Abstract
We have previously shown that herpes simplex virus type 1 (HSV-1) infection is associated with early destabilization/degradation of infected cell mRNAs and consequent shutoff of host protein synthesis by the activity of the virion-associated host shutoff (vhs) UL41 protein. Wild-type (wt) virus destabilized/degraded the housekeeping β-actin and α-tubulin mRNAs as well host stress functions, like the heat shock 70 protein induced postinfection. vhs mutants did not degrade the mRNAs. Elaborate studies by others have been concerned with the mode of mRNA degradation and the mRNAs affected. We now describe vhs activity in primary cultures of mouse cerebellar granule neurons (CGNs). Specifically, (i) upon infection in the presence of actinomycin D to test activity of input viral particles, there was a generalized inhibition of protein synthesis, which depended on the input multiplicity of infection (MOI). (ii) Low-MOI infection with vhs-1 mutant virus was associated with increased synthesis of all apparent proteins. Higher MOIs caused some shutoff, albeit significantly lower than that of wt virus. This pattern could reflect an interaction(s) of vhs-1 protein with host machinery involved in cellular mRNA destabilization/degradation, sequestering this activity. (iii) wt virus infection was associated with cell survival, at least for a while, whereas mutant virus induced apoptotic cell death at earlier times. (iv) wt virus replicated well in the CGNs, whereas there was no apparent replication of the vhs-1 mutant virus. (v) The vhs-1 mutant could serve as helper virus for composite amplicon vectors carrying marker genes and the human p53 gene. Ongoing studies test the use of vhs-1-based composite oncolytic vectors towards cancer gene therapy.
HSV-1 vhs activity.
The infection of cells with herpes simplex virus type 1 (HSV-1) is associated with a global shutoff of host protein synthesis early postinfection (p.i.) (15, 25, 26, 38, 46, 48). Analyses of the infected cells have revealed the destruction of polyribosomes and the degradation of infected cell mRNAs following infection with the wild type (wt) virus (9, 11, 25, 26, 46, 48, 50). We have previously derived viral mutants which did not shut off host protein synthesis and degrade the mRNAs (38). Employing marker rescue analyses with fragments from the entire genome, we have mapped the virion host shutoff (“vhs”) phenotype within a 265-bp segment of the UL41 gene (26). The mRNA degradation function was also found to map in this region. Sequencing analyses of the corresponding 265-bp segments within the wt and the vhs-1 mutant virus revealed a threonine-to-isoleucine mutation in amino acid 214 of the vhs protein (A. D. Kwong and N. Frenkel, unpublished results). All in all, the UL41 gene product corresponds to a 489-amino-acid phosphoprotein localized in the virion tegument, from where it is released upon viral entry into the cell (25, 38-41). It is expressed as a late (γ1) gene product which is incorporated into the structural particles of progeny virus. We have shown that in the presence of actinomycin D the UL41 protein was involved in the degradation of mRNAs transcribed prior to and during infection, including the housekeeping genes β-actin and α-tubulin, which were persistently present in the cells, and the heat shock protein 70 (HSP70), induced p.i. (46). These mRNAs were stable in cells infected with the vhs-1 mutant virus.
Several studies examined whether the vhs protein had an intrinsic nucleolytic activity. Read and coworkers (36) have shown that the transfection of cells with a plasmid containing the UL41 gene inhibited the expression of a cotransfecting chloramphenicol acetyltransferase reporter gene. This activity was absent in cells transfected with mutant plasmids (22, 36). Furthermore, Everly and Read (12) provided genetic and biochemical evidence that the vhs protein has an mRNase activity. Smiley and coworkers (7, 8) documented that the vhs protein produced in vitro employing the rabbit reticulocyte (RRL) translation system possessed an endonucleolytic activity on added exogenous RNA substrates. Furthermore, when vhs was expressed in the budding yeast Saccharomyces cerevisiae there was inhibition of colony formation, while vhs mutants tested in the system had no activity. Cell extracts of yeast expressing vhs displayed an endoribonuclease activity if the extracts were mixed with RRL. These results suggested that there was a mammalian macromolecular factor required for the vhs activity (30).
Mode of vhs nuclease activity.
Several studies have examined the direction of the vhs nucleolytic activity: Karr and Read (23) showed that sequences at the 5′ mRNA end were degraded earlier than those at the 3′ end. Smiley and coworkers reported that the vhs protein synthesized in vitro by RRL displayed an endonucleolytic activity (7, 8). The placing of an internal ribosome entry site from encephalomyocarditis virus or poliovirus into test RNA substrates yielded endonucleolytic cleavage clustering downstream of the element. The first endonucleolytic cleavage event appears to be followed by a 5′-to-3′ exonucleolytic degradation (37). Finally, recent studies by Roizman and coworkers have suggested that the vhs degradation involved, in some cases, the recognition of AU-rich elements at the 3′ termini of mRNA (9, 11, 49, 50).
Proteins that interact with vhs.
Several proteins were found to interact with the vhs protein, including the following. (i) The eukaryotic initiation factor 4A (eIF4A), eIF4B, and eIF4H translation factors were found to form complexes with the vhs protein (6, 13, 14). These interactions might be involved in transporting the vhs protein to compartments where mRNA translation initiation takes place. (ii) VP16, responsible for the trans-activation of the immediate-early (α) genes. The two proteins were found to coprecipitate with anti-vhs antibodies and to interact in the yeast two-hybrid system (41). It has been suggested that vhs interaction with VP16 attenuated the vhs mRNA degradation late p.i. (28, 31). A segment of 20 amino acids previously suggested to contain a binding domain for VP16 was shown to be required for mRNA degradation by tegument-derived vhs and for the viral replication in mice cornea, trigeminal ganglia, and brains (44). (iii) The vhs protein was found to physically interact with the cellular tristetraprolin, which has been previously reported to recruit AU-rich element-containing RNAs to the exosome for degradation (10).
mRNAs targeted by the vhs protein.
