Abstract
The herpes simplex virus genome can enter a repressed transcriptional state (latency) in sensory neurons of the host nervous system. Although reduced permissiveness of the neuronal environment is widely accepted as a causal factor, the molecular pathway(s) directing and maintaining the viral genome in the latent state remains undefined. Over the past decade, the field has been strongly influenced by the observations of Kosz-Vnenchak et al., which have been interpreted to indicate that, in sensory neurons in vivo, a critical level of viral DNA synthesis within the neuron is required for sufficient viral immediate-early (IE) and early (E) gene expression (M. Kosz-Vnenchak, J. Jacobson, D. M. Coen, and D. M. Knipe, J. Virol. 67:5383-5393, 1993). The levels of IE and E genes are, in turn, thought to regulate the decision to enter the lytic cycle or latency. We have reexamined this issue using new strategies for in situ detection and quantification of viral gene expression in whole tissues. Our results using thymidine kinase-null and rescued mutants as well as wild-type strains in conjunction with viral DNA synthesis blockers demonstrate that (i) despite inhibition of viral DNA replication, many neurons express lytic viral proteins, including IE proteins, during acute infection in the ganglion; (ii) at early times postinoculation, the number of neurons expressing viral proteins in the ganglion is not reduced by inhibition of viral DNA replication; and (iii) following a reactivation stimulus, the numbers of neurons and apparent levels of lytic viral proteins, including IE proteins, are not reduced by inhibition of viral DNA replication. We conclude that viral DNA replication in the neuron per se does not regulate IE gene expression or entry into the lytic cycle.
The current understanding of the biology underlying the complex life cycle of herpes simplex virus (HSV) is incomplete (recently reviewed [16]). A central remaining question is how a virus geared for lytic replication enters a latent state in a sensory neuron and subsequently resumes lytic replication in this same neuron at a future time. A decade ago, Kosz-Vnenchak et al. proposed that neuronal latency is directed by a novel HSV gene expression regulatory pathway (10). Their hypothesis was based on two observations, both suggesting that, in trigeminal ganglion (TG) neurons, synthesis of viral DNA is a prerequisite for effective levels of immediate-early (IE) and early (E) gene expression. First, during acute infection with two viral thymidine kinase (TK)-null mutants, IΕ and Ε gene expression was virtually undetectable in TGs despite evidence of transport to the ganglia (9). In a second study, latently infected ganglia undergoing reactivation in explant were examined. In this case, a TK-null mutant as well as a wild-type (wt) strain in the presence or absence of viral DNA replication inhibitors was examined. Again, expression of IE and E genes appeared to be dependent on viral DNA replication (10). These investigators argued that, since functional viral TK had been shown to be required for efficient replication in cultured growth-arrested cells (3, 7) and the HSV TK is believed to provide deoxyribonucleoside triphosphate precursors for viral DNA replication in resting cells not expressing the host enzyme, the defect in replication in resting cells for TK-null mutant viruses would be at the level of viral DNA replication (10). Thus, the investigators suggested that the expression of IE and E viral genes was linked to viral DNA replication. These observations led to the hypothesis that some replication of the viral genome preceded detectable levels of IΕ and Ε gene expression and full progression into the lytic cycle. In contrast to the lytic replication cycle in cultured cell lines, where viral IE and E gene expression is independent of viral DNA replication, in the TG amplification of the viral genome was hypothesized to be required for optimum IE gene expression and progression into lytic replication.
Supporting this hypothesis, Nichol et al. (14) demonstrated that the HSV replication cycle was prolonged in primary sympathetic neuronal cultures compared to Vero cells and that blocking viral DNA synthesis with acyclovir (ACV) resulted in reduced levels of IE and E gene expression, again in contrast to Vero cells. Thus, the progression of HSV lytic infection was indeed measurably different in cultured neurons compared to Vero cells and the differences observed were consistent with the notion that viral DNA synthesis could be a critical regulatory event between lytic and latent pathways in neurons. An additional component to the studies of Nichol et al. dealt with the mechanism by which viral DNA replication was stimulating IE and E gene expression. On the basis of observations with a ribonucleotide reductase mutant and a UL9 mutant, both containing an E gene (β6)/promoter lacZ cassette, the authors concluded that template amplification (genome copy number) was not the primary stimulus of activation of viral gene expression. Instead, these authors proposed that regulation was linked to the activities initiating viral DNA synthesis at the origins of replication by the stimulation of transcriptional activity of promoters surrounding these regions (14).
In a more recent study, Summers and Leib (25) confirmed the earlier observations of Nichol et al. regarding the effect of DNA synthesis inhibitors on IE and E gene expression in cultured neurons and showed that this was not exclusive to primary cultured neurons but characteristic of viral replication in primary mouse embryo fibroblasts as well. In addition, using an ectopic reporter cassette, these investigators demonstrated that, contrary to the proposal of Nichol et al. (14), the origins of replication have no significant role in the regulation of flanking promoters in vitro or in vivo (25).
Despite these studies, in the context of acute or latent infection in vivo there are only limited data supporting the hypothesis that entry into the lytic cycle is regulated by viral DNA replication. Recent advances in detecting and quantifying viral gene expression in TG neurons in vivo (20) justify reexamination of this central issue. We repeated and extended earlier in vivo studies (9, 10) using TK-null and genomically restored mutants in HSV strains 17syn+ and KOS with new methodologies to detect and quantify the number of cells expressing viral protein in whole ganglia (20). Our results demonstrate (i) that, following a reactivation stimulus, the numbers of neurons and apparent levels of lytic viral proteins, including IE proteins, are not directly influenced by viral DNA replication; (ii) that many neurons express lytic viral proteins, including IE proteins but not true late proteins, during acute infection with TK-null mutants in the TG; and (iii) at early times postinoculation (p.i.), the number of neurons expressing viral proteins in the TG is equivalent irrespective of viral DNA replication capacity. These findings strongly indicate that viral DNA replication in the neuron per se is not required for the initiation of lytic viral protein expression, and thus, it is unlikely that viral DNA synthesis in the neuron functions as a major regulatory event required for entry into the lytic transcriptional program.
MATERIALS AND METHODS
Viral strains and stock production.
The wild-type HSV type 1 (HSV-1) strain 17syn+ was originally obtained from John H. Subak-Sharpe at the Medical Research Council Virology Unit in Glasgow, Scotland. All base pair designations are as reported for strain 17syn+ by McGeoch and colleagues (13, 15). The construction and characterization of the thymidine kinase-negative mutant 17/tBTK− and its genomically restored counterpart, 17/tBTKR, were described in detail previously (17, 28). This mutant is completely thymidine kinase negative as assayed by the most sensitive assays including the black plaque assay in TK-negative tissue culture cells and an enzymatic assay performed on protein extracts (17). Strain KOS was originally obtained from M. Levine, Ann Arbor, Michigan, and plaque purified to yield strain KOS(M). The TK-null mutants, KOS/tBTK-1(3.1.1) and KOS/tBTK-2(4.2.1), were generated in the background of KOS(M) using a strategy identical to that employed to generate 17/tBTK− (17, 28). In brief, the Escherichia coli beta-galactosidase gene, driven by the simian virus 40 early region promoter/enhancer, was inserted into the SnaBI site at bp 47560 in the KOS(M) HSV-1 genome by standard cotransfection techniques (17, 28). This insertion disrupted the open reading frame for TK (UL23, bp 47803 to 46672) and completely abolished viral TK activity, while permitting the continued expression of UL24 and UL24.5 (17, 28).
Two independently derived mutants from separate transfections were plaque purified by three rounds of limiting dilution and tested for ACV sensitivity (4). Confirmation that the promoter/reporter cassette recombined into the desired genomic location was performed by restriction fragment length polymorphism analysis (not shown). KOS/tBTK-1 was genomically restored to wild type by recombination with the wild-type HSV-1 genome fragment EcoRI N (bp 45570 to 47986). Genomically restored isolates were identified by the loss of beta-galactosidase activity, reversion of the restriction fragment length polymorphism analysis result to a wild-type profile, and the restoration of viral TK activity as evidenced by sensitivity to ACV (data not shown). Virus stocks were generated by routine propagation on rabbit skin cell (RSC) monolayers. Infected cells were harvested and sonicated, and the titer of each stock was determined by serial dilution plaque assay on RSC monolayers.
Inoculation of mice.
