Abstract
Two conserved herpes simplex virus 1 proteins, UL31 and UL34, form a complex at the inner nuclear membrane which governs primary envelopment and nuclear egress of the herpesvirus nucleocapsids. In mouse cytomegalovirus, a member of the betaherpesvirus subfamily, the homologous proteins M53/p38 and M50/p35 form the nuclear egress complex (NEC). Since the interaction of these proteins is essential for functionality, the definition of the mutual binding sites is a prerequisite for further analysis. Using a comprehensive random mutagenesis procedure, we have mapped the M53/p38 binding site of M50/p35 (A. Bubeck, M. Wagner, Z. Ruzsics, M. Lötzerich, M. Iglesias, I. R. Singh, and U. H. Koszinowski, J. Virol. 78:8026-8035). Here we describe a corresponding analysis for the UL31 homolog M53/p38. A total of 72 individual mutants were reinserted into the genome to test the complementation of the lethal M53 null phenotype. The mutants were also studied for colocalization and for coprecipitation with M50/p35. The analysis revealed that the nonconserved N-terminal one-third of M53/p38 provides the nuclear localization signal as an essential function. The collective results for many mutants localized the binding site for M50/p35 to amino acids (aa) 112 to 137. No single aa exchange for alanine could destroy NEC formation, but virus attenuation revealed a major role for aa K128, Y129, and L130. The lethal phenotype of several insertion and stop mutants indicated the functional importance of the C terminus of the protein.
Lytic replication of the herpesviruses gives rise to the production of infectious virus particles as a result of an assembly process that starts in the nucleus of the infected cell, where the viral genomes are packaged into the nucleocapsid. Nucleocapsid formation is followed by a complex process of nucleocapsid transitions through cellular membranes. First, the nucleocapsid buds from the inner nuclear membrane (INM) into the space between the INM and the outer nuclear membrane (ONM). This process is termed primary envelopment. By primary envelope fusion with the ONM, the nucleocapsids reach the cytoplasm. A secondary envelopment occurs at a not yet clearly defined compartment of the cellular exocytotic pathway. Finally, enveloped virus particles are released into the extracellular space (17, 18).
Virus maturation events are controlled by multiprotein assemblies which require the interaction of a number of viral and cellular proteins. Studies of alphaherpesviruses have shown that two conserved viral proteins, the prototypic UL31 and UL34 gene products, play a major role during primary envelopment (7, 8, 10, 25-27). In the betaherpesvirus mouse cytomegalovirus (MCMV), the products of the M53 and M50 genes (3, 7, 10, 19) have this function, and in the gammaherpesvirus Epstein-Barr virus, the BFRF1 and BFLF2 gene products (7, 10, 12) have this function. UL34 and related proteins are type II membrane proteins which, upon isolated expression, circulate in the contiguous membranes of the endoplasmic reticulum and the INM (UL34, M50, and BFRF1) unless the nuclear protein UL31 and UL31-related proteins (M53 and BFLF2) arrest the viral membrane protein in the INM. Apparently, complex formation by the two proteins is pivotal for the nuclear egress of herpesvirus nucleocapsids. The nuclear egress complex (NEC) executes this task by interacting with cellular proteins which are already residing in or recruited to the INM and presumably recruits the viral capsids to the egress sites. These interactions lead to the displacement of the rigid nuclear lamina along with nucleocapsid budding (7, 10).
In herpes simplex virus type 1 (HSV-1), the interaction between the UL34 and UL31 gene products is required for function. To study the function of the betaherpesvirus NEC in more detail, we started with an analysis of M50/p35 (3). M50/p35, an essential protein, is localized at the nuclear envelope in MCMV-infected cells and recruits its viral interaction partner M53/p38 and cellular protein kinase C to the INM. We identified the M53/p38 binding region of M50/p35 and showed that this interaction is essential for productive MCMV infection (3).
Here we report the first mutational analysis of a herpesvirus UL31 family member, the MCMV M53 gene. Deletion of the M53 gene from the MCMV bacterial artificial chromosome (BAC) abolishes virus replication, and reintroduction of the M53 open reading frame (ORF) at an ectopic position in the genome rescues the null phenotype. By an improved random Tn7-based linker-scanning mutagenesis method (2), a library of M53 insertion mutants was generated. A representative set of M53 mutants was reinserted into ΔM53-MCMV-BAC for functional analysis in the genomic context. A nuclear localization signal embedded in nonessential sequences was mapped to the N terminus of the protein. The sequence required for M50/p35 binding was localized to amino acids (aa) 107 to 136 of M53/p38. Lethal insertions accumulated within the C-terminal two-thirds of M53/p38. None of the C-terminal truncation mutants was able to rescue the M53 null phenotype, underscoring the functional importance of this part of the protein.
MATERIALS AND METHODS
Cells and viruses.
NIH 3T3 murine fibroblasts (ATCC CRL 1658), M2-10B4 cells (a bone marrow stromal cell line [ATCC CRL 1972]), human 293 cells (ATCC CRL 1573), and mouse embryonic fibroblasts (MEF) were propagated as described previously (15). The wild-type (wt) MCMV Smith strain was recovered from pSM3fr-BAC (32). All mutant viruses were derived from pSM3fr-16FRT17-BAC carrying an FRT site between the m16 and m17 genes. The growth properties of 16FRT17 MCMV are identical to those of wt MCMV both in vitro and in vivo (4). The wt and mutant MCMV BACs were reconstituted to viruses by the transfection of MEF, using Superfect transfection reagent (QIAGEN) according to the manufacturer's instructions. Reconstituted viruses were propagated on M2-10B4 cells (15) and titrated by a plaque assay (23).
Virus infection.
NIH 3T3 fibroblasts were infected with wt MCMV at a multiplicity of infection (MOI) of 0.5 to determine the expression kinetics of M53/p38. To block DNA replication, phosphonoacetic acid (PAA; Sigma) was used at a concentration of 300 μg/ml as described previously (9). To compare the growth of wt and mutant viruses, NIH 3T3 fibroblasts were infected at a MOI of 0.1. Supernatants of the infected cultures were collected daily for 5 days, and the released viruses were quantified by a plaque assay.
Plasmids.
The M53 ORF was cloned into the pOriR6K-zeo-ie rescue vector (3) from pCR3-M53 (3), using KpnI and NotI, resulting in pOriR6K-zeo-ie-M53, which was used as a wt M53 rescue and expression vector in this study. pOriR6K-zeo-ie-STM50, which was used for pull-down analysis, was constructed as follows. The synthetic oligonucleotides ieST1 and ieST2 were annealed and inserted into pOriR6K-zeo-ie by the use of ApaI and XhoI, resulting in pOriR6K-zeo-ie-ST. The M50 ORF was amplified with the AB6-02 and M50Strep primers and cloned into pOriR6K-zeo-ie-ST, using KpnI and XhoI.
To generate N-terminal M53 deletion mutants lacking aa 16 to 106, pOriR6K-zeo-ie-M53 was amplified by inverse touchdown PCR (20) using the 5′-SapI-delN and 3′-Ndel-SapI primers, which carried a nuclear localization sequence (NLS) derived from the simian virus 40 (SV40) large T antigen. The PCR product was treated with SapI and religated, generating the pM53-Δ16-106NLS construct, in which the deleted sequence was replaced with the SV40 NLS. The NLS sequence was removed from pM53-Δ16-106NLS by AgeI digestion, and religation generated the pM53-Δ16-106 construct carrying only the 16-106 deletion. An N-terminal M53 deletion mutant lacking aa 16 to 136 was generated by insertion of the NLS21 and NLS22 annealed synthetic oligonucleotides into AgeI- and BspHI-treated pM53-Δ16-106NLS, resulting in pM53-Δ16-136NLS.
Point mutations were introduced into the M53 ORF by mutated overlapping primers (see Table S1 in the supplemental material for a primer list), as follows. The M53 ORF was amplified by PCRs using AB6-02 and reverse mutagenesis primers as well as AB7-02 and forward mutagenesis primers, thereby generating 5′ and 3′ M53 fragments, respectively. The 5′ fragments were digested with KpnI/SapI, and the 3′ fragments were digested with SapI/MluI; the fragments were then inserted into KpnI- and MluI-treated pOriR6K-zeo-ie-M53 in a one-step reaction.
An M53-green fluorescent protein (GFP) fusion protein was generated as follows. The mutant M53-s106 ORF was amplified by a PCR using primers AB6-SpAs and AB7-02. The PCR product was inserted into Litmus28 (NEB) with BamHI and SpeI. The subcloned wt and mutant M53-s106 fragment was then isolated by SpeI and PmeI digestion and inserted into NheI- and SmaI-treated pEGFPN1 (Clontech).
Linker-scanning mutagenesis of the M53 ORF.