Previous analyses have documented that the UL41 destabilization/degradation activity targeted both host and viral mRNAs, including the following: (i) the “housekeeping” genes β-actin and α-tubulin, found to be expressed prior and during the infection (11, 46); (ii) stress functions, induced postinfection, including HSP70 (46) and the stress-inducible immediate-early response gene 1 (IEX1) (21, 50); (iii) host functions constituting a generalized antiviral immune response, as recently reviewed in reference 42, including interleukin-1β, interleukin-8, macrophage inflammatory protein-1α (47), and mRNAs encoding interferon and interferon-stimulated genes (29), as well as major histocompatibility complex class I and class II (20, 51); (iv) the α, β, and γ viral mRNAs which, as shown by us and by Oroscar and Read, were overexpressed in cells infected with the vhs-1 mutant virus (25, 34, 35). Transcriptional turning on of viral genes was coupled to the destabilization of viral mRNAs, resulting in limited and coordinated viral protein synthesis and better utilization of infected cell resources (25). (v) Roizman and coworkers (9) suggested that there were several sequence-specific mRNA degradation pathways. The first type of mRNA was rapidly degraded by the UL41 function and included the constitutively expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin, and the β5-tubulin genes. The second type included the stress-induced IEX-1 mRNA, which was induced p.i. and thereafter rapidly degraded. The third type of mRNA includes the tristetraprolin-inducible and the growth arrest and DNA damage-inducible gene 45β (GADD45β). These transcripts were upregulated but not degraded (9, 11, 50).
vhs activity in neuronal cells.
Several groups have reported analyses of vhs function in neuronal cells. Specifically, Olivo and coworkers (33) reported that there was no shutoff of protein synthesis in cultured sympathetic neurons prepared from rat superior cervical ganglion at embryonic day 21 and in sensory neurons prepared from rat dorsal root ganglia at embryonic day 15. Control cultures of primary rat fibroblasts and undifferentiated PC-12 and Vero cells exhibited the vhs shutoff. They suggested that the resistance of neurons to VHS might be important in the establishment of latent HSV-1 infection. In a later study, Leib and coworkers (45) analyzed vhs activity in superior cervical ganglion prepared from neonatal mice. They reported no vhs-related degradation of GAPDH mRNA in cultures infected with 20 PFU/cell, whereas infection at 100 PFU/cell resulted in 70% degradation.
We undertook the studies reported here for several reasons: (i) to characterize the vhs-related shutoff of protein synthesis in primary cultures of mature cerebellar neurons, (ii) to test the involvement of the vhs-1 mutant in cell death and apoptosis, towards the use of the vhs-1 mutant as an oncolytic vector for cancer gene therapy, and (iii) to test the use of the vhs-1 mutant virus as a helper virus with amplicon vectors.
MATERIALS AND METHODS
Cell cultures.
Primary cultures highly enriched for cerebellar granule neurons (CGNs) were prepared from 8-day-old BALB/c mice employing conditioned medium (CM) as previously described (32). Specifically, cultures prepared from dissociated cerebellar granule neurons secrete factors necessary for cell viability and growth. Furthermore, the cerebellar granule neurons become sensitive to newly added serum, since it contains glutamate, and replacement of the medium with standard medium results in a massive neuronal death unless the cells receive conditioned medium. After decapitation, the cerebella were removed, and the cells were trypsinized and plated in two types of culture: (i) for the production of CM, cells were seeded at a concentration of 5 × 107 cells per 75-cm2 flask in 15 ml of Eagle's basal medium supplemented with 25 mM KCl, 2 mM glutamine, 50 μg/ml gentamicin, 250 ng/ml amphotericin B, 1 mg/ml glucose, and 10% fetal calf serum. Supernatants were collected 3 to 4 days later. (ii) Cells to be used for viral infections were plated in 24- or 96-well dishes coated with poly-l-lysine in standard medium. To prevent replication of nonneuronal cells, 1-β-arabinosylcytosine (AraC) was added to the medium after 18 to 22 h at a concentration of 10 μM. After three additional days, the neurons in the wells were rinsed thoroughly with CM to remove the AraC. The cultures were then mock infected or infected with the virus in CM (lacking AraC). After viral adsorption, the medium containing the inoculum was removed, and the cultures were rinsed and further incubated with CM for different times as specified in the text.
Viruses.
HSV-1 strain KOS was isolated by K. O. Smith, Baylor University, Houston, Tex. The vhs-1 mutant was derived in our laboratory as previously described (38). Virus stocks were produced in Vero cells employing triply plaque-purified viruses with limited passaging at an input multiplicity of infection (MOI) of 0.01 PFU/cell.
Preparation of composite amplicon vector stocks.
Semiconfluent Vero cells were transfected with the appropriate amplicon plasmid by calcium phosphate precipitation. At 24 h the cells were superinfected with 1 PFU/cell of the helper virus. Virus stocks, designated as passage zero, were harvested 2 or 3 days later by three cycles of freezing (−80°C) and thawing (37°C) and were further passaged repeatedly in Vero cells at 1:4 dilutions. The different passages contained mixtures of helper virus and defective genomes (18, 24, 27, 43).
Infection of cerebellar granule neurons.
Four days after plating, the number of viable cells in the well was determined by the trypan blue exclusion assay. Neuronal cells were washed twice with CM to remove the AraC and then exposed to virus infection. The cells were infected in CM. Following virus absorption for 2 hours, the inoculum was removed and CM was added prior to further incubation for the time specified.
Assays of infectious virus yields.
The infected CGNs were harvested at different times p.i. by three cycles of freezing and thawing. The titer of released virus was obtained by plaque assays in Vero cells.
Shutoff of host protein synthesis.
Four days after the preparation of mouse cerebellar granule neurons, the cells were plated in 24-well dishes, at 1.5 × 106 cells per well, were rinsed four times with 200 μl CM, preincubated for 1 h in 0.5 ml CM containing 5 μg/ml actinomycin D, and then infected at the specified input MOIs. Absorption was for 2 hours in 100 μl CM containing actinomycin D, after which 300 μl CM with 5 μg/ml actinomycin D was added and incubation continued for an additional 4 hours. For the labeling, the cells were rinsed four times and then incubated for 30 min in Dulbecco's modified Eagle's medium lacking methionine (Met-) and containing 5 μg/ml actinomycin D. Labeling was for an additional hour in Met- medium containing actinomycin D and 50 μCi/ml [35S]methionine. For protein analyses, the cells were rinsed four times with 200 μl phosphate-buffered saline (PBS), scraped into 200 μl lysis buffer, and prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Quantification of the 35S-labeled proteins was performed using the Java-based public domain image-processing and analysis program ImageJ (W. S. Rasband, Image J, U.S. National Institutes of Health, Bethesda, Md.; http://rsb.info.nih.gov/ij/; 1997-2005).
