Abstract
Respiratory syncytial virus (RSV) is a primary cause of morbidity and life-threatening lower respiratory tract disease in infants and young children. Children with acute RSV bronchiolitis often develop respiratory sequelae, but the disease mechanisms are poorly understood. Mounting evidence suggests that RSV may mediate persistent infection. Using immunohistochemistry to identify RSV and RSV-infected cell types, we show that RSV infects primary neurons and neuronal processes that innervate the lungs through a process that involves RSV G protein and the G protein CX3C motif. These findings suggest a mechanism for disease chronicity and have important implications for RSV disease intervention strategies.
Respiratory syncytial virus (RSV) is the single most important cause of serious lower respiratory tract disease in infants and young children worldwide and is now recognized to be an important pathogen of the elderly and patients with compromised cardiac, pulmonary, and immune systems (7, 13, 19). The signature of RSV infection is bronchiolitis, but infection may also cause a range of illnesses including pneumonia, otitis media, and apnea (13). Natural RSV infection provides limited protective immunity, and humans may experience repeated infections and disease throughout life. The mechanisms that contribute to inadequate RSV resistance are not well understood; however, evidence indicates that RSV proteins may modulate immunity (19). The RSV NS1 and NS2 gene products have been shown to antagonize the alpha, beta, and gamma interferon response (6, 18), and the RSV G protein has been implicated with altered cytokine and chemokine expression by pulmonary leukocytes (19). In addition, RSV G protein contains an evolutionary conserved CX3C chemokine motif capable of interacting with CX3CR1 and antagonizing the activities of CX3CL1 (19, 20). It is likely that these immune evasion strategies benefit virus replication, but they may also affect viral persistence. RSV primarily infects respiratory epithelial cells; however, several studies have shown that RSV may infect and persist in alveolar macrophages of guinea pigs for up to 60 days postinfection (p.i.) (9), persist in bovine B cells infected with bovine RSV (23), and persist in the lungs of mice for more than 100 days postinfection (17). RSV persistence may be important in disease sequelae or chronicity and has important implications for the development of RSV vaccine candidates (15) and disease intervention strategies.
RSV infection is associated with exaggerated neurogenic inflammation in the airways, and studies in rodents have linked the tachykinin neuropeptide substance P as a primary mediator of neurogenic inflammation (14, 19). RSV G protein expression is associated with increased pulmonary levels of substance P (22), and sensory neurons in the lung express CX3CR1 on their cell surfaces (4, 8). On the basis that RSV G protein binds CX3CR1 (20), that CX3CR1 is expressed on neurons (8), and that RSV may mediate persistent infection (9, 17, 23), we postulated that RSV may infect immune-privileged neuronal cells in the lungs of BALB/c mice.
To determine whether RSV infects neuronal cells and the importance of G protein or the G protein CX3C motif for infection, primary cortical neuronal cultures were prepared from 16-day-old embryos of Swiss-Webster mice (Harlan Sprague-Dawley, Indianapolis, IN) as described previously (1, 10, 11). Briefly, mice were euthanized, the neocortexes were collected and digested with trypsin, and the neuronal cells were plated onto culture wells treated with poly-d-lysine (50 μg/ml). The primary neurons were grown in modified Eagle's medium (GIBCO BRL, Grand Island, NY) in 5% CO2 at 37°C. Ara-c (Sigma) was added to the cultures 3 days after plating at a final concentration of 1 μM to prevent the proliferation of nonneuronal cells. Neuronal cell cultures were infected at a multiplicity of infection (MOI) of 1 with RSV strain A2 (RSV/A2), recombinant RSV/A2 (6340WT), recombinant RSV/A2 lacking the G protein gene (6340ΔG), or with a RSV/A2 point mutant having a CYS→ARG amino acid change at position 186, ablating the CX3C motif (R10C7G) (16) termed RSV/ΔCX3C as previously described (21), or treated with uninfected Vero cell lysate (control). RSV infection was confirmed by immunohistochemistry using monoclonal antibody specific to RSV N protein (clone 130-12H) (3). Briefly, at day 5 p.i., neuronal cultures were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature, treated with 0.1% Triton X-100 for 10 min, and blocked for 1 h with 5% normal mouse serum (Sigma) diluted in PBS (blocking buffer). Anti-RSV N protein monoclonal antibody was diluted 1:500 in blocking buffer, added to the fixed cultures, and incubated at 4°C for 48 h. Cultures were washed three times with PBS and incubated with appropriate dilutions of secondary antibodies conjugated with fluorescent dyes for 1 h at room temperature. Where counterstaining was required, Nissl staining dye (Molecular Probes, Eugene, OR) was added for 10 min. After the PBS wash, stained cells were observed by using an epifluorescence microscope (Olympus, Japan).