For the mutagenesis procedure, the M53 ORF was cloned from pCR3-M53 (3) into Litmus28 (NEB) by using KpnI and XbaI, generating pL-M53, which was used as a target vector for transposon mutagenesis. pST76K-S4, a new transposon donor vector with a temperature-sensitive origin of replication, was generated by inserting a mini-transposon carrying the KpnI/SacI fragment of pGPS-4 (NEB) into pST76K (21). pLit28-M53 was subjected to in vitro random transposon insertion mutagenesis using TnsABC* transposase (NEB) and pST76K-S4 as the donor according to the manufacturer's instructions. Escherichia coli DH10B cells (Invitrogen) were transformed with the transposition mixture, and insertion mutants were selected by chloramphenicol and ampicillin at 43°C to remove both the intact acceptor and donor plasmids. Plasmid DNAs were prepared from this primary pool of insertion mutants, and the 1,084-bp KpnI/NsiI fragments containing the M53 ORF and the randomly inserted mini-transposons were isolated and recloned into the KpnI/NsiI-treated pOriR6K-zeo-ie rescue plasmid. Recombinants containing the M53 ORF with the mini-transposon insertions were selected by chloramphenicol and Zeocin. Plasmid DNAs were prepared from this secondary pool of insertion mutants, and the mini-transposon was removed by PmeI digestion and religation, generating a pOriR6K-zeo-ie-M53mut insertion library which was maintained in E. coli PIR1 (Invitrogen). Insertion sites were identified for selected clones by PCR screening and sequencing as described previously (3).
Generation and reconstitution of recombinant MCMV BACs.
ΔM53-BAC was generated on the basis of pSM3fr-16FRT17 (4). A linear recombination fragment carrying a kanamycin resistance marker was generated by PCR with a pACYC177 template (NEB) by using the 5′-M53del and 3′-M53del primers. The M53 ORF (nucleotide positions 78461 to 79459 of MCMV strain Smith, according to Rawlinson et al. [22]) was deleted from pSM3fr-16FRT17 by ET recombination in E. coli with this linear recombination fragment, as described previously (33). The wt and mutant rescue plasmids were inserted into ΔM53-BAC at the FRT site as described previously (3). For virus reconstitution, semiconfluent MEF in 6-cm dishes were transfected with 1.5 μg of purified DNA. Two independent BAC clones were always transfected in two replicates. Twenty-four hours after transfection, cells were replated onto 10-cm dishes and then refed weekly. Cultures were inspected for 6 weeks after transfection. As a control, pM53E DNA was transfected, and a reconstitution experiment was considered valid when pM53E DNA-derived virus plaques occurred during the second week posttransfection.
M53 transcomplementation.
To create an M53/p38 complementing cell line, the M53 ORF from pOriR6K-zeo-ie-M53 was isolated by BsaI and SpeI and inserted into the PvuII- and NheI-cleaved pTRE2Hyg vector (Clontech), resulting in pTRE-M53. Stable NIH 3T3 transfectants harboring pTRE-M53 were selected according to the manufacturer's instructions. Thirty-two independent cell clones were expanded and designated NT/M53-1 to -32.
An artificial transcription unit expressing tTA under the control of the HCMV immediate-early promoter was cloned into pOriR6K-zeo-ie by using SpeI and NotI, generating pO6-tTA, which was inserted into pSM3fr-16FRT17 by the Flp system in E. coli (3), resulting in pm16-17FRT-tTA. Subsequently, the M53 gene was deleted from m16-17FRT-tTA by ET recombination using the recombination cassette for M53 deletion described above, generating ΔM53-tTA-BAC. To reconstitute the ΔM53-tTA virus, eight of the NT/M53 cell clones were transfected with ΔM53-tTA-BAC. After the appearance of a significant cytopathic effect, the culture supernatants were harvested and pooled. The NT/M53 cell clones were tested for productivity, using the pooled supernatant for infection, and NT/M53-12 was chosen for the propagation of both m16-17FRT-tTA and ΔM53-tTA. The resulting virus stocks were titrated on NT/M53-12 by the tissue culture infective dose (TCID) method (15).
To determine virus production under multistep growth conditions, NIH 3T3 cells were infected with either ΔM53-tTA or m16-17FRT-tTA at an MOI of 0.1. Supernatants of the infected cells were harvested at day 5 postinfection, and the released infectious units were determined by the 50% TCID (TCID50) method, using NT/M53-12 cells. To determine virus production under single-step growth conditions, NIH 3T3 cells were infected with either ΔM53-tTA or m16-17FRT-tTA at an MOI of 3. Supernatants of the infected cells were harvested at day 3 postinfection, and the released infectious units were determined by the TCID50 method, using NT/M53-12 cells.
Metabolic labeling and coimmunoprecipitation.
Subconfluent 293 cells in 6-cm dishes were cotransfected with pOriR6K-zeo-ie-M53 or pOriR6k-zeo-ie-M53mut and with 3.5 μg of the pOriR6K-zeo-ie-M50 or pOriR6K-zeo-ie vector (3) by Ca2PO4 precipitation (29). Twenty-four hours after transfection, the newly synthesized proteins were labeled with 300 μCi/ml [35S]methionine-cysteine (Promix; Amersham Bioscience) for 1 hour. The cells were lysed, and coimmunoprecipitation was carried out as described previously (3). An M50/p35-specific polyclonal rabbit antiserum (19) and protein A-Sepharose (Amersham Biosciences) were used to precipitate the M50/p35-specific complexes. M53/p38 was precipitated on protein G-Sepharose beads (Amersham Biosciences) with a specific rat polyclonal antiserum raised against a synthetic peptide representing the 15 N-terminal amino acids of M53/p38 (3).
Strep tag pull-down assay.
Subconfluent 293 cells in 6-cm dishes were cotransfected with 3.5 μg of the construct pOriR6K-zeo-ie-STM50, expressing Strep-tagged M50/p35, and 3.5 μg of plasmid pOriR6K-zeo-ie-M53 or pOriR6k-zeo-ie-M53mut by Ca2PO4 precipitation (29). Twenty-four hours after transfection, the cells were washed with phosphate-buffered saline, scratched from the plates, and resuspended in phosphate-buffered saline. Five percent of the cell suspension was lysed directly in loading buffer (62.5 mM Tris, pH 6.8, 2% [vol/vol] sodium dodecyl sulfate [SDS], 10% [vol/vol] glycerol, 6 M urea, 5% [vol/vol] β-mercaptoethanol, 0.01% [wt/vol] bromophenol blue, 0.01% [wt/vol] phenol red), and the samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) to determine the total protein load. The rest of the cell suspension was collected by centrifugation, and pellets were lysed in hypotonic buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2) by sonication. The nuclei were collected by centrifugation and treated with M-Per mammalian protein extraction reagent (Pierce). Strep-Tactin Sepharose (IBA) was used to pull down Strep-tagged M50/p35 complexes according to the manufacturer's instructions. Desthiobiotin (IBA)-eluted samples were separated by SDS-PAGE. The proteins were transferred from the gels onto Hybond-P membranes (Amersham Biosciences) in the presence of blotting buffer (25 mM Tris, 192 mM glycine, 20% [vol/vol] methanol, pH 8.3). Membranes were blocked in TBS-T (Tris-buffered saline, 0.05% Tween 20) containing 5% nonfat dry milk overnight at 4°C. To detect M53/p38, the membrane was incubated at room temperature with TBS-T containing M53/p38-specific polyclonal rat antiserum (3). Membranes were washed with TBS-T and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (Dianova). The proteins were visualized with an ECL-Plus Western blot detection system (Amersham Biosciences).
Confocal laser scanning microscopy.
Transfected NIH 3T3 cells were grown on glass coverslips and fixed as previously described (19). M53/p38 was visualized with a specific polyclonal rat antiserum as the primary antibody and fluorescein isothiocyanate-conjugated goat anti-rat immunoglobulin G (Dianova) as the secondary antibody. M50/p35 was visualized with a polyclonal rabbit antiserum as the primary antibody and Texas red-conjugated donkey anti-rabbit immunoglobulin G (Dianova) as the secondary antibody.
RESULTS
M53/p38 is expressed with late kinetics and is essential for MCMV replication.
The expression kinetics of M53/p38 was determined with NIH 3T3 cells infected with wt MCMV at an MOI of 0.5. Cell lysates were prepared at different times after infection, and proteins were separated by SDS-PAGE. M53/p38 signals were visualized by Western blotting using rat antiserum specific to the N-terminal 15 aa of the M53/p38 ORF (3). The M53/p38-specific 38-kDa band became detectable at 18 h postinfection (Fig. 1A, lane 4). M53/p38 gradually accumulated at later time points (Fig. 1A, lanes 5 and 6). To confirm that M53/p38 is a true late protein, DNA replication was blocked in infected cells by PAA to prevent the expression of late MCMV genes (9). M53/p38 was clearly detectable 24 h after infection in untreated cells (Fig. 1B, lane 3), whereas PAA treatment abolished expression of the protein (Fig. 1B, lane 6). Accordingly, the expression of the major capsid protein (M86), another late gene product, was blocked by PAA treatment, while the expression of the immediate-early gene product pp89 was not affected. Therefore M53/p38 expression follows late kinetics.