Trypan blue viability assays.
Neuron survival was determined by the trypan blue exclusion assay. Cells were incubated for 10 min in 0.1% trypan blue in PBS, pH 7.4, and then washed twice with PBS. Three randomly chosen fields, which contained approximately 500 cells each, were analyzed by phase-contrast and bright-field microscopy. Cells excluding the dark blue dye (white cells) were counted as viable, whereas blue-stained cells were scored as dead.
MTT assay.
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] tests were based on a modification of the previously described procedure (6). Neuronal cultures seeded in 96-well plates were incubated for 60 min at 37°C with 0.5 mg/ml MTT in standard medium. The MTT solution was aspirated, and the cells were lysed in 200 μl dimethyl sulfoxide. The amount of MTT formazan was quantified by determining the absorbency at 490/690 in a Bio-Tek microplate reader (Wiooski, VT).
DAPI staining.
Cells were grown on glass coverslips coated with poly-l-lysine. The cells were infected with 10 PFU/cell of HSV-1 (KOS) or the vhs-1 mutant virus. At the indicated times p.i., the cells were washed with PBS, pH 7.4, and fixed for 10 min in 4% formaldehyde (in PBS). After fixation the neurons were rinsed with PBS, stained for 60 min with 10 μg/ml 4,6-diamino-2-phenylindole (DAPI), and rinsed twice with PBS; a drop of N-propyl gallate or glycerol was added to the slide to enhance fluorescence, which was detected by UV light microscopy.
The HSV-1 amplicon β-Gal vector.
The amplicon plasmid (pNF1142) was constructed by inserting the β-galactosidase (β-Gal) gene into the pF1′-pα amplicon containing the HSV-1 (F) oriS, the pac-1 and pac-2 signals, and the HSV-1 IE4/5 promoter (16, 24). The helper virus used in the present study corresponded to the temperature-sensitive mutant tsLB2, containing a ts ICP4 gene (19).
Tests of amplicon β-Gal expression.
For the β-Gal assay the infected cells were rinsed twice with PBS and fixed by incubation for 5 min at 4°C with 2% paraformaldehyde in 100 mM Na-phosphate, pH 7.3, 2 mM MgCl2, and 2 mM EGTA. The cells were then rinsed twice with PBS containing 2 mM MgCl2 and incubated for 30 min in 100 mM Na phosphate, pH 7.3, 1.3 mM MgCl2, 3 mM potassium ferricyanide, and 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside. The blue cells were viewed in a Zeiss inverted microscope and photographed with an MC camera.
HSV-1 amplicon-p53 vector.
The vector (pNF1224) was constructed in our laboratory (G. Kotliroff and N. Frenkel, unpublished data). A 1.8-kb SnaBI-SmaI fragment containing the p53 cDNA with the human cytomegalovirus promoter and the simian virus 40 polyadenylation signal was prepared from a pCDNA3 clone (a gift of Moshe Oren). The fragment was ligated into a blunted partial SnaBI and HpaI segment of the HSV-1 amplicon GFP (pNF1225) vector.
Tests of amplicon-p53 expression.
The cells were rinsed four times with 200 μl CM and infected with the appropriate virus stock at an MOI of 0.1 PFU/cell. At 5 h p.i. the cells were lysed and 20 μg of each sample was loaded on a 12.5% SDS-polyacrylamide gel. Proteins were transferred onto a nitrocellulose membrane and reacted with DO-1 antibody to p53 as primary antibody and mouse anti-human secondary antibodies conjugated to horseradish peroxidase. Following rinsing with Tris-buffered saline-Tween, the membrane was incubated with ECL (Amersham Pharmacia Biotech, Piscataway, NJ) and exposed to X-ray films.
RESULTS
The UL41-vhs protein present in the incoming virus particles causes efficient shutoff of host protein synthesis in primary mouse cerebellar neurons.
Earlier studies of vhs-UL41 activity revealed the pronounced shutoff of host protein synthesis in epithelial and fibroblastic cells. The shutoff was coupled to an extensive mRNA destabilization/degradation activity (9, 11, 15, 25, 26, 38, 46, 48, 50). In contrast to this pronounced activity, the infection of rat embryonic sympathetic and sensory neurons was reported to result in inefficient vhs-type shutoff and it required high-MOI infection to partly reduce host protein synthesis (33, 45).
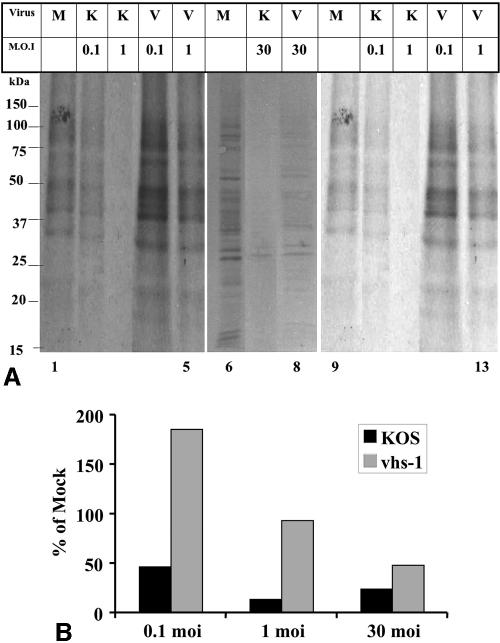
In the present study, we characterized the pattern of viral infection in mature mouse neuronal cells. Primary cultures of mouse CGNs were prepared from 8-day-old BALB/c mice and were infected with HSV-1 (KOS) and the vhs-1 mutant virus at different MOIs in the presence of actinomycin D. The infected cells were labeled with [35S]methionine, from 4 to 5 h p.i., and equal amounts of total cell lysates were loaded onto SDS-polyacrylamide gels (Fig. 1A). Due to the presence of actinomycin D throughout the infection, the shutoff of protein synthesis reflected the activity of the vhs protein present in the infecting particles on mRNAs which were transcribed prior to the infection. The proteins induced postinfection were not followed in this experiment. To allow better visual representation of the amounts of proteins synthesized in the infected cultures, the gel lanes containing the low-MOI infections are shown after long (lanes 1 to 5) and short (lanes 9 to 13) exposures of the X-ray film. The relative amounts of 35S-labeled proteins synthesized in the wt and mutant virus infections were quantified in comparison to the corresponding mock-infected cells (Fig. 1B).
