Abstract
Exocytosis in Saccharomyces cerevisiae requires the specific interaction between the plasma membrane t-SNARE complex (Sso1/2p;Sec9p)and a vesicular v-SNARE (Snc1/2p). While SNARE proteins drive membrane fusion, many aspects of SNARE assembly and regulation are ill defined. Plasma membrane syntaxin homologs (including Sso1p) contain a highly charged juxtamembrane region between the transmembrane helix and the “SNARE domain” or core complex domain. We examined this region in vitro and in vivo by targeted sequence modification, including insertions and replacements. These modified Sso1 proteins were expressed as the sole copy of Sso in S. cerevisiae and examined for viability. We found that mutant Sso1 proteins with insertions or duplications show limited function, whereas replacement of as few as three amino acids preceding the transmembrane domain resulted in a nonfunctional SNARE in vivo. Viability is also maintained when two proline residues are inserted in the juxtamembrane of Sso1p, suggesting that helical continuity between the transmembrane domain and the core coiled-coil domain is not absolutely required. Analysis of these mutations in vitro utilizing a reconstituted fusion assay illustrates that the mutant Sso1 proteins are only moderately impaired in fusion. These results suggest that the sequence of the juxtamembrane region of Sso1p is vital for function in vivo, independent of the ability of these proteins to direct membrane fusion.
Biological membrane fusion is imperative for cellular survival. Membrane fusion requires an energy-dependent reaction to overcome the repulsive nature of two opposing membranes (4, 10, 11). In cells, conformational rearrangements of specific fusion proteins drive this membrane merger (9, 44). Membrane fusion reactions in the secretory pathway have been extensively studied, and much is known about the molecular machinery, and yet many aspects of this process are not well understood. SNARE proteins (Soluble NSF attachment protein receptors) constitute the core fusion machinery (38, 49) and are the final arbiters of fusion specificity (23). SNAREs are operationally divided into two groups: those that are found primarily on the transport vesicle, called v-SNAREs, and those found primarily on the target membrane, called t-SNAREs. The founding members of the SNARE superfamily were identified from bovine brain and participate in synaptic transmission (38). The neuronal SNARE complex is comprised of two t-SNAREs localized to the plasma membrane, called syntaxin 1A (3) and SNAP25 (synaptosome-associated protein of 25 kDa [28]), as well as one v-SNARE located on the synaptic vesicle, known as VAMP (vesicle-associated membrane protein or synaptobrevin [40, 43]).
SNARE proteins also share structural and mechanistic commonalities with viral fusogens, such as the assembly of a coiled-coil bundle structure and a hemifusion transition-state intermediate (15, 22, 36, 39, 51). However, several elements that regulate membrane fusion, including proteins such as Rab GTPases and the exocyst complex, remain incompletely understood (27, 30, 31, 52). Similarly, the Sec1/Munc18 family of SNARE regulators has a variety of proposed functions, including direct stimulation of fusion through interactions with the yeast plasma membrane t-SNARE complex (18, 42).
The fusion of Golgi-derived transport vesicles with the plasma membrane requires the formation of a binary t-SNARE complex on the plasma membrane comprised of a syntaxin family member and the respective SNAP25 homolog. Complete membrane fusion occurs when a VAMP-like v-SNARE found on the transport vesicle associates with the t-SNARE complex to form a ternary SNARE complex. This association is thought to occur in a “zipper”-like fashion that pulls the membranes close together (7, 16, 17). The structure of the cytosolic portion of the four-stranded neuronal core complex shows that the proteins align in a parallel fashion with the transmembrane domains of both syntaxin 1A and VAMP emerging from the same end of the helical bundle (32, 41).
Membrane fusion of Golgi-derived transport vesicles with the plasma membrane in yeast utilizes homologous SNARE proteins. The plasma membrane t-SNARE complex is composed of the syntaxin 1A homolog Sso1p (1) or the functionally redundant Sso2p and the SNAP25-like Sec9p (6). The v-SNARE Snc1p (14) or the functionally redundant Snc2p completes the four-helix bundle.
The majority of SNARE proteins have a membrane-spanning region at their extreme carboxy terminus with a nearby core complex domain that has the propensity to form coiled coils. A common feature conserved in many if not all plasma membrane syntaxins is a polybasic juxtamembrane region between the core complex domain and the transmembrane domain (Fig. 1B) (50). Similar stretches of charge are also seen in plasma membrane v-SNAREs. Electron paramagnetic resonance measurements suggest that the juxtamembrane regions of the neuronal SNAREs syntaxin 1A and VAMP interact with and are embedded in the phospholipid bilayer (20, 21).
FIG. 1.
Domain structure and sequence of Sso1p, Sso1p juxtamembrane mutations, and other plasma membrane syntaxins. A. The general domain structure of Sso1p is depicted. HA, HB, and HC refer to predicted coiled-coil domains that form an autonomously folding N-terminal regulatory domain. Mutations were introduced into the primary sequence of Sso1p by engineering a parent construct that contains a unique restriction site for insertions by double-stranded oligonucleotides (1x linker and 3x linker) or overlap PCR (6AA replaced, 6AA insertion, and 2x juxtamembrane). The linker constructs (6AA replaced, 6AA insertion, 1x linker, and 3x linker Sso1p) contain glycine-glycine-serine repeats. The 2x juxtamembrane construct duplicates 12 endogenous amino acids in the juxtamembrane region. Single, double, and triple point mutations were introduced by overlap PCR at the indicated residues. B. Sequence alignment of the juxtamembrane region of syntaxins from various species. Sc, Saccharomyces cerevisiae; Cn, Cryptococcus neoformans; Um, Ustilago maydis; Ag, Ashbya gossypii; Ca, Candida albicans; Sp, Schizosaccharomyces pombe; Ce, Caenorhabditis elegans; Hs, Homo sapiens; Ma, Macaca mulatta; Bt, Bos tarus; Rn, Rattus norvegicus; Mm, Mus musculus; Lo, Loligo pealei; Lp, Limulus polyphemus; Ls, Lymnaea stagnalis; St, Strongylocentrotus purpuratus. Numbering is relative to the S. cerevisiae Sso1p sequence.
Previous in vitro studies have shown that additional sequence in the juxtamembrane of the neuronal t-SNARE syntaxin 1A and the v-SNARE VAMP is permitted, although the amount of fusion progressively decreases as the distance between the core complex domain and the transmembrane region increases (24). The introduction of sequential helix-breaking proline residues in the juxtamembrane region of syntaxin 1A reduced fusion ∼50% in a liposome mixing assay; however, the same insertion in VAMP2 did not significantly impair fusion in vitro (24). While this study examined the in vitro function of juxtamembrane linker mutants, no in vivo analysis was possible.