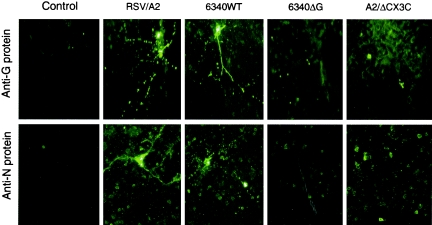
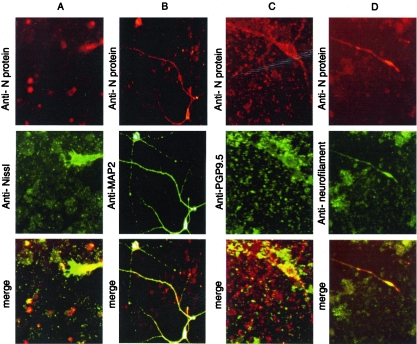
RSV infection of primary neuronal cell cultures was detected following infection with either RSV/A2 or 6340WT (Fig. 1). RSV infection of neuronal cell cultures was not detected following heat inactivation (60°C, 1 h) or following 1 μg/ml anti-CX3CR1 monoclonal antibody blockade (data not shown), and no staining was detected in negative control neuronal cells (control). To determine the distinguishing features of the neuronal cell types that were susceptible to RSV infection, primary neuronal cell cultures were infected with RSV/A2 (MOI of 1), fixed at day 5 p.i., and costained with anti-RSV N protein monoclonal antibody and Nissl counter staining (Fig. 2A) anti-MAP-2 (Chemicon, Temecula, California) (panel B), anti-PGP9.5 (Chemicon) (panel C), or antineurofilament antibodies (Sigma) (panel D). The results showed that RSV may infect cells containing Nissl substance (12) (Fig. 2A), microtubule-associated protein (MAP-2; Fig. 2B), neuron-specific protein-gene product 9.5 (PGP9.5) (5) (Fig. 2C), or cells with neurofilaments (Fig. 2D), indicating that RSV may infect neurons.
FIG. 1.
Expression of RSV G and N proteins in primary cortical neurons at day 5 post-RSV infection. Mouse primary neurons were infected (MOI of 1) with wild-type RSV strain A2 (RSV/A2), recombinant RSV derived from strain A2 (6340WT), 6340WT lacking the G protein gene (6340ΔG), or with a RSV strain A2 point mutant having a CYS→ARG amino acid change at position 186 (A2/ΔCX3C). At 5 day p.i., neurons were fixed with 4% paraformaldehyde and viral antigens were detected using anti-RSV N monoclonal antibodies. RSV N protein expression was detected in only cells infected with RSV/A2 and 6340WT, not in cells infected with mutant RSV, i.e., 6340ΔG and A2/ΔCX3C. Magnification, ×32.
FIG. 2.
Codetection of RSV N antigen and neuronal markers in primary neurons at day 5 post-RSV infection. Primary neurons infected with RSV/A2 were fixed at 5 day p.i. RSV N protein was detected together with neuronal markers Nissl (A), MAP2 (B), PGP9.5 (C), and neurofilament (D). RSV N antigen staining was merged with each of the neuronal markers. RSV N antigen staining overlapped with each of the neuronal markers, indicating that RSV infects primary neurons (Magnification, ×32). No staining was detected in negative control neuronal cells (Fig. 1).