FIG. 1.
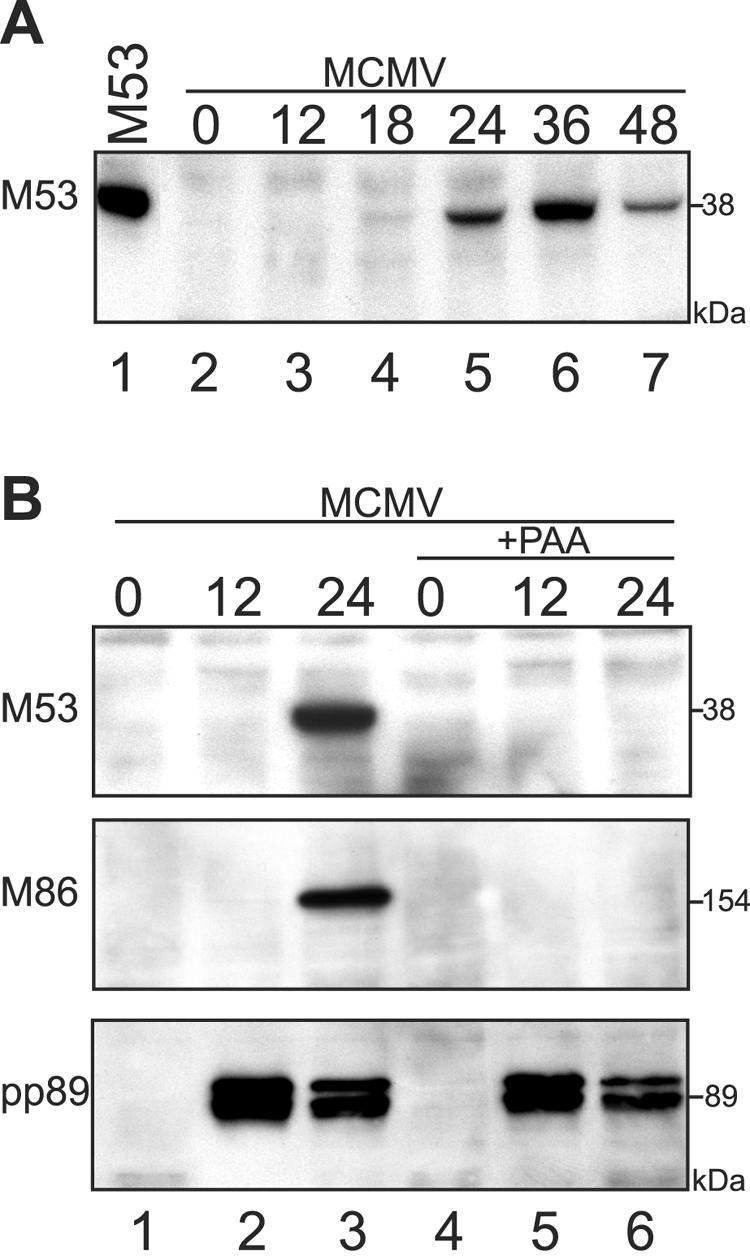
Expression kinetics of M53/p38. (A) NIH 3T3 fibroblasts were infected with wt MCMV, and cell lysates were prepared at the indicated time points (hours) after infection (lanes 2 to 7). As a control, NIH 3T3 cells were transfected with pOriR6k-ie-M53 (M53), and a cell lysate was prepared at 24 h posttransfection (lane 1). Proteins were separated by SDS-PAGE, and the M53/p38 signal was visualized by Western blotting using specific rat antiserum. (B) NIH 3T3 fibroblasts were infected with wt MCMV in the absence or presence of PAA (lanes 1 to 3 and lanes 4 to 6, respectively). Cell lysates were prepared from infected cells at the indicated time points (hours) after infection. Proteins were separated by SDS-PAGE, and signals for M53/p38, M86, and pp89 were visualized by Western blotting using specific antisera.
M50/p35 and M53/p38 colocalize to the nuclear membrane. As we have shown previously, M53/p38 is diffusely distributed in the nuclei of transfected 293 cells expressing only M53/p38, whereas isolated M50/p35 is found in nuclear and endoplasmic reticulum membranes (19). This phenomenon is reproducible after transfection of NIH 3T3 cells (3).
The deletion of UL31 or UL34 from the HSV-1 genome severely reduces viral growth, but the genes are not strictly essential (14). In contrast, the M50 gene is essential for virus reconstitution of MCMV (3). To test whether M53 is an essential gene, the M53 ORF was deleted from m16/17FRT-MCMV-BAC, using ET recombination in E. coli. The capacity to reconstitute MCMV from MCMV-BAC and the M53 deletion construct (m16/17FRT-ΔM53) was compared by transfection of the purified BAC DNAs into MEF. The transfection of m16/17FRT-MCMV-BAC resulted in MCMV progeny, whereas the transfection of m16/17FRT-ΔM53-BAC did not.
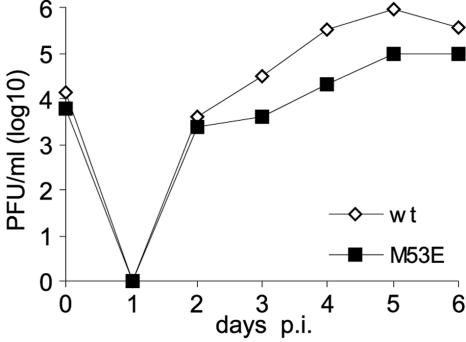
The null phenotype of m16/17FRT-ΔM53-BAC was reverted in order to ensure that the inability to gain infectious virus after the transfection of m16/17FRT-ΔM53-BAC was solely due to the deletion of the M53 gene. By using the rescue plasmid pOriR6K-ie-zeo-M53 carrying an FRT site (3), the M53 ORF was reinserted into m16/17FRT-ΔM53-BAC at its FRT site in Flp-expressing E. coli. The Flp recombinase directs site-specific recombination between the FRT sites located on the rescue plasmid and the BAC (for construction principles, see reference 3). The rescue inserted the M53 ORF into the deletion BAC at a position between the m16 and m17 genes, resulting in pM53E-BAC. The predicted genome sequence of pM53E-BAC was confirmed by restriction pattern analysis and sequencing (data not shown). The transfection of MEF cells with pM53E-BAC DNA resulted in viral progeny (M53E-MCMV). The growth of M53E-MCMV was slightly attenuated. Ectopic expression of the M53 gene in M53E-MCMV resulted in moderately (0.7 log) lower end titers at 5 days postinfection under multistep growth conditions than those of the parental BAC-derived virus (Fig. 2). The slight attenuation of M53E-MCMV compared to wt MCMV was observed in four independent experiments and may be connected to the altered expression kinetics of ectopic M53/p38. Thus, the M53 gene is essential for MCMV growth, and the M53 null phenotype could be reverted by ectopic expression of the M53 ORF.
FIG. 2.
Ectopic rescue of M53 deletion mutant. The growth kinetics of the viruses derived from pSMfr3 (wt) or pM53E (M53E) were determined on NIH 3T3 cells. Subconfluent cultures were infected with the respective viruses. Supernatants of the infected cells were harvested on the indicated days, and the infectious progeny was quantified by a plaque assay.
In order to trans-complement the M53 deletion, NIH 3T3-derived cell lines were generated that harbored the M53 ORF under the control of the artificial TRE promoter (NT/M53 clones). The TRE promoter is practically silent in mammalian cells in the absence of its fusion transactivator tTA, allowing the establishment of stable cell lines even if the expression of the gene of interest would be deleterious for the host cell (11). A tTA expression cassette was introduced into m16/17FRT-MCMV-BAC, generating m16/17FRT-tTA, which possesses all essential functions of MCMV and expresses the tTA protein constitutively. Subsequently, the M53 gene was deleted from this recombinant, as described above for m16/17FRT-ΔM53, resulting in ΔM53-tTA-BAC, which lacks the M53 gene but expresses tTA. m16/17FRT-tTA and ΔM53-tTA-BAC were reconstituted by transfecting different NT/M53 cell clones. Both were able to produce infectious viruses, which were then propagated on the complementing cell line NT/M53-12. ΔM53-tTA and m16/17FRT-tTA were tested for viability under both multi- and single-step growth conditions, using noncomplementing NIH 3T3 and complementing NT/M53 cells. ΔM53-tTA was unable to produce progeny under noncomplementing conditions at both low and high MOIs (Table 1), confirming that M53 is an essential MCMV gene.
TABLE 1.