Saccharomyces cerevisiae is amenable to both in vitro and in vivo studies, allowing mechanistic models developed in vitro to be directly tested in vivo. The present work correlates in vivo mutational analysis of Sso1p function with in vitro fusion activity to examine the function of the juxtamembrane region of Sso1p. Modifications introducing distance and flexibility were used to test bilayer-coupling models of fusion in a reconstituted fusion assay, similar to prior work performed with neuronal SNAREs, and to examine the effects of the same mutations on in vivo SNARE function. Additional mutations examined the specific sequence requirements of the juxtamembrane region necessary to maintain in vivo function.
MATERIALS AND METHODS
Reagents.
All lipids were purchased from Avanti Polar lipids, detergents were bought from Calbiochem (n-octyl β-glucopyranoside) and Fisher Scientific (Triton X-100), 5-fluoroorotic acid (5-FOA) was from Zymo Research, and G418 sulfate was from GIBCO. Bacterial and yeast media components including yeast extract-peptone-dextrose, synthetic complete media, raffinose, and amino acid and nucleotide supplements were obtained from Qbiogene, while yeast nitrogen base was from Difco, Bacto agar from BD Bioscience, and the carbon sources glucose and galactose were from Fisher. Restriction endonucleases were purchased from New England Biolabs, Tgo polymerase from Roche, and oligonucleotides from Integrated DNA Technologies. The monoclonal antihemagglutinin (HA) 16B12 antibody was purchased from Covance. Anti-Sso1/2p antiserum (rabbit R3174) was described previously (37). Secondary antibodies included goat antimouse immunoglobulin G (IgG) horseradish peroxidase from Rockland Immunochemicals, goat antirabbit IgG Fc horseradish peroxidase from Pierce, and Alexafluor 488 goat antimouse IgG from Molecular Probes.
Strains.
JMY128 was described previously (47). All other strains in this study are derivatives of JMY128 with an additional plasmid transformed into the yeast (Table 1).
TABLE 1.
Yeast strains
| Strain name | Genotype | Source (reference) |
|---|---|---|
| JMY128 | MATaade2-1 leu2-3,112 ura3-1 trp1-1 his3-11,15 can1-100 sso1::KanMX2 sso2-d1::LEU2 [pJM198] | Van Komen et al., 2005 (47) |
| JMY303 | JMY128(pJM198)(pJM290) | Van Komen et al., 2005 (47) |
| JMY367 | JMY128(pJM198)(pJM251) | This study |
| JMY368 | JMY128(pJM198)(pJM252) | This study |
| JMY369 | JMY128(pJM198)(pJM253) | This study |
| JMY370 | JMY128(pJM198)(pJM210) | This study |
| JMY371 | JMY128(pJM198)(pJM299) | This study |
| JMY372 | JMY128(pJM198)(pJM377) | This study |
| JMY373 | JMY128(pJM198)(pJM371) | This study |
| JMY374 | JMY128(pJM198)(pJM374) | This study |
| JMY375 | JMY128(pJM198)(pJM314) | This study |
| JMY376 | JMY128(pJM198)(pJM431) | This study |
| JMY377 | JMY128(pJM198)(pJM432) | This study |
| JMY378 | JMY128(pJM198)(pJM433) | This study |
| JMY379 | JMY128(pJM198)(pJM434) | This study |
| JMY380 | JMY128(pJM198)(pJM394) | This study |
| JMY381 | JMY128(pJM198)(pJM395) | This study |
| JMY382 | JMY128(pJM198)(pJM396) | This study |
| JMY383 | JMY128(pJM198)(pJM436) | This study |
| JMY384 | JMY128(pJM198)(pJM355) | This study |
| JMY385 | JMY128(pJM198)(pJM437) | This study |
| JMY386 | JMY128(pJM198)(pJM358) | This study |
| JMY387 | JMY128(pJM198)(pJM444) | This study |
| JMY388 | JMY128(pJM198)(pJM357) | This study |
| JMY389 | JMY128(pJM198)(pJM356) | This study |
| JMY390 | JMY128(pJM198)(pJM443) | This study |
| JMY391 | JMY128(pJM198)(pJM384) | This study |
| JMY392 | JMY128(pJM198)(pJM385) | This study |
| JMY393 | JMY128(pJM198)(pJM359) | This study |
| JMY394 | JMY128(pJM198)(pJM448) | This study |
| JMY395 | JMY128(pJM198)(pJM445) | This study |
| JMY396 | JMY128(pJM198)(pJM446) | This study |
| JMY397 | JMY128(pJM198)(pJM447) | This study |
| JMY398 | JMY128(pJM198)(pJM438) | This study |
| JMY399 | JMY128(pJM198)(pJM439) | This study |
| JMY400 | JMY128(pJM198)(pJM440) | This study |
| JMY401 | JMY128(pJM198)(pJM441) | This study |
| JMY402 | JMY128(pJM198)(pJM424) | This study |
Plasmids.
Plasmids used in this study are listed in Table 2. Details regarding the constructions of plasmids are presented in the supplemental material.
TABLE 2.
SNARE constructs
| Plasmid | Protein | Vector | Origin | Marker | Promoter |
|---|---|---|---|---|---|
| pJM88 | His8-Sso1p | pET24 | ColE1 | Kan | T7 |
| BB442 | GST-Sec9c | pGEX 2T | ColE1 | AMP | Ptac |
| pJM90 | Snc1p-His6 | pET28a | ColE1 | Kan | T7 |
| pJM198 | Sso1p | pRS316 | CEN6 | URA3 | SSO1 |
| pJM210 | Sso1p-2x juxtamembrane-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM251 | Sso1p-KL-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM252 | Sso1p-3x linker-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM253 | Sso1p-1x linker-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM280 | ΔNRD-Sso1p-KL-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM281 | ΔNRD-Sso1p-3x linker-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM282 | ΔNRD-Sso1p-1x linker-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM283 | ΔNRD-Sso1p-KLGPP-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM286 | H8-Sso1p-KL-HA | pET24a | ColE1 | Kan | T7 |
| pJM287 | H8-Sso1p-3x linker-HA | pET24a | ColE1 | Kan | T7 |
| pJM288 | H8-Sso1p-1x linker-HA | pET24a | ColE1 | Kan | T7 |
| pJM289 | H8-Sso1p-2x juxtamembrane-HA | pET24a | ColE1 | Kan | T7 |
| pJM290 | Sso1p-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM299 | Sso1-6AA replaced-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM314 | Sso1p-6AA replaced-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM355 | Sso1p-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM356 | Sso1p-2x juxtamembrane-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM357 | Sso1p-1x linker-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM358 | Sso1p-6AA insertion-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM359 | Sso1p-6AA replaced-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM360 | Sso1p-6AA replaced-HA | pET24a | ColE1 | Kan | T7 |
| pJM371 | Sso1p-N262G, K263G, I264S-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM374 | Sso1p-A259G, R260G, K261S-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM377 | Sso1p-KLGPP-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM378 | Sso1p-KLGPP-HA | pET24a | ColE1 | Kan | T7 |
| pJM384 | Sso1p-N262G, K263G, I264R-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM385 | Sso1p-A259G, R260G, K261S-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM394 | Sso1p-R260G-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM395 | Sso1p-K261G-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM396 | Sso1p-K263G-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM431 | Sso1p-R260G, K261G-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM432 | Sso1p-R260G, K263G HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM433 | Sso1p-R261G, K263G-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM434 | Sso1p-R260G, K261G, K263G-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM436 | Sso1p-A259G-HA | pYX223 | 2 μm | HIS3 | GAL1-10 |
| pJM437 | Sso1p-KL-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM438 | Sso1p-R260G, K261G-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM439 | Sso1p-R260G, K263G HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM440 | Sso1p-K261G, K263G-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM441 | Sso1p-R260G, K261G, K263G-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM443 | Sso1p-3x linker-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM444 | Sso1p-KLGPP-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM445 | Sso1p-R260G-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM446 | Sso1p-K261G-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM447 | Sso1p-K263G-HA | pRS424 | 2 μm | TRP1 | SSO1 |
| pJM448 | Sso1p-A259G-HA | pRS424 | 2 μm | TRP1 | SSO1 |
5-FOA counterselection.