All procedures involving animals were approved by the Children's Hospital Institutional Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animals. Animals were housed in American Association for Laboratory Animal Care-approved quarters. Male, outbred, Swiss Webster mice (Harlan Laboratories), 4 to 5 weeks of age, were used throughout these studies. Prior to inoculation, mice were anesthetized by intraperitoneal injection of sodium pentobarbital (Nembutal [50 mg/kg of body weight]). A 10-μl drop containing 1 × 105 PFU was placed onto each scarified corneal surface. In our laboratory, this inoculum titer of 17syn+ results in ∼80% survival of mice with 100% of ganglia being latently infected. Mice inoculated with 17/tBTK− received a total of 5 × 106 PFU in a total of 50 μl. Each scarified cornea received a 10-μl drop, and 15 μl was gently rubbed onto each shaved and abraded whisker pad (23, 28). Mice were inoculated on eyes and whisker pads with KOS-based viruses (wild-type strain KOS and TK− mutants KOS/tBTK-1, KOS/tBTK-2, and KOS/tBTK-1R) using a total of 5 × 106 PFU, divided between surfaces as detailed for 17/tBTK−.
Replication in vivo.
In order to confirm the efficiency of inoculation and progression of acute infection, three mice from each inoculation group were sacrificed on days 2, 4, 6, 8, and 10 p.i. Eyes and TGs were removed and assayed for infectious virus titer by serial dilution plaque assay on RSC monolayers as described previously.
Drugs, dosage, and treatment regimen.
Viral DNA synthesis was blocked in vivo using ACV (Glaxo Wellcome). ACV solution was prepared in sterile saline just prior to use. Fifty milligrams/kilogram was delivered in a 200-μl volume by intraperitoneal injection. In addition to ACV, phosphonoacetic acid (PAA; Sigma) was utilized to block viral DNA replication in explanted ganglia. The compounds were dissolved in medium to achieve a final concentration of 1 μg/ml ACV and 300 μg/ml PAA as reported previously (10). Mice were dosed as detailed in Results.
In vivo reactivation.
HSV was induced to reactivate in the ganglia of mice in vivo using hyperthermic stress (HS). This procedure has been described in detail previously (23). In brief, mice were placed into restrainers, suspended in a 43°C water bath for 10 min, and subsequently towel dried and placed in a 37°C incubator to prevent hypothermia. At the indicated times posttreatment, mice were euthanized and the TGs were removed and processed for detection of HSV proteins (described below) or detection of infectious virus. Virus was detected by homogenizing ganglia individually on ice in minimal essential medium containing 5% newborn calf serum. Following centrifugation to pellet cellular debris, the entire homogenate (∼1.5 ml) from each ganglion was placed onto a 60-mm RSC monolayer and absorbed for 2 h with gentle rocking at 37°C in a 5% CO2 incubator. Plates were then rinsed with medium and overlaid with medium containing 1% carboxymethyl cellulose. Plaques appeared within 24 to 48 h postplating. For quantification of plaques, carboxymethyl cellulose overlay was removed from plates, which were then rinsed in phosphate-buffered saline (PBS) and stained with crystal violet. Plaques were counted under a dissecting microscope.
Explant reactivation.
Latent HSV was induced to reactivate in the ganglia of mice in vitro by aseptically removing the ganglia and placing them in minimal essential medium supplemented with glutamine, antibiotics, and 5% newborn calf serum in a 5% CO2 incubator at 37°C for the time indicated. At the indicated times postexplant, ganglia were processed for detection of either viral protein (described below) or infectious virus as described above.
DNA extraction and quantification.
The infected TGs were homogenized in a 25 mM EDTA-0.2% sodium dodecyl sulfate lysis solution containing 1 mm zirconia-silica beads (BioSpec Products) using a MiniBeadbeater (BioSpec Products) for 3 min, and the homogenate was incubated with proteinase K at a concentration of 100 ng/ml. DNA was extracted from the homogenate by standard phenol-chloroform extraction, resuspended in 30 μl of PCR-grade water, and quantified using the PicoGreen double-stranded DNA quantification kit according to the manufacturer's instructions (Molecular Probes). A 1:10 serial dilution of DNA provided with the kit was used to set up a standard curve ranging from 0 ng/ml to 1 μg/ml, and from preliminary experiments it was determined that the concentration of a 1:4,000 dilution of DNA extracted from mouse TGs would fall within the standard curve. The PicoGreen was read using a SpectraMax Gemini XS (Molecular Devices) fluorescence plate reader at an excitation of 480 nm and an emission of 520 nm. The data were then analyzed using SOFTmax Pro version 3.1.2 software (Molecular Devices) and Prism software (GraphPad).
Real-time PCR.
A real-time PCR assay using the LightCycler system (Roche) was developed to detect HSV. For standards, 10 μl of plasmid DNA containing the HSV TK locus was serially diluted and 106 to 101 viral genome copy equivalents together with 50 ng of mouse DNA was added to 10 μl of QuantiTect SYBR Green PCR mix (QIAGEN) containing 10 pmol of each TK primer as previously described (8). DNA extracted from infected or control TGs was analyzed along with the standards as follows. Five microliters containing 50 ng of TG DNA was added to 15 μl of QuantiTect SYBR Green PCR mix (QIAGEN) containing 10 pmol of each TK primer described above. The assay was run under the following conditions: one cycle of 95°C for 15 min and 55 cycles of 95°C for 15 s, 57°C for 20 s, and 72°C for 10 s with a single fluorescence detection point taken at the end of the extension segment and a temperature transition rate of 20°C/s. This was followed by one cycle of 95°C for 0 s, 65°C for 15 s, and 65 to 95°C by a temperature transition rate of 0.1°C/s with continuous fluorescence detection to determine melting curve followed by a cooling cycle of 40°C for 30 s.
Analysis of data was performed with the LightCycler 3.5 software (Roche) and Prism software (GraphPad). The number of genome copies per 50 ng of sample was determined using the “second derivative maximum” function and arithmetic baseline adjustment. The melting curve analysis was used to determine the melting temperature of the standards and samples. Samples were considered HSV positive if their melting temperature (Tm) matched the Tm determined in preliminary experiments, in which the PCR product was analyzed and found to be a single band of the predicted size on an acrylamide gel.
Viral genome copy number in ganglia was determined by analyzing 50 ng of total DNA harvested from TGs. The total genome copies in the TG were calculated from the number of viral genomes in the 50-ng sample and the total amount of DNA recovered from the TG.
Antibodies and immunohistochemistry.
Western blot analysis of HSV-infected cell lysates with the antibodies utilized in this study demonstrated that, as anticipated, the anti-HSV antibody detected many lytic viral proteins, while the remaining antibodies detected single bands consistent with the molecular weight of the protein (not shown).
Immunohistochemistry on whole ganglia (WGIHC) was carried out using a procedure previously reported by Luque et al. (12) and adapted for detection of HSV (20).
For fixation, ganglia were fixed for 2 h in 0.5% paraformaldehyde, rinsed in PBS twice for 15 min, and postfixed overnight in methanol containing 20% dimethyl sulfoxide (DMSO). Ganglia were incubated for 1 h in a solution of methanol containing 20% DMSO and 10% H2O2. Following two 15-min rinses in 100% methanol, ganglia were stored overnight in methanol at −70°C. Ganglia were allowed to equilibrate at room temperature for 15 min, rinsed twice in PBS, and incubated for 2 h at 37°C in PBS containing 3.6-mg/ml β-d(+)-glucose (Sigma), 100-μg/ml glucose oxidase (Sigma), and 130-μg/ml sodium azide (Mallinckrodt). Ganglia were rinsed twice in PBS and incubated overnight at 37°C in the primary antibody diluted in PBS containing 2% bovine serum albumin, 5% DMSO, and 5% normal horse serum. The rabbit anti-HSV type 1/2 antibody (Accurate) was used at 1:3,000, the rabbit anti-HSV-ICP0 antibody (Zymed) was used at 1:500, and the rabbit anti-HSV-ICP27 antibody (Zymed) was used at 1:500. Following rinsing for 5 h which included five changes of PBS, ganglia were incubated overnight at room temperature in a 1:500 dilution of anti-rabbit horseradish peroxidase conjugate (Vector) diluted in PBS containing 2% bovine serum albumin, 5% DMSO, and 5% normal horse serum. Tissue was again rinsed in PBS, with five changes over a period of 5 h, followed by a final rinse in 0.05 M Tris Cl, pH 8.2. Ganglia were then incubated in a solution containing 250 μg/ml diaminobenzidine (Aldrich) and 0.004% H2O2 in 0.1 M Tris (pH 8.2). The reaction was carefully monitored by visualizing color development and stopped by rinsing the reaction mixture in distilled H2O. Rinsed ganglia were cleared in glycerol, mounted between two slides, and viewed and photographed under an Olympus BX40 microscope with a digital DP-10 camera.
