Abstract
When eukaryotic proteins with multiple disulfide bonds are expressed at high levels in Escherichia coli, the efficiency of thiol oxidation and isomerization is typically not sufficient to yield soluble products with native structures. Even when such proteins are secreted into the oxidizing periplasm or expressed in the cytoplasm of cells carrying mutations in the major intracellular disulfide bond reduction systems (e.g., trxB gor mutants), correct folding can be problematic unless a folding modulator is simultaneously coexpressed. In the present study we explored whether the bacterial twin-arginine translocation (Tat) pathway could serve as an alternative expression system for obtaining appreciable levels of recombinant proteins which exhibit complex patterns of disulfide bond formation, such as full-length human tissue plasminogen activator (tPA) (17 disulfides) and a truncated but enzymatically active version of tPA containing nine disulfides (vtPA). Remarkably, targeting of both tPA and vtPA to the Tat pathway resulted in active protein in the periplasmic space. We show here that export by the Tat translocator is dependent upon oxidative protein folding in the cytoplasm of trxB gor cells prior to transport. Whereas previous efforts to produce high levels of active tPA or vtPA in E. coli required coexpression of the disulfide bond isomerase DsbC, we observed that Tat-targeted vtPA and tPA reach a native conformation without thiol-disulfide oxidoreductase coexpression. These results demonstrate that the Tat system may have inherent and unexpected benefits compared with existing expression strategies, making it a viable alternative for biotechnology applications that hinge on protein expression and secretion.
During the production of heterologous proteins in bacteria, failure to rapidly reach a native conformation typically results in the formation of insoluble aggregates known as inclusion bodies or in degradation. A primary factor contributing to inclusion body formation is the inability of bacteria to perform certain posttranslational modifications necessary for a protein to fold properly. For instance, the oxidation of protein thiols is disfavored in the reducing cytoplasm of wild-type Escherichia coli owing to the combined action of thioredoxins and glutathiones (12, 38). Consequently, proteins whose native state requires one or more disulfide bonds (e.g., mouse urokinase, tissue plasminogen activator) accumulate predominantly in a misfolded form when they are expressed in the E. coli cytoplasm and are highly prone to aggregation (14, 34).
Secretion into the periplasm using a bacterial signal peptide can often alleviate the tendency of multidisulfide proteins to aggregate (3). This is because the periplasmic space of E. coli and other gram-negative bacteria is highly oxidizing, a state established primarily by the powerful disulfide oxidoreductase DsbA (12, 38). However, while this oxidative environment is sufficient for folding host proteins with relatively simple patterns of disulfide bonds (e.g., alkaline phophatase, outer membrane protein OmpA), periplasmic expression of bacterial and eukaryotic proteins with more complex patterns of disulfide bonds (e.g., tissue plasminogen activator) often results in very low yields or completely inactive products (5, 9, 37, 50).
The low yields observed in studies typically arise from (i) aberrant formation of disulfide bonds in the target protein that remain uncorrected due to low activity of the periplasmic disulfide isomerase DsbC and/or (ii) inefficient secretion of the protein into the periplasm. Proteins that are misoxidized can often be corrected by overexpressing DsbC, a strategy which has been shown to enhance disulfide bond-isomerizing activity in a manner that often leads to appreciable yields of correctly folded proteins (27, 37). This is best exemplified by human tissue plasminogen activator (tPA), a serine protease with 17 disulfide bonds, which is misfolded and completely inactive following secretion into the periplasm but can exhibit its native structure upon overexpression of DsbC (37). Similarly, periplasmic folding of other proteins with nonconsecutive disulfide bonds, including mouse urokinase, bovine pancreatic trypsin inhibitor, and E. coli phytase (AppA), hinges critically on the presence of DsbC (5, 9). Overcoming secretion limitations can be circumvented by transporting the protein through altered secretion machinery (33), through coexpressing factors that promote secretion (e.g., the molecular chaperone SecB) (4), or through bypassing secretion altogether via expression of the target gene in cells with a cytoplasm favorable for disulfide bond formation (16). In the latter case, E. coli strains lacking the enzyme thioredoxin reductase (encoded by the trxB gene) possess a more oxidizing cytoplasm and are capable of forming stable disulfide bonds in normally secreted proteins that are expressed without a signal peptide. An even higher yield of oxidized proteins can be obtained in the cytoplasm of trxB gor mutants, in which the reduction of both thioredoxins and glutathione is impaired (8). However, similar to the situation in the periplasm, cytoplasmic expression of multidisulfide proteins in trxB gor cells, including, for instance, expression of single-chain Fv and Fab antibody fragments (25, 28) and full-length and truncated versions of tPA (8), requires coexpression of folding modulators, such as Skp and DsbC, that remain cytoplasmic by removal of their N-terminal signal peptides.
In this study, we explored the use of an alternate translocation system, namely, the E. coli twin-arginine translocation (Tat) pathway, for the production of complex eukaryotic proteins containing multiple disulfide bonds. The bacterial Tat pathway is a secretion mechanism for the transport of proteins across the inner cytoplasmic membrane of gram-negative bacteria (6, 45, 49). Whereas the Sec- and signal recognition particle (SRP)-dependent pathways deal exclusively with proteins that have not yet folded, the hallmark of the Tat pathway is its ability to transport proteins of various sizes (up to ∼120 kDa) that have already folded (13, 22, 41). Recent evidence suggests that substrates are targeted to the Tat pathway via an N-terminal signal peptide and must satisfy a proofreading requirement prior to transport (13, 18). The latter feature makes the Tat pathway an appealing system for biotechnology applications that hinge on protein biosynthesis in bacteria. However, owing to its relative novelty, the potential of the Tat pathway for heterologous protein expression has yet to be fully explored.