The transformed JMY128 shuffle strain was grown in synthetic complete media minus histidine and uracil with 2% galactose at 30°C for 2 days, followed by a subsequent back dilution and another overnight growth. Cells (optical density at 600 nm = 10) were spun down and resuspended in 1 ml sterile water. A threefold serial dilution with 20 μl spots was plated onto synthetic complete media minus histidine plates containing 2% galactose and 1 g/liter 5-fluoroorotic acid and incubated for 3 days at 30°C.
Microscopy.
Microscopy was performed as described previously (34) with the exception that the strains were grown in synthetic complete media with galactose for expression of the mutant Sso1 proteins.
Protein production.
All H8-Sso1p proteins were expressed in 4 liters of Super Broth media and induced with 1 mM isopropyl-β-d-thiogalactopyranoside at 30°C for 4 h. Protein was purified by nickel chelate chromatography as described previously (23). His8-Sso1p (pJM88), Snc1p-His6 (pJM90), and glutathione S-transferase (GST)-Sec9c (BB442) were expressed and purified as described previously (23).
Reconstitution into liposomes and fusion assays.
All proteoliposomes were prepared by detergent dilution and dialysis and used in standard fusion assays as described previously (35).
Western blotting of whole-cell extracts.
Total cell extracts were made by glass bead lysis of trichloroacetic acid-killed cells. The amount of total cell extract indicated in the figure legends was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and probed with anti-HA antibodies. Primary antibodies were at a 1:1,000 dilution (anti-HA or anti-Sso1/2p). The horseradish peroxidase-conjugated secondary antibodies were used at a 1:10,000 dilution. Immunoblots were developed using ECL detection (Amersham).
RESULTS
Helical continuity between the SNARE core helix and the transmembrane domain may be beneficial but is not required in vivo.
Previous work has shown that increasing the physical distance between the SNARE transmembrane domain and the core helical bundle progressively inhibits membrane fusion in vitro (24). However, this work also demonstrated that substantial distance, as well as increased flexibility, is largely tolerated. While these results confirm that helical continuity and strict coupling of the SNARE core helix and transmembrane domain are not required in vitro, the in vivo implications were untested. We now examined specific mutations in the juxtamembrane domain of the yeast plasma membrane syntaxin, Sso1p, to determine the in vivo requirements for mechanical coupling (Fig. 1). We began by addressing the requirement for helical continuity in vivo. A haploid yeast strain that contains a genomic deletion in both the SSO1 and SSO2 genes was constructed for use as a plasmid shuffle strain (JMY128). Disruption of both SSO loci is required, since these genes form a functionally redundant pair. The viability of this strain is maintained by a low-copy URA3 plasmid expressing Sso1p under the control of its endogenous promoter (pJM198) (47). The mutant Sso1p plasmids containing modifications to their juxtamembrane domain were transformed into the plasmid shuffle strain and maintained on glucose. The functionality of the mutant plasmid is determined by growth on plates containing the drug 5-FOA. This drug is metabolized to a toxic intermediate in cells that express the URA3 gene product, thereby counterselecting for this marker (5). Since wild-type Sso1p on the URA3 plasmid is required for viability, only coexpressing plasmids that produce functional Sso1p will survive in the presence of 5-FOA. An initial construct with two specific point mutations at the membrane delimiting charge (R265K and C266L) was made for cloning purposes. At both high and low expression levels, these two mutations have no observable in vivo defect, with the mutant protein behaving like wild-type Sso1p (Fig. 2A).
FIG. 2.
Conversion of R265 and C266 to KL does not affect Sso1p function. A. Growth on 5-FOA. Left: threefold serial dilutions of JMY303 (Sso1p-HA) or JMY367 (Sso1p-KL-HA) were spotted onto synthetic complete media with 2% galactose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. Right: threefold serial dilutions of JMY384 (Sso1p-HA) or JMY385 (Sso1p-KL-HA) were spotted onto synthetic complete media with 2% glucose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. B. Kinetic fusion assay comparing different acceptor t-SNARE liposomes containing t-SNARE complexes composed of GST-Sec9c and the indicated Sso1 protein, H8-Sso1p (pJM88) or H8-Sso1p-KL-HA (pJM286). t-SNARE liposomes (45 μl) were mixed with fluorescent donor v-SNARE liposomes containing Snc1p (5 μl), and NBD fluorescence was monitored in a fluorescent plate reader for 2 h. C. Coomassie blue-stained gel of the liposomes used in panel B, indicating that very similar amounts of various Sso1p mutants were reconstituted.
We next addressed the potential requirement for helical continuity between the SNARE core domain and the transmembrane segment in vivo by introducing helix-breaking tandem proline residues. Figure 3A shows that Sso1p interrupted with the sequence KLGPP added to the cytosolic side of the transmembrane domain is capable of providing Sso1p function in vivo when overexpressed under the control of the strong GAL1-10 promoter (Fig. 3A, left). Yeast expressing the KLGPP insertion yield a similar number of colonies on 5-FOA medium, but the colony size is much smaller, suggesting impaired growth. Somewhat surprisingly, growth is not seen when the KLGPP insertion is expressed at lower levels closer to endogenous Sso1p (Fig. 3A, right). These results suggest that while helical continuity may be beneficial in vivo, it is not absolutely required, since the insertion of two helix-breaking proline residues in the juxtamembrane region can provide Sso1p function when overexpressed.
FIG. 3.