Immunohistochemistry to detect the true late viral protein, glycoprotein C, was performed on fresh-frozen sectioned ganglia as follows. Tissue was removed, snap frozen in OCT compound (Tissue Tec), and sectioned using a Minotome Plus cryostat (Triangle Park Biosciences). Serial sections were cut and picked up on Superfrost Plus slides (Fisher), air dried, and fixed for 10 min in Streck tissue fixative (Streck Laboratories). Endogenous peroxidase activity was blocked by incubating sections in a methanol bath containing 0.15% hydrogen peroxide for 15 min. Sections were then rinsed in PBS and incubated for 1 h with primary antibody (rabbit anti-gC [Zymed], anti-HSV [Accurate], anti-ICP27 [Zymed], or anti-ICP4 [Zymed]). Sections were again rinsed in PBS and incubated for 1 h with conjugated secondary antibody (horseradish peroxidase-labeled anti-rabbit secondary antibody [Vector]). Antigen-antibody complexes were detected using either Vector Red or the VIP substrate kit (Vector) as directed by the manufacturer.
RESULTS
The absence of viral DNA replication within the neuron does not directly influence the number of latently infected neurons entering the lytic cycle in response to a reactivation stimulus.
The potential regulatory role of viral DNA replication in the transition from latent to lytic gene transcription was examined as follows. First, we asked whether blocking viral DNA synthesis with ACV alters the initiation of lytic viral protein expression induced from the latent viral genome either in vivo by hyperthermic stress or in vitro by explant. If viral DNA synthesis is a key regulatory event preceding the initiation of lytic gene expression from the wild-type latent viral genome, as has been hypothesized (10), then inhibition of viral DNA synthesis would be expected to block or reduce the number of neurons entering the lytic cycle.
Male Swiss Webster mice were inoculated on scarified corneas with 1 × 105 to 2 × 105 PFU of HSV-1 strain 17syn+. Quantification of replication in eyes and TGs of three separate animals on day 4 confirmed that infection was within the expected range with modest variation (eyes, mean, 7 × 104; range, 5 × 104 to 1 × 105; TGs, mean, 2 × 104; range, 1 × 104 to 2.5 × 104). This group of infected mice was maintained for 60 days prior to reactivation studies. Additional groups of mice were inoculated on scarified corneas with 2 × 106 PFU of HSV-1 strain KOS. As with 17syn+, replication in the eyes and TGs of these mice was monitored on day 4 and these titers confirmed the quality of the inoculation (data not shown).
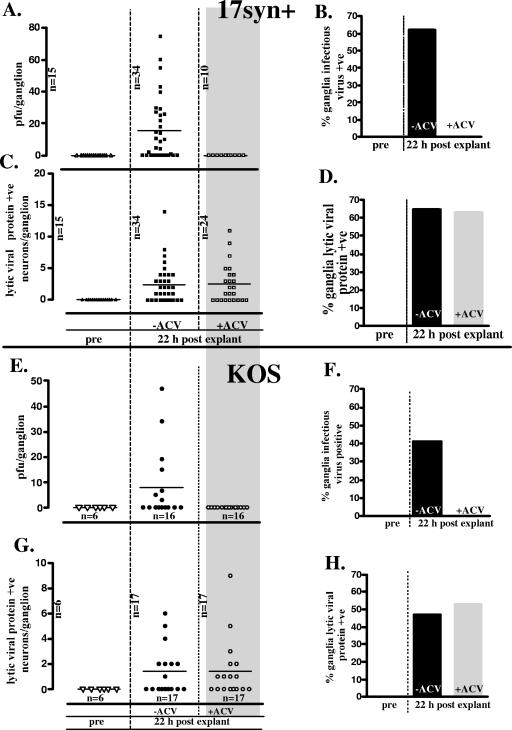
Mice latently infected (>60 days p.i.) with strain 17syn+ were induced to reactivate in vivo using HS as described previously (23) and dosed at 0, 6, 12, and 18 h post-HS with an intraperitoneal injection of 50-mg/kg ACV or sham inoculated with sterile saline. This ACV treatment regimen was shown to completely eliminate detectable infectious virus in TGs following HS (21). Ganglia from additional latently infected mice were explanted in the presence of ACV or in medium alone. At 22 h post-HS or -explantation, TGs were either assayed for infectious virus on RSC monolayers or processed for WGIHC, and neurons positive for lytic viral proteins were quantified (20). Treatment with ACV prevented the production of infectious virus in both reactivation settings. In vivo, 0% (0/16) of TGs from ACV-treated 17syn+-infected mice contained detectable infectious virus at 22 h post-HS compared to 63% (10/16) of TGs from the sham-treated group. Similar results were observed in ganglia explanted from these mice with 0% (0/10) of ACV-treated TGs and 61% (21/34) of untreated TGs being positive for infectious virus at 22 h postexplantation (Fig. 1A and B).
FIG. 1.
Quantification of infectious virus and lytic viral protein-expressing neurons in ganglia latently infected with either HSV-1 strain 17syn+ (squares) or KOS (circles) 22 h postexplant in the presence (open symbols) or absence (closed symbols) of the viral DNA replication inhibitor ACV. (A and E) Infectious virus titers in individual latently infected TGs before and 22 h postexplant. (B and F) Percentage of ganglia positive for infectious virus. (C and G) Number of lytic viral protein-expressing neurons in individual latently infected TGs before and 22 h postexplant. (D and H) Percentage of ganglia containing lytic viral protein-containing neurons.
In contrast, the number of neurons in which lytic viral proteins were detected in TGs following HS in vivo or in explanted TGs remained unchanged. In vivo following hyperthermic stress, 11/16 and 10/16 TGs from ACV- and sham-treated 17syn+-infected mice, respectively, contained one or more lytic viral protein-positive neurons. The mean number of neurons positive per TG was 2.2 and 2.5 in ACV-treated and sham-treated mice, respectively. Similar results were observed for explanted TGs (Fig. 1C and D).
Similarly, TGs from mice latently infected with strain KOS were induced to reactivate by being explanted in the presence or absence of ACV. These results are shown in Fig. 1E and F. As observed in TGs infected with strain 17syn+, while ACV treatment inhibited the production of infectious virus, there was no effect on the number of neurons in the TG that expressed lytic viral proteins at 22 h postinduction. Thus, as measured by this initiation-of-reactivation assay (22) the onset of lytic gene expression was not reduced when viral DNA replication was inhibited with ACV. An additional viral DNA synthesis inhibitor, PAA, was also tested. As with ACV, production of infectious virus was inhibited but no reduction in the number of neurons initiating the lytic cycle was observed (data not shown). With regard to IE protein expression specifically, when an antibody directed against an IE gene, ICP27, was utilized, again, at 22 h postexplant there was no difference in the number of neurons expressing this IE gene in the presence or absence of ACV (32 and 37 positive neurons per 10 TGs, respectively).
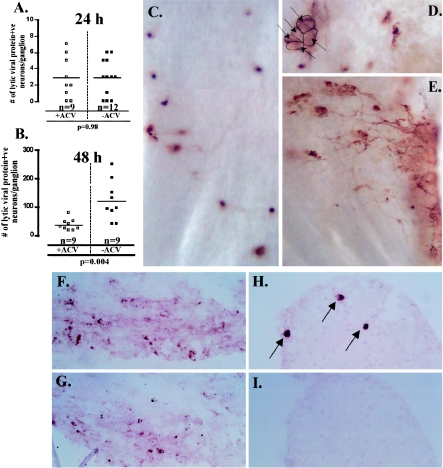
In the two studies addressing this issue previously, TGs were examined at 48 to 72 h postexplant (10) and at 36 h postexplant (25). We have demonstrated that, even as early as 36 h postexplant, in the absence of ACV, the infectious virus produced from reactivation that occurs as soon as 18 h earlier has spread to neighboring cells within the ganglia (22), thus increasing the number of infected cells within the ganglia. Figure 2 shows that, as reported previously (10), there is a significant difference in the number of neurons expressing lytic genes in the presence or absence of ACV at 48 h postexplant. However, as is made clear using WGIHC (Fig. 2C to E), spread of reactivated virus in the absence of ACV is a significant factor at this time and the explanation for the difference observed.
FIG. 2.
