermitidae) and Termitogeton planus (Rhinotermitidae) were collected in Shizuoka prefecture in Japan and in Sabah state in Malaysia, respectively. Only the worker caste was used. The protist genus Pseudotrichonympha was identified on the basis of morphological characteristics (5) after protargol impregnation, and a single species was detected in each termite (Pseudotrichonympha grassii in C. formosanus and Pseudotrichonympha sp. in T. planus). Three and one protist species inhabited the guts of C. formosanus and T. planus, respectively. The three protist species in C. formosanus were easily recognized by their cell sizes and the presence of a rostrum (5). The gut (hindgut plus midgut) of C. formosanus was extracted from live specimens as previously described (26). Acetone-preserved specimens of T. planus were reconstituted with solution U (20) for 5 min and then dissected. A protist cell was physically isolated using a micromanipulator (TransferMan; Eppendorf), as described previously (20). The isolation step was repeated three times to remove contaminated protist cells and free-swimming bacteria. A single cell or a pool of three to five cells of the isolated protist was placed in acetone and used for PCR amplification after the acetone was evaporated at 94°C for 30 s.
T-RFLP.
The gut contents were fractionated into protists and free-swimming bacteria by centrifugation; the optimal conditions for centrifugation were 23 × g for 15 s. The precipitated protist cells were resuspended in solution U and washed three times to remove the contaminated free-swimming bacterial cells. The free-swimming bacteria in the supernatant were transferred to a new tube, and the centrifugation and transfer procedure was repeated three times. The cells in each fraction were used directly as PCR templates. Bacterial 16S rRNA genes were amplified using ExTaq DNA polymerase (Takara) with 6-carboxyfluorescein (6-FAM)-labeled primer Eub27F+ (5′-TTGACCGTTTGATCMTGGCTCAG) and primer 520R (24). The PCR conditions were 25 cycles of 94°C for 30 s, 50°C for 45 s, and 72°C for 2 min. The PCR products were purified with a multiscreen PCR purification filter (Millipore), digested with AccII (Takara), and analyzed using an ABI3700 sequencer with a GeneScan-500 ROX size standard (PE Applied Biosystems). Terminal restriction fragment length polymorphism (T-RFLP) electropherograms were analyzed with GeneScan v.3.5.1 (PE Applied Biosystems).
Gene analyses.
Bacterial genes for 16S rRNA, DNA gyrase subunit B (gyrB), elongation factor Tu (EF-Tu), and chaperonin (groEL) were amplified by PCR with ExTaq DNA polymerase (Takara) using the isolated protist cells as templates. The PCR conditions were 20 cycles for the 16S rRNA gene and 30 cycles for the other three genes; each cycle consisted of 94°C for 30 s, 52°C for 45 s, and 72°C for 2 min. The PCR primers used are shown in Table 1. The PCR products were cloned into the pCR2.1-TOPO vector (Invitrogen), and clones containing inserts of the expected size were picked and sequenced. The primer used for sequencing of the partial 16S rRNA gene in all clones was Eub750R (18), and the primer set described previously (18) was used for complete sequencing of the 16S rRNA gene in representative clones. Primers T7 and Sp6 (Promega) were used for the other three genes. Nucleotide sequences were determined using ABI dye terminator chemistry with an ABI3700 sequencer.
TABLE 1.
PCR primers used in this study
| Primer | Target gene | Sequence (5′ to 3′) | Reference |
|---|---|---|---|
| Eub27F | 16S rRNA | ATTGGATCCGTTTGATCMTGGCTCAG | 18 |
| 1392R | 16S rRNA | CGGGCGGTGTGTRC | 18 |
| gyrB-UP1A | gyrB | CAYGCNGGNGGNAARTTYGA | 36a |
| gyrB-UP2A | gyrB | CCRTCNACRTCNGCRTCNGTCAT | 36a |
| EB-EFTu-Fw1 | EF-Tu | GCXGAYTAYRTXAARAAYATG | This study |
| EB-EFTu-Rv1 | EF-Tu | TGXCKXCCXCCYTCXTCYTT | This study |
| groEL-F | groEL | GCNCCIGAYGGNACNACNAC | This study |
| groEL-R | groEL | CCRAAICCNGGNGCYTTNAC | This study |
Primers gyrB-UP1A and gyrB-UP2A were slightly modified from the primers described in a previous report (36).
The sequence data used to infer phylogenetic trees were retrieved from the public sequence databases. The accession numbers are shown below (see Fig. 2 and 3). Sequences were aligned with the CLUSTAL X package (32) and were corrected manually. Phylogenetic analyses were restricted to unambiguously aligned positions. To infer the 16S rRNA gene phylogeny, we used a general time-reversible model with gamma-distributed rate variation and a portion of invariable sites that was selected as the appropriate model for sequence evolution with Modeltest 3.06 (27). The phylogenetic trees were constructed by the maximum-likelihood (ML) method using the program PHYML v2.4.4 (7). The robustness of the branching pattern was confirmed by bootstrap analysis of 500 replicates. Bayesian inference was performed using MrBayes v3.0b4 (11), which was run for 100,000 generations, and the posterior probabilities of each node were measured after the first 10,000 generations were discarded. PAUP*4.10b (31) was used for the maximum-parsimony method, and bootstrapping with 200 replicates was conducted using a heuristic search with TBR branch swapping and 10 random additions of sequences. For protein phylogeny, the ML method was applied using PHYML v2.4.4 with the WAG substitution model and a gamma distribution rate across sites with four categories, plus invariant sites estimating the shape parameter and fraction of invariable sites from each data set. A bootstrap analysis was performed with 500 replicates.