Several recent studies provide strong evidence that the Tat system is highly capable of secreting heterologous proteins (13, 26, 43). Interestingly, complex proteins with intra- or intermolecular disulfide bonds, such as scFv and Fab antibody fragments, become competent for export via the Tat pathway only when they are expressed in strains in which the cytoplasm promotes oxidative protein folding (13). This provides strong evidence that the bacterial Tat system requires its substrates to be correctly folded prior to transport. The further observation that DsbC was not required for cytoplasmic folding or subsequent Tat transport of scFv or Fab antibody fragments is consistent with these relatively simple pattern of disulfide bond formation in these two proteins. Indeed, disulfide isomerization does not appear to be a rate-limiting step in the folding and association of scFv or Fab fragments (29, 48). In the present work, we report the construction of plasmid vectors for Tat translocation of recombinant proteins exhibiting more complex patterns of disulfide bond formation. As a model protein, we selected a truncated version of human tPA (vtPA) that consists of the kringle 2 and protease domains and requires the formation of nine disulfide bonds, eight of which are nonconsecutive, to become enzymatically active. Expression of a Tat signal peptide-vtPA fusion in trxB gor cells resulted in significant yields of active protein in both the cytoplasm and periplasm. Similarly, full-length tPA (17 disulfides) was also capable of being routed through the Tat system. Contrary to our expectations, accumulation of active vtPA and tPA was not dependent on DsbC coexpression. The ability to produce functional recombinant vtPA and tPA via the Tat system, in the absence of thiol-disulfide oxidoreductase coexpression, raises interesting questions about the requirements for efficient disulfide bond formation in the E. coli cytoplasm and about the inherent features of Tat translocation that make it a valuable tool for heterologous eukaryotic protein expression in bacteria.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are described in Table 1. For Tat-dependent expression of all recombinant proteins tested in this study, plasmid pTorA-cassette was first constructed by inserting a 162-bp cDNA encoding the Tat-specific signal peptide plus the next eight residues of E. coli trimethlyamine N-oxide reductase (ssTorA) into pTrc99A (Amersham Pharmacia) between NcoI and EcoRI sites. Next, the vtPA gene was amplified from the pTrcvtPA plasmid (8) and cloned into plasmid pTorA-cassette between XbaI and HindIII sites. A similar cloning procedure was used to construct ssTorA fusions to full-length human tissue plasminogen activator (8). Finally, plasmids for expressing vtPA with a C-terminal six-histidine tag were constructed as follows. Sequences encoding vtPA lacking an N-terminal signal peptide (Δss-vtPA), vtPA fused in frame to the Sec-specific heat-stable enterotoxin signal peptide (stII-vtPA), and ssTorA-vtPA were PCR amplified using a reverse primer containing the polyhistidine DNA sequence; the resulting PCR products were digested with NcoI/HindIII, and each product was inserted into pTrc99A between the same sites. All plasmids constructed in this study were confirmed by DNA sequencing.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant features | Source or reference |
|---|---|---|
| E. coli strains | ||
| DHB4 | MC1000 phoR Δ(phoA) PvuII Δ(malF)3 F′[lacIqZYA pro] | Laboratory stock |
| FÅ113 | DHB4 trxB::Kan gor552 Tn10Tc ahpC* | Gift from J. Beckwith |
| FUDDY | FÅ113 tatC::Spec | 13 |
| DR473 | DHB4 ΔtrxB gor552 Tn10Tc ahpC* Tn10Cm (araC Para-trxB) | Gift from J. Beckwith |
| DRA | DR473 dsbA::Kan | 13 |
| Plasmids | ||
| pTrc99A | trc promoter, ColE1 ori, Ampr | Amersham Pharmacia |
| pBAD33 | ara promoter, pACYC184 ori, Cmr | 20 |
| pTorA-cassette | E. coli TorA signal peptide in pTrc99A | This study |
| pTrcTorAvtPA | vtPA with E. coli TorA signal in pTrc99A | This study |
| pTrcvtPA-h6 | Truncated human tPA (Δ6-175) with C-terminal six-His tag in pTrc99A | This study |
| pTrcStIIvtPA-h6 | vtPA with stII signal with C-terminal six-His tag in pTrc99A | This study |
| pTrcTorAvtPA-h6 | vtPA with E. coli TorA signal with C-terminal six-His tag in pTrc99A | This study |
| pTrctPA | Human tPA in pTrc99A | 8 |
| pTrcStIItPA | tPA with stII signal in pTrc99A | 8 |
| pTrcTorAtPA | tPA with E. coli TorA signal in pTrc99A | This study |
| pBADdsbC | DsbC in pBAD33 with optimized ribosome binding site | 8 |
| pBADΔSSdsbC | DsbC(Δ2-20) in pBAD33 with optimized ribosome binding site | 8 |
| pABS-tatABC | tatABC under control of constitutive tatA promoter in pABS | 7 |
Protein expression in shake flasks.
For monitoring the production of vtPA and tPA, overnight cultures of cells containing pTrc99A derivatives were subcultured in fresh LB at a starting optical density at 600 nm (OD600) of 0.1 and then incubated at 30°C with shaking. At an OD600 of 0.5, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM to induce protein synthesis, and cells were grown for an additional 6 h. For DsbC coexpression studies, cells were grown to an OD600 of 0.5, and this was followed by addition of 0.2% (wt/vol) arabinose to induce the synthesis of DsbC with or without its native signal peptide from pBAD33. After 30 min, IPTG was added to a concentration of 1 mM, and the culture was grown for an additional 6 h. Antibiotic selection was maintained for all markers on plasmids at the following concentrations: ampicillin, 100 μg/ml; and chloramphenicol, 25 μg/ml.
Protein expression in fermentors.
All fermentations were conducted in 14-liter BioFlow 3000 fermentors. For inoculum preparation, FÅ113 cells carrying a pTrc99A-based plasmid were grown overnight in 200 ml of defined medium [13.3 g/liter KH2PO4, 4 g/liter (NH4)2HPO4, 1.2 g/liter MgSO4 · 7H2O, 1.7 g/liter citric acid, 10 g/liter yeast extract, 1% (vol/vol) trace metal solution] supplemented with 0.2% glucose and 50 μg/ml ampicillin. The entire 200 ml of the overnight culture was added to 5 liters of defined medium in a 14-liter fermentor vessel. Fermentors were operated at 30°C, pH 6.8, and 250 rpm with a flow rate of air of 2.0 liters/min in order to maintain the dissolved oxygen level at 30% of air saturation. The cell growth was fed-batch growth with an initial batch phase lasting until the initial glucose was consumed (∼7.5 h), followed by feeding of a solution containing 330 g/liter glucose, 150 g/liter yeast extract, and 20 g/liter MgSO4 · 7H2O at a constant feeding rate of 11 ml/liter · h. Cells were induced 11.5 h postinoculation with 1 mM IPTG and grown for an additional 10 h after induction. The entire fermentation process was ∼21.5 h long.
Cell fractionation.
Equivalent numbers of cells expressing vtPA or tPA were harvested following induction, pelleted by centrifugation, and fractionated by an ice-cold osmotic shock procedure (10), with one modification. Specifically, cells were treated with 100 mM iodoacetamide for 30 min prior to centrifugation to prevent spontaneous activation of free thiols (15). To analyze total cellular proteins, collected cells were centrifuged, resuspended in cold phosphate-buffered saline containing 100 mM iodoacetamide, and lysed by sonication. The insoluble fractions were removed by centrifugation (12,000 × g, 10 min, 4°C), and soluble protein was quantified by the Bio-Rad protein assay with bovine serum albumin as the standard. Only data from fractionation experiments in which ≥95% of the β-galactosidase activity was in the cytoplasmic fraction are reported below. An identical protocol was employed for samples harvested from fermentations.
Activity assays for vtPA and tPA.
Plasminogen activation was quantified by an indirect chromogenic assay as described by Bessette et al. (8). Briefly, in a microtiter plate, 25-μl fractionation samples generated from equivalent numbers of cells were added to wells containing 50 mM Tris-HCl (pH 7.4), 0.01% Tween 80, 0.04 mg/ml human glu-plasminogen (American Diagnostica, Greenwich, CT), and 0.4 mM Spectrozyme PL (American Diagnostica) (final volume, 200 μl). The plate was then incubated at 37°C, and absorbance at 405 nm was monitored. The activity was directly proportional to the absorbance after subtraction of the background value for strain FÅ113 lacking a vector expressing vtPA (ΔA405). Relative activities were calculated as described by Bessette et al. (8) by normalizing the ΔA405 obtained for each sample to the ΔA405 measured for FÅ113 cells expressing Δss-vtPA or Δss-tPA or coexpressing Δss-vtPA and empty pBAD33.
Western blot analysis.