Comparison of the effect of ACV treatment on the number of lytic viral protein-expressing neurons in TGs latently infected with 17syn+ at 24 h (A) and 48 h (B) postexplant. At 24 h postexplant there is no difference between ACV-treated (□) and untreated (▪) ganglia. However, at 48 h postexplant, the number of lytic viral protein-expressing neurons in untreated ganglia (▪) is significantly greater than that observed for ganglia treated with ACV (□) (P = 0.004, Student's t test). Panels C to E are photomicrographs of whole ganglia probed with a polyclonal antibody directed against HSV lytic proteins. This method allows for a comprehensive assessment of HSV lytic gene expression in a single view of the ganglia. As shown in panel C, at 48 h postexplant, ACV-treated ganglia contain discrete viral protein-expressing neurons with no evidence of lateral spread within the TG. In contrast, in the absence of ACV, clusters of viral protein-expressing neurons (D) and spread of lytic viral protein expression within the TG (E) are readily apparent. Panels F to I show serial sectioned frozen ganglia at 5 days postexplant in the absence (F and G) and presence (H and I) of ACV, immunostained for ICP4 (F and H) and gC (G and I). There is abundant ICP4 and gC detected in the TG after 5 days in the absence of ACV (F and G). In contrast, in the presence of ACV, only discrete neurons express ICP4 (H, arrows) and gC expression is not detected in these neurons (I).
To further test the efficacy of the ACV treatment to inhibit viral DNA synthesis in this setting, the expression of gC, a true late gene, was examined (5). In preliminary studies it was determined that optimum immunoreactivity in tissue with the anti-gC antibody was obtained in fresh-frozen tissue postfixed in a precipitating fixative. Ganglia were removed from mice latently infected with 17syn+ and explanted in the presence or absence of ACV as detailed above. On day 5 postexplant, ganglia were snap frozen and serial sections from both ACV-treated and untreated groups were examined for IE (ICP4) and true late (gC) gene expression. It is clear that, in the absence of ACV, expression of gC can be readily detected in regions in which IE gene expression is detected (Fig. 2F and G). In contrast, in the presence of ACV, IE gene but not gC expression can be detected (Fig. 2H and I).
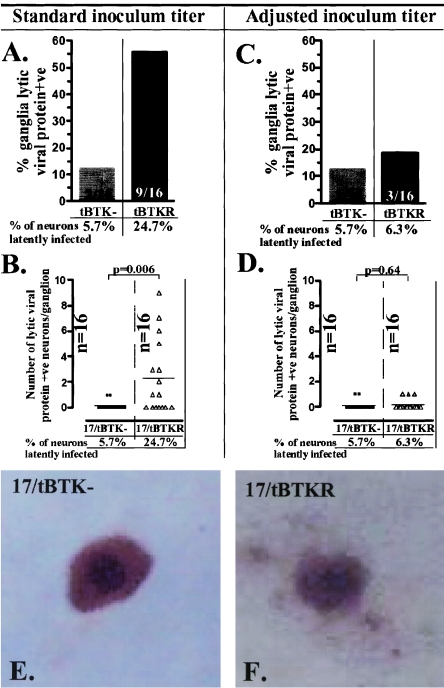
We next asked whether viral protein expression could be detected in vivo following hyperthermic stress or in vitro following explant in ganglia latently infected with TK-null mutants. Viral thymidine kinase activity is required for viral replication in neurons in vivo, and mutants lacking this enzyme function do not reactivate (1). This is attributed to a requirement for TK function to support viral DNA synthesis in neurons in vivo. At equal input titers, ganglia from KOS-based TK-null mutant-infected mice contained on average 50-fold-less HSV DNA than wild-type-infected ganglia (8), and ganglia from 17syn+-based TK-null mutant-infected mice contained ∼10-fold-fewer latently infected neurons than ganglia from mice infected with the rescue or parental wild-type strain (28). Since the size of the latent pool influences reactivation frequency (19, 21, 24), viral input titers were adjusted to achieve equivalent numbers of latently infected neurons in the TGs of 17/tBTKR- and 17/tBTK−-infected mice using a strategy described previously (26, 27). Mice were inoculated with 5 × 106 PFU of the TK-null mutant and either 700 or 1 × 105 PFU of the rescued virus. At times greater than 40 days p.i., the numbers of neurons containing the viral genome in the TG were quantified using CXA (18) and determined to be 5.7% (7/122) in 17/tBTK−-infected ganglia and 6.3% (9/143) and 24.7% (18/73) in mice infected with 700 and 105 PFU of 17/tBTKR, respectively. Mice were subjected to HS, and 22 h later, ganglia were removed and examined for lytic viral protein expression using WGIHC (20). Additional mice were sacrificed, and ganglia were removed and explanted for 22 h, at which time these ganglia were also examined for lytic viral protein expression using WGIHC.
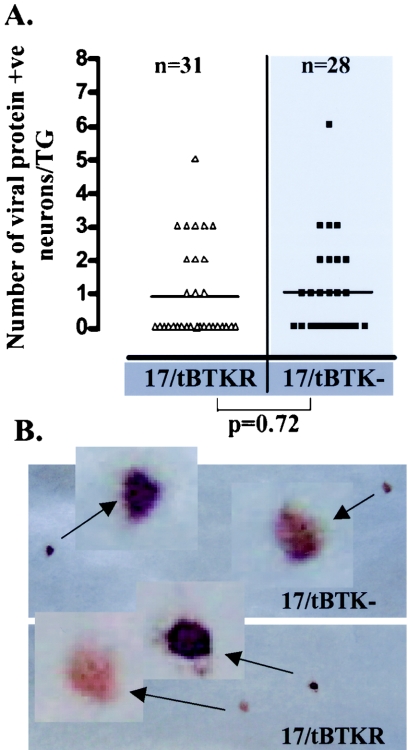
At 22 h post-HS, the percentage of ganglia that contained lytic viral protein-positive neurons was 56% (9/16) in 17/tBTKR- and 12.5% (2/16) in 17/tBTK−-infected mice when standard inoculum titers were utilized (Fig. 3A). As shown in Fig. 3B, the number of neurons expressing lytic viral proteins in these ganglia was significantly different, with a mean of 0.125 neurons per TG in 17/tBTK−- and 2.3 neurons per TG in 17/tBTKR-infected ganglia. This significant difference in the number of neurons entering lytic cycle between TK-null and TK-rescued mutants is consistent with previously published findings (10). However, when inoculum titer was adjusted such that the percentage of neurons latently infected was similar in 17/tBTKR- and 17/tBTK−-infected ganglia, the percentage of 17/tBTKR-infected ganglia positive for lytic viral proteins was now 18.8% (3/16), a value similar to that observed in 17/tBTK−-infected ganglia (Fig. 3C). Importantly, there was also no difference in the number of viral protein-expressing neurons detected at 22 h post-HS (Fig. 3D) or postexplant (not shown). In addition, the intensity of the signal in the positive neurons in 17/tBTK−- and 17/tBTKR-infected ganglia was similar, indicating that there were no large differences in the amounts of lytic viral protein expressed (Fig. 3E and F). Taken together, these findings indicate (10) that a significant inequality in the level of latency established by TK-null and wt strains was an important but unconsidered factor underlying the differences in lytic gene expression reported previously (10).
FIG. 3.
Quantification of lytic viral protein-expressing neurons 22 h post-hyperthermic stress in ganglia latently infected with 17/tBTK− (gray bars and squares) or 17/tBTKR (black bars and triangles). Mice were inoculated as detailed in Materials and Methods, and the percentage of neurons latently infected in the TG was determined using CXA. Using standard inoculum titers (A and B), the percentage of neurons latently infected in 17/tBTK−-infected ganglia was more than fourfold lower than the percentage in 17/tBTKR-infected ganglia (A), and the number of neurons expressing lytic viral proteins post-hyperthermic stress was different (P = 0.006, Student's t test) in these two groups (B). When the inoculum titer was adjusted such that levels of latency were similar in the two groups, both the percentage of ganglia and the number of neurons per TG expressing lytic viral protein at 22 h post-HS were similar (C and D). Representative neurons expressing lytic viral proteins in 17/tBTK−- and 17/tBTKR-infected ganglia at 22 h post-HS are shown in panels E and G, demonstrating that the intensity of staining is not different between the groups.
Absence of viral TK does not prevent lytic viral protein expression in neurons on day 3 during acute infection in TGs, but the number of positive neurons is reduced compared to that with TK+ virus.