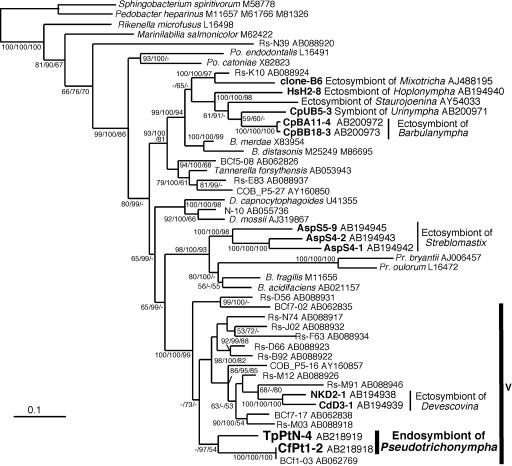
FIG. 2.
Phylogenetic positions of the endosymbiotic bacteria of the Pseudotrichonympha protists. The tree was inferred by the ML method based on comparisons of 1,254 unambiguously aligned positions of 16S rRNA gene sequences. The numbers at the nodes indicate the levels of support for ML, Bayesian inference, and maximum-parsimony results (from left to right, divided by slashes). Scale bar = 0.1 nucleotide substitution per position. Cluster V of the Bacteroidales sequences from termite guts is indicated by the vertical bar on the right. Sequences from termite guts are indicated by the prefixes Rs (Reticulitermes speratus), BCf (Coptotermes formosanus), and COB (Cubitermes orthognathus). The sequences obtained in this work and the sequences reported to be sequences of ectosymbionts of protists in other termites are indicated by boldface type. The database accession numbers are indicated after the names of taxa. Abbreviations: B., Bacteroides; Po., Porphyromonas; Pr., Prevotella; D., Dysgonomonas.
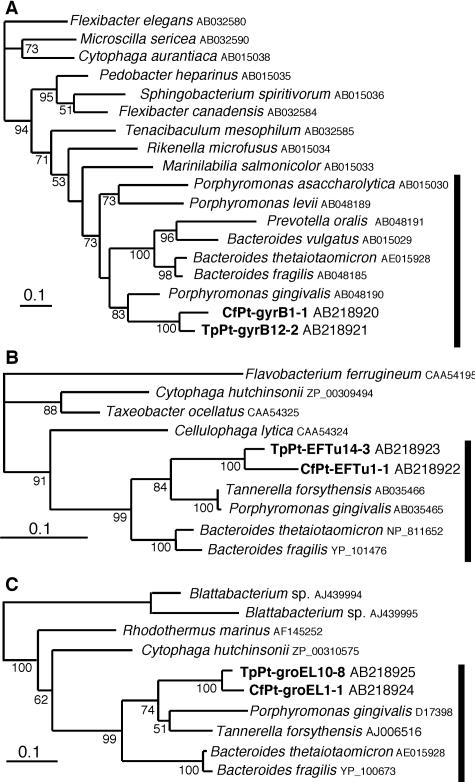
FIG. 3.
Phylogenetic analyses of the endosymbiotic bacteria of the Pseudotrichonympha protists based on the partial amino acid sequences of GyrB (A), EF-Tu (B), and GroEL (C), using 226, 222, and 185 unambiguously aligned positions, respectively. The trees were inferred by the ML method. The numbers at the nodes indicate ML bootstrap values. Scale bar = 0.1 nucleotide substitution per position. The sequences obtained from P. grassii in C. formosanus are indicated by the prefix CfPt, and the sequences obtained from Pseudotrichonympha sp. in T. planus are indicated by the prefix TpPt. The database accession numbers are indicated after the names of taxa.
FISH and enumeration.
Fluorescent in situ hybridization (FISH) was performed by using the method described by Noda et al. (20). The previously described general eubacterial probe (12) was labeled at the 5′ end with Texas Red and used as a control for the permeability of the cells. Probe CfPt-729 for the identified 16S rRNA gene sequence of the P. grassii endosymbiont was designed, and its sequence was 5′-AGATATGGTCTGGTAAGCAGTC-3′; this probe was labeled at the 5′ end with 6-FAM. The probes were incubated with the fixed gut contents for 3 h at 48°C in the buffer described previously (20), and the signal was observed with an Olympus epifluorescence microscope (BX-60). For enumeration of FISH-positive cells, the gut contents were suspended in solution U containing 0.5% NP-40 for 10 min at 4°C, and after vortexing, dispersed cells were fixed and used for hybridization. After washing, the hybridized cells were stained with 4,6-diamidino-2-phenylindole (DAPI) and enumerated. The total bacterial cells in the gut contents were enumerated after dispersion, as described above, by using DAPI staining. One hundred cells of P. grassii were pooled, and after dispersion the numbers of endosymbiont cells per protist cell were also determined.