Western blotting was performed as previously described (13). All lanes of sodium dodecyl sulfate (SDS)-12% polyacrylamide gels were loaded with samples generated from equivalent numbers of cells harvested from each experiment. The following primary antibodies were used: monoclonal mouse antipolyhistidine (Sigma) diluted 1:2,000 and polyclonal rabbit anti-GroEL (Sigma) diluted 1:20,000. The secondary antibody was goat anti-mouse and goat anti-rabbit horseradish peroxidase diluted 1:2,000. Membranes were first probed with antipolyhistidine antibody and, following development, were stripped in Tris-buffered saline-2% SDS-0.7 M β-mercaptoethanol. Stripped membranes were reblocked and probed with anti-GroEL antibody.
Quantification of vtPA and tPA expression.
vtPA and tPA were quantified using an IMUBIND tPA enzyme-linked immunosorbent assay (ELISA) kit (American Diagnostica). Samples (20 μl) containing vtPA or tPA were added to 50 μl of phosphate-buffered saline-EDTA-Tween 20 buffer in 96-well plates, mixed, and incubated for 1 h at room temperature. Following incubation, 50 μl of detection antibody conjugate was added to each microwell and incubated for 15 min. After each well was washed four times with phosphate-buffered saline-EDTA-Tween 20 buffer, 100 μl of substrate was added to each well and incubated for 15 min, and this was followed by addition of 50 μl of 1.5 M sulfate acid to stop the reaction. Plates were stored for 10 min in the dark to allow the color to stabilize, after which the absorbance at 490 nm was read. All samples were assayed at two different dilutions, and each dilution was assayed in duplicate. vtPA and tPA concentrations were calculated from a standard curve generated using purified vtPA (kindly provided by Elisabeth Schwarz) or tPA (American Diagnostica).
RESULTS
Production of vtPA by the E. coli Tat pathway.
To address whether the E. coli Tat pathway was a viable mechanism for the production of complex eukaryotic proteins, we fused a cDNA encoding the complete amino acid sequence (amino acids 1 to 46) of the E. coli Tat-dependent TorA signal peptide (ssTorA) plus the first eight residues of mature TorA in frame to the 5′ end of the gene encoding truncated tPA (vtPA) downstream from a trc promoter in pTrc99A. The eight additional amino acids were added to ensure proper signal peptide cleavage, but they do not appear to be required for Tat transport as ssTorA lacking these eight residues resulted in productive transport (32). The ssTorA-vtPA fusion was further modified by introduction of a C-terminal six-histidine sequence for immundetection, resulting in plasmid pTrcTorAvtPA-h6. For comparison, we constructed two additional plasmids: pTrcvtPA-h6, which expressed vtPA lacking an N-terminal signal peptide for cytoplasmic expression (8), and pTrcStIIvtPA-h6, which expressed the Sec-specific heat-stable enterotoxin signal peptide fused in frame to vtPA (8, 37). Both fusion constructs carried a C-terminal six-histidine epitope.
Whole-cell protein extracts prepared from IPTG-induced E. coli wild-type cells (strain DHB4) expressing either Δss-vtPA or ssTorA-vtPA yielded vtPA activity that was indistinguishable from that of DHB4 cells carrying empty pTrc99A, as revealed by a quantitative assay that measured the activation of plasminogen to plasmin (data not shown). This was not surprising since the cytoplasm of DHB4 is maintained at a low redox potential (−270 mV) and is unfavorable for the formation of disulfide bonds necessary for correct folding of vtPA (8). Furthermore, since the folding of Tat substrates occurs in the cytoplasm and is a prerequisite for transport (13), ssTorA-vtPA expressed in the reducing DHB4 cytoplasm was inactive and was transport incompetent. We did observe a very low level of activity in DHB4 cells expressing stII-vtPA (data not shown), which is consistent with previous reports that the periplasm of DHB4 is sufficiently oxidizing for disulfide bond formation but the low efficiency of disulfide bond isomerization in this compartment is inadequate for obtaining high levels of active vtPA (37).
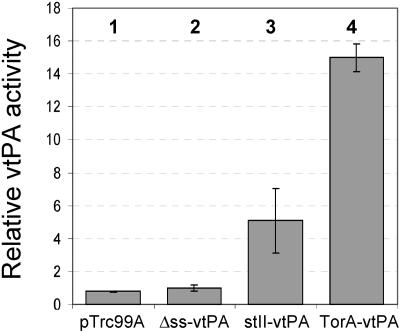
In order to select conditions that allowed proper folding of vtPA in both the cytoplasm and periplasm of E. coli, the pTrcvtPA-h6, pTrcStIIvtPA-h6, and pTrcTorAvtPA-h6 plasmids were introduced into strain FÅ113 (DHB4 trxB::Kan gor522 Tn10Tc ahpC*) (8). FÅ113 cells expressing Δss-vtPA exhibited weak whole-cell activity that was nearly indistinguishable from the background activity measured in control cells (Fig. 1). FÅ113 cells expressing stII-vtPA exhibited a moderate level of whole-cell vtPA activity (Fig. 1). Remarkably, expression of the ssTorA-vtPA fusion protein in FÅ113 cells revealed a significant level of activity which was ∼10-fold greater than cytoplasmic expression of vtPA and ∼3-fold greater than vtPA activity arising from Sec transport.
FIG. 1.
Expression of vtPA in E. coli. Plasminogen activation was measured for total cell extracts from cultures expressing Δss-vtPA, stII-vtPA, and ssTorA-vtPA. Equivalent numbers of cells were harvested 6 h after induction and lysed by sonication, and this was followed by quantification of plasminogen activation using an indirect chromogenic assay described in Materials and Methods. Relative activity is expressed as the amount of plasminogen activated in each lysate sample normalized to the amount activated by lysates from FÅ113 cells expressing Δss-vtPA. Bar 1, FÅ113/pTrc99A (empty vector); bar 2, FÅ113 /pTrcvtPA-h6; bar 3, FÅ113/pTrcStIIvtPA-h6; bar 4, FÅ113/pTrcTorAvtPA-h6.
vtPA targeted to the Tat pathway is localized in the periplasm.
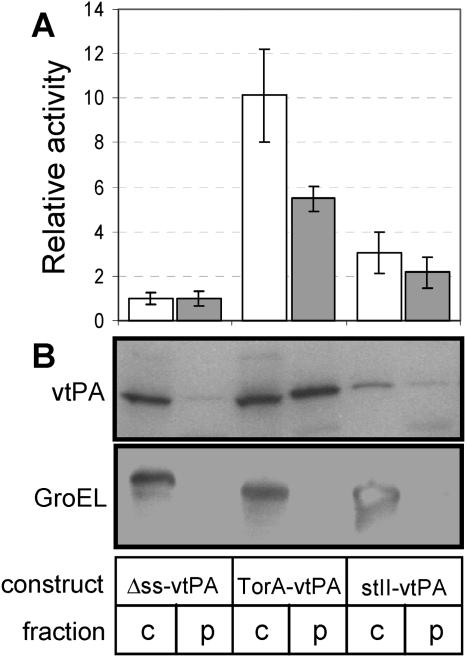
We next determined whether any of the activity arising from strain FÅ113 expressing ssTorA-vtPA was localized in the periplasm. Indeed, an appreciable level of active ssTorA-vtPA corresponding to ∼37% of the whole-cell activity was found in the periplasm of FÅ113 cells, while the remainder of the cellular activity was associated with the cytoplasmic fraction (Fig. 2A). This partitioning of vtPA activity was supported by Western blot data for the same subcellular fractions (Fig. 2B) and was reminiscent of the partitioning observed for other complex eukaryotic proteins expressed via the E. coli Tat pathway (13). The degree of leakage of cytoplasmic components during fractionation was <5%, as determined by the subcellular distribution of β-galactosidase activity (data not shown) and of GroEL protein (detected by Western blotting) (Fig. 2B).