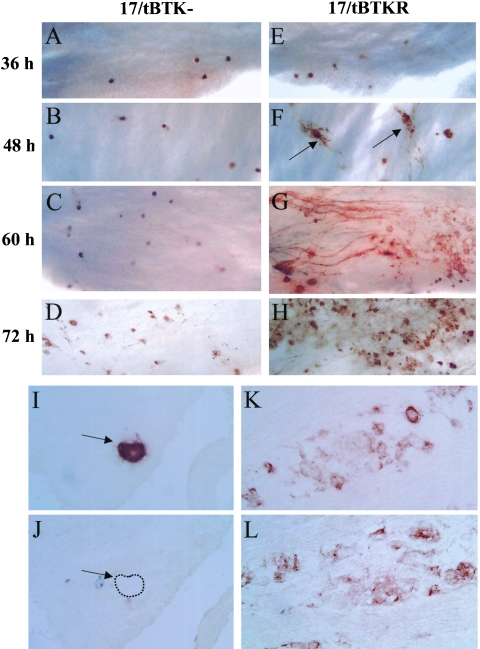
The preceding data demonstrate that the inhibition of viral DNA replication does not alter entry into the lytic cycle from the latent viral genome. A more difficult issue to approach experimentally is the role of viral DNA replication in the entry into lytic gene expression during acute infection in the nervous system. In a previous report absent or dramatically reduced levels of lytic viral transcripts were observed on day 3 p.i. in TGs infected with TK-null mutants compared to the wild-type strain (9). In a later study using reverse transcription-coupled PCR, viral IE and E gene transcripts were detected on day 3 but at a level ∼1,000-fold reduced from that of wt (11). We previously reported a reduction in corneal replication by the TK− mutant, 17/tBTK−, compared to the rescue strain, 17/tBTKR, or wild-type 17syn+ at times beyond day 2 p.i. (28). In contrast, replication of the TK-null mutant in the whisker pad remained comparable to that of wild type through day 4 p.i. (28). To test the possibility that the reduced capacity of the TK-null mutant to sustain replication on the eye could account for the very low or undetectable levels of viral gene expression in the TGs reported previously, mice were inoculated bilaterally on scarified corneas or shaved and abraded whisker pads with 5 × 106 PFU of 17/tBTK−. For comparison, mice were also inoculated on scarified corneas with 2 × 105 PFU of 17/tBTKR. A portion of the 17/tBTK−-infected animals were treated with ACV, 50 mg/kg, 2×/day starting 24 h p.i., to confirm that replication of this mutant was not sensitive to ACV. TGs and eyes or snouts were harvested on day 3 p.i. from the infected mice. TGs were examined for expression of HSV protein using WGIHC, and viral titers were determined in the eyes or snouts. In mice infected with 17/tBTK−, the mean viral titer in the snouts was 3 × 105 PFU (range, 1.4 × 105 to 4.5 × 105, n = 4) and in the eyes was 2.4 × 103 (range, 4.3 × 102 to 4.0 × 103, n = 4). In mice infected with 17/tBTKR, the mean viral titer in the eyes was 2.3 × 104 (range, 1.4 × 104 to 3.3 × 104, n = 4). Consistent with our previous results, titers of 17/tBTK− in snouts were 100-fold greater than those in eyes on day 3 (28). Using WGIHC, neurons expressing lytic viral proteins were readily detected in 17/tBTK−-infected TGs (Fig. 4). On day 3, a mean of 33 neurons/TG (range, 17 to 52, n = 6) and 96.3 neurons/TG (range, 58 to 130, n = 10) was detected in eye- and snout-inoculated mice, respectively. As anticipated, neither the titers of virus in eyes or snouts nor the numbers of neurons expressing viral proteins in the TG were altered by ACV treatment (not shown), confirming the TK-null phenotype of 17/tBTK−. TGs from snout-inoculated mice had ∼3-fold-more viral protein-expressing neurons than did TGs from mice inoculated in the eye. However, compared to TGs from 17/tBTKR-infected animals, there were many fewer viral protein-expressing neurons in 17/tBTK−-infected ganglia regardless of inoculation site (Fig. 4A and E). Considering that there are in the range of 22,000 neurons per ganglion (18), only 0.15% (eye inoculation) or 0.44% (snout inoculation) of neurons in ganglia infected with 17/tBTK− expressed lytic viral proteins compared to ∼2.3% of neurons (average of 500/TG) in ganglia infected with 17/tBTKR. This large difference in the number of viral protein-expressing neurons in TGs infected with 17/tBTK− compared to the number in TGs infected with 17/tBTKR is consistent with the observations reported previously (9), but it is not consistent with our findings during reactivation from latency reported above. Further characterization of viral gene expression during lytic infection in vivo is detailed below.
FIG. 4.
Lytic viral protein expression in 17/tBTK−-infected (A to D) and 17/TBTKR-infected (E to H) ganglia 36 h (A and E), 48 h (B and F), 60 h (C and G), and 72 h (D and H) p.i. using WGIHC as described in Materials and Methods. In 17/tBTK−-infected ganglia, lytic viral protein-expressing neurons remain discrete with no evidence of lateral spread. In contrast, spread of virus in TGs of 17/tBTKR-infected mice is apparent by 48 h p.i. (arrows). Panels I to L show serial sectioned frozen ganglia from 17/tBTK− (I and J)- and 17/tBTKR (K and L)-infected mice harvested on day 4 day p.i. and immunostained for ICP27 (I and K) and gC (J and L). Arrows in panels I and J indicate neurons containing detectable ICP27 but no gC (dashed line indicates cell boundary).
To characterize the extent to which viral DNA synthesis was impaired in the TK-null mutants, the expression of gC was examined. Mice were inoculated on scarified corneas with 5 × 106 PFU of the TK-null mutant 17/tBTK− (n = 8) or 2 × 105 PFU of its rescue strain, 17/tBTKR (n = 4). On day 4 p.i., the mice were sacrificed and the ganglia were removed and snap frozen. Serial sections were examined using antibodies specific for ICP27 and gC. In 17/tBTKR-infected ganglia, both IE (ICP27) and true late (gC) gene expression was detected (Fig. 4K and L). In contrast, ICP27 but not gC was detected in TK-null virus-infected ganglia (Fig. 4I and J).
The difference in the number of neurons expressing lytic viral proteins in TK-null and TK+ strain-infected TGs is inversely proportional to time p.i.
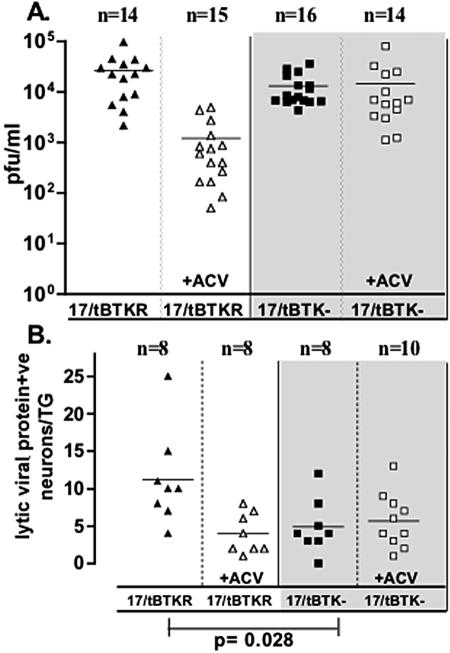
The above results could mean that the inhibition of DNA replication in the TG neurons infected with 17/tBTK− directly resulted in a reduced number of viral protein-expressing neurons. However, the relationship between replication at the surface site of inoculation and replication in the innervating ganglia must be considered. In vivo, virus replicated at the surface feeds into the ganglia, replicates there, and travels back to the surface, infecting additional cells and broadening the geographic area of surface infection. This, in turn, results in the virus exposure to additional innervating axonal endings and infection of additional neurons in the ganglia (28). In the ganglia, lateral spread of the virus to neighboring neurons also occurs and virus is then transported back to the surface innervated by these neurons and replicates in this region. Thus, viral replication in the ganglia influences the number of neurons infected by (i) lateral spread within the ganglion and (ii) expansion of the geographic boundaries of surface infection/replication. For this reason, the extent to which the inhibition of DNA replication in neurons per se contributes to the difference in the number of neurons expressing lytic viral proteins observed in TGs infected with TK-null compared to rescued virus is not possible to discern on day 3. We reasoned that the effect of this intersite feedback loop could be minimized by looking at earlier times p.i., before significant transport of virus from the ganglion back to the surface, replication, and return to the ganglion as well as lateral spread of virus within the ganglia could occur. Because we have the ability to detect and quantify low numbers of positive neurons in the TGs using WGIHC, this experiment was practical. Mice infected with 1 × 106 PFU of either 17/tBTK− or 17/tBTKR on scarified corneas or 5 × 106 PFU of 17/tBTK− on scarified snouts were examined for viral protein expression 48 h p.i. The number of viral protein-positive neurons in TGs harvested on day 2 from mice infected on the corneas with either 17/tBTK− or 17/tBTKR rescued viruses is shown in Fig. 5. The mean number of neurons expressing lytic viral proteins in ganglia from mice infected on the corneas with 17/tBTKR was greater than that in ganglia of mice infected with 17/tBTK− (11.3 versus 5.7); however, the difference was only twofold compared to the 15-fold difference observed on day 3. This strongly suggests that spread of infectious virus produced within the ganglia did influence the magnitude of the difference in the number of lytic viral protein-positive neurons observed between the two groups (Fig. 4C and G).