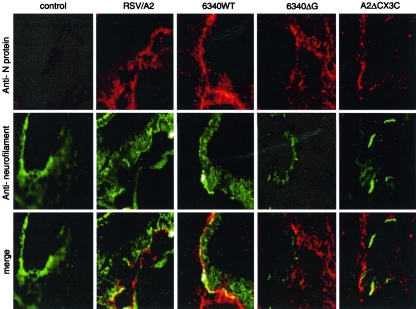
Based on the findings that RSV infects up to 5% of primary neuronal cells (Fig. 1) and because the lungs are innervated with sensory neurons (2), we determined whether RSV infected neurons in the lungs of six-week-old, specific-pathogen-free female BALB/c mice (Harlan Sprague-Dawley). Mice were anesthetized with Avertin (2,2,2-tribromoethanol) and intranasally challenged with 106 PFU of RSV/A2, 6340WT, 6340ΔG, or RSV/ΔCX3C. At day 5 p.i., lungs were removed, fixed, sectioned as previously described (10), and costained with anti-RSV N protein monoclonal antibody and antineurofilament antibodies (Fig. 3). Briefly, fresh frozen lung tissues were sectioned at 20 μm by Cryostat, fixed in cold acetone for 10 min, treated with 0.1% Triton X-100 for 10 min, and blocked for 1 h with 10% normal serum diluted in PBS. Primary antibodies were diluted in blocking buffer and incubated with the lung sections for 48 h at 4°C. Lung sections were washed three times with PBS and incubated with secondary antibodies conjugated with a fluorescent dye for 1 h at room temperature. Sections were examined under an epifluorescence microscope.
FIG. 3.
Detection of RSV N antigen in neuronal processes in lung tissue. Lung tissues from mice infected with 106 PFU RSV/A2, 6340WT, 6340ΔG, or A2/ΔCX3C virus were fixed and subjected to immunohistochemistry using monoclonal antibodies to RSV N protein or neurofilament. RSV N protein staining was merged with neurofilament staining. Overlapping staining was observed in only the lungs of mice infected with wild-type RSV (RSV/A2 and 6340WT), not in lungs of mice infected with mutant RSV (6340ΔG and A2/ΔCX3C) (Magnification, ×16).
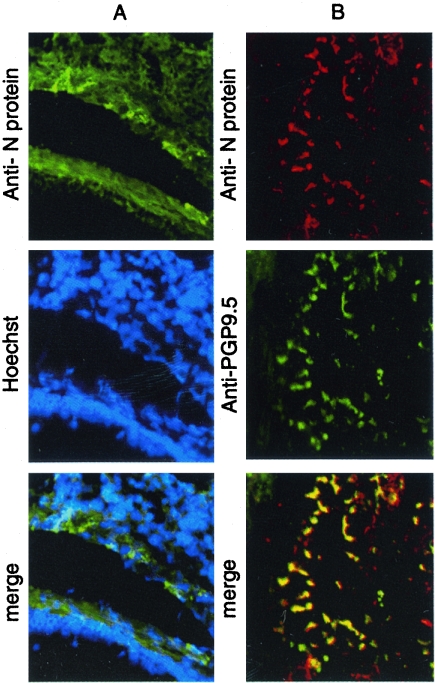
The immunohistochemistry results showed that only RSV/A2 or 6340WT infected neuronal cells in the lungs of mice, which was primarily observed around the small bronchi, suggesting that RSV infection is reduced in the absence of RSV G protein and the G protein CX3C motif. To determine the distinguishing features of lung neuronal cells that may be infected with RSV, 20-μm lung sections were prepared from RSV/A2-infected mice at day 5 p.i. and costained with anti-RSV N protein monoclonal antibody and Hoechst's to counterstain (Fig. 4A) or with antibodies against PGP9.5 (panel B). The results showed that RSV infects PGP9.5+ neurons in the lungs of mice. The proportion of neuronal cells in the lungs infected with RSV/A2 was relatively low (1 to 5%) but similar to that of primary neuronal cell cultures (Fig. 1).
FIG. 4.