FIG. 2.
Subcellular distribution of vtPA. Equivalent numbers of cells were harvested 6 h after induction and fractionated into cytoplasmic (c) (open bars) and periplasmic (p) (gray bars) fractions using the ice-cold osmotic shock method described in Materials and Methods. (A) Quantification of plasminogen activation; (B) Western blot analysis of cultures expressing Δss-vtPA, stII-vtPA, and ssTorA-vtPA. Relative activity is expressed as the activity in the cytoplasmic or periplasmic sample normalized to the activity measured in the cytoplasm or periplasm of FÅ113 cells expressing Δss-vtPA from pTrcvtPA-h6. Western blot samples were resolved on SDS-12% polyacrylamide gels. GroEL was used as a fractionation marker by probing with anti-GroEL serum. Only data from fractionation experiments in which ≥95% of the β-galactosidase activity was in the cytoplasmic fraction are shown.
We reasoned that the significant accumulation of ssTorA-vtPA in the cytoplasm might be due to saturation of endogenous Tat transporters. Consistent with this notion, previous reports have demonstrated that increased expression of the tatABC genes encoding the minimal Tat translocase can partially suppress this saturation effect (2). However, upon coexpression of the tatABC operon from its native promoter in plasmid pABS-tatABC (7), we observed no change in the subcellular partitioning of vtPA (Table 2). A concomitant decrease in total cellular activity was observed, which we speculated was a consequence of the additional burden placed on the cell when both vtPA and the TatABC proteins were coexpressed, although at present we cannot rule out other explanations for this observation.
TABLE 2.
Summary of vtPA expression in various strains of E. colia
| Strain/plasmid | Relative cytoplasmic vtPA activityb | Relative periplasmic vtPA activityc |
|---|---|---|
| FÅ113/pTrcvtPA-h6 | 1.0 | 1.0 |
| FÅ113/pTrcStIIvtPA-h6 | 3.0 | 2.2 |
| FÅ113/pTrcTorAvtPA-h6 | 10.1 | 5.5 |
| FÅ113/pTrcTorAvtPA-h6/pABS-tatABC | 6.5 | 2.8 |
| FUDDY/pTrcTorAvtPA-h6 | 5.6 | 0.8 |
| DR473/pTrcTorAvtPA-h6 | 8.9 | 4.6 |
| DRA/pTrcTorAvtPA-h6 | 8.7 | 3.9 |
All vtPA activity values are the averages of three replicate experiments (standard error, <0.5%).
The relative cytoplasmic activity for all samples was normalized to the activity measured in the cytoplasm of FÅ113 cells expressing Δss-vtPA from pTrcvtPA-h6.
The relative periplasmic activity for all samples was normalized to the activity measured in the periplasm of FÅ113 cells expressing Δss-vtPA from pTrcvtPA-h6.
For comparison, we performed a similar subcellular analysis for FÅ113 cells expressing Δss-vtPA and FÅ113 cells expressing stII-vtPA. In the former case, we observed a large quantity of Δss-vtPA in the cytoplasmic fraction, as determined by Western blotting (Fig. 2A), but it was predominantly inactive, as shown in Fig. 2A. In the case of stII-vtPA, weak bands were seen in the cytoplasmic and periplasmic fractions, which correlated directly with the moderate activity levels seen in these fractions. It is noteworthy that the periplasmic stII-vtPA activity observed in FÅ113 was found to be slightly increased relative to that associated with the periplasm of wild-type DHB4 cells, indicating that the relatively oxidizing cytoplasm of strain FÅ113 does not disrupt Sec transport. Rather, the activity of stII-vtPA seen in the cytoplasm likely represented translocation-incompetent vtPA that arose due to Sec translocon saturation and, by virtue of its residence time in the cytoplasm, underwent productive folding. This is consistent with the relative half-lilves of these two competing processes; transport out of the cytoplasm via the Sec pathway is completed in a few seconds (35), whereas oxidative protein folding in the cytoplasm of FÅ113 cells requires >1 min (8).
vtPA transport is dependent on a functional Tat system and an oxidizing cytoplasm.
The observation that a significant portion of ssTorA-vtPA is localized in the periplasm prompted us to investigate whether periplasmic localization of vtPA was due solely to transport via the Tat pathway. It has been firmly established that deletion of the tatC gene abolishes transport of natural and also heterologous precursors bearing twin-arginine signal peptides (10, 13). Therefore, we introduced pTrcTorAvtPA-h6 into a tatC::Spec derivative of FÅ113 (strain FUDDY) and analyzed subcellular fractions for plasminogen activation. As expected, the periplasm of FUDDY cells contained no measurable vtPA activity (Table 2) and produced no detectable band corresponding to the vtPA protein (data not shown). It should be noted that the amount of vtPA activity in the cytoplasm of strain FUDDY was reproducibly smaller than the amount of activity observed for FÅ113 cells expressing the same ssTorA-vtPA construct (Table 2). This was not entirely surprising as we and others have previously suggested that there is a yet-to-be-identified “housecleaning” mechanism whereby nontransported Tat substrates are inactivated or degraded in the cytoplasm (13, 42).
Next, we explored whether ssTorA-vtPA was folded in the cytoplasm prior to transport or, instead, was transported in a partially folded or unfolded state and subsequently oxidized by DsbA upon arrival in the periplasm. We reasoned that if ssTorA-vtPA was fully oxidized in the cytoplasm prior to transport, then the periplasmic vtPA activity measured for a trxB gor mutant lacking the potent oxidant dsbA gene would be equivalent to the activity in an isogenic dsbA+ strain background. For this purpose, we employed a trxB gor mutant strain which possessed a nonoxidizing periplasm owing to an additional mutation that inactivated the dsbA gene (strain DRA) and its isogenic parent (strain DR473). Consistent with previous findings (13), the periplasmic vtPA activity was nearly identical in dsbA cells and dsbA+ cells (Table 2), indicating that oxidation of vtPA occurs predominantly in the cytoplasm before export.
Effect of cysteine oxidoreductase DsbC coexpression on vtPA activity.