FIG. 5.
Comparison of viral titers in the eyes (A) and the number of lytic viral protein-expressing neurons in the TGs (B) of mice infected with 17/tBTKR (triangles) and 17/tBTK− (squares) at 48 h p.i. Open symbols indicate groups of mice treated with ACV as detailed in Materials and Methods (+ACV). The difference in the number of lytic protein-positive neurons in TGs of 17/tBTKR- and 17/tBTK−-infected ganglia was significant (P = 0.028, Student's t test).
Acyclovir treatment did not alter replication or the number of lytic viral protein-positive neurons in 17/tBTK−-infected mice at 48 h p.i.
A portion of the mice from each inoculation group were treated with ACV (50 mg/kg, intraperitoneally) starting at 24 h p.i. Again, in mice infected with 17/tBTK−, treatment with ACV did not influence the mean viral titers in eyes (1.3 × 104 and 1.4 × 104, P = 0.76, unpaired t test) (Fig. 5A). However, replication of the TK-restored virus in the eyes was reduced by 25-fold, from 26,600 to 1,200 PFU/ganglion pair (P = 0.0005, unpaired t test) as a result of three doses of ACV administered between 24 and 42 h p.i. Importantly, there was no difference between the number of viral protein-positive neurons in ganglia from ACV-treated and that from untreated mice inoculated with 17/tBTK− (ACV treated, mean, 5.7; range, 1 to 13; n = 10; untreated, mean, 4.9; range, 0 to 12; n=8) (Fig. 5B). In 17/tBTKR-infected mice, ACV treatment administered from 24 to 42 h p.i. resulted in significantly fewer positive neurons than in the untreated group (ACV treated, mean, 4.0; range, 1 to 8; n = 8; untreated, mean, 11.3; range, 4 to 25; n = 8; P = 0.01, unpaired t test) (Fig. 5B).
There is no difference in the number of lytic viral protein-expressing neurons in 17/tBTK−- and 17/tBTKR-infected ganglia through 36 h p.i.
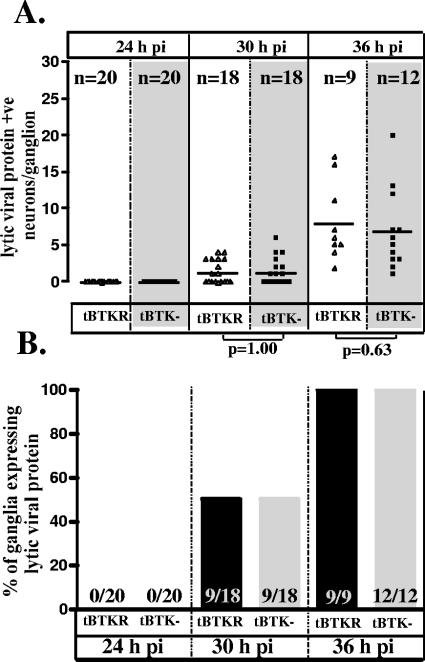
As shown in Fig. 4G, by 48 h there was evidence of lateral spread of virus within the TGs of 17/tBTKR-infected mice that was not observed in 17/tBTK−-infected TGs. In order to further reduce the contribution of overall replication to the number of neurons expressing lytic viral proteins, mice were inoculated and examined at earlier times p.i., including 24, 30, and 36 h (Fig. 6). At 24 h p.i., lytic viral protein was not observed in ganglia infected with either TK-null (0 of 20) or TK-restored (0 of 20) mutants. At 30 and 36 h, viral protein expression was detected in equivalent numbers of neurons in 17/tBTK−- and 17/tBTKR-infected ganglia (P = 1.00 and 0.063 at 30 and 36 h p.i., respectively, Student's t test) (Fig. 6). These data demonstrate that, at early times after inoculation with equal input titers, the number of neurons expressing viral protein in the TG is not different between 17/tBTK− and the rescue strain, 17/tBTKR. Although not shown, the parental strain 17syn+ was indistinguishable from 17/tBTKR.
FIG. 6.
Comparison of the number of lytic viral protein-expressing neurons (A) and the percentage of TGs expressing viral proteins (B) in mice infected with 17/tBTK− (squares) and 17/tBTKR (triangles) at 24 h, 30 h, and 36 h p.i. There is no significant difference between the groups at any of these times p.i. (Student's t test).
Inhibition of viral DNA synthesis resulting from the absence of viral TK function in strain KOS does not reduce the number of neurons expressing lytic viral protein in TGs at early times p.i. or following reactivation stimulus.
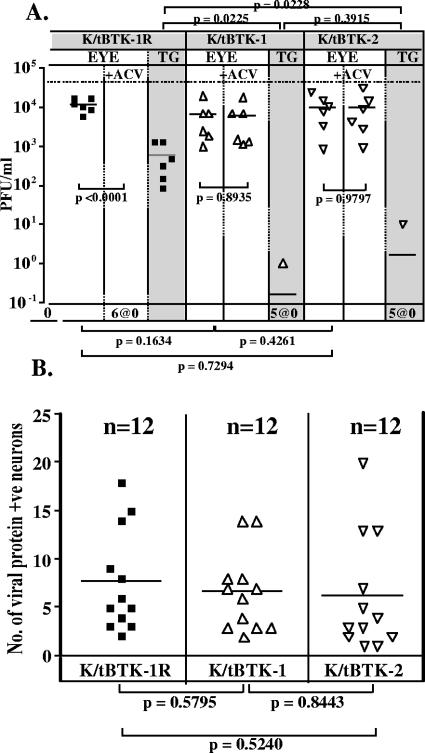
Previous studies were performed using strain KOS (9, 10). In order to test whether the absence of viral gene expression observed in vivo was viral strain related, experiments similar to those described above using 17syn+ were performed using strain KOS. Two independent isolates of a KOS-based TK-null mutant, K/tBTK-1 and K/tBTK-2, and the genomically restored version of K/tBTK-1, named K/tBTK-1R, were examined. The construction and characterization of these mutants are described in Materials and Methods. The mutants were ACV insensitive and exhibited the predicted genomic structures by Southern blot analysis (not shown). Mice were inoculated with 2 × 106 PFU on the eyes with the wild-type strain KOS, the TK-null mutants, and the restored virus. One-half of the mice from each inoculation group were treated with ACV as detailed above. Eyes and TGs were harvested at 42 h p.i., and the viral titers in the eyes and TGs (Fig. 7A) and the number of lytic viral protein-positive neurons in the TGs (Fig. 7B) were determined. As anticipated, ACV treatment had no effect on viral titers of K/tBTK-infected eyes, with mean titers of 7 × 103 and 1 × 104 PFU without treatment and 6 × 103 and 1 × 104 with ACV treatment for K/tBTK-1- and -2-infected mice, respectively. In contrast, titers from K/tBTK-1R-infected eyes were reduced from a mean of 1×104 PFU in untreated mice to undetectable levels when infected mice were treated with ACV. In the TGs of K/tBTK-1- and K/tBTK-2-infected mice, a single positive ganglion was detected, containing 1 and 10 PFU, respectively. The near-absence of lytic virus in the TG is consistent with previous reports (1, 6, 11, 28) and confirms the replication impairment of our TK-null mutants in the nervous system. In the TK rescue strain-infected mice, TGs at 42 h p.i. contained a mean of 586 PFU. Analysis of the number of lytic viral protein-expressing neurons within the TG at this time is shown in Fig. 7B. There was no difference, with a mean of 6.5 and 6.2 positive neurons in TGs from TK− strain-infected mice and a mean of 7.7 positive neurons in the TGs of K/tBTK-1R-infected mice. Although not shown, wild-type-infected mice were not different from those infected with the rescue strain in the parameters measured. These data demonstrate that, as observed with 17syn+, viral TK function (and thereby viral DNA replication) in KOS-infected TG neurons did not in and of itself alter the number of TG neurons expressing viral protein.
FIG. 7.
Comparison of viral titers (A) (n = 6 mice) and the number of lytic viral protein-expressing neurons in the TGs (B) (n = 12 TGs) at 42 h p.i. in mice infected with the TK-null and TK-restored mutants, K/tBTK-1 (triangles), K/tBTK-2 (inverted triangles), and K/tBTK-1R (squares). Groups of mice were inoculated with 106 PFU of the indicated virus, and at 42 h p.i. tissues were harvested and processed as detailed in Materials and Methods. Some mice (open symbols) were treated with ACV as detailed in Materials and Methods.