Growth of trans-complemented M53 deletion virus under noncomplementing conditions
| Virus | Virus stock titera | Titer on NIH 3T3 cells at 5 days postinfection
|
|
|---|---|---|---|
| MOI of 0.1 | MOI of 3.0 | ||
| ΔM53-tTA | 0.8 × 107 | <101 | <101 |
| m16/17FRT-tTA | 2.0 × 107 | 2.4 × 105 | 6.2 × 106 |
The ΔM53-tTA and m16/17FRT-tTA virus stocks were titrated on the NT/M53-12 cell line by the TCID50 method. The titers of wt MCMV determined by the TCID50 method are 0.5 to 1 log higher than the titers determined by the standard plaque assay using MEF.
Construction of an insertion mutant library of M53 ORFs and analysis of the mutants in the context of the MCMV genome.
A library of M53 insertion mutants was generated. The M53 ORF was first subcloned into the Litmus28 vector, which served as the acceptor for Tn7-based transposon-mediated mutagenesis (2). We constructed a new transposon donor plasmid, which carries the Tn7-derived mini-transposon and a conditional origin of replication to allow donor plasmid elimination after the transposition reaction at a nonpermissive temperature (21). After in vitro transposition, acceptor plasmids carrying the mini-transposon were selected and purified as a pool. The M53 ORF, which was increased in size due to the inserted transposon, was isolated and inserted into the rescue plasmid pOriR6K-ie-zeo. PmeI digestion removed the mini-transposon, and subsequent religation resulted in the insertion of 5 aa or a stop codon into the coding sequence. From the library of 28,000 pOriR6K-ie-zeo-M53 mutants, 986 independent clones were screened by PCR, and 498 plasmids with an insertion in the M53 ORF were sequenced. Depending on the primary insertion site in the codon, M53 readthrough and truncation mutants were found, some of which were mutated at the same amino acid position. From this set, a total of 46 in-frame insertion mutants, representing a distribution of about one insertion per 10 to 15 aa, and 8 stop mutants were selected for the complementation assays and were reinserted one by one into m16/17FRT-ΔM53-BAC.
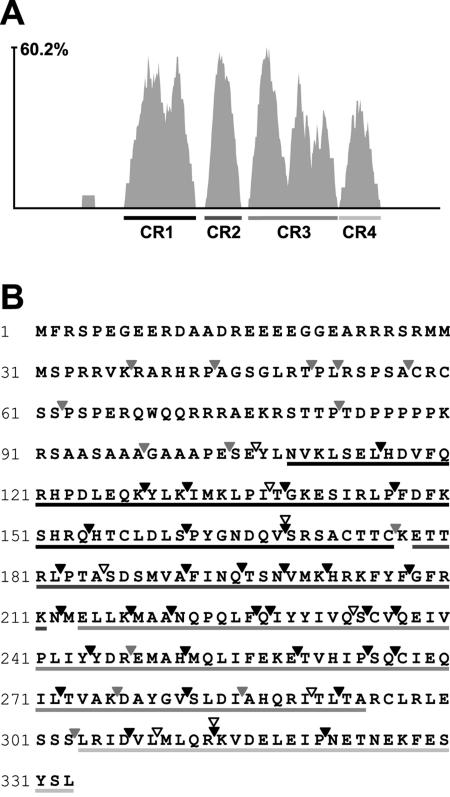
Using the AlignX program (Invitrogen), the aa sequences of a total of 36 members of the UL31 family (accession number PF02718) (1; www.sanger.ac.uk/Software/Pfam) were aligned (Fig. 3). The average similarity between betaherpesvirus UL31 members was 47.5%, with an average of 25.1% within the whole family. However, the homology was not equally distributed along the sequences. A variable N-terminal region and a conserved region including the central and C-terminal two-thirds of the protein were identified, with a similarity of 84.6% among the betaherpesviruses and of 44% for the whole family. Furthermore, when the entire UL31 family was aligned, the similarity plot indicated four peaks along the conserved region, which may reflect strictly conserved functional domains and were designated conserved regions 1 to 4 (CR1 to CR4). We assumed that, according to the sequence conservation, lethal mutations should accumulate in the conserved central and C-terminal regions (corresponding to aa 115 to 333 of M53/p38) rather than in the variable N-terminal region (corresponding to aa 1 to 114 of M53/p38). We expected to find the binding site(s) for M50/p35 within one of the four conserved regions.
FIG. 3.
Analysis of M53 insertion mutants by ectopic cis-complementation of ΔM53-BAC. (A) Sequence comparison of M53/p38 homologues. The amino acid sequence of M53/p38 was aligned with the sequences of all 36 UL31 family members by use of the Vector NTI Align X program (Invitrogen) via the BLOSUM 62 similarity matrix. The depicted similarity plot was calculated using a 5-aa window size, with scores for identity and strong and weak similarities of 1.0, 0.5, and 0.2, respectively. The x axis represents the number of amino acids in the consensus sequence. Conserved regions 1 to 4 (CR1-4) are indicated below the diagram. (B) M53 mutants and their ability to rescue virus growth of the ΔM53 genome. The sequence displayed is the amino acid sequence of M53/p38. Arrowheads indicate transposon insertion sites. Open arrowheads indicate insertions leading to a stop codon. Light gray arrowheads indicate in-frame insertion mutations that rescued the ΔM53 phenotype. Black arrowheads indicate in-frame insertion mutations that were not able to rescue the ΔM53 null phenotype. Underlined parts of the M53 amino acid sequence indicate the conserved regions described in panel A.
First, the ability of the selected mutant set to rescue the M53 null phenotype was tested. To this end, ΔM53-MCMV-BAC with reinserted M53 mutants was transfected into MEF. The results of the virus reconstitution screen are summarized in Fig. 3B. All in-frame insertions within the N-terminal part of the M53 ORF (aa 1 to 114) gave rise to viral progeny. In contrast, 32 of 37 insertion mutations in the central- and C-terminal domains caused a lethal phenotype. The clusters of lethal mutants are interspersed with single viable insertion mutants. The 14 viable insertion mutants showed viral plaques comparable to those of the MCMV-M53E virus. A set of eight truncation mutants was chosen to test the essentiality of the four conserved sequence clusters. All truncation mutants turned out to be nonfunctional. Altogether, loss-of-function mutants accumulated in the conserved central- and C-terminal parts of M53/p38. The less-conserved N-terminal domain tolerated a number of insertions, suggesting that this domain has a less stringent function, as predicted from sequence homology.
The N-terminal one-third of M53/p38 contains a nuclear localization signal.
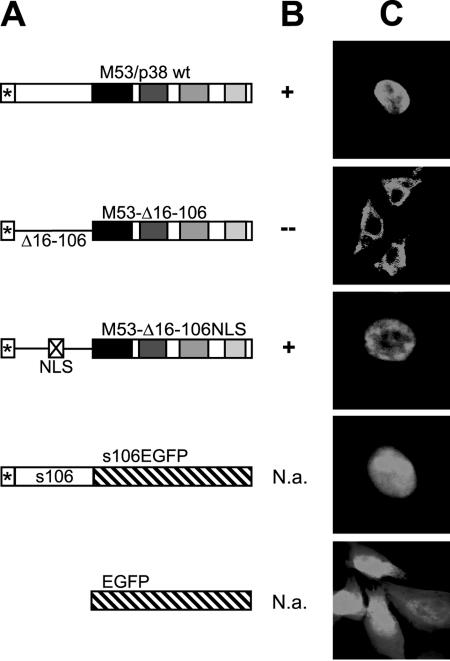
Within the nonconserved N-terminal region of M53/p38, two overlapping bipartite NLSs are predicted at aa 24 to 41. In order to test this, the N-terminal domain of the wt M53 protein from aa 16 to 106 was deleted, thereby creating mutant M53-Δ16-106. The very N-terminal 15 aa were kept as a target sequence for the M53/p38 peptide-specific antibody (3). After insertion into m16/17FRT-ΔM53-BAC, M53-Δ16-106 was not able to rescue the null phenotype of the genome (Fig. 4). After the transfection of NIH 3T3 cells with pOriR6K-ie-zeo expressing M53-Δ16-106, cytoplasmic staining was observed instead of the diffuse nuclear staining typical for wt M53/p38 (Fig. 4). To prove that the loss of function of the M53-Δ16-106 mutant was due to protein mislocation, we inserted the 9-aa NLS from the SV40 large T antigen into M53-Δ16-106 to create M53-Δ16-106NLS. The heterologous NLS restored the correct nuclear localization of the protein (Fig. 4). The existence of an NLS within the N-terminal part of M53/p38 was tested by a fusion between aa 1 to 106 of M53/p38 and the enhanced GFP (EGFP) ORF. NIH 3T3 cells expressing EGFP showed fluorescent protein in both the cytoplasm and the nucleus, whereas the M53/1-106-EGFP fusion construct was restricted to the nucleus. Furthermore, M53-Δ16-106NLS, but not M53-Δ16-106, colocalized with M50/p35 upon coexpression (data not shown). Finally, the mutant M53-Δ16-106NLS, lacking aa 16 to 106 but carrying the SV40 NLS, but not M53-Δ16-106, rescued the null phenotype of m16/17FRT-ΔM53-BAC. Thus, nuclear targeting of the protein is the only essential function of the N-terminal part of M53/p38.