Electron microscopy.
Electron microscopy was performed by methods described previously (18). Thin sections were poststained with uranyl acetate and lead citrate and viewed with a JEOL 1230 transmission electron microscope at 80 kV.
Quantification of rRNA.
To quantify the endosymbiotic bacteria, we used sequence-specific cleavage of rRNA with RNase H (34). The probes used were EUB338 (34), which is universal for eubacterial 16S rRNA genes, and CfPt-729-18 (5′-ATGGTCTGGTAAGCAGTC-3′), which is specific for the endosymbiotic bacteria of P. grassii. The reference clones of bacterial, archaeal, and protist small-subunit (SSU) rRNA used were Rs-D73 and Rs-G65 for spirochetes, Rs-A34 for β-proteobacteria, Rs-N31 for δ-proteobacteria, Rs-M09 for clostridia, Rs-N74 and NkD2-1 for Bacteroidales, Rs-D95 for termite group I, Hj1 for methanogens, and Cf14 for protists (9, 25, 26). The insert of the SSU rRNA gene sequence in conjunction with the RNA polymerase promoters was amplified by PCR, and the transcript was generated in vitro using a T7 or Sp6 RiboMAX express kit (Promega). For clones Rs-D95 and Rs-A34, T3 RNA polymerase (Promega) was used for the in vitro transcription. Cleavage reactions were carried out at 50°C for 15 min in the hybridization buffer described previously (34) with appropriate formamide concentrations. For the scissor probe EUB338 the hybridization stringency was adjusted to 25% formamide, and for the CfPt-729-18 probe the stringency was optimal without formamide. The resultant RNA fragments were electrophoresed and quantified by using an Agilent 2100 bioanalyzer with an RNA 6000 nano kit (Agilent). All quantifications were replicated in triplicate in different digestion experiments. The cleavage efficiency of each bacterial reference RNA was 95.3 to 100% for the EUB338 probe. The archaeal and protist SSU reference rRNAs were not cleaved by the EUB338 probe at all. The cleavage efficiency of probe CfPt-729-18 for target SSU rRNA was 44.2%, and the cleavage efficiency of probe CfPt-729-18 for reference SSU rRNA was 0 to 2.6%. Extraction of the total RNA from the termite gut was carried out by using the method described previously (19). The SSU rRNAs of prokaryotes and protists were not separated from each other, because the protist SSU rRNA in the termite was almost the same size as the SSU rRNA of the prokaryotes (26). Thus, we estimated the amount of SSU rRNA of all the gut microbiota (prokaryotes plus protists) as the total amount of RNA in the size range from 1,400 to 1,650 bases.
Nucleotide accession numbers.
The sequences reported in this study have been deposited in the DDBJ database under accession numbers AB218918 to AB218925, AB231932, and AB231933.
RESULTS
T-RFLP analysis.
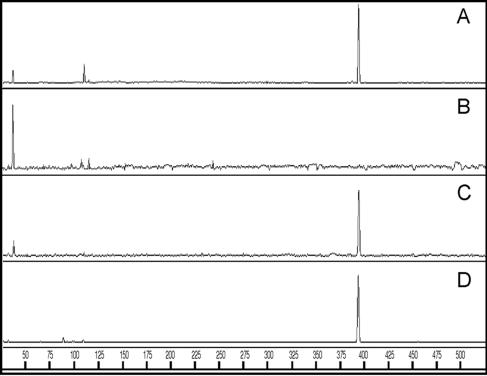
The gut contents of C. formosanus were carefully fractionated into the protists and free-swimming bacteria, and the T-RFLP profiles of PCR-amplified fragments of the bacterial 16S rRNA gene were compared with each other and with those of the whole gut community (Fig. 1). One major terminal restriction fragment (T-RF) peak at 394 bases was detected in the whole gut and the protist fraction (Fig. 1A and C). The T-RF profile of the free-swimming bacterial fraction was clearly distinct from the profiles of the whole gut and the protist fraction, and there was no peak at 394 bases (Fig. 1B). The results indicate that a major bacterial population in the gut community was associated with protists. Three species of gut protists were found in C. formosanus, and numerous intracellular bacteria were observed in the protist P. grassii with DAPI staining (data not shown). The P. grassii cells were physically isolated and analyzed by T-RFLP (Fig. 1D), and the results indicated that the P. grassii-associated bacteria corresponded to the major population in the gut.
FIG. 1.