FIG. 1.
A. Shutoff of host protein synthesis in cells infected with the wt HSV-1 (KOS) and vhs-1 mutant virus. CGNs plated at 1.5 × 106 cells per well in 24-well plates were infected with the indicated virus at the indicated MOIs in the presence of 5 μg/ml actinomycin D. The cells were labeled from 4 to 5 h p.i. with 50 μCi/ml [35S]methionine. The cells were lysed, and 20-μg protein samples were loaded in each lane of 12.5% SDS-polyacrylamide gels. The gels were transferred to nitrocellulose membranes and exposed to film. Shown in this figure are longer (lanes 1 to 5) and shorter (lanes 9 to 13) exposures of the X-ray films for the gel containing the 0.1- and 1-PFU samples. Lanes 6 to 8 are from a gel with 30-PFU infections. B. Quantitative analysis of the shutoff of protein synthesis. The ImageJ program was used to quantitate the 35S-labeled proteins in the samples shown in panel A. The data are shown as percent relative to mock infected. M, mock; K, KOS; V, vhs1; moi, MOI measured in PFU/cell.
The results can be summarized as follows. (i) Infection of the CGNs with the wt HSV-1 (KOS) resulted in pronounced shutoff of host protein synthesis, ranging from 55% shutoff at 0.1 PFU/cell to 80 to 90% shutoff at 1 and 30 PFU/cell. Because the shutoff was measured in the presence of actinomycin D, it reflected the activity of incoming particles. Moreover, the finding that wt virus infections at an MOI of 0.1 and 1 PFU/cell reduced protein synthesis by approximately 55 and 85% suggests that the shutoff was caused also by viral particles incapable of plaque formation. (ii) The shutoff affected the majority of proteins seen in the gels and ranging in size from 15 to >150 kDa. From inspection of the gels, it appears that the majority of proteins were similarly affected. (iii) The level of protein synthesis in CGNs infected with the vhs-1 mutant appeared also to be MOI dependent. Most interestingly, the infection with 0.1 PFU/cell was associated with a 1.8-fold increase in total protein synthesis. Again, the effect was generalized, and an increase in protein synthesis was noted for all the labeled proteins visible in the gels. (iv) Mutant virus infection at 1 PFU/cell was associated with protein synthesis at a level similar to that in the mock-infected cells, whereas some reduction of protein synthesis was evident during infections at the MOI of 30 PFU/cell. (v) Two additional experiments employing 0.1, 1, and 10 PFU/cell of HSV-1 (KOS) and the vhs-1 mutant virus reproduced the results above (data not shown). A model whereby the incoming vhs-nucleolytic protein interacts with a given amount of cellular “machinery” required for mRNase activity in the cell will be further outlined in the Discussion section below.
Induction of cell death by wt and vhs-1 mutant viruses.
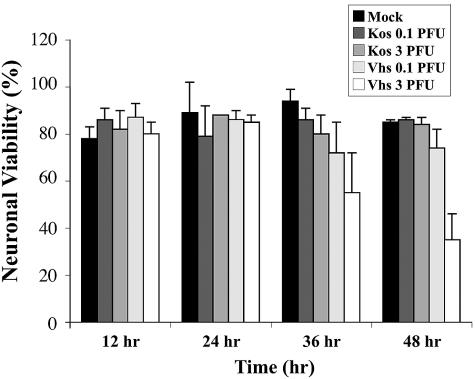
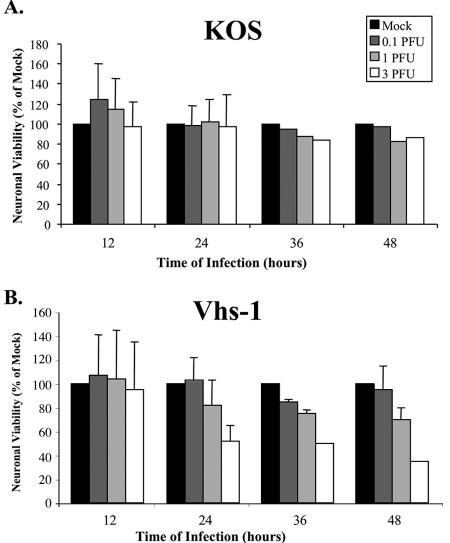
Because the vhs function causes destabilization/degradation of host cell mRNAs, it was of interest to determine whether the infections were accompanied by neuronal cell death. Primary cultures of the CGNs were infected with 0.1 and 3 PFU/cell of the viruses, and neuronal viability was determined at the indicated times, using first the trypan blue assay. As shown in Fig. 2, up to 48 h postinfection, there was no substantial cell death above the mock-infected control in the wt HSV-1 (KOS)-infected cells. In contrast, 42% and 68% of the cells were scored as dead by 36 and 48 h p.i. with 3 PFU/cell of the vhs-1 mutant virus. Cell viability was also tested employing the MTT assay, measuring mitochondrial dehydrogenase activity. As shown in Fig. 3, following wt HSV-1 (KOS) infection there was no significant loss of cell viability, even at the input MOI of 3 PFU/cell by 24 h p.i. By 36 and 48 h. p.i. viability was scored as 82 and 85% of the mock-infected control. In contrast, in the vhs-1 mutant infections there was an MOI-dependent reduction in cell viability, starting by 12 h p.i. and increasing throughout the test. At 24, 36, and 48 h p.i. with 3 PFU/cell, 53%, 49%, and 35% of the cells, respectively, were scored as viable compared to the mock-infected cultures. The experiment was repeated twice with similar results.
FIG. 2.
Cell death in infected cerebellar granule neurons. CGNs were infected with wt or vhs-1 mutant viruses at the indicated MOI. Neuronal viability was determined using trypan blue assay as described in Materials and Methods.
FIG. 3.
Viability of infected cerebellar granule neurons. CGNs were mock infected or infected with the wt HSV-1 (KOS) (A) or the vhs-1 mutant virus (B) at different MOIs. Neuronal viability was determined at the indicated times, using MTT assays as described in Materials and Methods.
vhs-1 mutant virus infection induces apoptosis.