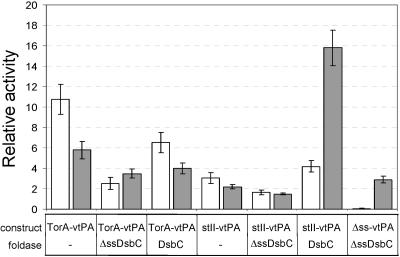
Coexpression of a cysteine oxidoreductase, such as E. coli DsbC or yeast protein disulfide isomerase, is required to obtain appreciable levels of active vtPA expressed in the cytoplasm or in the periplasm via the Sec mechanism; however, no such coexpression was needed for obtaining active ssTorA-vtPA expression. Nonetheless, we reasoned that coexpression of such foldases might improve the periplasmic yield of ssTorA-vtPA, a strategy that was previously observed to increase the periplasmic accumulation of recombinant antibody fragments targeted to the Tat pathway (13). Specifically, we hypothesized that coexpression of E. coli DsbC in the cytoplasm would be expected to increase the yield of correctly folded ssTorA-vtPA in this compartment, leading to a greater number of export-competent molecules. Upon coexpression of ssTorA-vtPA with cytoplasmic DsbC (ΔssDsbC), we observed that the subcellular partitioning of vtPA in the periplasm was improved from ∼35% to nearly 60% of the total cellular activity (Fig. 3). However, this improvement came at the expense of total activity as the vtPA activity measured in both the periplasmic and cytoplasmic fractions was considerably less than that in control cells expressing ssTorA-vtPA and empty pBAD33. The results were nearly identical regardless of whether ΔssDsbC coexpression was induced simultaneously with ssTorA-vtPA induction or preinduced 30 min prior to ssTorA-vtPA induction (data not shown). For comparison and in agreement with previous findings (8, 37), coexpression of ΔssDsbC led to a nearly 3-fold increase in the quantity of active Δss-vtPA in the cytoplasm, while expression of periplasmic DsbC resulted in a nearly 16-fold increase in the quantity of stII-vtPA in the periplasm (Fig. 3). It is noteworthy that the periplasmic activity of ssTorA-vtPA was not affected by coexpression of periplasmic DsbC, providing additional indirect evidence that the majority of ssTorA-vtPA folding occurs in the cytoplasm prior to transport.
FIG. 3.
Effect of DsbC coexpression on subcellular localization and activity of vtPA. Expression of foldase was induced with 0.2% arabinose 30 min prior to vtPA induction. Equivalent numbers of cells were harvested 6 h after vtPA induction and fractionated into cytoplasmic (open bars) and periplasmic (gray bars) fractions. FÅ113 cells carried a vtPA plasmid plus one of the following coexpression vectors: empty pBAD33 (−), pBADΔSSdsbC (ΔssDsbC), or pBADdsbC (DsbC). Relative activity is expressed as the activity in the cytoplasmic or periplasmic samples normalized to the activity measured in the cytoplasm or periplasm of FÅ113 cells expressing Δss-vtPA plus the pBAD33 empty vector. Only data from fractionation experiments in which ≥95% of the β-galactosidase activity was in the cytoplasmic fraction are shown.
vtPA expression in high-cell-density fermentors.
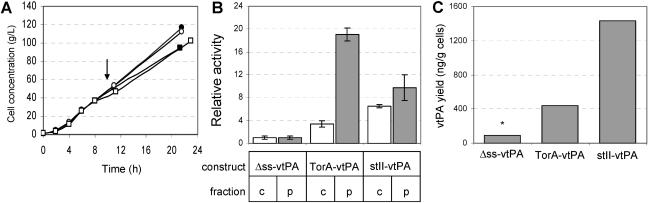
FÅ113 cells carrying pTrcvtPA-h6, pTrcStIIvtPA-h6, or pTorATrc-vtPA-h6 were grown in a 14-liter bioreactor in order to determine the efficiency of Tat secretion. Interestingly, while measurable reductions in cell growth were observed for FÅ113 cells expressing stII-vtPA and ssTorA-vtPA in shake flask cultures (data not shown), induction of vtPA expression in FÅ113 cells grown under controlled bioreactor conditions resulted in no measurable cessation of cell growth (Fig. 4A). Fermentor-cultivated cells expressing ssTorA-vtPA accumulated a large quantity of vtPA in the periplasmic fraction, as shown by Western blotting (data not shown) and activity assays in which approximately 93% of the total ssTorA-vtPA activity was localized in the periplasm (Fig. 4B). For comparison, expression of Δss-vtPA was not detected in either fraction by Western blotting (data not shown), and the corresponding activities were barely detectable above the background levels (Fig. 4B). Finally, stII-vtPA was expressed in a manner similar to ssTorA-vtPA, with most of the vtPA and its corresponding activity associated with the periplasmic fraction. It is noteworthy that the steady-state level of active periplasmic vtPA expressed via the Tat pathway was more than twofold greater than the level of activity obtained for expression via Sec. On the other hand, quantitative ELISA indicated that the yield of periplasmic ssTorA-vtPA was about threefold less than the yield of periplasmic stII-vtPA (Fig. 4C). Thus, the relative specific activity for ssTorA-vtPA was nearly 10-fold greater than the stII-vtPA activity, suggesting that there may be a difference in the relative stabilities of ssTorA-vtPA and stII-vtPA. While the reasons for this difference in stability are not currently known, we speculate that either (i) periplasmic folding of stII-vtPA (as opposed to cytoplasmic folding of ssTorA-vtPA) may be inefficient, resulting in a large quantity of partially folded, inactive vtPA that still retains cross-reactivity during ELISA; and/or (ii) Sec-targeted vtPA is more sensitive to proteolytic degradation, resulting in inactive protein fragments that are still detected by ELISA.
FIG. 4.
Production of vtPA in a high-cell-density fermentation. (A) The cell density, expressed in grams (wet weight) of cells, is shown for four fermentations: FÅ113/pTrcvtPA-h6, induced (•); FÅ113/pTrcStIIvtPA-h6, induced (○); FÅ113/pTrcTorAvtPA-h6, induced (▪); and FÅ113/pTrcTorAvtPA-h6, uninduced (□). The arrow indicates the time of induction with 1 mM IPTG. (B) Relative plasminogen activation for cytoplasmic (c) (open bars) and periplasmic (p) (gray bars) fractions. All data were normalized to the activity measured for the cytoplasmic or periplasmic fraction of FÅ113 expressing Δss-vtPA. (C) Quantitative periplasmic vtPA yield assessed by ELISA as described in Materials and Methods. The ELISA data are the averages of two replicate experiments. The asterisk indicates that the yield was measured for the cytoplasmic fraction since no Δss-vtPA was localized in the periplasm. Only data from fractionation experiments in which ≥95% of the β-galactosidase activity was in the cytoplasmic fraction are shown.
Full-length tPA is translocated by the Tat system.