T-RFLP profiles of bacterial 16S rRNA gene fragments amplified from the whole gut (A), the free-swimming bacterial fraction (B), the protist fraction (C), and isolated cells of the protist P. grassii (D). The profiles obtained after AccII digestion are shown. The horizontal axis indicates the sizes of the T-RFs (bases). The major T-RF at 394 bases in panels A, C, and D was predicted from the sequences of the P. grassii endosymbiont described in this study and corresponds to clone BCf1-03 obtained from the gut community of C. formosanus (29). The major T-RF at 40 bases in panel B was likely derived from spirochetes since all the sequences identified as spirochetes in the gut of C. formosanus (16, 29) had a restriction site at the same position. In fact, microscopic observation of this fraction revealed the presence of a large number of spirochete-like cells.
Phylogeny of the endosymbiont as determined with multiple genes.
The bacterial 16S rRNA gene amplified from physically isolated P. grassii was cloned and analyzed. We constructed three clone libraries of the PCR products, two of which were amplified from a single protist cell and one of which was amplified from a pool of five protist cells. Eleven, 12, and 19 clones were analyzed from these libraries, respectively, and 40 clones comprised one major phylotype (CfPt1-2) that exhibited more than 99.3% sequence identity. The other two clones (from two independent libraries) were affiliated with Lactobacillus and the TM7 phylum, and these two minor clones were not included in further analyses.
Phylogenetic analyses based on the 16S rRNA gene sequence (Fig. 2), indicated that the CfPt1-2 phylotype from the endosymbiont of P. grassii was clearly affiliated with the order Bacteroidales and, with significant statistical support, belonged to cluster V of this order, which was described previously in a comparison of Bacteroidales members from diverse termites (24). This cluster did not contain any cultivable species, and it was composed exclusively of sequences identified from termite guts and thus probably represented a novel Bacteroidales genus. This cluster included the sequences identified from ectosymbionts attached to the cells of the gut protist genus Devescovina (18), but these sequences did not form a monophyletic group with the P. grassii endosymbiont. The sequence of the P. grassii endosymbiont was closely related to the clone BCf1-03 sequence obtained from the gut community of the same termite species (29), and these sequences formed a unique lineage in cluster V.
We also identified the sequences of the gyrB, EF-Tu, and groEL genes of the P. grassii endosymbiont. As in the case of the 16S rRNA gene, three clone libraries derived from a single protist (in two libraries) and from five cells of the physically isolated protist were constructed for each gene, and approximately 10 and 20 clones were analyzed, respectively, to obtain a very similar (>99.1%), unique sequence for each gene. Phylogenetic analyses showed that each of the three genes grouped with members of the Bacteroidales with significant statistical support (Fig. 3), although the reported sequences for Bacteroidales members were limited.
In situ identification and morphology.
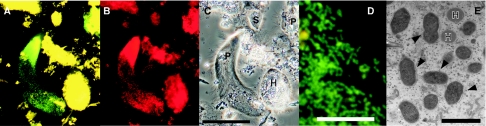
We designed a sequence-specific oligonucleotide probe (CfPt-729) targeting 16S rRNA of the P. grassii endosymbiont and used it for FISH (Fig. 4A to D). This probe gave strong signals with the numerous rod-shaped bacteria associated intracellularly with P. grassii. Probe CfPt-729 did not detect any free-swimming bacteria in the gut contents of C. formosanus. However, the endosymbiont of P. grassii was also detected by the previously described probe for most members of cluster V in the Bacteroidales (18) (data not shown). The control hybridizations with other Bacteroidales bacteria, which are known to attach to several protist species in different termite species (18), resulted in no positive signals (data not shown).
FIG. 4.
In situ detection and morphology of endosymbiotic bacteria in P. grassii. The specific probe CfPt-729 (A and D) labeled with 6-FAM and the eubacterial consensus probe labeled with Texas Red (B) were used simultaneously. (C) Phase-contrast micrograph of the same sample. The protist species are labeled P (Pseudotrichonympha grassii), S (Spirotrichonympha leidyi), and H (Holomastigotes mirabile). Panel D is a magnified view of endosymbiotic bacteria in P. grassii detected with probe CfPt-729. The green fluorescence derived from the probe labeled by 6-FAM enabled us to distinguish the positive signals from amorphous yellow backgrounds of autofluorescence or wood particles in the gut. (E) Transmission electron micrograph of the endosymbionts (indicated by arrowheads) in a P. grassii cell. Probable hydrogenosomes of the host protist are labeled H. The scale bars in panels C, D, and E are 100 μm, 10 μm, and 1 μm, respectively.
Transmission electron microscopy of ultrathin sections revealed that the cytoplasm of P. grassii contained numerous endosymbiont cells (Fig. 4E). The endosymbionts were 0.36 ± 0.03 μm wide (mean ± standard deviation) and 0.71 ± 0.07 μm long and had a single cell membrane (Fig. 4E). The endosymbiont cell was surrounded by an external membrane, probably derived from the host protist.
Abundance of the endosymbiont in the gut community.