Inhibition of viral DNA synthesis resulting from absent viral TK function does not reduce the number of neurons expressing IE genes at early times p.i.
In the above experiments, a reagent that detects a broad spectrum of lytic HSV proteins was utilized. While this reagent would be anticipated to reflect the sum of lytic viral proteins and thus indicate entry into the lytic cycle, reduction or absence of one or more specific lytic viral proteins would likely not be apparent. In order to test whether IE gene expression in TG neurons was influenced by the absence of TK activity, ganglia from mice infected with 17/tBTK− or17/tBTKR were harvested at 30 h p.i. and WGIHC was performed using antibodies to detect the IE proteins ICP0 and ICP27. As shown in Fig. 8, neither the intensity of staining nor the number of neurons positive for ICP0 and ICP27 was influenced by the absence of viral TK function. Thus, by quantifying lytic viral expression in TG neurons before the feedback loop between replication at the surface and in the ganglion comes into play, it was possible to demonstrate that the defect in viral DNA synthesis in TK− strain-infected neurons does not directly alter lytic viral protein expression in these cells.
FIG. 8.
(A) Comparison of the number of neurons expressing IE proteins in TGs infected with 17/tBTK− (squares) and 17/tBTKR (triangles) 30 h p.i. TGs were probed with antibodies directed against ICP27 and ICP0 as described in Materials and Methods. The difference between the groups was not significant (Student's t test). (B) Positive neurons representative of the intensity of staining in 17/tBTK−- and 17/tBTKR-infected ganglia.
The amount of viral DNA in ganglia from TK− strain- and TK+ strain-infected animals is not different at early times p.i.
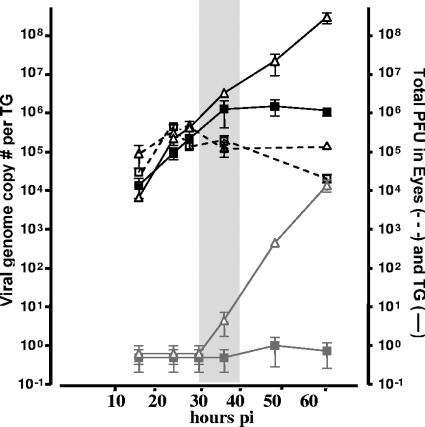
Our interpretation of the preceding data is dependent upon the assumption that there is no difference in the amount of viral DNA that is transported to the ganglia of TK-null strain- or TK+ strain-infected mice. In order to test this, mice were inoculated on scarified corneas with 2 × 106 PFU of either 17/tBTK− or 17/tBTKR. Eyes and TGs were harvested at 16, 24, 28, 36, 48, and 60 h p.i. from three or four mice per time point per inoculation group. Infectious virus titers in the eyes of each animal were determined by standard plaque assay, and viral genome copy number in the TGs of each animal was determined by real-time PCR as described in Materials and Methods. Ganglia from additional mice from each inoculation group were analyzed for infectious viral titers. These results are shown in Fig. 9. Replication in the eyes of TK− mutant- and rescue strain-infected mice did not differ from 16 to 36 h, ranging from ∼5 × 104 to 5 × 105 PFU. However, replication in the eyes of 17/tBTK− mutant-infected mice was 10-fold less than that observed in the 17/tBTKR-infected mice at 60 h p.i., a finding consistent with our previous report (28). Through the 36-h time point, the number of viral genomes in the TG did not differ significantly between 17/tBTK−- and 17/tBTKR-infected mice. By 60 h p.i., 17/tBTKR-infected TGs contained more than 100-fold-more viral genomes than TGs infected with the 17/tBTK− strain, consistent with a previous report (11). The number of viral genomes in the TGs of 17/tBTK−- and 17/tBTKR-infected mice paralleled viral replication in the ganglia, which increased sharply from ∼10 PFU in 17/tKTBR-infected ganglia at 36 h p.i. to 104 PFU at 60 h p.i. In contrast, detection of virus in the TGs of 17/tBTK−-infected mice was at most 1 to 2 PFU. Importantly, at the time when the number of lytic viral protein-expressing neurons in the TGs of TK-null strain- and TK rescue strain-infected TGs was the same, the number of viral genomes was also equivalent (Fig. 9).
FIG. 9.
Viral genome copies in the ganglia of 17/tBTK− (squares)- and 17/tBTKR (triangles)-infected mice from 16 to 60 h p.i. (black solid line). The viral replication contributing to this total number of viral genomes in the ganglia is also shown (eyes, open symbols and black dashed line; ganglia, gray solid symbols and gray solid line). A minimum of three mice per time point was examined. The means ± standard errors of the means of values from three to four mice per time point per group are plotted. The central shaded region indicates the time p.i. during which ganglia from mice infected with 17/tBTK− and 17/tBTKR contain equivalent numbers of the viral protein-expressing neurons.
DISCUSSION
HSV enjoys two distinct lifestyles. In a variety of tissue culture cells and in mucotaneous epithelial cells at the body surface, HSV engages in an aggressive replicative cycle, producing thousands of genome copies and hundreds of infectious progeny and cell death within 18 h of entry. In contrast, the outcome following axonal transport and entry into the sensory neuronal cell bodies can be either (i) lytic transcriptional activity, infectious progeny, and cell death or (ii) transcriptional repression, no progeny, and cell survival. This second outcome results in the permanent presence of viral genetic information within the neurons of the host nervous system. The molecular interactions determining entry of HSV into either the lytic or latent transcriptional programs in the nervous system are central to the in vivo behavior of the virus but remain unclear. In this paper, we present the results from experiments designed to test the hypothesis that has dominated thinking in the field for over the past decade, namely that, in sensory neurons in vivo, entry into the lytic cycle is regulated by viral DNA synthesis (10). Our data demonstrate that (i) viral TK-dependent viral DNA amplification is required for late viral protein expression but is not required for robust expression of HSV immediate-early and early gene expression in neurons during acute infection in TGs; (ii) at early times p.i., the number of neurons that express lytic viral proteins is influenced by input titer but not viral TK function; (iii) following a reactivation stimulus in vivo or in explant, lytic viral proteins are detected in the number of neurons predicted from the level of latency established and are independent of viral TK function; and (iv) chemical blockades of viral DNA replication following a reactivation stimulus in vivo or in explant do not influence the intensity or number of neurons expressing immediate-early and early viral proteins (but do block expression of a late protein) in the TGs of latently infected mice. We conclude that viral DNA replication in the neurons per se is not required for robust viral lytic gene expression and thus does not function as a regulator in the decision between latent and lytic transcriptional programs in neurons in vivo.
This conclusion is not in agreement with the hypothesis that viral DNA replication is a key regulatory step in the initiation of the HSV lytic cycle in the nervous system (9, 10). We report here that a rapid increase in viral DNA synthesis occurred in the 17/tBTKR-infected ganglion between 36 and 48 h p.i. that did not occur in ganglia infected with 17/tBTK− (Fig. 9). Kramer et al. (11) observed a similar increase in viral DNA in the TG between 32 and 48 h p.i. and concluded that the 100- to 1,000-fold increase in ICP4 and TK RNA levels that they observed in the TG at this same time was consistent with their proposal that IE and E gene expression is activated by viral DNA synthesis in neurons. However, these investigators acknowledged the fact that their data could not exclude the possibility that the increase in IE and E RNA levels was due to viral spread (11). In order to understand the basis for these opposing conclusions, two factors need to be considered, timing and sensitivity of approach. In vivo, HSV travels between two interconnected but distinct replication compartments, the surface site of inoculation and the ganglia harboring the innervating sensory neuronal cell bodies. Wild-type virus can replicate in both compartments, and a positive feedback loop between them is set up, increasing virus in both places and influencing the size and quality of the residual latent pool (28). In contrast, TK-null mutants are conditionally impaired and replicate only at the surface. Replication in the ganglia is absent or extremely reduced (1). Although virus is transported from the surface to the ganglia, it is not amplified there and positive feedback is not established. Over time, this results in fewer neurons receiving virus, and thus, fewer neurons would have the potential to express lytic viral genes. That fewer neurons do express lytic genes in ganglia infected with TK-null mutants has been observed and reported by us and others (9, 11, 28) and shown here (Fig. 4). There is also a measurable difference in the replication of TK-null mutants in the eye at times beyond 48 h (1, 28). This could be explained by the absence of the positive feedback loop caused by replication in the TG, but regardless of the cause, this reduced replication would reduce the potential input of virus into the TG.