Effects of insertions in the Sso1p juxtamembrane in vivo. Constructs containing Sso1p insertion mutations described in Fig. 1 were assayed for in vivo function by plasmid shuffling. A. Growth on 5-FOA. Left: threefold serial dilutions of JMY303 (Sso1p-HA), JMY372 (Sso1p-KLGGP-HA), JMY371 (Sso1p-6AA insertion-HA), JMY369 (Sso1p-1x linker-HA), JMY370 (Sso1p-2x juxtamembrane-HA), JMY368 (Sso1p-3x linker-HA), or JMY305 (Sso1pΔNRD) were spotted onto synthetic complete media with 2% galactose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. Right: threefold serial dilutions of JMY384 (Sso1p-HA), JMY387 (Sso1p-KLGGP-HA), JMY386 (Sso1p-6AA insertion-HA), JMY388 (Sso1p-1x linker-HA), JMY389 (Sso1p-2x juxtamembrane-HA), JMY390 (Sso1p-3x linker-HA), or JMY402 (empty vector) were spotted onto synthetic complete media with 2% glucose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. B. Plasma membrane localization. Differential interference contrast (DIC) images and indirect immunofluorescence images are shown for JMY303 (Sso1p-HA), JMY371 (Sso1p-6AA insertion-HA), JMY369 (Sso1p-1x linker-HA), and JMY370 (Sso1p-2x juxtamembrane-HA). Localization was determined by staining with an anti-HA antibody. Scale bar equals 5 μm. C. Expression. Whole-cell extracts of JMY303 (Sso1p-HA), JMY372 (Sso1p-KLGGP-HA), JMY371 (Sso1p-6AA insertion-HA), JMY369 (Sso1p-1x linker-HA), JMY370 (Sso1p-2x juxtamembrane-HA), JMY368 (Sso1p-3x linker-HA), JMY384 (Sso1p-HA), JMY387 (Sso1p-KLGGP-HA), JMY386 (Sso1p-6AA insertion-HA), JMY388 (Sso1p-1x linker-HA), JMY389 (Sso1p-2x juxtamembrane-HA), and JMY390 (Sso1p-3x linker-HA) were resolved by SDS-PAGE on a 4 to 10% bis-Tris NuPAGE gel and blotted with an anti-HA antibody (∼16 μg total protein per lane or ∼83 μg total protein per lane). Five times the amount of extract was used for strains expressing Sso1p from the CEN plasmids. D. Relative Sso1p expression. A whole-cell extract of JMY384 (Sso1p-HA) was resolved by SDS-PAGE on a 4 to 10% bis-Tris NuPAGE gel and blotted with an anti-Sso antibody (∼30 μg total protein).
Moderate increases in distance and flexibility are tolerated in vivo.
In contrast to the KLGPP mutant, insertion of six amino acids consisting of GGSGGS is fully acceptable in vivo. Figure 3A demonstrates that while the insertion of the helix-breaking proline residues is only moderately tolerated when overexpressed, the GGSGGS insertion, which is one additional amino acid longer, is well tolerated when overexpressed (Fig. 3A, left) and, more importantly, at lower expression levels (Fig. 3A,right). Further extension of the flexible linker by an additional 5 amino acids (11 in total; 1x linker in Fig. 3) can replace Sso1 when overexpressed (Fig. 3A, left) but cannot provide enough Sso1p function when expressed at roughly endogenous levels (Fig. 3A, right). When we increased the distance between the SNARE core helix and the transmembrane domain by a simple duplication of the endogenous juxtamembrane domain, a different result was seen. In this context, a similar length extension of 12 amino acids, but a different sequence composition, yielded marginal growth when overexpressed but no growth at near-endogenous expression. Finally, an even longer insertion of 33 amino acids (3x linker) is virtually dead under all expression conditions. The limited function or lack of function of the 1x linker and 2x juxtamembrane mutants is not due to inappropriate expression or localization. Fluorescence microscopy (Fig. 3B) and immunoblot analysis (Fig. 3C) with an anti-HA antibody confirm that the mutant Sso1 proteins are all well expressed and properly localized to the yeast plasma membrane. Controls using an anti-Sso1/2p antibody, no HA-tagged protein, or no primary antibody indicate that the detected fluorescence is specifically Sso1p (data not shown). Furthermore,immunofluorescence microscopy without cell permeabilization confirms correct topology (data not shown). The degree of overexpression seen with the GAL1-10uas was only about five times greater that with SSO1uas (Fig. 3C). Figure 3D illustrates the amount of Sso1p-HA expressed from the endogenous SSO1uas on a centromeric plasmid relative to both endogenous Sso1p and Sso2p for purposes of comparison.
Taken together, these results suggest that while helical continuity is not required when Sso1p is overexpressed, it may be beneficial in vivo. Furthermore, increased distance and flexibility are somewhat tolerated in vivo, but the specific sequence of the inserted linker also plays a role.
Requirements for Sso1 function in vivo are more stringent than for in vitro fusion.
Next, we examined the behavior of the insertion mutant Sso1p proteins, employing a reconstituted in vitro fusion assay. This assay utilizes recombinant SNARE proteins purified from Escherichia coli and proteoliposomes formed by detergent dilution and dialysis with synthetic phospholipids (35, 49). The v-SNARE liposome population contains two fluorescently labeled lipids that are used to monitor membrane fusion by the loss of fluorescence resonance energy transfer as fusion with nonfluorescent t-SNARE proteoliposomes occurs. Similar to results in the in vivo studies (Fig. 2A), introduction of two point mutations in Sso1p (R265K and C266L) has no effect on fusion efficiency. Neither the presence of the HA tag nor the KL mutation affects membrane fusion in vitro (Fig. 2B and C; also data not shown). Figure 4 documents the performance of the insertion mutants tested in vitro when expressed and purified from E. coli and reconstituted into POPC/DOPS liposomes at approximately equivalent concentrations (35). In contrast to the in vivo setting, mutant Sso1p that contains the KLGPP insertion is only marginally impaired (∼23%) compared with wild-type Sso1p in vitro (Fig. 4A, open circles versus filled circles). As the core helical domain is extended further away from the transmembrane domain by sequential addition of flexible amino acids, progressively diminished fusion is observed (Fig. 4A). These results are qualitatively similar to those with mutations in syntaxin 1A (24). The 3x linker and the 2x juxtamembrane mutants are the most impaired in vitro, which corresponds well with the in vivo results.
FIG. 4.
In vitro fusion reactions with recombinant Sso1p juxtamembrane insertions. A. Kinetic fusion assay comparing different acceptor t-SNARE liposomes containing t-SNARE complexes composed of GST-Sec9c and the indicated Sso1 protein. Each t-SNARE liposome population (45 μl) was mixed with fluorescent donor v-SNARE liposomes containing Snc1p (5 μl) and NBD fluorescence monitored in a fluorescent plate reader for 2 h. B. Coomassie blue-stained gel of the liposomes used in panel A, indicating that very similar amounts of various Sso1p mutants were reconstituted.