We measured the abundance of the P. grassii endosymbiont in the gut community using sequence-specific cleavage of rRNA with RNase H (34). The probe designed for the P. grassii endosymbiont specifically cleaved the control target RNA that was transcribed in vitro from the clone DNA, whereas it cleaved other reference RNAs very little, indicating that the probe could be used for quantitative detection of the 16S rRNA of the P. grassii endosymbiont. The eubacterial universal probe almost completely cleaved all eubacterial reference RNAs, but no cleavage was observed for RNAs of methanogenic archaea and protists. The level of eubacterial 16S rRNA was estimated to be 29.9% ± 2.1% of the total SSU rRNA in the gut symbiotic community of C. formosanus. Since a large portion of the gut contents was shared by voluminous gut protists and the number of methanogenic archaea was far less than the number of bacteria, most of the remaining 70% of the SSU rRNA was probably SSU rRNA of the gut protists. The rRNA of the P. grassii endosymbiont was estimated to account for 24.5% ± 2.7% of the total SSU rRNA, which corresponded to 81.9% of the total bacterial 16S rRNA in the gut.
The abundance of the P. grassii endosymbiont was also estimated by cell counting after FISH with the specific probe. The P. grassii endosymbiont was estimated to account for 71.3% ± 6.0% of the total eubacterial cells in the gut, while the general eubacterial probe detected 93.7% ± 3.3% of DAPI-stained prokaryotic cells.
Single cells of P. grassii harbored 1.08 × 105 ± 0.04 × 105 cells of DAPI-stained endosymbionts, and it has been reported that up to 800 cells of P. grassii are present in each termite gut (37); thus, the number of endosymbionts in each termite gut was calculated to be 8.6 × 107 cells. Because the total prokaryote count was 1.30 × 108 ± 0.13 × 108 cells per gut, the P. grassii endosymbiont was estimated to account for 66% of the prokaryotic cells in each gut. This estimate was in good agreement with the data obtained by sequence-specific cleavage of rRNA and FISH counting (Table 2). The endosymbiont of P. grassii was the predominant bacterial population in the gut of C. formosanus.
TABLE 2.
Abundance of an endosymbiotic bacterial population of P. grassii measured by different methods
| Quantitative method | % of endosymbiont (mean ± SD)a |
|---|---|
| rRNA cleavage | 81.9 ± 9.0 |
| FISH counting | 71.3 ± 6.0 |
| Estimated numberb | 66 |
The percentages of the endosymbiont population are the percentages of the total eubacteria in the gut for the rRNA cleavage and FISH counting methods and the percentage of the total DAPI-stained prokaryotic cells for the estimated number method. See text for details.
The number of endosymbiont bacteria was estimated from the number of endosymbiont cells per protist, the reported number of protist cells per gut (36), and the total number of prokaryote cells per gut.
Endosymbiont in another Pseudotrichonympha species.
We also investigated the endosymbiotic bacteria in another species of Pseudotrichonympha in the gut of the termite T. planus. The 16S rRNA, gyrB, EF-Tu, and groEL genes from the isolated protist cells exhibited significant sequence similarity to the genes of the endosymbiont of P. grassii (89.2%, 83.3%, 83.9%, and 85.4% nucleotide identity, respectively). Each of the genes from the two Pseudotrichonympha species formed a stable monophyletic group in the phylogenetic tree (Fig. 2 and 3). The results indicate that closely related Bacteroidales species are associated with Pseudotrichonympha protists as their endosymbionts.
DISCUSSION
In this study, we identified a previously undescribed, unique endosymbiont belonging to the Bacteroidales in the cells of a cellulolytic parabasalid protist. The protist-associated bacteria were characterized not only in terms of phylogenetic position and in situ identification but also, for the first time in the gut microbial community of termites, in terms of genetic heterogeneity using multiple protein-encoding genes and abundance as determined by polyphasic approaches. The results clearly indicate that the endosymbiont of the gut protist P. grassii in C. formosanus, a member of the Bacteroidales that is genetically very homogeneous, is very abundant and is the predominant organism in the gut bacterial community.
Identification of endosymbiotic bacteria that fill the cells of Pseudotrichonympha spp. showed that a species belonging to the Bacteroidales lives intracellularly in eukaryotic cells. Only a few bacteria belonging to the Bacteroidetes or the Cytophaga-Flavobacterium-Bacteroides phylum have been identified as intracellular endosymbionts of eukaryote cells. In cockroaches and the termite Mastotermes darwiniensis, intracellular Blattabacterium spp. are found in bacteriocytes of the insect fat body (2). Intracellular bacteria of acanthamoebae are affiliated with either the genus Flavobacterium or “Candidatus Amoebophilus asiaticus”; the latter organism is closely related to endosymbionts of a tick and a white fly and represents a novel phylogenetic lineage (10). Based on 16S rRNA gene comparisons, these endosymbionts form coherent clusters in this phylum but are clearly not members of the order Bacteroidales. Thus, at least to our knowledge, this is the first description of an intracellular endosymbiont belonging to the order Bacteroidales.