FIG. 4.
Functional analysis of N-terminal deletion mutants of M53. (A) Schematic representation of the different deletion constructs. The epitope for the anti-M53/p38 serum is indicated by a star at the very N terminus of each diagram. The variable sequences are represented by open boxes. The conserved regions are shown as shaded boxes. The deleted sequences are indicated by a line. The inserted SV40 NLS is indicated by a crossed box. The EGFP sequence is shown as a diagonally hatched box. (B) wt M53 and mutants M53-Δ16-106 and M53-Δ16-106NLS were reinserted into ΔM53-BAC. BAC DNA was isolated, transfected into MEF cells, and screened for plaque formation, and the rescue results are indicated (+, −, or N.a. [not applicable]). (C) Subcellular localization of expressed proteins with N-terminal deletions. NIH 3T3 fibroblasts were transfected with the indicated constructs. The localization of the expressed proteins was visualized by indirect immunofluorescence for wt M53, M53-Δ16-106, and M53-Δ16-106NLS. Endogenous fluorescence was recorded for EGFP and mutant s106EGFP.
M50/p35 binding site of M53/p38.
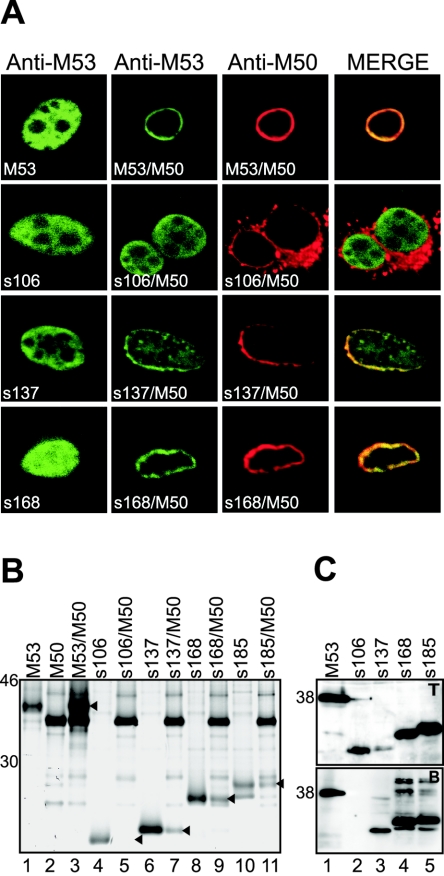
Recently, we showed that M53/p38 binds directly to M50/p35 and that the binding region of M50/p35 is essential for MCMV growth (3). We predicted that the binding of M53/p38 to M50/p35 should also represent an essential function. Therefore, the pool of lethal M53 mutants should contain mutants which have lost the ability to bind to M50/p35. For a first rough mapping of the binding region, the selected set of M53/p38 truncation mutants was analyzed. To this end, NIH 3T3 fibroblasts were transfected with either wt M53/p38 or M53/p38 mutants in the presence or absence of M50/p35 coexpression. The subcellular localization of wt and mutated M53/p38 was analyzed by confocal microscopy after indirect immunofluorescence staining with specific antisera (Fig. 5A). After isolated expression, all truncation mutants as well as wt M53/p38 (M53) showed the typical diffuse nuclear staining pattern (Fig. 5A, first column). After cotransfection with M50/p35, the same diffuse nuclear staining was observed only for the stop mutant s106, which lacks all four conserved regions (Fig. 5A, second column). The other stop mutants, i.e., s137, lacking CR2 to -4 and the C-terminal part of CR1, s168, lacking CR2 to -4 (Fig. 5A, third and fourth columns), s233, s290, and s309 (data not shown), colocalized with M50/p35 at the nuclear rim.
FIG. 5.
Analysis of interaction of M53 stop mutants with M50/p35. (A) Localization of M53 stop mutants. NIH 3T3 cells were transfected with M53 stop mutants s106, s137, and s168 alone (first column) or together with wt M50/p35 (second to fourth columns). Single transfected cells were stained with a specific rat antiserum against M53/p38. For detection, a fluorescein isothiocyanate-conjugated secondary antibody was used (green). Cotransfected cells were treated as described above and costained with an M50/p35-specific rabbit serum, which was detected by a Texas red-coupled secondary antibody (red). As a control, wt M53/p38 was used (first row). (B) Coimmunoprecipitation of M53 stop mutants and M50/p35. Plasmids expressing wt M53/p38 (lane 1), wt M50/p35 (lane 2), and M53 stop mutants (lanes 4, 6, 8, and 10) were transfected into 293 cells. In parallel, wt M53/p38 and M53 stop mutants were cotransfected with wt M50/p35 (lanes 3, 5, 7, 9, and 11). Cells were radioactively labeled, and singly transfected probes were precipitated with anti-M53/p38-specific rat serum on protein G-Sepharose. Protein complexes with wt M50 were precipitated with protein A-Sepharose, using anti-M50/p35-specific rabbit serum. Samples were analyzed by SDS-PAGE followed by autoradiography. (C) Pull-down assay of M53 stop mutants with Strep-tagged M50/p35. wt M53/p38 and M53 stop mutants were cotransfected with M50ST into 293 cells. Total cell lysates were analyzed to test the protein expression by Western blotting using a specific antiserum against M53/p38 (upper panel, total protein [T]). Proteins in complex with M50ST were precipitated by Strep-Tactin-Sepharose. Desthiobiotin eluates were analyzed by SDS-PAGE and blotted onto membranes. wt M53/p38 and mutant proteins were detected by Western blotting with M53/p38-specific antiserum (lower panel, bound protein [B]).
These colocalization experiments indicated a critical role of M53/p38 aa 1 to 137 for interaction with M50/p35. To confirm the results obtained by subcellular localization of the M53 truncation mutants, we performed coimmunoprecipitation experiments with the stop mutants. 293 cells were cotransfected with expression plasmids containing mutant M53 constructs and with pM50, expressing wt M50/p35. Controls were transfected with plasmids expressing wt M50, wt M53, or M53 truncation mutants alone. Cell lysates were precipitated by specific antisera. The wt M53/p38 protein was coprecipitated in the presence of M50/p35 when M50-specific antiserum was used (Fig. 5B, lane 3). The M53s106 stop mutant was unable to bind to M50/p35 (lane 5). The other stop mutants, M53s137, M53s168, and M53s185, bound M50/p35, as shown by the specific bands corresponding to the expected molecular masses after coprecipitation with anti-M50/p35 (lanes 7, 9, and 11). The same result was found with the s233, s290, and s309 stop mutants (data not shown). The binding pattern was also tested in pull-down assays after cotransfection of pM50ST, expressing Strep-II-tagged M50/p35, with wt and mutant M53 constructs. From the lysates of cotransfected 293 cells, proteins bound to M50ST were recovered by Strep-Tactin-Sepharose beads. The M50ST complexes were eluted from the beads by desthiobiotin, separated by SDS-PAGE, and subjected to Western blot analysis using M53/p38-specific antiserum. The data were in full agreement with the results obtained by coimmunoprecipitation with wt M50/p35. This convinced us that the tagged M50/p35 protein had kept the properties of wt M50/p35 with regard to M53/p38 binding (Fig. 5C).