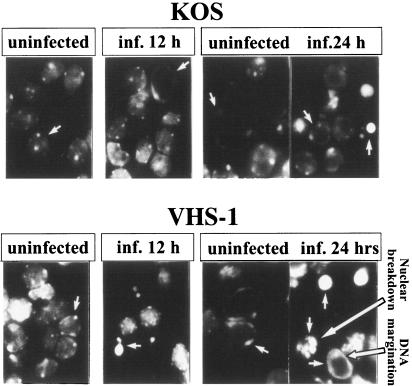
To examine whether the vhs-1 mutant induced apoptotic cell death, the neuronal cells grown on microscope slide coverslips were infected with the wt and the vhs-1 mutant viruses for different times. The infected cells fixed and stained with DAPI were examined under the fluorescence microscope 12 and 24 h p.i. The results (Fig. 4) can be summarized as follows. (i) The mock-infected cells at both 12 and 24 h p.i. contained homogeneously stained oval-shaped nuclei, with spotted glowing areas. This type of staining is typical in DAPI-stained mouse CGNs (32). (ii) By 12 h and 24 h p.i., the majority of the cells infected with wt HSV-1 (KOS) were similar to cells in the mock-infected cultures. (iii) In contrast, 12 h after exposure to the vhs-1 mutant virus, many nuclei lost their oval shape and appeared like bright round condensed fragmented nuclei. Twenty-four hours p.i. the number of the disintegrated cells increased. The fragmented nuclei contained condensed fragments with the appearance of round balls and nuclear DNA margination. These patterns were indistinguishable from the reported DAPI staining of CGNs deprived of high potassium concentrations, leading to apoptotic neuronal death (32). Taken together, the results revealed that the vhs-1 mutant induced apoptotic death in the infected CGNs. It is noteworthy that infection of cells with the vhs-1 mutant virus also produces DNA ladder breakage (test of Fermentas MBI) in human neuronal and nonneuronal cells, as will be elaborated upon separately (Kotliroff and Frenkel, unpublished).
FIG. 4.
Apoptosis of cerebellar granule neurons infected with wt HSV-1 (KOS) or the vhs-1 mutant virus. CGNs grown on glass coverslips were infected with 10 PFU/cell of the viruses. At the indicated time points, the cells were fixed and stained with DAPI, as described in Materials and Methods. Shown are fluorescent figures in the UV light microscope.
Viral replication in mouse cerebellar granule neurons.
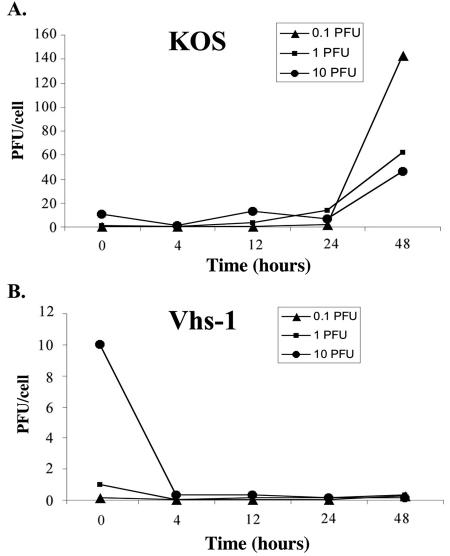
The replication of the wt HSV-1 (KOS) and the vhs-1 mutant virus in mouse cerebellar granule neurons was measured at different MOIs. Figure 5 compares infectious virus yields in the neuronal cells up to 48 h p.i. with input MOIs of 0.1, 1, and 10 PFU/cell. Titration of the resultant virus stocks was done in Vero cells. The results showed the following. (i) The wt virus replicated efficiently in the neuronal cells, producing 45 to 143 PFU/cell. Thus, in the cultures that were infected with an input MOI of 0.1 PFU/cell, there was 1,430-fold amplification of the input virus by 48 h p.i., whereas the infections with 1 and 10 PFU/cell resulted in 60- and 5-fold infectious virus production, respectively. (ii) In contrast, the vhs-1 mutant virus barely replicated in the cells, and maximal infectious virus yield produced by 48 h reached 0.3 PFU/cell in the cells tested with different viral MOIs.
FIG. 5.
Infectious virus yields in the CGN infections. CGNs were infected with 0.1, 1, and 10 PFU/cell. (A) HSV-1 (KOS); (B) vhs-1 mutant virus. At the indicated times the infected cells were freeze-thawed three times, and resultant virus stock titers were determined in Vero cells, yielding infectious virus yields calculated in PFU/cell.
Transgene expression in CGNs by composite amplicon-based defective virus vectors.
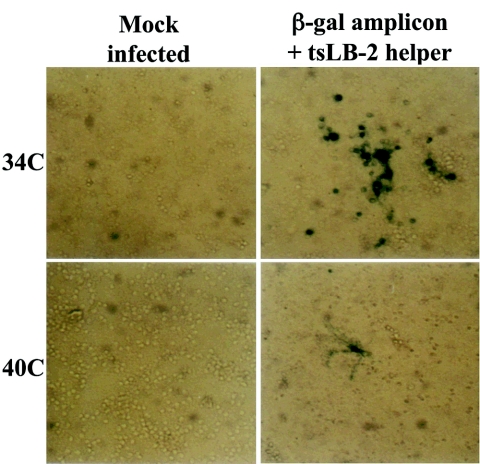
We showed above that the vhs mutant has the capability of inducing cell death while producing reduced infectious virus yields. As a prelude for the potential use of the vhs-1 mutant as an oncolytic viral vector for cancer gene therapy, we tested the ability of composite amplicon-based vectors to obtain transgene expression in infected CGNs. The composite vector stocks were derived in Vero cells as previously described (18, 24, 27, 43) and contained (i) the test helper virus and (ii) a defective viral genome with multiple repeats of the test amplicon units, each with a viral DNA replication origin, the pac-1 and pac-2 packaging signals, and the test transgene(s). The composite amplicon vectors were produced in Vero cells and were used to infect the CGNs. Two exemplary vectors were tested: (i) an HSV-1 amplicon-β-Gal vector (pNF1142) employing our prototype amplicon (24) containing the oriS DNA replication origin, the pac-1 and pac-2 signals, and the transgene placed under the HSV-1 ICP4/5 promoter. The HSV-1 ts LB2 mutant virus served as the helper virus. This helper virus contains a ts mutation in ICP4, and the infection does not progress beyond immediate-early (α) gene expression at the nonpermissive temperature (19). Four days after the preparation of CGNs, they were mock infected or infected with the composite vector, at 34°C or at 40°C. At 48 h p.i. the cells were tested for β-Gal expression. As shown in Fig. 6, there was significant β-Gal expression at 34°C compared to limited expression at 40°C. This basic experiment demonstrates the ability to express the transgene in the CGNs in a helper-dependent regulation.