Members of the Bacteroidales are common in termite guts (24), and several members, which form at least three distinct lineages in this order, have been identified as ectosymbionts of gut protists (18). The genus Pseudotrichonympha belongs to the order Trichonymphida in the parabasalian class Hypermastigea, and the monophyletic grouping of this order was shown in our molecular phylogenetic study (22). The ectosymbionts identified from this protist order (the genera Hoplonympha, Barburanympha, Urinympha, and Staurojoenina) form a monophyletic lineage in the phylogenetic tree (Fig. 2), while the endosymbionts of Pseudotrichonympha branch in a lineage that is distinct from them. Rather, the Pseudotrichonympha endosymbiont exhibits a closer relationship, although not a monophyletic relationship, with the ectosymbionts of Devescovina protists, which belong to the order Cristamonadida in the parabasalid class Trichomonadea. In addition to these associations with the protists, members of cluster V are also known to free swimmers in the gut contents and to be attached on the gut wall (17).
There is a striking difference in ultrastructure between the P. grassii endosymbiont and ectosymbionts attached to the cell surfaces of various gut protists, although each organism belongs to the Bacteroidales. A typical gram-negative cell wall with inner and outer membranes having an external surface layer is the common feature for ectosymbiotic members of the Bacteroidales (18, 30, 35). However, transmission electron microscopy observation (Fig. 4E) revealed a single cell membrane for the endosymbiont, and no outer surface layer was found. The endosymbiont probably lost the cell wall during adaptation in the cytoplasm of the host protist. The ectosymbionts on the protist cell surface are often incorporated into vacuoles in the host cytoplasm, and the attachment structures with the host cell membrane are still observed in the vacuoles (18, 30). In the case of the P. grassii endosymbiont, however, neither such an attachment structure with the host membrane nor cells receiving digestion in food vacuoles occur in the host cytoplasm. Based on these observations, it can be hypothesized that the endosymbiont originated from an incorporated ectosymbiont; however, at least among known ectosymbionts, there is no closely related candidate.
Now that we have identified the sequences of the gyrB, EF-Tu, and groEL genes from the endosymbiont, we can estimate the genetic variation. In general, these protein-encoding genes, each of which exists as a single copy in the genome, show a much higher frequency of base substitutions than the 16S rRNA gene. For instance, the level of divergence of gyrB sequences among different strains of Pseudomonas putida was up to 8.4% (percentage of base substitutions), although the sequences of the 16S rRNA gene differed less than 1.1% (36). The sequence variation of the 16S rRNA gene of the P. grassii endosymbiont detected in the analysis of 40 clones was less than 0.7% (<5 bases in 700 bp), which is in the range for the divergence that occurs in the multiple operons of a single genome (1). In the gyrB, EF-Tu, and groEL genes, only small sequence variations were found in the clones from the endosymbiont examined (about 40 clones in each gene); the frequencies of base substitutions in a single clone library were less than 0.6% (<4 bases in 680 bp), 0.9% (<6 bases in 666 bp), and 0.9% (<5 bases in 556 bp), respectively. In each gene, the frequency did not increase when we compared the three libraries, one of which was derived from a pool of five P. grassii cells instead of the single cells that were used for the other two libraries. Although some amplification biases may occur and artificial base substitutions may be introduced during PCR, at least the majority of the endosymbiont population is almost homogeneous genetically, but the microheterogeneity, although very small, is apparent even in a single host cell.
The great abundance of the P. grassii endosymbiont in the gut community of C. formosanus was estimated by sequence-specific cleavage of rRNA and FISH or direct cell counting. The results of the semiquantitative T-RFLP analysis also supported the predominance of this organism (Fig. 1). Very recently, the diversity of the bacterial community in the gut of C. formosanus was described, and the sequence of BCf1-03, a close relative of the P. grassii endosymbiont, represented the most abundant clones (67% of the clones examined) (29). This predominance is due to its high density in the host cell and the significant volume of the host protist in the gut lumen. In fact, P. grassii cells are very large (approximately 200 by 50 μm). Two other protist species, Holomastigotoides mirabile and Spirotrichonympha leidyi, have been found in the gut of C. formosanus. Endosymbionts are rarely found within the cells of H. mirabile, while this protist harbors attached spirochetes (ectosymbionts) that have been identified as organisms that belong to cluster I of termite treponemas (20). However, the concentration of ectosymbiotic spirochetes is less than 200 cells per H. mirabile cell. The cells of S. leidyi harbor an endosymbiotic methanogen, probably belonging to the genus Methanobrevibacter (28), but no more than 300 cells of the methanogen have been detected in a single cell. Thus, the prokaryotic populations associated with H. mirabile and S. leidyi are considerably smaller (each <0.5% of the total prokaryotes) than the P. grassii endosymbiont population.
Since the P. grassii endosymbiont is predominant in the gut and is active based on the significant amount of its rRNA, it must play important roles in the gut symbiotic system. The roles of the ectosymbiotic members of the Bacteroidales of various gut protists have been discussed elsewhere (6, 15, 18), but no definitive conclusion has been drawn. Moreover, the intracellular location differs from the location of the ectosymbionts. Further studies on the functions of the Bacteroidales symbionts are necessary to understand their relationships with the host protists, the real nature of the termite gut symbiotic systems, and the evolution of these systems.