While the in vitro fusion assay provides unparalleled access to the constituent components of the fusion reaction, allowing for mechanistic dissection, comparison of the in vitro and in vivo results suggests that the in vitro assay does not fully recapitulate the in vivo environment. For example, the helix-breaking KLGPP mutant is moderately impaired in vitro and nonfunctional in vivo unless it is overexpressed, and even then it is not fully wild type. These results illustrate the benefit of combining in vitro and in vivo analysis. The behavior of the 2x juxtamembrane mutant relative to that of the 11-amino-acid 1x linker mutant also suggests that sequence determinants in this region may also be important in vivo. While the 1x linker mutant is clearly impaired in vitro (∼53% of wild type) (Fig. 4A,open diamonds), the similar-length 2x juxtamembrane mutant is essentially nonfunctional in vitro (Fig. 4A, filled diamonds). Close inspection of the 15 amino acids preceding the transmembrane domain of many syntaxin homologs shows a high conservation of amino acid sequence in this region, specifically the number and distribution of positively charged residues (Fig. 1B).
Specific sequence determinants are required in the juxtamembrane domain in vivo.
We next examined the sequence requirements of the juxtamembrane region by replacing the endogenous sequence rather than inserting amino acids. When the six-amino-acid sequence ARKNKI immediately preceding the transmembrane segment (Fig. 1) is replaced with the sequence GGSGGS, Sso1p is completely nonfunctional at any expression level (Fig. 5A). This result suggests that specific amino acids are required in the juxtamembrane domain in vivo, since this mutation produced a fully functional Sso1p protein in vitro (Fig. 5D). This six-amino-acid region was further refined by making two different three-amino-acid replacements. While the more C-terminal three amino acids (N262G, K263G, and I264S) were functional when overexpressed, these replacements were lethal at lower expression levels. The more N-terminal three amino acids (A259G, R260G, and K261S) were nonfunctional under all expression conditions. The in vivo deficit with the six-amino-acid replacement (as well as the ARK replacement) was not due to expression or localization, since similar amounts of these mutant proteins are made relative to the case with the functional N262G K263G I264S mutant (Fig. 5B). Additionally, the six-amino-acid replacement mutant protein was properly localized to the plasma membrane (Fig. 5C). It is unlikely that the in vivo deficiency is due to the inability to assemble SNARE complexes and drive membrane fusion, since a recombinant version of the GGSGGS insertion mutant protein repeatedly promoted membrane fusion at nearly wild-type levels, 84% ± 3% (n = 7), in vitro (Fig. 5D; also data not shown).
FIG. 5.
In vivo effects of Sso1p juxtamembrane amino acid replacements. A. Constructs containing Sso1p replacement mutations described in Fig. 1 were assayed for in vivo function by plasmid shuffling. Growth on 5-FOA. Left: threefold serial dilutions of JMY303 (Sso1p-HA), JMY373 (N262G, K263G, I264S-Sso1p-A-HA), JMY374 (A259G, R260G, K261S-Sso1p-HA), JMY375 (Sso1p-6AA replaced-HA), or JMY305 (Sso1pΔNRD) were spotted onto synthetic complete media with 2% galactose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. Right: threefold serial dilutions of JMY384 (Sso1p-HA), JMY391 (Sso1p-N262G, K263G, I264S-HA), JMY392 (Sso1p-A259G, R260G, K261S-HA), JMY393 (Sso1p-6AA replaced-HA), or JMY402 (vector) were spotted onto synthetic complete media with 2% glucose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. B. Expression. Whole-cell extracts of JMY303 (Sso1p-HA), JMY373 (Sso1p-N262G, K263G, I264S-HA), JMY374 (Sso1p-A259G, R260G, K261S-HA), JMY375 (Sso1p-6AA replaced-HA), JMY384 (Sso1p-HA), JMY391 (Sso1p-N262G, K263G, I264S-HA), JMY392 (Sso1p-A259G, R260G, K261S-HA), or JMY393 (Sso1p-6AA replaced-HA) were resolved by SDS-PAGE on a 4 to 10% bis-Tris NuPAGE gel and blotted with an anti-HA antibody (∼29 μg total protein per lane or ∼137 μg total protein per lane). Five times the amount of extract was used for strains expressing Sso1p from the CEN plasmids. C. Plasma membrane localization. Differential interference contrast (DIC) image and indirect immunofluorescence image of JMY375 (Sso1p-6AA replaced-HA). Localization was determined by staining with an anti-HA antibody. Scale bar equals 5 μm. D. Kinetic fusion assay comparing different acceptor t-SNARE liposomes containing t-SNARE complexes composed of GST-Sec9c and the indicated Sso1 protein. t-SNARE liposomes (45 μl) were mixed with fluorescent donor v-SNARE liposomes containing Snc1p (5 μl) and NBD fluorescence monitored in a fluorescent plate reader for 2 h. E. Coomassie blue-stained gel of the liposomes used in panel D, indicating that very similar amounts of various Sso1p mutants were reconstituted.
The polybasic character of the juxtamembrane region is required in vivo.
An interesting and conserved feature of these six juxtamembrane residues is the cluster of positive charge (Fig. 1B). In fact, lysine and arginine residues within the juxtamembrane region of rat syntaxin 1A (R262-K265) have been shown by electron paramagnetic resonance (EPR) spectroscopy to be embedded in the hydrophobic region of the phospholipid bilayer (20). This observation prompted us to focus on the charged residues within the six-amino-acid sequence. Figure 6 shows the functional effects of all possible single, double, and triple mutations of these charged residues. When overexpressed, none of the individual changes cause a growth defect, including the uncharged A259G mutation. Additionally, no growth defect was seen when these single mutants were expressed at closer to endogenous levels (Fig. 6A, right).
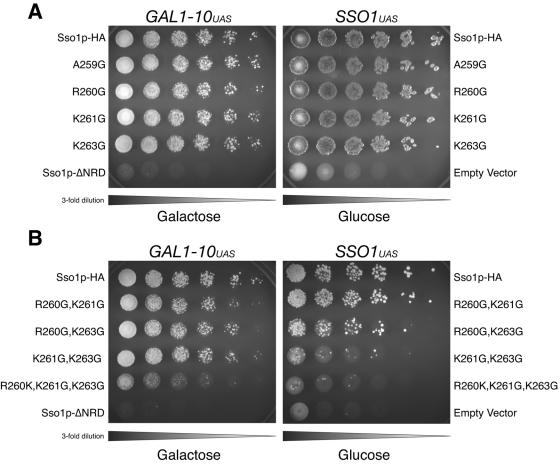
FIG. 6.