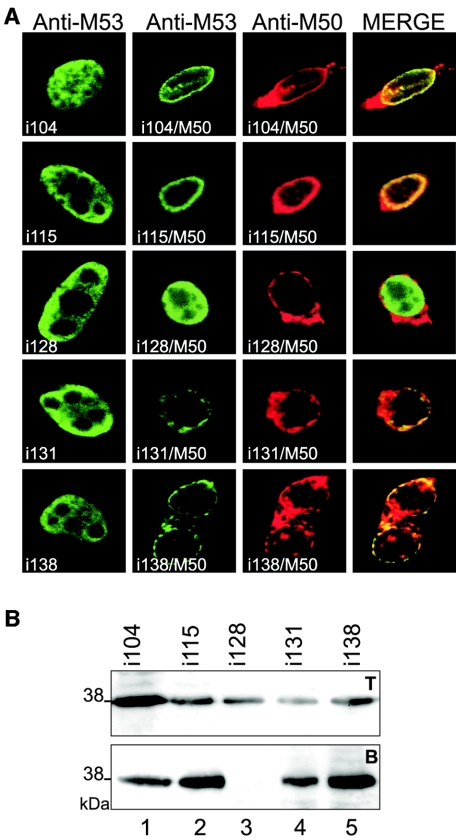
These data, together with the functionality of the M53Δ16-106NLS mutant in the context of the virus, suggested that a short sequence of M53/p38 CR1 is required for M50/p35 binding. To describe the contribution of specific sequences involved in the M50/p35-M53/p38 interaction in more detail, we analyzed M53/p38 mutants with insertions in the region between aa 106 and aa 138 by confocal microscopy and by the M50ST-mediated protein pull-down assay. NIH 3T3 cells were transfected with different M53/p38 insertion mutants alone or together with pM50. As expected, all analyzed M53 insertion mutants showed diffuse nuclear staining after isolated expression (Fig. 6A, first column). After cotransfection with pM50, only mutant M53i128 was not able to colocalize with M50/p35 at the nuclear rim (Fig. 6A, third column). All other insertion mutants tested, i.e., i104, i115, i131, and i138, tolerated 5-aa insertions inasmuch as they were recruited to the nuclear envelope by M50/p35 coexpression. In addition, 293 cells were cotransfected with pM50ST and the same set of M53/p38 insertion mutants which was used for the colocalization experiments. In parallel, the total cell lysates were analyzed to determine the expression of the M53/p38 mutants by Western blotting. Among the mutants tested, only the insertion mutant M53i128 was not pulled down by M50ST (Fig. 6B, lane 3), which is in line with the results of the colocalization studies described above.
FIG. 6.
Analysis of interaction of M53 insertion mutants with M50/p35. (A) Localization of M53 insertion mutants. NIH 3T3 cells were transfected with M53 insertion mutants i104, i115, i128, i131, and i138 alone (first column) or together with wt M50 (second to fourth columns). Single transfected cells were stained with a specific rat antiserum against M53/p38. For detection, a fluorescein isothiocyanate-conjugated secondary antibody was used (green). Cotransfected cells were treated as described above and costained with an M50/p35-specific rabbit serum, which was detected by a Texas red-coupled secondary antibody (red). (B) Pull-down analysis of M53 insertion mutants with Strep-tagged M50/p35. 293 cells were cotransfected with M53 insertion mutants and M50ST. Total cell lysates were analyzed by SDS-PAGE and Western blotting using an M53/p38-specific antiserum (upper panel, T). Proteins in complex with M50ST were precipitated with Strep-Tactin-Sepharose, and desthiobiotin-eluted proteins were separated by SDS-PAGE. Signals for M53/p38 insertion mutants were visualized by Western blotting using a specific antiserum against M53/p38 (lower panel, B).
Taken together, colocalization and protein-protein interaction assays using several C-terminal truncation and insertion mutants indicated that the region of M53/p38 which is necessary for M50/p35 binding is located at the beginning of CR1 and that the most important residues are probably represented by aa 115 to 131 of the M53/p38 protein.
Characterization of the M50/p35 binding site of M53/p38.
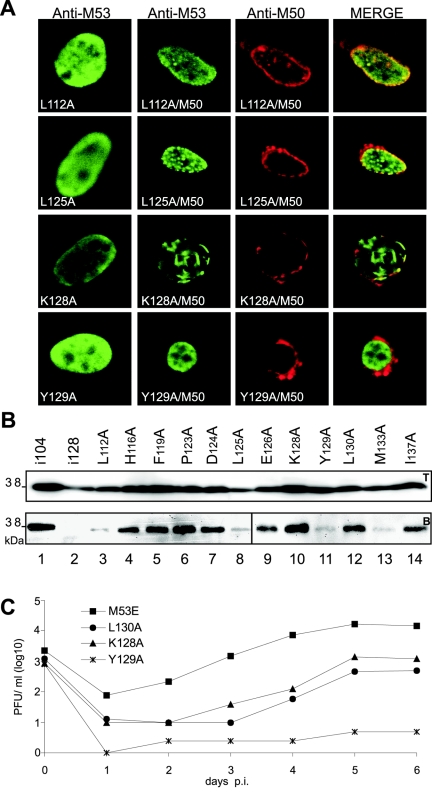
Insertions of five amino acids helped to identify a binding motif, but the contribution of the inserted amino acids that differed between the different insertion mutants was difficult to evaluate. In order to define the relative roles of individual amino acids with respect to M50/p35 binding, the 12 amino acids which are conserved in betaherpesviruses within the N-terminal half of CR1 (aa 112 to 137) were replaced one by one with alanine. After the transfection of NIH 3T3 cells with the M53/p38 point mutants alone or cotransfection with M50/p35, the subcellular localization of the M53/p38 mutants was analyzed. All M53/p38 point mutants showed diffuse nuclear staining when expressed alone (Fig. 7, first column) (not all data are shown; see Fig. S1 in the supplemental material). After coexpression with M50/p35, the intranuclear distribution of M53 point mutants fell into three classes of phenotypes: (i) mutants that redistributed to the nuclear rim and recruited M50/p35 similar to wt M53/p38 (M53P123A, M53D124A, and M53E126A), (ii) mutants that had apparently lost the ability to redistribute to the nuclear rim in the presence of M50/p35 (M53Y129A [Fig. 7A], M53L130A, and M53I133A), and (iii) mutants with intermediate phenotypes. Several mutants fell into the last class, and their phenotypic appearance was variable. One subset showed abundant diffuse intranuclear staining in addition to colocalization with M50/p35 at the nuclear rim (M53L112A [Fig. 7A], M53H116A, M53F119A, and M53I137A). The second type of mutants colocalized with M50/p35, at least to some extent. The remarkable phenotype, however, was a new distribution of M53/p38 mutants upon coexpression of M50/p35 resulting in the formation of discrete intranuclear aggregates (M53L125A) and fibrous structures (M53K128A) (Fig. 7A) which did not colocalize with M50/p35. In the absence of M50/p35, the intranuclear distribution of the mutants was indistinguishable from that of wt M53/p38.
FIG. 7.
Functional analysis of M53 point mutants. (A) Subcellular localization of M53 point mutants. NIH 3T3 cells were transfected alone with the M53 point mutants L112A, L125A, K128A, and Y129A (first column) or cotransfected with wt M50/p35 (second to fourth columns). Single transfected cells were stained with a specific rat antiserum against M53/p38. For detection, a fluorescein isothiocyanate-conjugated secondary antibody was used (green). Cotransfected cells were treated as described above and costained with an M50/p35-specific rabbit serum, which was detected by a Texas red-coupled secondary antibody (red). (B) Pull-down analysis of 12 M53 point mutants with Strep-tagged M50/p35. 293 cells were cotransfected with the indicated M53 point mutants and M50ST. Total cell lysates were analyzed to test protein expression by Western blotting using a specific antiserum against M53/p38 (upper panel, T). Proteins in complex with M50ST were precipitated by Strep-Tactin-Sepharose. Desthiobiotin eluates were analyzed by SDS-PAGE and blotted onto membranes. Signals for M53 point mutants were visualized by Western blotting with M53/p38-specific antiserum (lower panel, B). As a positive control for precipitation, the functional insertion mutant M53i104 was used (lane 1), and the M53 insertion mutant M53i128 served as a negative control (lane 2). (C) Rescue of ΔM53-BAC by M53 point mutants K128A, Y129A, and L130A. The growth kinetics of the viruses derived from pM53E, pM53K128AE, pM53Y129AE, and pM53L130AE are shown. NIH 3T3 cells were infected with the respective viruses. Supernatants of the infected cells were harvested on the indicated days, and virus titers were determined by a plaque assay.
Next, 293 cells were cotransfected with M50ST and the set of alanine-scanning M53/p38 mutants. Strep-Tactin-Sepharose pull-down assays were performed to analyze the binding capacity of the M53 point mutants to M50ST. Remarkably, despite the different distribution phenotypes, all M53 point mutants could be retrieved by M50ST pull-down assays, including those that did not show a detectable interaction in the colocalization assay (Fig. 7B). Notably, binding was found to be significantly reduced for the mutants M53L112A, M53L125A, M53Y129A, and M53M133A.
Since there was a substantial degree of variability in the colocalization assay and a certain heterogeneity of interactions detectable by the pull-down assay, we tested the M53 point mutants for functionality in the viral context. Interestingly, the null phenotype of ΔM53FRT-BAC could be reverted by all M53 point mutants. About 11 days are usually required to detect the first virus plaques after transfection of the ectopically complemented deletion genome. The virus reconstitution time and plaque formation were significantly delayed, to 3 weeks, for mutants M53K128A and M53L130A. Virus reconstitution from the BAC carrying M53Y129A required 6 weeks. Accordingly, the growth of mutants M53K128A and M53L130A was reduced by 1 order of magnitude, and M53Y129A was attenuated >3 orders of magnitude, compared with a genome complemented by the wt M53 ORF (Fig. 7C). These data show that already the exchange of a single aa within the binding region of M53/p38 can strongly affect M50/p35 binding but cannot completely abolish the M50/p35-M53/p38 interaction and thus can rescue the null phenotype.