Acknowledgments
This work was partially supported by grants for the Bioarchitect Research Program and the Eco Molecular Science Research Program from RIKEN and by a grant-in-aid for scientific research from JSPS (grant 16380065). H.N. was supported by a grant for the Junior Research Associate Program from RIKEN.
We thank T. Inoue for useful discussions and M. Kawai for assistance with nucleotide sequencing. We especially thank Y. Hongoh for providing the reference clones and M. Maryati (University of Malaysia, Sabah) for providing termite material.
REFERENCES
- 1.Acinas, S. G., L. A. Marcelino, V. Klepac-Ceraj, and M. F. Polz. 2004. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J. Bacteriol. 186:2629-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandi, C., M. Sironi, G. Damiani, L. Magrassi, C. A. Nalepa, U. Laudani, and L. Sacchi. 1995. The establishment of intracellular symbiosis in an ancestor of cockroaches and termite. Proc. R. Soc. Lond. B 259:293-299. [DOI] [PubMed] [Google Scholar]
- 3.Berchtold, M., A. Chatzinotas, W. Schönhuber, A. Brune, R. Amann, D. Hahn, and H. König. 1999. Differential enumeration and in situ localization of microorganisms in the hindgut of the lower termite Mastotermes darwiniensis by hybridization with rRNA-targeted probes. Arch. Microbiol. 172:407-416. [DOI] [PubMed] [Google Scholar]
- 4.Breznak, J. A. 2000. Ecology of prokaryotic microbes in the guts of wood- and litter-feeding termites, p. 209-231. In T. Abe, D. E. Bignell, and M. Higashi (ed.), Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 5.Brugerolle, G., and J. J. Lee. 2000. Phylum Parabasalia, p. 1196-1250. In J. J. Lee, G. F. Leedale, and P. Bradbury (ed.), An illustrated guide to the protozoa, 2nd ed., vol. II. Allen Press, Lawrence, KS. [Google Scholar]
- 6.Dyer, B. D., and O. Khalsa. 1993. Surface bacteria of Streblomastix strix are sensory symbionts. BioSystems 31:169-180. [DOI] [PubMed] [Google Scholar]
- 7.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 8.Hara, K., N. Shinzato, T. Oshima, and A. Yamagishi. 2004. Endosymbiotic Methanobrevibacter species living in symbiotic protists of the termite Reticulitermes speratus detected by fluorescent in situ hybridization. Microbes Environ. 19:120-127. [Google Scholar]
- 9.Hongoh, Y., M. Ohkuma, and T. Kudo. 2003. Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera; Rhinotermitidae). FEMS Microbiol. Ecol. 44:231-242. [DOI] [PubMed] [Google Scholar]
- 10.Horn, M., M. D. Harzenetter, T. Linner, E. N. Schmid, K.-D. Müller, R. Michel, and M. Wagner. 2001. Members of the Cytophaga-Flavobacterium-Bacteroides phylum as intracellular bacteria of acanthamoebae: proposal of ‘Candidatus Amoebophilus asiaticus.’ Environ. Microbiol. 3:440-449. [DOI] [PubMed] [Google Scholar]
- 11.Huelsenbeck, J. P., and F. Ronquist. 2001. MrBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754-755. [DOI] [PubMed] [Google Scholar]
- 12.Iida, T., M. Ohkuma, K. Ohtoko, and T. Kudo. 2000. Symbiotic spirochetes in the termite hindgut: phylogenetic identification of ectosymbiotic spirochetes of oxymonad protists. FEMS Microbiol. Ecol. 34:17-26. [DOI] [PubMed] [Google Scholar]
- 13.Inoue, T., O. Kitade, T. Yoshimura, and I. Yamaoka. 2000. Symbiotic associations with protists, p. 275-288. In T. Abe, D. E. Bignell, and M. Higashi (ed.), Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 14.Kitade, O., and T. Matsumoto. 1998. Characteristics of the symbiotic flagellate composition within the termite family Rhinotermitidae (Isoptera). Symbiosis 25:271-278. [Google Scholar]
- 15.Leander, B. S., and P. J. Keeling. 2004. Symbiotic innovation in the oxymonad Streblomastix strix. J. Eukaryot. Microbiol. 51:291-300. [DOI] [PubMed] [Google Scholar]
- 16.Lilburn, T. G., T. M. Schmidt, and J. A. Breznak. 1999. Phylogenetic diversity of termite gut spirochaetes. Environ. Microbiol. 4:331-345. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima, H., Y. Hongoh, R. Usami, T. Kudo, and M. Ohkuma. 2005. Spatial distribution of bacterial phylotypes in the gut of the termite Reticulitermes speratus and the bacterial community colonizing the gut epithelium. FEMS Microbiol. Ecol. 54:247-255. [DOI] [PubMed]