In vivo effects of single, double, and triple point mutations in the Sso1p juxtamembrane region. A. Growth on 5-FOA with single point mutation. Left: threefold serial dilutions of JMY303 (Sso1p-HA), JMY384 (Sso1p-A259G-HA), JMY380 (Sso1p-R260G-HA), JMY381 (Sso1p-K261G-HA), JMY382 (Sso1p-K263G-HA), or JMY305 (Sso1pΔNRD) were spotted onto synthetic complete media with 2% galactose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. Right: threefold serial dilutions of JMY384 (Sso1p-HA), JMY394 (Sso1p-A259G-HA), JMY395 (Sso1p-R260G-HA), JMY396 (Sso1p-K261G-HA), JMY397 (Sso1p-K263G-HA), or JMY402 (vector) were spotted onto synthetic complete media with 2% glucose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. B. Growth on 5-FOA with double and triple point mutations. Left: threefold serial dilutions of JMY303 (Sso1p-HA), JMY376 (Sso1p-R260G, K261G-HA), JMY377 (Sso1p-R260G, K263GHA), JMY378 (Sso1p-K261G, K263G-HA), JMY379 (Sso1p-R260G, K261G, K263G-HA), or JMY305 (Sso1pΔNRD) were spotted onto synthetic complete media with 2% galactose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h. Right: threefold serial dilutions of JMY384 (Sso1p-HA), JMY398 (Sso1p-R260G, K261G-HA), JMY399 (Sso1p-R260G, K263G-HA), JMY400 (Sso1p-K261G, K263G-HA), JMY401 (Sso1p-R260G, K261G, K263G-HA), or JMY402 (vector) were spotted onto synthetic complete media with 2% glucose containing 1 mg/ml 5-fluoroorotic acid and grown at 30°C for 72 h.
However, more-subtle phenotypes are exposed when double and triple mutations are introduced. Figure 6B shows that all three of the double mutants are very similar to wild-type Sso1p when overexpressed by the GAL1-10 promoter, although differences are observed at lower expression levels. Both the R260G K261G Sso1p mutant and the R260G K263G Sso1p mutant are similar to the wild type, but the K261G K263G Sso1p mutant is virtually nonviable under lower expression conditions. Similarly, the R260G K261G K263G Sso1p triple mutant is also nonfunctional in vivo at lower expression levels and also shows significant growth impairment when overexpressed.
DISCUSSION
A large body of experimental evidence strongly suggests that SNARE proteins provide the mechanical energy that drives membrane fusion. However, the precise mechanism by which force is generated and lipid mixing promoted remains unclear. One proposed model for SNARE-mediated fusion based on the crystal structure of the neuronal SNARE complex suggested that a rigid connection between the SNARE transmembrane domains and the core helical domains that form the four-helix bundle would stress the lipid bilayer in the region of the transmembrane domains as SNARE assembly proceeds, leading to molecular contact of merging outer leaflets and initiation of lipid mixing (41). However, increasing evidence suggests that helical continuity does not exist between the core complex domain and the transmembrane region. Site-directed spin-labeled EPR measurements of syntaxin 1A suggest some helical continuity extends from the core complex domain into the juxtamembrane region, but the last five residues before the transmembrane region (260 to 264) remain unstructured (20). Molecular dynamics simulations of syntaxin 1A concur that this region is likely unstructured (19).
Specific experiments were designed to determine the need for helical continuity between the core complex domain and the transmembrane domain of Sso1p. Previous work using neuronal SNAREs in an in vitro liposome mixing assay has shown an insertion of KLGPP in the syntaxin 1A juxtamembrane reduces the rate of fusion by 50% (24). Similar experiments with yeast SNAREs show that insertion of KLGPP in the juxtamembrane region reduces the in vitro rate of fusion only slightly compared to results with wild-type Sso1p (Fig. 4A).Overexpression of this protein in S. cerevisiae as the only source of Sso1p allows growth in vivo, albeit at a reduced rate (Fig. 3A). When the proline-containing Sso1 protein is expressed at near-endogenous levels, no growth occurs. This in vivo impairment is not likely attributable to the inability of the protein to drive membrane fusion (Fig. 4). These data suggest that helical continuity might be preferred for some interaction(s) in vivo but is not required when elevated levels of the protein are present. A series of insertions that introduce flexibility and physically separate the transmembrane domain from the SNARE “core complex” were also produced. The insertions contained glycine-glycine-serine repeats. This sequence was chosen because glycine contains no side chain and thus is highly flexible. These mutant proteins address two related questions with respect to the distance between the core complex domain and the transmembrane domain: is the distance between the core complex domain and the transmembrane domain critical for function, and what degree of flexibility is permitted in the juxtamembrane region to maintain function? Progressive insertions in the juxtamembrane of Sso1p enable a large degree of flexibility, likely reducing the ability of Sso1p to translate the energy generated by SNARE complex formation to the membrane anchors. At the highest expression level, some colonies grew when as many as 33 amino acids (3x linker) were inserted into the juxtamembrane region (Fig. 3A). These survivors may represent the highest overexpressors that permit very minimal growth under these extreme circumstances. It is unlikely that these survivors represent mutation in the URA3 gene, because the “post shuffle” strains are completely dependent on galactose for growth; hence, they require the GAL-driven mutant Sso1p plasmid (data not shown). An insertion of six amino acids in the juxtamembrane results in behavior like that of the wild-type protein at near-endogenous expression (Fig. 3). These results imply that the distance from the transmembrane domain to the core complex domain is not critical to maintain function.
The 1x linker and 2x juxtamembrane proteins have 11 and 12 amino acids introduced into the juxtamembrane region, respectively. While these two insertions likely provide a similar distance increase, the degree of flexibility is probably different due to amino acid composition. The average extent of fusion at 120 min for the 1x linker is 61% ± 8% (mean ± standard deviation; n = 6) of that of the wild type and is 23% ± 8% (n = 6) of that of the wild type for the 2x juxtamembrane mutant (Fig. 4A; also data not shown). These results indicate that an insertion that is more flexible can more readily fuse liposomes in vitro. An argument can be made that more-flexible insertions are favored in vivo as well, based on the data presented in Fig. 3A; however, this conclusion is tempered by the possibility of sequence specific effects.
One other study has examined the influence of inserting residues in the juxtamembrane region of Vam3p, a syntaxin protein required for vacuolar fusion (26, 29). In this case, addition of as few as three amino acids in the juxtamembrane marginally inhibits fusion, but addition of 12 amino acids abolishes SNARE-mediated fusion in an in vitro vacuolar fusion assay (48). This apparent difference can likely be attributed to differences in the SNARE proteins that form the vacuolar t-SNARE complex. The Vam3p heavy chain complexes with two membrane-anchored t-SNARE protein light chains, Vti1p and Vam7p, while the membrane integral Sso1p binds to a soluble protein, Sec9p, that contributes both t-SNARE light chains (13, 23). Thus, the lack of growth and fusion with the Vam3p mutants might simply reflect an inability to form a t-SNARE complex due to steric constraints, that is, the two membrane-integral SNAREs, Vti1p and mutant Vam3p, could not form a helical bundle because the insertion in Vam3p throws the heptad repeat sequence out of register with the t-SNARE light chains. Conversely, at the plasma membrane, soluble Sec9p can easily form a t-SNARE complex irrespective of the distance found in the juxtamembrane segment.