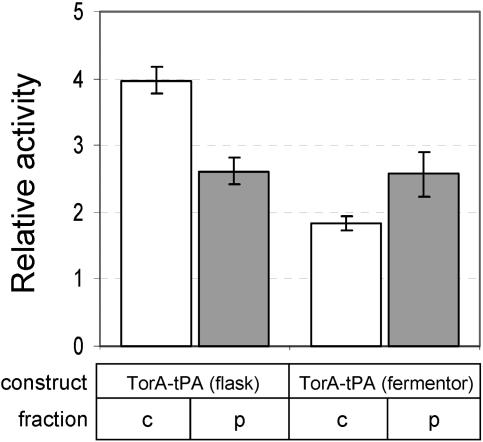
Finally, we explored whether full-length tPA, which requires 17 disulfides in its native structure, could be similarly transported by the Tat mechanism. FÅ113 cells were transformed with pTrcTorAtPA and fractionated in order to determine the subcellular distribution of active tPA. As was observed for vtPA, full-length tPA accumulated in both the cytoplasm and the periplasm of FÅ113 cells, albeit at a much lower level than was observed for truncated tPA (Fig. 5). In agreement with previous studies, the tPA activity in FÅ113 cells expressing tPA or stII-tPA without a signal sequence was virtually indistinguishable from the activity in FÅ113 cells carrying an empty vector (data not shown). Following fermentation in which the cell growth was similar to that described above for cells expressing ssTorA-vtPA (data not shown), we again observed active ssTorA-tPA in both the cytoplasmic and periplasmic fractions (Fig. 5). Finally, the periplasmic yield of tPA from FÅ113 cells grown in fermentors was 115 ng/g cells (data not shown), a quantity that was nearly fourfold less than the quantity of vtPA obtained under identical conditions. This was likely a consequence of the more complex pattern of disulfide bonds in tPA relative to vtPA (17 and 9 disulfides, respectively). Nonetheless, the observation that expression via the Tat pathway can yield active, full-length tPA is quite significant considering that most strategies for producing recombinant tPA result in enzymatically inactive protein that must be recovered from intracellular inclusion bodies (37). Studies are now under way to improve the yield of full-length tPA that can be obtained using the Tat pathway.
FIG. 5.
Expression of full-length tPA by the Tat pathway. Equivalent numbers of FÅ113/pTrcTorAtPA cells grown in shake flasks and fermentors were harvested 6 and 10 h, respectively, after induction and fractionated into cytoplasmic (c) (open bars) and periplasmic (p) (gray bars) fractions. The plasminogen activation data shown are the averages of three replicate experiments. Relative activity is expressed as the activity in the cytoplasmic or periplasmic samples normalized to the activity measured in the cytoplasm or periplasm of FÅ113 cells expressing Δss-tPA in shake flasks or fermentors. Only data from fractionation experiments in which ≥95% of the β-galactosidase activity was in the cytoplasmic fraction are shown.
DISCUSSION
Here we present evidence that the Tat system is capable of transporting complex eukaryotic proteins to the bacterial periplasm. In particular, a truncated version of human tissue plasminogen activator (vtPA) comprising the kringle 2 and serine protease domains was found to transit the E. coli inner membrane when it is affixed to an N-terminal Tat signal peptide (ssTorA). The observed transport of vtPA was dependent on two critical factors: (i) the targeting of vtPA to the periplasm via the Tat pathway was dependent upon a functional Tat system, as shown by the complete blockage of transport in FÅ113 cells lacking a copy of the tatC gene; and (ii) it required a relatively oxidizing cytoplasm capable of promoting correct assembly of the nine nonconsecutive disulfide bonds in vtPA (Table 2). The latter observation was not entirely unexpected as the Tat system requires its protein substrates to be correctly folded (6, 13). In particular, proteins whose native structures required the formation of disulfides (e.g., E. coli alkaline phosphatase, murine scFv and Fab antibody fragments) were excluded from transport when they were expressed in the reducing cytoplasm of wild-type E. coli cells (13, 46). Such proteins become competent for transport only when they are expressed in a trxB gor ahpC* strain (e.g., FÅ113) whose cytoplasmic compartment favors the formation of disulfide bonds (13). As the proteins tested in the previous study exhibited relatively simple patterns of disulfide bonding, it was extremely satisfying in the present study to discover that eukaryotic proteins with significantly more complex disulfide bond patterns are indeed compatible with the Tat system.
Our finding that vtPA must be oxidized in order to be translocated (i.e., no transport was observed in wild-type DHB4 cells) and our finding that oxidation occurs predominantly in the cytoplasm prior to transport (based on a comparison of the periplasmic vtPA activities in DR473 and DRA) provide further evidence that a folding quality control mechanism is an inherent component of the Tat transport process (13). While it is not currently known how such a process works, it is intriguing to speculate that the “proofreading” of Tat substrates is carried out directly by the Tat transporter itself. In support of this notion, recent cross-linking studies indicate that the mature portion of a Tat preprotein interacts specifically with TatB but not with any of the other Tat proteins (1). Another possibility is that a portion of the proofreading is handled at an earlier stage of the translocation process by a cytoplasmic accessory factor(s). For instance, chaperone binding of a misfolded Tat substrate may shield it from the Tat transporter until it is either sufficiently folded or shunted to the cell's proteolytic machinery. To date, two Tat-specific chaperones, DmsD and TorD, have been identified (23, 31), but whether these or additional generalized molecular chaperones broadly interact with all (or most) Tat-targeted proteins is still an open question. Finally, we cannot rule out the possibility that the proofreading mechanism is the result of a kinetic competition that arises between productive folding and degradation.
One unanticipated finding of our study was that expression of ssTorA-vtPA in FÅ113 cells resulted in a significant level of plasminogen activation (∼37% of the activity localized in the periplasm and the remaining ∼63% localized in the cytoplasm), even though previous attempts to obtain appreciable yields of correctly folded, active vtPA required (i) coexpression of a folding modulator, such as the disulfide isomerase DsbC (37), (ii) secretion entirely outside of the cell, where presumably the free thiols in vtPA are readily oxidized (44), (iii) refolding from inclusion bodies (19), or (iv) expression in higher eukaryotes that provide a more favorable environment for the formation of disulfide bonds (19).
Perhaps even more surprising was the finding that ssTorA-vtPA accumulated in an active form at very high levels in the cytoplasm (and was subsequently transported), while vtPA without a signal peptide expressed in the same compartment was largely inactive. The N-terminal sequences of these two constructs differ enough to suggest that transcriptional efficiency and/or mRNA stability may account for this discrepancy. Indeed, bioinformatics predicts that certain Tat signals, including ssTorA (unpublished data), fold into stable stem-loop structures that can result in dramatic overproduction of fusion proteins (36). However, this does not explain our data as both Δss-vtPA and ssTorA-vtPA proteins were relatively well expressed (Fig. 2B). Another possibility is that the Tat signal peptide itself might have a chaperone function. However, this seems unlikely as recent structural studies demonstrated that Tat signal peptides are unstructured in aqueous solvents and have essentially no stable secondary structure, suggesting that they do not interact directly with the passenger protein prior to transport (40). Yet another possible explanation is that a Tat pathway-specific chaperone, such as TorD (23), recognizes the TorA signal peptide and contributes to productive folding of vtPA either directly or via recruitment of other cytoplasmic molecular chaperones. While we cannot rule out this possibility, the hypothesis that we currently favor is that the observed differential activities are related to the folding kinetics of each protein in the cytoplasm. That is, ssTorA-vtPA likely folds more slowly than Δss-vtPA owing to the presence of its moderately hydrophobic N-terminal signal peptide, and this slow folding is likely to be favorable for correct disulfide bond formation. In support of this notion, numerous studies have shown that signal peptides can dramatically decrease the rate of folding of the passenger protein to which they are attached. For instance, Chun and coworkers have shown that the presence of a Sec-specific signal peptide decreases the rate at which maltose binding protein folds by as much as 30-fold compared to folding of mature maltose binding protein (11). As a result of slower folding and a concurrent lower oxidation rate for ssTorA-vtPA, disulfide bond formation is determined by the conformational preferences of the polypeptide chain, resulting in the correct alignment of cysteine residues. Indeed the rate of oxidation in the cytoplasm, which was slower than that in the periplasm, was previously implicated in the higher yields of active disulfide-containing proteins in the cytoplasm compared to the yields when the same proteins were expressed in the periplasm (8).