- 18.Noda, S., T. Inoue, Y. Hongoh, M. Kawai, C. A. Nalepa, C. Vongkaluang, T. Kudo, and M. Ohkuma. Identification and characterization of ectosymbionts of distinct lineages in Bacteroidales attached to flagellated protists in the gut of termites and a wood-feeding cockroach. Environ. Microbiol., in press. [DOI] [PubMed]
- 19.Noda, S., M. Ohkuma, and T. Kudo. 2002. Nitrogen fixation genes expressed in the symbiotic microbial community in the gut of the termite Coptotermes formosanus. Microbes Environ. 17:139-143. [Google Scholar]
- 20.Noda, S., M. Ohkuma, A. Yamada, Y. Hongoh, and T. Kudo. 2003. Phylogenetic position and in situ identification of ectosymbiotic spirochetes on protists in the termite gut. Appl. Environ. Microbiol. 69:625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkuma, M. 2003. Termite symbiotic systems: efficient bio-recycling of lignocellulose. Appl. Microbiol. Biotechnol. 61:1-9. [DOI] [PubMed] [Google Scholar]
- 22.Ohkuma, M., T. Iida, K. Ohtoko, H. Yuzawa, S. Noda, E. Viscogliosi, and T. Kudo. 2005. Molecular phylogeny of parabasalids inferred from small subunit rRNA sequences, with emphasis on the Hypermastigea. Mol. Phylogenet. Evol. 35:646-655. [DOI] [PubMed] [Google Scholar]
- 23.Ohkuma, M., and T. Kudo. 1996. Phylogenetic diversity of the intestinal bacterial community in the termite Reticulitermes speratus. Appl. Environ. Microbiol. 62:461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohkuma, M., S. Noda, Y. Hongoh, and T. Kudo. 2002. Diverse bacteria related to the Bacteroides subgroup of the CFB phylum within the gut symbiotic communities of various termites. Biosci. Biotechnol. Biochem. 66:78-84. [DOI] [PubMed] [Google Scholar]
- 25.Ohkuma, M., S. Noda, and T. Kudo. 1999. Phylogenetic relationships of symbiotic methanogens in diverse termites. FEMS Microbiol. Lett. 171:147-153. [DOI] [PubMed] [Google Scholar]
- 26.Ohkuma, M., K. Ohtoko, T. Iida, M. Tokura, S. Moriya, R. Usami, K. Horikoshi, and T. Kudo. 2000. Phylogenetic identification of hypermastigotes, Pseudotrichonympha, Spirotrichonympha, Holomastigotoides and parabasalian symbionts in the hindgut of termites. J. Eukaryot. Microbiol. 47:249-259. [DOI] [PubMed] [Google Scholar]
- 27.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 28.Shinzato, N., T. Matsumoto, I. Yamaoka, T. Oshima, and A. Yamagishi. 2001. Methanogenic symbionts and the locality of their host lower termites. Microbes Environ. 16:43-47. [Google Scholar]
- 29.Shinzato, N., M. Muramatsu, T. Matsui, and Y. Watanabe. 2005. Molecular phylogenetic diversity of the bacterial community in the gut of the termite Coptotermes formosanus. Biosci. Biotechnol. Biochem. 69:1145-1155. [DOI] [PubMed] [Google Scholar]
- 30.Stingl, U., A. Maass, R. Radek, and A. Brune. 2004. Symbionts of the gut flagellate Staurojoenina sp. from Neotermes cubanus represent a novel, termite-associated lineage of Bacteroidales: description of ‘Candidatus Vestibaculum illigatum’. Microbiology 150:2229-2235. [DOI] [PubMed] [Google Scholar]
- 31.Swofford, D. L. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA.
- 32.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tool. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokura, M., M. Ohkuma, and T. Kudo. 2000. Molecular phylogeny of methanogens associated with flagellated protists in the gut and with the gut epithelium of termites. FEMS Microbiol. Ecol. 33:233-240. [DOI] [PubMed] [Google Scholar]
- 34.Uyeno, Y., Y. Sekiguchi, A. Sunaga, H. Yoshida, and Y. Kamagata. 2004. Sequence-specific cleavage of small-subunit (SSU) rRNA with oligonucleotides and RNase H: a rapid and simple approach to SSU rRNA-based quantitative detection of microorganisms. Appl. Environ. Microbiol. 70:3650-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenzel, M., R. Radek, G. Brugerolle, and H. König. 2003. Identification of the ectosymbiotic bacteria of Mixotricha paradoxa involved in movement symbiosis. Eur. J. Protistol. 39:11-23. [Google Scholar]
- 36.Yamamoto, S., and S. Harayama. 1995. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl. Environ. Microbiol. 61:1104-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura, T., K. Tsunoda, and M. Takahashi. 1992. Distribution of the symbiotic protozoa in the hindgut of Coptotermes formosanus Shiraki (Isoptera; Rhinotermitidae). Jpn. J. Environ. Entomol. Zool. 4:115-120. [Google Scholar]