FIG. 6.
β-Gal expression in CGNs infected with composite amplicon β-Gal vector and the tsLB-2 helper virus. CGNs were infected or mock infected at 34 and 40°C with composite vector containing a mixture of the amplicon β-Gal and tsLB-2 helper virus. Shown is the β-Gal histochemical staining examined at 8 h p.i. in a Zeiss inverted microscope, photographed with an MC100 camera. Magnification, ×200.
A second test included an HSV-1 amplicon carrying the p53 transgene under the human cytomegalovirus promoter in conjunction with the wt and the vhs-1 mutant helper viruses. The cultured CGNs were infected with the indicated viral stocks at an MOI of 0.1 PFU/cell, and at 5 h p.i. the cells were lysed and tested for p53 protein expression in Western blot assays employing the DO-1 antibody. As seen in Fig. 7, the p53 gene was expressed in the neuronal cultures infected with the amplicon-p53 vector and both helper viruses. There was somewhat higher p53 gene expression in the vhs-1-infected cells than in the wt helper virus infection. The level of expression is predicted to depend on the exact ratio of the helper and amplicon-defective genomes in addition to having less mRNA degradation in the vhs-1 mutant infections, as documented previously (18, 24, 27).
FIG. 7.
Expression of the amplicon p53 vector in CGNs infected with composite amplicon vectors. CGN cells were plated at 1.5 × 106 cells per well in 24-well plates. Infection was with 0.1 PFU/cell of the indicated viral stocks. Protein samples prepared at 5 h p.i. were electrophoresed in a 12.5% SDS-PAGE gel, blotted, and reacted with anti-p53 DO-1 antibody.
DISCUSSION
The UL41-vhs protein is functional in mouse CGNs.
We have shown that wt HSV-1 infections of primary cultures of mouse CGNs at input MOIs of 0.1, 1, and 30 PFU/cell were associated with the shutoff of host protein synthesis. The shutoff occurred in the presence of actinomycin D and, therefore, reflected the activity of proteins present in the virus inoculum. The results corroborated the early studies showing that the shutoff did not require the production of progeny plaque-forming virus (15, 38). It could occur following exposure of cells to UV-irradiated virus particles and in cytoplasts that were enucleated before infection (15). It is noteworthy that virus stocks such as the ones employed in the present study are routinely produced in our laboratory by infections at 0.01 PFU/cell employing triply plaque-purified virus. Such stocks were shown to have a particle-to-PFU ratio of 50 (17), and it can thus be estimated that the cells infected as described in Fig. 1 were exposed to multiple particles per cell even at the lower MOI of 0.1 PFU/cell.
The results of our study in CGN cultures differed somewhat from the earlier studies by Nichol et al. (33), who reported inefficient vhs activity following infections of sympathetic and sensory neurons. Furthermore, Strand and coworkers (45) observed degradation of GAPDH mRNA in mouse superior cervical ganglion cells only at 100 PFU/cell input virus compared to efficient shutoff in primary fibroblasts. It is possible that the different susceptibility to vhs reflected different properties of the neuronal cells employed in the studies: the sympathetic and sensory neurons employed earlier (33, 45) are part of the autonomic nerve system, whereas the CGNs employed here make up most of the cerebellum and form part of the central nervous system. Furthermore, whereas the sympathetic and sensory neurons were prepared at embryonic days 15 and 21 (33, 45), the CGN cultures were prepared postnatally from 8-day-old mice and represented fully mature neurons. It is possible that host elements required for vhs activity differ in these systems.
UL41 function induces a generalized shutoff of host protein synthesis.
Inspection of the proteins affected by the vhs function revealed a generalized effect. wt virus infections were accompanied by reduced synthesis of the majority of proteins visible in the gel (Fig. 1A). Similarly, the alterations in protein synthesis observed during the vhs-1 mutant infections were generalized, including the induction of protein synthesis by 0.1 PFU/cell up to partial shutoff at 30 PFU/cell. All proteins across the gel appeared to be affected (Fig. 1A). It is noteworthy that a similar global shutoff of host protein synthesis was observed in the early studies of vhs employing Vero cells and mouse Ltk- epithelial cell lines in the presence and absence of actinomycin D (25, 26, 46). Furthermore, the α, β, and γ proteins produced p.i. were overexpressed in vhs-1 mutant virus infections compared to their expression during wt virus infection. Altogether, the vhs-related alterations in the rate of protein synthesis appeared to affect the synthesis of the majority of host and viral proteins.
The shutoff might involve an interaction(s) of the vhs-UL41 protein with host mRNA destabilization machinery.
The vhs-1 mutation in amino acid 214 of UL41 was shown to affect simultaneously the shutoff of protein synthesis and the destabilization/degradation of infected cell mRNAs (26). During the vhs-1 mutant virus infection, we observed MOI-dependent stimulatory (low MOI) and inhibitory (high MOI) effects on protein synthesis. The results can be explained by a model whereby the wt as well as the mutant vhs-1 proteins interact directly with a generalized host machinery which usually destabilizes/degrades cell mRNAs as dictated by their respective half-lives. We suggest the formation of a complex, termed here the “RNA-degradon,” consisting of two components: (i) a cellular function providing “machinery” which is essential for mRNA degradation (e.g., a compartment, “cutting board,” and/or factors) and (ii) a viral or cellular enzyme with a basic mRNase activity. Also in the model are the relative efficiencies of the mRNase binding and/or enzyme efficiency: wt vhs mRNase > host mRNase > vhs-1 mRNase. Finally, the putative RNA-degradons are brought to polysomes or compartments containing mRNAs for degradation by the translation initiation factors eIF4A, eIF4B, and eIF4H (6, 13, 14). The binding zones for these translation factors are outside the site defined for the vhs-1 mutation in amino acid 214.