Recent studies have shown that the yeast SNAREs Snc1p, Syn8p, and Tlg1p are palmitoylated at a cysteine near the cytoplasmic end of their transmembrane domain, and it has been suggested that Sso1p may be similarly modified (45). The function of this posttranslational modification is believed to protect the proteins from degradation. Conversion of cysteine 266 to leucine (in Sso1p) shows no in vivo or in vitro effect on the protein function (Fig. 2). Palmitoylation also has a role in targeting of proteins to lipid rafts (25). This probably accounts for SNAP25 accumulation within lipid rafts in mammalian cells (8). Our results indicate that if Sso1p is palmitoylated, then it is not required for in vivo function.
Since many residues of the juxtamembrane are well conserved among a broad class of species (Fig. 1B), experiments were designed to examine the specific sequence requirements of the juxtamembrane. We found that changing six residues in the juxtamembrane sequence (6AA replace) is not permitted in vivo under any expression conditions but can be fully tolerated in vitro, yielding fusion rates still near wild-type levels (Fig. 5D) (84% ± 8%; n = 7). Further experiments found that as few as three residue changes were not functional in vivo (Fig. 5A and B), while changing two residues in the juxtamembrane showed some impaired growth (Fig. 6B). These results suggest that the specific sequence of the juxtamembrane region is critical for Sso1p function in vivo but likely at a step independent of membrane fusion itself, since these mutants are capable of membrane fusion in vitro.
Several possibilities can be suggested to explain the differences between in vitro fusion and in vivo function. The most obvious possibility, given the charged nature of the critical residues and their proximity to the phospholipid bilayer, is an electrostatic interaction with lipid headgroups. Others have shown that lysine and arginine residues in the rat syntaxin 1A juxtamembrane region (R262-K265) are embedded in the hydrophobic region of the phospholipid bilayer by EPR spectroscopy (20). This type of protein-lipid interaction with bulk phospholipid or possibly with specific anionic lipids, such as phosphorylated phosphatidylinositols, may help destabilize the bilayer during membrane fusion and promote lipid mixing. Evidence that VAMP2 interacts with lipids in both the vesicle membrane and the plasma membrane has also been documented (12). Our specific point mutations made in the Sso1p juxtamembrane would decrease the general electrostatic interaction with the lipid headgroups and likely minimize these potential in vivo functions. Additionally, SNARE-lipid interactions may be required in native membranes but not in the artificial environment of a synthetic liposome in the in vitro-reconstituted assay.
Alternatively, an interaction between SNAREs and lipids may facilitate the formation of the proper lipid microenvironment for in vivo fusion. SNAREs may need to be spatially located to the correct lipid environment, whether it be into lipid rafts, cholesterol, or sphingolipid-rich microdomains (33). This causes SNAREs to concentrate at specific regions of the lipid bilayer, and it has been shown that for regulated exocytosis, this spatial organization into lipid rafts is critical for regulation. Although the existence of lipid rafts in yeast is debated (2, 46), a requirement for SNARE localization to specialized lipid microdomains could also explain our data.
We also must consider the possibility that a specific interaction with a regulatory protein may depend on the juxtamembrane charge and changing the charge could influence the activity of the putative regulatory factor. This potential involvement of a regulatory protein would be missing from the in vitro fusion assay, and its role influenced by the juxtamembrane domain charge would go unfulfilled.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (IBN-0212605 to J.A.M.), The Robert A. Welch Foundation (C-1517 to J.A.M), and an NSF IGERT training grant (DGE-0114264 to J.V.K.).
We thank Robert Johnston for initial Sso1p DNA constructs.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Aalto, M. K., H. Ronne, and S. Keranen. 1993. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 12:4095-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagnat, M., and K. Simons. 2002. Lipid rafts in protein sorting and cell polarity in budding yeast Saccharomyces cerevisiae. Biol. Chem. 383:1475-1480. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, M. K., N. Calakos, and R. H. Scheller. 1992. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257:255-259. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal, R., M. J. Clague, S. R. Durell, and R. M. Epand. 2003. Membrane fusion. Chem. Rev. 103:53-69. [DOI] [PubMed] [Google Scholar]
- 5.Boeke, J. D., J. Trueheart, G. Natsoulis, and G. R. Fink. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154:164-175. [DOI] [PubMed] [Google Scholar]
- 6.Brennwald, P., B. Kearns, K. Champion, S. Keranen, V. Bankaitis, and P. Novick. 1994. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell 79:245-258. [DOI] [PubMed] [Google Scholar]
- 7.Bruns, D., and R. Jahn. 2002. Molecular determinants of exocytosis. Pflugers Arch. 443:333-338. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain, L. H., R. D. Burgoyne, and G. W. Gould. 2001. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc. Natl. Acad. Sci. USA 98:5619-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Y. A., and R. H. Scheller. 2001. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2:98-106. [DOI] [PubMed] [Google Scholar]
- 10.Chernomordik, L. V., and M. M. Kozlov. 2003. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72:175-207. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, F. S., and G. B. Melikyan. 2004. The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement. J. Membr. Biol. 199:1-14. [DOI] [PubMed] [Google Scholar]
- 12.De Haro, L., S. Quetglas, C. Iborra, C. Leveque, and M. Seagar. 2003. Calmodulin-dependent regulation of a lipid binding domain in the v-SNARE synaptobrevin and its role in vesicular fusion. Biol. Cell 95:459-464. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda, R., J. A. McNew, T. Weber, F. Parlati, T. Engel, W. Nickel, J. E. Rothman, and T. H. Sollner. 2000. Functional architecture of an intracellular membrane t-SNARE. Nature 407:198-202. [DOI] [PubMed] [Google Scholar]