Recently, Schaerlaekens et al. demonstrated that human proteins (tissue necrosis factor and interleukin-10) could be secreted by the Streptomyces lividans Tat system (43); however, it was not clear whether this is also true in E. coli given recent evidence that the Tat pathway in gram-positive bacteria may be mechanistically dissimilar to that in gram-negative bacteria (24). Lutz and coworkers used the E. coli Tat pathway to identify the correct reading frame of human glycinamide ribonucleotide formyltransferase sequences by targeting these sequences to the Tat pathway; however, whether transport was solely through the Tat system was not demonstrated (30). Thus, to our knowledge, the present study is the first successful demonstration of Tat-specific translocation of a human protein in E. coli. Not only was the Tat system capable of transporting human vtPA, but it provided intrinsic advantages over traditional methods used for secretory expression of recombinant proteins (e.g., Sec expression). First, as mentioned above, both ssTorA-vtPA and ssTorA-tPA accumulated in active conformations and at high levels in the bacterial cytoplasm and periplasm without the need for a folding modulator, such as DsbC. Second, the periplasmic activity and yield of ssTorA-vtPA were both significantly higher relative to the amount of Sec-transported vtPA found in the periplasm of FÅ113 cells cultured under shake flask and high-cell-density conditions. While the reasons for this improvement in yield were not explicitly revealed by our work, we believe that the Tat system offers a number of significant advantages for protein expression. First, since only extended polypeptides can fit through the Sec protein conducting channel, it is imperative that Sec substrates are maintained in an unfolded state via interactions with chaperones or by cotranslational protein synthesis (17). Often, Sec preproteins fold prematurely in the cytoplasm and are not readily exported. Instead, such proteins typically suffer a variety of fates, including jamming in the inner membrane, accumulation in preprotein inclusion bodies, or rapid degradation within the cytoplasm. For example, cell toxicity due to membrane jamming is one of the key problems in the expression of recombinant antibody fragments in bacteria (21). Another shortcoming is that Sec substrates which are translocated correctly often fold slowly or incorrectly in the periplasmic space, in large part because this compartment lacks the complex ATP-dependent chaperone systems, such as GroEL/GroES and DnaK/DnaJ/GrpE, which are essential in the folding of many proteins (3, 47). Finally, certain complex proteins that consist of several polypeptide chains or require certain posttranslational modifications, such as cofactor incorporation, may be intrinsically incompatible with Sec export (13, 39). Consequently, while there have been many remarkable technological achievements related to existing protein expression platforms (3, 47), protein secretion via the Tat pathway is likely to have a number of distinct advantages for expression and engineering of heterologous proteins.
Acknowledgments
We thank Thomas Brüser for providing pABS-tatABC, George Georgiou for providing pBADdsbC and pBADΔssdsbC, and Elisabeth Schwarz for providing purified vtPA.
Funding was provided by NIH grant 1R41AI063709-01 (to M.P.D. and L.A.H.) and by a NYSTAR James D. Watson Investigator Award (to M.P.D.).
REFERENCES
- 1.Alami, M., I. Luke, S. Deitermann, G. Eisner, H. G. Koch, J. Brunner, and M. Muller. 2003. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell 12:937-946. [DOI] [PubMed] [Google Scholar]
- 2.Alami, M., D. Trescher, L. F. Wu, and M. Muller. 2002. Separate analysis of twin-arginine translocation (Tat)-specific membrane binding and translocation in Escherichia coli. J. Biol. Chem. 277:20499-20503. [DOI] [PubMed] [Google Scholar]
- 3.Baneyx, F., and M. Mujacic. 2004. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22:1399-1408. [DOI] [PubMed] [Google Scholar]
- 4.Berges, H., E. Joseph-Liauzun, and O. Fayet. 1996. Combined effects of the signal sequence and the major chaperone proteins on the export of human cytokines in Escherichia coli. Appl. Environ. Microbiol. 62:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkmen, M., D. Boyd, and J. Beckwith. 2005. The nonconsecutive disulfide bond of Escherichia coli phytase (AppA) renders it dependent on the protein-disulfide isomerase, DsbC. J. Biol. Chem. 280:11387-11394. [DOI] [PubMed] [Google Scholar]
- 6.Berks, B. C., F. Sargent, and T. Palmer. 2000. The Tat protein export pathway. Mol. Microbiol. 35:260-274. [DOI] [PubMed] [Google Scholar]
- 7.Berthelmann, F., and T. Bruser. 2004. Localization of the Tat translocon components in Escherichia coli. FEBS Lett. 569:82-88. [DOI] [PubMed] [Google Scholar]
- 8.Bessette, P. H., F. Aslund, J. Beckwith, and G. Georgiou. 1999. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc. Natl. Acad. Sci. USA 96:13703-13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bessette, P. H., J. Qiu, J. C. Bardwell, J. R. Swartz, and G. Georgiou. 2001. Effect of sequences of the active-site dipeptides of DsbA and DsbC on in vivo folding of multidisulfide proteins in Escherichia coli. J. Bacteriol. 183:980-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogsch, E. G., F. Sargent, N. R. Stanley, B. C. Berks, C. Robinson, and T. Palmer. 1998. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J. Biol. Chem. 273:18003-18006. [DOI] [PubMed] [Google Scholar]
- 11.Chun, S. Y., S. Strobel, P. Bassford, Jr., and L. L. Randall. 1993. Folding of maltose-binding protein. Evidence for the identity of the rate-determining step in vivo and in vitro. J. Biol. Chem. 268:20855-20862. [PubMed] [Google Scholar]
- 12.Collet, J. F., and J. C. Bardwell. 2002. Oxidative protein folding in bacteria. Mol. Microbiol. 44:1-8. [DOI] [PubMed] [Google Scholar]
- 13.DeLisa, M. P., D. Tullman, and G. Georgiou. 2003. Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc. Natl. Acad. Sci. USA 100:6115-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derman, A. I., and J. Beckwith. 1991. Escherichia coli alkaline phosphatase fails to acquire disulfide bonds when retained in the cytoplasm. J. Bacteriol. 173:7719-7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derman, A. I., and J. Beckwith. 1995. Escherichia coli alkaline phosphatase localized to the cytoplasm slowly acquires enzymatic activity in cells whose growth has been suspended: a caution for gene fusion studies. J. Bacteriol. 177:3764-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derman, A. I., W. A. Prinz, D. Belin, and J. Beckwith. 1993. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science 262:1744-1747. [DOI] [PubMed] [Google Scholar]