In wt virus infection the host mRNase is displaced from the machinery, resulting in an apparent generalized higher degradation than in the mock-infected cells. The wt interaction involves repeated association and then dissociation of the mRNase with the machinery and with new mRNAs, leading to increased degradation. During vhs-1 mutant infection, the vhs-mRNase sequesters irreversibly the machinery and no mRNA degradation can occur. At higher MOI, the vhs-1 mRNase exhibits some residual activity, leading to some reduction in protein synthesis. Further studies are required to identity the details of the interacting proteins. Parameters that could play a role in the process include the quantities of incoming UL41 protein relative to the existing host degradative functions and the abundance of mRNAs as well as the secondary mRNA structures, which might affect the rate of nucleolytic activity across the mRNAs.
Induction of apoptotic cell death.
Of great interest has been the finding that the infections of primary neuronal cells with the wt virus did not lead to immediate cell death at MOIs of 0.1, 1.0, and 3 PFU/cell, whereas the parallel infections with the vhs-1 mutant virus induced apoptotic death in the majority of the cells by 48 h p.i. The extent of cell death depended on the input MOI. A possible explanation for these observations could be that viral infections led to the induction of suicidal host response, which the wt virus evaded readily by mRNA degradation, at least during the initial days of infection. In contrast, the vhs-1 mutant virus stimulated and allowed the induced suicidal mRNAs. In accordance with this suggestion are the earlier studies documenting the induction of stress response genes, such as the HSP70 (46), IEX1, and GADD45β (9, 11, 49, 50).
The vhs antiapoptotic function is essential for viral replication in cerebellar granule neurons.
We have shown that the vhs-1 mutant virus has greatly reduced replication capability, whereas the wt virus replicated well. A potential explanation for these observations could be the induction of host suicidal functions during vhs-1 mutant virus infections, resulting in cell death and no virus replication. In the wt virus infections, the mRNAs encoding the apoptotic functions were degraded and the cells survived, allowing the utilization of cellular functions essential for viral replication. Cell survival continues until viral replication has progressed and cell death is desirable in order to spread viral progeny. It is noteworthy that the vhs-1 mutant virus can replicate with severalfold (three- to fivefold) lower yields in Vero cells (38; Kotliroff et al., unpublished).
Several HSV functions were previously described as serving antiapoptotic roles (1-5). However, the involvement of the UL41 gene product in negating apoptosis has not been extensively characterized. Our present study documenting the induction of apoptosis in mouse cerebellar neurons by the vhs-1 mutant virus differs from the reports by Blaho and coworkers (1-4), who found no effect of vhs deletion on induced apoptosis in HEp-2 cells. The two studies differ with respect to the cells examined, corresponding to primary cell culture versus continuous cell lines. Additionally, the studies differed in the type of vhs mutants employed as well as the MOI used.
Potential use of vhs-based oncolytic HSV amplicon vectors.
The studies described in this paper form the grounds for the derivation of oncolytic viral vectors containing the vhs-1 mutant helper virus and the HSV-1 amplicon vector(s). We have shown successful transgene expression in mouse CGNs. The use of the vhs-1-based composite amplicon vector as a potential oncolytic vector for cancer gene therapy is predicted to be advantageous in several respects. (i) The induction of suicidal host response is initiated by the mutant virus without the necessity to express viral genes postinfection. (ii) Mutant virus stocks can be easily produced in Vero cells. (iii) The vhs-1 mutant vector can target brain cells with limited viral replication. (iv) The vhs-1 mutant virus can serve as a helper virus for composite HSV-1 amplicon vectors carrying other toxic functions. Ongoing studies have been designed to further characterize the mechanism of the vhs function and its involvement in the apoptotic cellular response, as well as the potential use of the mutant as an oncolytic vector for cancer gene therapy.
Acknowledgments
We thank Gabriela Kotliroff, who derived the amplicon-p53 vector, and Moshe Oren of the Weizmann Institute of Science, Rehovot, Israel, for the gift of the p53 gene and the DO-1 antibodies. We thank Israel Nir from Omrix Biopharmaceuticals Ltd., Weizmann Science Park, Rehovot, Israel, for the gift of pooled human gamma globulin employed in viral titrations.
The studies were supported by the Israel Academy of Science, the S. Daniel Abraham Institute of Molecular Virology, and the S. Daniel Abraham Chair (to N.F.) for Molecular Virology and Gene Therapy, Tel Aviv University.
REFERENCES
- 1.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, M., and J. A. Blaho. 2001. Modulation of apoptosis during herpes simplex virus infection in human cells. Microbes Infect. 3:859-866. [DOI] [PubMed] [Google Scholar]
- 3.Aubert, M., J. O'Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 73:10359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 75:1013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou, J., and B. Roizman. 1994. Herpes simplex virus 1 gamma134.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc. Natl. Acad. Sci. USA 91:5247-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doepker, R. C., W. L. Hsu, H. A. Saffran, and J. R. Smiley. 2004. Herpes simplex virus virion host shutoff protein is stimulated by translation initiation factors eIF4B and eIF4H. J. Virol. 78:4684-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elgadi, M. M., C. E. Hayes, and J. R. Smiley. 1999. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J. Virol. 73:7153-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgadi, M. M., and J. R. Smiley. 1999. Picornavirus internal ribosome entry site elements target RNA cleavage events induced by the herpes simplex virus virion host shutoff protein. J. Virol. 73:9222-9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esclatine, A., B. Taddeo, L. Evans, and B. Roizman. 2004. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc. Natl. Acad. Sci. USA 101:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esclatine, A., B. Taddeo, and B. Roizman. 2004. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J. Virol. 78:8582-8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esclatine, A., B. Taddeo, and B. Roizman. 2004. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc. Natl. Acad. Sci. USA 101:18165-18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, P., D. N. Everly, Jr., and G. S. Read. 2005. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol. 79:9651-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, P., D. N. Everly, Jr., and G. S. Read. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 75:10272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenwick, M. L., and M. J. Walker. 1978. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J. Gen. Virol. 41:37-51. [DOI] [PubMed] [Google Scholar]
- 16.Frenkel, N., and R. Sarid. 1996. Herpes simplex virus-1 amplicons, p. 143-148. In P. R. Lowenstein and L. W. Enquist (ed.), Protocols for gene transfer in neuroscience: towards gene therapy of neurological disorders. John Wiley & Sons, New York, N.Y.