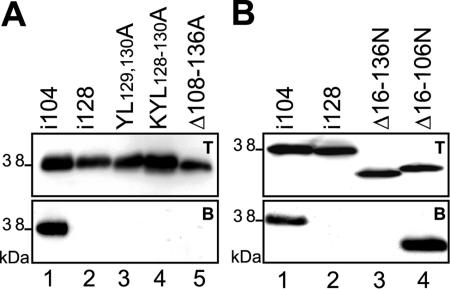
To rigorously test the binding site, we replaced either two adjacent critical amino acids (M53YL129-130A) or three adjacent amino acids (M53KYL128-130A) by alanine. In addition, we deleted the sequence of aa 108 to 136 of M53 (M53Δ108-136). As expected, each of these mutants completely failed to colocalize with M50/p35 (see Fig. S2 in the supplemental material), failed to interact with M50ST in the pull-down assay (Fig. 8A), and also failed to rescue the M53 null phenotype. The N-terminal deletion mutant M53-Δ16-106NLS, carrying a deletion of aa 16 to 106 and an artificial NLS, rescued the M53 deletion after ectopic reinsertion into the mutant MCMV-BAC, indicating that this mutant can interact with M50/p35. To confirm the M50/p35 interaction site mapping data, the N-terminal deletion was increased to aa 16 to aa 136 (M53-Δ16-136NLS). As expected, M53-Δ16-106NLS did interact with M50ST in the pull-down assay, whereas M53-Δ16-136NLS did not (Fig. 8B). Also, in contrast to M53-Δ16-106NLS, the N-terminal deletion mutant M53-Δ16-136NLS was not able to rescue the M53 null phenotype upon insertion into the deletion BAC.
FIG. 8.
Analysis of interaction of M50/p35 with M53 point and N-terminal deletion mutants. (A) Pull-down analysis of M53 point mutants YL129,130A, KYL128-130A, and M53-Δ108-136 with Strep-tagged M50. 293 cells were cotransfected with M53 point mutants and M50ST. The expression of the constructs was tested by SDS-PAGE with total cell lysates and by Western blotting using an M53/p38-specific antiserum (upper panel, T). Proteins in complex with M50ST were precipitated by Strep-Tactin-Sepharose. Desthiobiotin eluates were analyzed by SDS-PAGE and blotted onto membranes. Signals for M53 point mutants were visualized by Western blotting with an M53/p38-specific antiserum (lower panel, B). As a positive control, a functional insertion mutant, M53i104, was used (lane 1), and M53i128, which failed to bind to M50/p35, served as a negative control (lane 2). (B) Pull-down analysis of N-terminal deletion mutants of M53/p38. 293 cells were cotransfected with the M53 N-terminal deletion mutant M53-Δ16-106NLS or M53-Δ16-136NLS and M50ST. Analyses of total cell lysates (upper panel, T) and protein complex formation (lower panel, B) were performed as described above.
DISCUSSION
The comparative analysis of herpesvirus common protein functions is an interesting area of research. Although the three subgroups of herpesviruses diverged from a common ancestor probably more than 50 million years ago, the amino acid sequences of proteins involved in virus morphogenesis still indicate similar functions. However, sequence homology does not predict function, and proteins with no detectable sequence homology can still carry out similar functions. Since proteins of viruses and hosts may change motifs and domains during evolution, the spectrum of activities executed by related proteins may differ considerably.
We owe our basic knowledge of the herpesvirus common proteins governing the egress of the herpesvirus nucleocapsid from the cell nucleus to pioneering studies of alphaherpesviruses. To achieve nucleocapsid egress, the UL31 and UL34 proteins need to form a complex at the nuclear membrane. Deletion of either protein severely compromises virus replication but still gives rise to progeny (5, 8, 14, 25, 26, 35). For beta- and gammaherpesviruses, the conditions are even more stringent. The lack of either protein abolishes virus replication (3, 7, 10). Therefore, these studies could only be initiated after cloning and mutagenesis of complete infectious herpesvirus genomes as BACs in E. coli (16, 34). Published work and data presented in this study converge to the conclusion that UL31 and UL34 homologues of alpha-, beta-, and gammaherpesviruses have to interact at the inner nuclear membrane to execute their function as NECs (3, 5, 7, 10, 12, 14, 19, 24, 25, 31, 35). This might involve contact with several unknown cellular and viral proteins. The mutual binding sites need to be determined to address the question of which functions of these proteins are conducted in isolation and which are executed subsequent to NEC formation. In a previous paper, we reported on the binding domain in M50/p35, the UL34 homolog of a betaherpesvirus (3). The M50 gene was subjected to random mutagenesis followed by reintroduction into the viral genome (3). Due to frequent insertions into the rescue vector backbone, the efficiency of random insertion mutagenesis of the M50 ORF was <20% and required laborious screening. Here we excluded vector insertions by subcloning the primary pool of transposon-labeled M53 ORFs into the rescue vector, and the use of a temperature-sensitive transposon donor raised the successful insertion frequency to 50%. We created a library of about 28,000 insertion/stop mutants in the M53 ORF, screened 986 by PCR, and sequenced 498 PCR-positive clones. A total of 54 random mutants (46 M53 insertion mutants and 8 truncation mutants) were tested both at the level of isolated expression and for complementation of the M53 null phenotype in the viral context. In addition, a total of 18 targeted mutants were also tested in the genomic context. Altogether, we give the first report of the binding site of a UL31 protein family member, M53/p38.
We report the following observations. (i) A sequence comparison of 36 known members of the UL31 family of herpesvirus proteins revealed an N-terminal variable region and four conserved regions (CR) located within the C-terminal two-thirds of the sequences. (ii) The only essential function of the nonconserved N-terminal end of M50/p35 is to provide an NLS. (iii) Within CR1 (aa 115 to 174 in MCMV M53/p38), we found the binding motif for M50/p35. This motif is represented by aa 115 to 137, which are necessary for binding to M50/p35. (iv) Mutation of all conserved amino acids in this region into alanines resulted in different phenotypes with regard to NEC formation, revealing a prominent role for the residues K128, Y129, and L130. Nevertheless, all single point mutants were able to bind to M50/p35 and to rescue the M53 null phenotype of the genome. However, when two or three of the critical residues were replaced, the mutants failed to bind to M50/p35 and did not rescue the M53 null phenotype. (v) The C-terminal conserved regions 2 to 4 (CR2-4) bear an as yet unidentified essential function(s). Insertion mutants in these regions could bind to M50/p35 (data not shown) but lacked functionality.
An alignment of M53 with members of the UL31 family demonstrates a conserved central and C-terminal part within UL31 family homologues, whereas the N-terminal region is variable. The results of the functional analysis of M53 insertion mutants were in line with the in silico predictions. Nonfunctional insertion mutants accumulated only within the conserved two-thirds of the M53 ORF. Within the N-terminal variable region of M53/p38, two overlapping NLSs were predicted between aa 24 and 42. The deletion mutant of M53/p38 lacking the region from aa 16 to 106 failed to rescue virus growth in the absence of the wt M53 gene; however, the introduction of an artificial 9-aa NLS could restore the functionality of this mutant, demonstrating that the variable N-terminal domain of M53/p38 contains an NLS as a functional element. The consensus sequence for a nuclear targeting signal (6) is present in only 14 of 36 members of the UL31 family. However, if an NLS is predicted, it is always located within the N-terminal variable region. All analyzed members of the UL31 protein family are localized exclusively in the nucleus upon isolated expression, indicating that they have an active NLS, and we believe that this is the function of the N-terminal variable domain.
A conserved feature in all herpesvirus subgroups is the interaction between M50/p35 homologues (UL34) and M53/p38 homologues (UL31). A stop mutant suggested that the N-terminal 136 aa of M53/p38 are necessary and sufficient for M50/p35 binding. The N-terminal sequence of aa 16 to 106 was replaced without a loss of functionality and placed the M50/p35 binding region between aa 106 and 136. The nonfunctional insertion mutants M53i115 and i131 further localized sequences necessary for M50/p35 binding within aa 115 to 131 of M53/p38. Alanine-scanning mutagenesis for the 12 conserved aa of this essential region confirmed and extended these observations. All point mutants showed wt nuclear distribution upon isolated expression. However, a variety of phenotypes occurred in the presence of M50/p35, including mutants which formed intranuclear aggregates (M53L125A) or filamentous structures (M53K128A). Since these structures were only seen in the presence of M50/p35, we assume that a transient M50/p35-M53/p38 interaction is responsible for the observed phenotypic changes. This transient interaction was apparently sufficient to cause the aggregation of M53/p38 alone or in complexes with thus far unknown cellular partners. Surprisingly, all tested point mutants could bind to M50/p35 to some extent in pull-down assays, and more importantly, all rescued the M53 null phenotype in the viral context, including those without apparent colocalization. This indicates that using only one assay to study a protein-protein interaction might not be sufficient. Colocalization studies show the major steady-state phenotype, and residual low-affinity interactions may be overlooked. Coimmunoprecipitation reveals the potential to bind.