- 17.Driessen, A. J., E. H. Manting, and C. van der Does. 2001. The structural basis of protein targeting and translocation in bacteria. Nat. Struct. Biol. 8:492-498. [DOI] [PubMed] [Google Scholar]
- 18.Fisher, A. C., and M. P. DeLisa. 2004. A little help from my friends: quality control of presecretory proteins in bacteria. J. Bacteriol. 186:7467-7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgiou, G., and P. Valax. 1996. Expression of correctly folded proteins in Escherichia coli. Curr. Opin. Biotechnol. 7:190-197. [DOI] [PubMed] [Google Scholar]
- 20.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayhurst, A., and G. Georgiou. 2001. High-throughput antibody isolation. Curr. Opin. Chem. Biol. 5:683-689. [DOI] [PubMed] [Google Scholar]
- 22.Hynds, P. J., D. Robinson, and C. Robinson. 1998. The sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J. Biol. Chem. 273:34868-34874. [DOI] [PubMed] [Google Scholar]
- 23.Jack, R. L., G. Buchanan, A. Dubini, K. Hatzixanthis, T. Palmer, and F. Sargent. 2004. Coordinating assembly and export of complex bacterial proteins. EMBO J. 23:3962-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jongbloed, J. D., U. Grieger, H. Antelmann, M. Hecker, R. Nijland, S. Bron, and J. M. van Dijl. 2004. Two minimal Tat translocases in Bacillus. Mol. Microbiol. 54:1319-1325. [DOI] [PubMed] [Google Scholar]
- 25.Jurado, P., D. Ritz, J. Beckwith, V. de Lorenzo, and L. A. Fernandez. 2002. Production of functional single-chain Fv antibodies in the cytoplasm of Escherichia coli. J. Mol. Biol. 320:1-10. [DOI] [PubMed] [Google Scholar]
- 26.Kang, D. G., G. B. Lim, and H. J. Cha. 2005. Functional periplasmic secretion of organophosphorous hydrolase using the twin-arginine translocation pathway in Escherichia coli. J. Biotechnol. 118:379-385. [DOI] [PubMed] [Google Scholar]
- 27.Kurokawa, Y., H. Yanagi, and T. Yura. 2001. Overproduction of bacterial protein disulfide isomerase (DsbC) and its modulator (DsbD) markedly enhances periplasmic production of human nerve growth factor in Escherichia coli. J. Biol. Chem. 276:14393-14399. [DOI] [PubMed] [Google Scholar]
- 28.Levy, R., R. Weiss, G. Chen, B. L. Iverson, and G. Georgiou. 2001. Production of correctly folded Fab antibody fragment in the cytoplasm of Escherichia coli trxB gor mutants via the coexpression of molecular chaperones. Protein Expr. Purif. 23:338-347. [DOI] [PubMed] [Google Scholar]
- 29.Lilie, H., R. Rudolph, and J. Buchner. 1995. Association of antibody chains at different stages of folding: prolyl isomerization occurs after formation of quaternary structure. J. Mol. Biol. 248:190-201. [DOI] [PubMed] [Google Scholar]
- 30.Lutz, S., W. Fast, and S. J. Benkovic. 2002. A universal, vector-based system for nucleic acid reading-frame selection. Protein Eng. 15:1025-1030. [DOI] [PubMed] [Google Scholar]
- 31.Oresnik, I. J., C. L. Ladner, and R. J. Turner. 2001. Identification of a twin-arginine leader-binding protein. Mol. Microbiol. 40:323-331. [DOI] [PubMed] [Google Scholar]
- 32.Paschke, M., and W. Hohne. 2005. A twin-arginine translocation (Tat)-mediated phage display system. Gene 350:79-88. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Perez, J., G. Marquez, J. L. Barbero, and J. Gutierrez. 1994. Increasing the efficiency of protein export in Escherichia coli. Bio/Technology 12:178-180. [DOI] [PubMed] [Google Scholar]
- 34.Prinz, W. A., F. Aslund, A. Holmgren, and J. Beckwith. 1997. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J. Biol. Chem. 272:15661-15667. [DOI] [PubMed] [Google Scholar]
- 35.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Punginelli, C., B. Ize, N. R. Stanley, V. Stewart, G. Sawers, B. C. Berks, and T. Palmer. 2004. mRNA secondary structure modulates translation of Tat-dependent formate dehydrogenase N. J. Bacteriol. 186:6311-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu, J., J. R. Swartz, and G. Georgiou. 1998. Expression of active human tissue-type plasminogen activator in Escherichia coli. Appl. Environ. Microbiol. 64:4891-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritz, D., and J. Beckwith. 2001. Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol. 55:21-48. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigue, A., A. Chanal, K. Beck, M. Muller, and L. F. Wu. 1999. Co-translocation of a periplasmic enzyme complex by a hitchhiker mechanism through the bacterial Tat pathway. J. Biol. Chem. 274:13223-13228. [DOI] [PubMed] [Google Scholar]
- 40.San Miguel, M., R. Marrington, P. M. Rodger, A. Rodger, and C. Robinson. 2003. An Escherichia coli twin-arginine signal peptide switches between helical and unstructured conformations depending on the hydrophobicity of the environment. Eur. J. Biochem. 270:3345-3352. [DOI] [PubMed] [Google Scholar]
- 41.Sanders, C., N. Wethkamp, and H. Lill. 2001. Transport of cytochrome c derivatives by the bacterial Tat protein translocation system. Mol. Microbiol. 41:241-246. [DOI] [PubMed] [Google Scholar]
- 42.Santini, C. L., B. Ize, A. Chanal, M. Muller, G. Giordano, and L. F. Wu. 1998. A novel sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaerlaekens, K., E. Lammertyn, N. Geukens, S. De Keersmaeker, J. Anne, and L. Van Mellaert. 2004. Comparison of the Sec and Tat secretion pathways for heterologous protein production by Streptomyces lividans. J. Biotechnol. 112:279-288. [DOI] [PubMed] [Google Scholar]
- 44.Schaffner, J., J. Winter, R. Rudolph, and E. Schwarz. 2001. Cosecretion of chaperones and low-molecular-size medium additives increases the yield of recombinant disulfide-bridged proteins. Appl. Environ. Microbiol. 67:3994-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Settles, A. M., A. Yonetani, A. Baron, D. R. Bush, K. Cline, and R. Martienssen. 1997. Sec-independent protein translocation by the maize Hcf106 protein. Science 278:1467-1470. [DOI] [PubMed] [Google Scholar]
- 46.Stanley, N. R., F. Sargent, G. Buchanan, J. Shi, V. Stewart, T. Palmer, and B. C. Berks. 2002. Behaviour of topological marker proteins targeted to the Tat protein transport pathway. Mol. Microbiol. 43:1005-1021. [DOI] [PubMed] [Google Scholar]
- 47.Swartz, J. R. 2001. Advances in Escherichia coli production of therapeutic proteins. Curr. Opin. Biotechnol. 12:195-201. [DOI] [PubMed] [Google Scholar]
- 48.Venturi, M., C. Seifert, and C. Hunte. 2002. High level production of functional antibody Fab fragments in an oxidizing bacterial cytoplasm. J. Mol. Biol. 315:1-8. [DOI] [PubMed] [Google Scholar]
- 49.Weiner, J. H., P. T. Bilous, G. M. Shaw, S. P. Lubitz, L. Frost, G. H. Thomas, J. A. Cole, and R. J. Turner. 1998. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell 93:93-101. [DOI] [PubMed] [Google Scholar]
- 50.Wulfing, C., and A. Pluckthun. 1994. Protein folding in the periplasm of Escherichia coli. Mol. Microbiol. 12:685-692. [DOI] [PubMed] [Google Scholar]