- 17.Frenkel, N., R. J. Jacob, R. W. Honess, G. S. Hayward, H. Locker, and B. Roizman. 1975. Anatomy of herpes simplex virus DNA. III. Characterization of defective DNA molecules and biological properties of virus populations containing them. J. Virol. 16:153-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenkel, N., O. Singer, and A. D. Kwong. 1994. Minireview: the herpes simplex virus amplicon—a versatile defective virus vector. Gene Ther. 1(Suppl. 1):S40-S46. [PubMed] [Google Scholar]
- 19.Halliburton, I. W., R. E. Randall, R. A. Killington, and D. H. Watson. 1977. Some properties of recombinants between type 1 and type 2 herpes simplex viruses. J. Gen. Virol. 36:471-484. [DOI] [PubMed] [Google Scholar]
- 20.Hill, A. B., B. C. Barnett, A. J. McMichael, and D. J. McGeoch. 1994. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J. Immunol. 152:2736-2741. [PubMed] [Google Scholar]
- 21.Hsu, W. L., H. A. Saffran, and J. R. Smiley. 2005. Herpes simplex virus infection stabilizes cellular IEX-1 mRNA. J. Virol. 79:4090-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, F. E., C. A. Smibert, and J. R. Smiley. 1995. Mutational analysis of the herpes simplex virus virion host shutoff protein: evidence that vhs functions in the absence of other viral proteins. J. Virol. 69:4863-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karr, B. M., and G. S. Read. 1999. The virion host shutoff function of herpes simplex virus degrades the 5′ end of a target mRNA before the 3′ end. Virology 264:195-204. [DOI] [PubMed] [Google Scholar]
- 24.Kwong, A. D., and N. Frenkel. 1985. The herpes simplex virus amplicon. IV. Efficient expression of a chimeric chicken ovalbumin gene amplified within defective virus genomes. Virology 142:421-425. [DOI] [PubMed] [Google Scholar]
- 25.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong, F. A. 1995. Biology of herpes simplex virus (HSV) defective viruses and development of the amplicon system. Academic Press, San Diego, Calif.
- 28.Lam, Q., C. A. Smibert, K. E. Koop, C. Lavery, J. P. Capone, S. P. Weinheimer, and J. R. Smiley. 1996. Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J. 15:2575-2581. [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu, P., F. E. Jones, H. A. Saffran, and J. R. Smiley. 2001. Herpes simplex virus virion host shutoff protein requires a mammalian factor for efficient in vitro endoribonuclease activity. J. Virol. 75:1172-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mossman, K. L., R. Sherburne, C. Lavery, J. Duncan, and J. R. Smiley. 2000. Evidence that herpes simplex virus VP16 is required for viral egress downstream of the initial envelopment event. J. Virol. 74:6287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nardi, N., G. Avidan, D. Daily, R. Zilkha-Falb, and A. Barzilai. 1997. Biochemical and temporal analysis of events associated with apoptosis induced by lowering the extracellular potassium concentration in mouse cerebellar granule neurons. J. Neurochem. 68:750-759. [DOI] [PubMed] [Google Scholar]
- 33.Nichol, P. F., J. Y. Chang, E. M. Johnson, Jr., and P. D. Olivo. 1994. Infection of sympathetic and sensory neurones with herpes simplex virus does not elicit a shut-off of cellular protein synthesis: implications for viral latency and herpes vectors. Neurobiol. Dis. 1:83-94. [DOI] [PubMed] [Google Scholar]
- 34.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oroskar, A. A., and G. S. Read. 1987. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol. 61:604-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pak, A. S., D. N. Everly, K. Knight, and G. S. Read. 1995. The virion host shutoff protein of herpes simplex virus inhibits reporter gene expression in the absence of other viral gene products. Virology 211:491-506. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Parada, J., H. A. Saffran, and J. R. Smiley. 2004. RNA degradation induced by the herpes simplex virus vhs protein proceeds 5′ to 3′ in vitro. J. Virol. 78:13391-13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Read, G. S., B. M. Karr, and K. Knight. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 67:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smibert, C. A., D. C. Johnson, and J. R. Smiley. 1992. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J. Gen. Virol. 73:467-470. [DOI] [PubMed] [Google Scholar]
- 41.Smibert, C. A., B. Popova, P. Xiao, J. P. Capone, and J. R. Smiley. 1994. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J. Virol. 68:2339-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spaete, R. R., and N. Frenkel. 1982. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell 30:295-304. [DOI] [PubMed] [Google Scholar]
- 44.Strand, S. S., and D. A. Leib. 2004. Role of the VP16-binding domain of vhs in viral growth, host shutoff activity, and pathogenesis. J. Virol. 78:13562-13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strand, S. S., T. K. Vanheyningen, and D. A. Leib. 2004. The virion host shutoff protein of herpes simplex virus type 1 has RNA degradation activity in primary neurons. J. Virol. 78:8400-8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strom, T., and N. Frenkel. 1987. Effects of herpes simplex virus on mRNA stability. J. Virol. 61:2198-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzutani, T., M. Nagamine, T. Shibaki, M. Ogasawara, I. Yoshida, T. Daikoku, Y. Nishiyama, and M. Azuma. 2000. The role of the UL41 gene of herpes simplex virus type 1 in evasion of non-specific host defence mechanisms during primary infection. J. Gen. Virol. 81:1763-1771. [DOI] [PubMed] [Google Scholar]
- 48.Sydiskis, R. J., and B. Roizman. 1966. Polysomes and protein synthesis in cells infected with a DNA virus. Science 153:76-78. [DOI] [PubMed] [Google Scholar]
- 49.Taddeo, B., A. Esclatine, and B. Roizman. 2002. The patterns of accumulation of cellular RNAs in cells infected with a wild-type and a mutant herpes simplex virus 1 lacking the virion host shutoff gene. Proc. Natl. Acad. Sci. USA 99:17031-17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taddeo, B., A. Esclatine, W. Zhang, and B. Roizman. 2003. The stress-inducible immediate-early responsive gene IEX-1 is activated in cells infected with herpes simplex virus 1, but several viral mechanisms, including 3′ degradation of its RNA, preclude expression of the gene. J. Virol. 77:6178-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trgovcich, J., D. Johnson, and B. Roizman. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the gamma134.5 and UL41 genes of herpes simplex virus 1. J. Virol. 76:6974-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]