However, the growth of reconstituted viruses was poor for mutants M53K128A, M53Y129A, and M53L130A. These data imply that wt M50/p35-M53/p38 complexes are required for efficient productive infection and that a small number of transient complexes may suffice for virus production. However, if two or more aa within the predicted binding region were exchanged, these mutants neither colocalized with nor bound to M50/p35 and were not able to rescue the M53 null phenotype. Our data show that a loss of M53/p38 binding to M50/p35 is associated with the inability to replicate. However, M50/p35 binding might not be the only function of CR1. Notably, there are M53 insertion mutants with mutations within the identified binding motif (i115 and i131) which bind to M50/p35 but are nevertheless lethal.
Interestingly, the M50/p35 binding region of M53/p38 is strictly conserved only in betaherpesviruses. The homology of alpha- or gammaherpesvirus UL31 family members to the betaherpesvirus sequence (aa 115 to 136) is lower than the average similarity of the conserved regions. The sequence conservation is considerably high only within subfamilies. Therefore, we expect that sequences in the conserved regions, but not necessarily the same sequences in CR1, functionally define the binding region in alpha- and gammaherpesvirus homologues. This divergence is already experimentally proven for the binding regions of UL34 members from the alpha- and betaherpesvirus subfamilies. We showed that MCMV M50/p35 aa 53 to 57 and aa 114 are important for M53/p38 binding (3). Using a set of nine HSV-1 UL34 mutants, one of which was introduced into the genomic context, the binding region was localized to a different region, namely, aa 137 to 181 of UL34 (which correspond to aa 129 to 173 of M50/p35) (13).
The C-terminal regions CR2 to CR4 are conserved in the UL31 family, and several insertion mutants have a null phenotype in the genomic context, although they bind to M50/p35, indicating that the C-terminal half of M53/p38 bears as yet unidentified essential functions. Under certain conditions, HSV-1 UL31 interacts with lamins A/C and is involved in chromatin reorganization (24, 30, 31). M50/p35 and BFRF1 have indirect effects on the nuclear lamina (7, 19), and in Epstein-Barr virus, the complex of BFLF2 and BFRF1 interacts with lamin B (10). These features, and also capsid recruitment and regulation of the subsequent budding event, may involve CR2-4 of the UL31 family proteins. Since the mutual binding sites have been defined for the M50/p35-M53/p38 interaction, the decisive features dependent on complex formation can now be addressed. Experimental approaches such as testing mutants for dominant negative effects (28) or protein pull-down assays using a functional or nonfunctional NEC may pave the way to the elucidation of the herpesvirus NEC functions.
Supplementary Material
Acknowledgments
We thank S. Boos for excellent technical assistance and L. Dölken for a critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft through SFB 455, “Viral functions and immune modulation.”
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138-D141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biery, M. C., F. J. Stewart, A. E. Stellwagen, E. A. Raleigh, and N. L. Craig. 2000. A simple in vitro Tn7-based transposition system with low target site selectivity for genome and gene analysis. Nucleic Acids Res. 28:1067-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bubeck, A., M. Wagner, Z. Ruzsics, M. Lötzerich, M. Iglesias, I. R. Singh, and U. H. Koszinowski. 2004. Comprehensive mutational analysis of a herpesvirus gene in the viral genome context reveals a region essential for virus replication. J. Virol. 78:8026-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bubic, I., M. Wagner, A. Krmpotic, T. Saulig, S. Kim, W. M. Yokoyama, S. Jonjic, and U. H. Koszinowski. 2004. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J. Virol. 78:7536-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, Y. E., C. Van Sant, P. W. Krug, A. E. Sears, and B. Roizman. 1997. The null mutant of the U(L)31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J. Virol. 71:8307-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingwall, C., and R. A. Laskey. 1991. Nuclear targeting sequences—a consensus? Trends Biochem. Sci. 16:478-481. [DOI] [PubMed] [Google Scholar]
- 7.Farina, A., R. Feederle, S. Raffa, R. Gonnella, R. Santarelli, L. Frati, A. Angeloni, M. R. Torrisi, A. Faggioni, and H. J. Delecluse. 2005. BFRF1 of Epstein-Barr virus is essential for efficient primary viral envelopment and egress. J. Virol. 79:3703-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs, W., B. G. Klupp, H. Granzow, N. Osterrieder, and T. C. Mettenleiter. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 76:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold, M. C., M. W. Munks, M. Wagner, U. H. Koszinowski, A. B. Hill, and S. P. Fling. 2002. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo. J. Immunol. 169:359-365. [DOI] [PubMed] [Google Scholar]
- 10.Gonnella, R., A. Farina, R. Santarelli, S. Raffa, R. Feederle, R. Bei, M. Granato, A. Modesti, L. Frati, H. J. Delecluse, M. R. Torrisi, A. Angeloni, and A. Faggioni. 2005. Characterization and intracellular localization of the Epstein-Barr virus protein BFLF2: interactions with BFRF1 and with the nuclear lamina. J. Virol. 79:3713-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lake, C. M., and L. M. Hutt-Fletcher. 2004. The Epstein-Barr virus BFRF1 and BFLF2 proteins interact and coexpression alters their cellular localization. Virology 320:99-106. [DOI] [PubMed] [Google Scholar]
- 13.Liang, L., and J. D. Baines. 2005. Identification of an essential domain in the herpes simplex virus 1 UL34 protein that is necessary and sufficient to interact with UL31 protein. J. Virol. 79:3797-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang, L., M. Tanaka, Y. Kawaguchi, and J. D. Baines. 2004. Cell lines that support replication of a novel herpes simplex virus 1 UL31 deletion mutant can properly target UL34 protein to the nuclear rim in the absence of UL31. Virology 329:68-76. [DOI] [PubMed] [Google Scholar]
- 15.Menard, C., M. Wagner, Z. Ruzsics, K. Holak, W. Brune, A. E. Campbell, and U. H. Koszinowski. 2003. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. J. Virol. 77:5557-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messerle, M., I. Crnkovic, W. Hammerschmidt, H. Ziegler, and U. H. Koszinowski. 1997. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA 94:14759-14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 19.Muranyi, W., J. Haas, M. Wagner, G. Krohne, and U. H. Koszinowski. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297:854-857. [DOI] [PubMed] [Google Scholar]
- 20.Padgett, K. A., and J. A. Sorge. 1996. Creating seamless junctions independent of restriction sites in PCR cloning. Gene 168:31-35. [DOI] [PubMed] [Google Scholar]
- 21.Posfai, G., M. D. Koob, H. A. Kirkpatrick, and F. R. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawlinson, W. D., H. E. Farrell, and B. G. Barrell. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 70:8833-8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddehase, M. J., F. Weiland, K. Munch, S. Jonjic, A. Luske, and U. H. Koszinowski. 1985. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 55:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds, A. E., L. Liang, and J. D. Baines. 2004. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes U(L)31 and U(L)34. J. Virol. 78:5564-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds, A. E., B. J. Ryckman, J. D. Baines, Y. Zhou, L. Liang, and R. J. Roller. 2001. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roller, R. J., Y. Zhou, R. Schnetzer, J. Ferguson, and D. DeSalvo. 2000. Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J. Virol. 74:117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rupp, B., Z. Ruzsics, T. Sacher, and U. H. Koszinowski. 2005. Conditional cytomegalovirus replication in vitro and in vivo. J. Virol. 79:486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Scott, E. S., and P. O'Hare. 2001. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection. J. Virol. 75:8818-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson-Holley, M., J. Baines, R. Roller, and D. M. Knipe. 2004. Herpes simplex virus 1 U(L)31 and U(L)34 gene products promote the late maturation of viral replication compartments to the nuclear periphery. J. Virol. 78:5591-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner, M., S. Jonjic, U. H. Koszinowski, and M. Messerle. 1999. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J. Virol. 73:7056-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner, M., and U. H. Koszinowski. 2004. Mutagenesis of viral BACs with linear PCR fragments (ET recombination). Methods Mol. Biol. 256:257-268. [DOI] [PubMed] [Google Scholar]
- 34.Wagner, M., Z. Ruzsics, and U. H. Koszinowski. 2002. Herpesvirus genetics has come of age. Trends Microbiol. 10:318-324. [DOI] [PubMed] [Google Scholar]
- 35.Ye, G. J., and B. Roizman. 2000. The essential protein encoded by the UL31 gene of herpes simplex virus 1 depends for its stability on the presence of UL34 protein. Proc. Natl. Acad. Sci. USA 97:11002-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.