The issue then becomes dissecting apart the contribution of the generalized decrease in replication and input of virus to the ganglia from that of a direct effect of impaired viral DNA replication within neurons upon their entry into lytic viral gene expression. We dealt with this complexity by quantifying the number of neurons expressing lytic viral proteins in the ganglia very early postinoculation, when surface replication and viral DNA within the TG are not different between TK-null and restored mutants, and before the feedback loop between these compartments became dominant. In order to do this, an approach was utilized that allowed unequivocal detection and quantification of all of the viral protein-positive cells within the ganglia (20). Because the total number of positive cells in the ganglia at early times p.i. is very low regardless of genotype, serial sectioning through the entire ganglia coupled with immunostaining of all sections would be required for an accurate evaluation by conventional approaches. The observations of Kosz-Vnenchak et al. during acute infection could very well be explained by the fact that ganglia were analyzed at 2 to 3 days p.i., a time when reduced surface replication was observed by these investigators (1) and by others (28). Since the day 2 to 3 data were combined, the number of positive ganglia observed in each group on day 2 is not clear. Certainly by day 3, which is shown in their figures, the >2-log difference in surface replication and ∼1,000-fold difference in infectious virus titers (and ensuing spread of this virus) in the TG would influence the number of positive neurons observed, as we demonstrate in the present study. In addition, only 3 to 10 sections from each ganglion were examined in this previous study (8), a number too small to provide an accurate in situ picture of viral gene expression in ganglia on day 2.
Of what significance are the observations of reduced IE and E gene expression in the presence of replication inhibitors in primary neuronal cultures (14, 25) and primary mouse embryo fibroblasts (25)? If indeed the hypothesis that viral DNA replication regulated entry into the lytic cycle in neurons were valid, then these findings could be viewed as supportive data. However, as demonstrated here, viral DNA replication in the neuron in vivo does not influence entry into lytic viral gene expression as determined by viral protein expression. In addition, it should be kept in mind that the virus does not enter latency in these cultures and, further, that there is no evidence that the level of reduction observed in IE gene expression would influence entry into lytic cycle. To the contrary, Davido and Leib reported that the ICP0 promoter deletion mutant delta Tfi, despite a seven- to eightfold reduction in ICP0 RNA levels, exhibited no deficit in replication in the TG in vivo (2), and we also have observed similar results with this mutant (unpublished data).
How can differences in the conclusions about the role of viral DNA replication during reactivation from latency be explained? In this case again, timing and sensitivity are critical, but also differences in the levels of establishment between the TK-null mutants and wild type or restored mutants were not considered in prior studies but are critical to the outcome. Within 36 h after explant, virus that has reactivated during the first 22 h has spread within the ganglion (22; this report). While this is very apparent in a view of the whole ganglion, this spread would not be evident in sectioned material and might not be considered. Since Kosz-Vnenchak et al. examined ganglia at 48 h postexplant, in the absence of a DNA replication blockade the secondary spread would make it seem as if more neurons were reactivating compared to the number observed when secondary spread was prevented (i.e., in the presence of these inhibitors).
Secondary spread could also explain the difference in the promoter activity observed by Summers and Leib in explanted ganglia in the presence and absence of ACV (25). From examining ganglia before spread occurs (22 h postinduction) and enumerating the number of viral protein-expressing cells, it is very clear that blocking viral DNA replication with ACV or other viral DNA replication inhibitors did not decrease the number of neurons initiating lytic cycle gene expression, either in vivo or in explant. Because the level of establishment by TK-null mutants is notably less than that of wild-type or rescued viruses at equivalent input titers (reference 28 and this study) and the level of establishment is directly correlated with reactivation (19, 24), lytic gene expression in TK-null mutants can be compared only to the rescuant or wild type at equivalent levels of establishment. When this was done in the present study, there was no difference in the number of neurons expressing lytic genes either in vivo or in explant reactivation at 22 h. In the previous study differences in the levels of establishment between wild type and the TK-null mutants were not considered (9).
Importantly, we now have an assay to detect viral protein expression during the initiation of reactivation independent of infectious virus production. The ability to detect and quantify neurons exiting the latent transcriptional program on the basis of lytic gene expression rather than infectious virus production will enable us to address many questions regarding the role of viral genes at all points along the reactivation pathway. Findings presented here demonstrate that, in sensory neurons in vivo, viral DNA replication in the neuron per se is not required for the expression of immediate-early viral genes as was previously hypothesized (9).
Acknowledgments
This work was supported by the National Institutes of Health (RO1 AI32121 and EY13168).
Support was also provided by the Astellas USA Foundation.
REFERENCES
- 1.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davido, D. J., and D. A. Leib. 1996. Role of cis-acting sequences of the ICPO promoter of herpes simplex virus type 1 in viral pathogenesis, latency and reactivation. J. Gen. Virol. 77:1853-1863. [DOI] [PubMed] [Google Scholar]
- 3.Field, H. J., and P. Wildy. 1978. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J. Hyg. (London) 81:267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gage, P. J., B. Sauer, M. Levine, and J. C. Glorioso. 1992. A cell-free recombination system for site-specific integration of multigenic shuttle plasmids into the herpes simplex virus type 1 genome. J. Virol. 66:5509-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson, J. G., K. L. Ruffner, M. Kosz-Vnenchak, C. B. Hwang, K. K. Wobbe, D. M. Knipe, and D. M. Coen. 1993. Herpes simplex virus thymidine kinase and specific stages of latency in murine trigeminal ganglia. J. Virol. 67:6903-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson, A. T., G. A. Gentry, and J. H. Subak-Sharpe. 1974. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J. Gen. Virol. 24:465-480. [DOI] [PubMed] [Google Scholar]
- 8.Katz, J. P., E. T. Bodin, and D. M. Coen. 1990. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J. Virol. 64:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosz-Vnenchak, M., D. M. Coen, and D. M. Knipe. 1990. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. J. Virol. 64:5396-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosz-Vnenchak, M., J. Jacobson, D. M. Coen, and D. M. Knipe. 1993. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J. Virol. 67:5383-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer, M. F., S. H. Chen, D. M. Knipe, and D. M. Coen. 1998. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J. Virol. 72:1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luque, J. M., W. B. Adams, and J. G. Nicholls. 1998. Procedures for whole-mount immunohistochemistry and in situ hybridization of immature mammalian CNS. Brain Res. Brain Res. Protoc. 2:165-173. [DOI] [PubMed] [Google Scholar]
- 13.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 14.Nichol, P. F., J. Y. Chang, E. M. Johnson, Jr., and P. D. Olivo. 1996. Herpes simplex virus gene expression in neurons: viral DNA synthesis is a critical regulatory event in the branch point between the lytic and latent pathways. J. Virol. 70:5476-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry, L. J., and D. J. McGeoch. 1988. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:2831-2846. [DOI] [PubMed] [Google Scholar]
- 16.Preston, C. M. 2000. Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol. 81:1-19. [DOI] [PubMed] [Google Scholar]
- 17.Pyles, R. B., and R. L. Thompson. 1994. Mutations in accessory DNA replicating functions alter the relative mutation frequency of herpes simplex virus type 1 strains in cultured murine cells. J. Virol. 68:4514-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawtell, N. M. 1998. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J. Virol. 72:6888-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawtell, N. M. 2003. Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation. J. Virol. 77:4127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawtell, N. M., D. I. Bernstein, and L. R. Stanberry. 1999. A temporal analysis of acyclovir inhibition of induced herpes simplex virus type 1 in vivo reactivation in the mouse trigeminal ganglia. J. Infect. Dis. 180:821-823. [DOI] [PubMed] [Google Scholar]
- 22.Sawtell, N. M., and R. L. Thompson. 2004. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J. Virol. 78:7784-7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawtell, N. M., and R. L. Thompson. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 66:2150-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawtell, N. M., R. L. Thompson, L. R. Stanberry, and D. I. Bernstein. 2001. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J. Infect. Dis. 184:964-971. [DOI] [PubMed] [Google Scholar]
- 25.Summers, B. C., and D. A. Leib. 2002. Herpes simplex virus type 1 origins of DNA replication play no role in the regulation of flanking promoters. J. Virol. 76:7020-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson, R. L., and N. M. Sawtell. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J. Virol. 75:6660-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson, R. L., and N. M. Sawtell. 1997. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 71:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson, R. L., and N. M. Sawtell. 2000. Replication of herpes simplex virus type 1 within trigeminal ganglia is required for high frequency but not high viral genome copy number latency. J. Virol. 74:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]