- 14.Gerst, J. E., L. Rodgers, M. Riggs, and M. Wigler. 1992. SNC1, a yeast homolog of the synaptic vesicle-associated membrane protein/synaptobrevin gene family: genetic interactions with the RAS and CAP genes. Proc. Natl. Acad. Sci. USA 89:4338-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giraudo, C. G., C. Hu, D. You, A. M. Slovic, E. V. Mosharov, D. Sulzer, T. J. Melia, and J. E. Rothman. 2005. SNAREs can promote complete fusion and hemifusion as alternative outcomes. J. Cell Biol. 170:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson, P. I., R. Roth, H. Morisaki, R. Jahn, and J. E. Heuser. 1997. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90:523-535. [DOI] [PubMed] [Google Scholar]
- 17.Hay, J. C., and R. H. Scheller. 1997. SNAREs and NSF in targeted membrane fusion. Curr. Opin. Cell Biol. 9:505-512. [DOI] [PubMed] [Google Scholar]
- 18.Jahn, R. 2000. Sec1/Munc18 proteins: mediators of membrane fusion moving to center stage. Neuron 27:201-204. [DOI] [PubMed] [Google Scholar]
- 19.Knecht, V., and H. Grubmuller. 2003. Mechanical coupling via the membrane fusion SNARE protein syntaxin 1A: a molecular dynamics study. Biophys. J. 84:1527-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kweon, D. H., C. S. Kim, and Y. K. Shin. 2002. The membrane-dipped neuronal SNARE complex: a site-directed spin labeling electron paramagnetic resonance study. Biochemistry 41:9264-9268. [DOI] [PubMed] [Google Scholar]
- 21.Kweon, D. H., C. S. Kim, and Y. K. Shin. 2003. Regulation of neuronal SNARE assembly by the membrane. Nat. Struct. Biol. 10:440-447. [DOI] [PubMed] [Google Scholar]
- 22.Lu, X., F. Zhang, J. A. McNew, and Y. K. Shin. 2005. Membrane fusion induced by neuronal SNAREs transits through hemifusion. J. Biol. Chem. 280:30538-30541. [DOI] [PubMed] [Google Scholar]
- 23.McNew, J. A., F. Parlati, R. Fukuda, R. J. Johnston, K. Paz, F. Paumet, T. H. Sollner, and J. E. Rothman. 2000. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407:153-159. [DOI] [PubMed] [Google Scholar]
- 24.McNew, J. A., T. Weber, D. M. Engelman, T. H. Sollner, and J. E. Rothman. 1999. The length of the flexible SNAREpin juxtamembrane region is a critical determinant of SNARE-dependent fusion. Mol. Cell 4:415-421. [DOI] [PubMed] [Google Scholar]
- 25.Melkonian, K. A., A. G. Ostermeyer, J. Z. Chen, M. G. Roth, and D. A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910-3917. [DOI] [PubMed] [Google Scholar]
- 26.Nichols, B. J., C. Ungermann, H. R. Pelham, W. T. Wickner, and A. Haas. 1997. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature 387:199-202. [DOI] [PubMed] [Google Scholar]
- 27.Novick, P., and W. Guo. 2002. Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol. 12:247-249. [DOI] [PubMed] [Google Scholar]
- 28.Oyler, G. A., G. A. Higgins, R. A. Hart, E. Battenberg, M. Billingsley, F. E. Bloom, and M. C. Wilson. 1989. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J. Cell Biol. 109:3039-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelham, H. R. 1999. SNAREs and the secretory pathway—lessons from yeast. Exp. Cell Res. 247:1-8. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer, S. 2001. Vesicle tethering factors united. Mol. Cell 8:729-730. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer, S. R. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487-491. [DOI] [PubMed] [Google Scholar]
- 32.Poirier, M. A., W. Xiao, J. C. Macosko, C. Chan, Y. K. Shin, and M. K. Bennett. 1998. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 5:765-769. [DOI] [PubMed] [Google Scholar]
- 33.Salaun, C., D. J. James, and L. H. Chamberlain. 2004. Lipid rafts and the regulation of exocytosis. Traffic 5:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott, B. L., J. S. Van Komen, H. Irshad, S. Liu, K. A. Wilson, and J. A. McNew. 2004. Sec1p directly stimulates SNARE-mediated membrane fusion in vitro. J. Cell Biol. 167:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott, B. L., J. S. Van Komen, S. Liu, T. Weber, T. J. Melia, and J. A. McNew. 2003. Liposome fusion assay to monitor intracellular membrane fusion machines. Methods Enzymol. 372:274-300. [DOI] [PubMed] [Google Scholar]
- 36.Skehel, J. J., and D. C. Wiley. 1998. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell 95:871-874. [DOI] [PubMed] [Google Scholar]
- 37.Sogaard, M., K. Tani, R. R. Ye, S. Geromanos, P. Tempst, T. Kirchhausen, J. E. Rothman, and T. Sollner. 1994. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell 78:937-948. [DOI] [PubMed] [Google Scholar]
- 38.Sollner, T., S. W. Whiteheart, M. Brunner, H. Erdjument-Bromage, S. Geromanos, P. Tempst, and J. E. Rothman. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature 362:318-324. [DOI] [PubMed] [Google Scholar]
- 39.Sollner, T. H. 2004. Intracellular and viral membrane fusion: a uniting mechanism. Curr. Opin. Cell Biol. 16:429-435. [DOI] [PubMed] [Google Scholar]
- 40.Sudhof, T. C., M. Baumert, M. S. Perin, and R. Jahn. 1989. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron 2:1475-1481. [DOI] [PubMed] [Google Scholar]
- 41.Sutton, R. B., D. Fasshauer, R. Jahn, and A. T. Brunger. 1998. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395:347-353. [DOI] [PubMed] [Google Scholar]
- 42.Toonen, R. F., and M. Verhage. 2003. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol. 13:177-186. [DOI] [PubMed] [Google Scholar]
- 43.Trimble, W. S., D. M. Cowan, and R. H. Scheller. 1988. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc. Natl. Acad. Sci. USA 85:4538-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ungar, D., and F. M. Hughson. 2003. SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 19:493-517. [DOI] [PubMed] [Google Scholar]
- 45.Valdez-Taubas, J., and H. Pelham. 2005. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J. 24:2524-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valdez-Taubas, J., and H. R. Pelham. 2003. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr. Biol. 13:1636-1640. [DOI] [PubMed] [Google Scholar]
- 47.Van Komen, J. S., X. Bai, B. L. Scott, and J. A. McNew. An intramolecular t-SNARE complex functions in vivo without the syntaxin N-terminal regulatory domain. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 48.Wang, Y., I. Dulubova, J. Rizo, and T. C. Sudhof. 2001. Functional analysis of conserved structural elements in yeast syntaxin Vam3p. J. Biol. Chem. 276:28598-28605. [DOI] [PubMed] [Google Scholar]
- 49.Weber, T., B. V. Zemelman, J. A. McNew, B. Westermann, M. Gmachl, F. Parlati, T. H. Söllner, and J. E. Rothman. 1998. SNAREpins: minimal machinery for membrane fusion. Cell 92:759-772. [DOI] [PubMed] [Google Scholar]
- 50.Weimbs, T., K. Mostov, S. H. Low, and K. Hofmann. 1998. A model for structural similarity between different SNARE complexes based on sequence relationships. Trends Cell Biol. 8:260-262. [DOI] [PubMed] [Google Scholar]
- 51.Xu, Y., F. Zhang, Z. Su, J. A. McNew, and Y. K. Shin. 2005. Hemifusion in SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 12:417-422. [DOI] [PubMed] [Google Scholar]
- 52.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.