Codetection of RSV N protein and neuronal marker PGP9.5 in RSV-infected lung tissue. (A) RSV N protein was detected primarily in the epithelial walls surrounding small bronchioles (longitudinal section), and Hoechst staining shows only a few RSV-positive cells (most likely epithelial cells). (B) RSV N protein staining overlapped with neuronal marker PGP9.5 in nervous fibers in a small bronchiole (transversal section). These nerve fibers may come from one neuron (Magnification, ×32).
Together, these observations show that RSV may infect lung neuronal cells by a process associated with RSV G protein and that RSV infection of primary neuronal cell cultures is linked to expression of RSV G protein and the G protein CX3C motif. These findings suggest that RSV infection of immune-privileged cell types in the lung may be a mechanism that contributes to persistence and have important implications in understanding RSV disease chronicity as well as in RSV disease intervention and live-attenuated vaccine strategies.
Acknowledgments
This work was supported by the Georgia Research Alliance and National Institutes of Health grant R01 051560.
We thank Peter Collins, NIH, and Jose Melero, Instituto de Salud Madrid, Spain, for their generous gifts of recombinant RSV viruses and RSV strain A2 point mutant, respectively.
REFERENCES
- 1.Adamec, E., F. Yang, G. M. Cole, and R. A. Nixon. 2001. Multiple-label immunocytochemistry for the evaluation of nature of cell death in experimental models of neurodegeneration. Brain Res. Brain Res. Protoc. 7:193-202. [DOI] [PubMed] [Google Scholar]
- 2.Adriaensen, D., I. Brouns, J. Van Genechten, and J. P. Timmermans. 2003. Functional morphology of pulmonary neuroepithelial bodies: extremely complex airway receptors. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 270:25-40. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, L. J., J. C. Hierholzer, C. Tsou, R. M. Hendry, B. F. Fernie, Y. Stone, and K. McIntosh. 1985. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect. Dis. 151:626-633. [DOI] [PubMed] [Google Scholar]
- 4.Combadiere, C., K. Salzwedel, E. D. Smith, H. L. Tiffany, E. A. Berger, and P. M. Murphy. 1998. Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J. Biol. Chem. 273:23799-23804. [DOI] [PubMed] [Google Scholar]
- 5.Day, I. N. 1992. Enolases and PGP9.5 as tissue-specific markers. Biochem. Soc. Trans. 20:637-642. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh, B., T. Komatsu, K. Takeuchi, and J. Yokoo. 2001. Paramyxovirus accessory proteins as interferon antagonists. Microbiol. Immunol. 45:787-800. [DOI] [PubMed] [Google Scholar]
- 7.Handforth, J., J. S. Friedland, and M. Sharland. 2000. Basic epidemiology and immunopathology of RSV in children. Paediatr. Respir. Rev. 1:210-214. [DOI] [PubMed] [Google Scholar]
- 8.Hatori, K., A. Nagai, R. Heisel, J. K. Ryu, and S. U. Kim. 2002. Fractalkine and fractalkine receptors in human neurons and glial cells. J. Neurosci. Res. 69:418-426. [DOI] [PubMed] [Google Scholar]
- 9.Hegele, R. G., S. Hayashi, A. M. Bramley, and J. C. Hogg. 1994. Persistence of respiratory syncytial virus genome and protein after acute bronchiolitis in guinea pigs. Chest 105:1848-1854. [DOI] [PubMed] [Google Scholar]
- 10.Li, X., L. Sarmento, and Z. F. Fu. 2005. Degenerative changes of mouse neuronal processes after rabies infection. J. Virol. 79:10063-10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, Y., D. Brewer, R. E. Burke, and G. A. Ascoli. 2005. Developmental changes in spinal motoneuron dendrites in neonatal mice. J. Comp. Neurol. 483:304-317. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien, L., K. Shelley, J. Towfighi, and A. McPherson. 1980. Crystalline ribosomes are present in brains from senile humans. Proc. Natl. Acad. Sci. USA 77:2260-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogra, P. L. 2004. Respiratory syncytial virus: the virus, the disease and the immune response. Paediatr. Respir. Rev. 5(Suppl. A):S119-S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piedimonte, G. 2003. Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 22:S66-S74. (Discussion, S74-S75.) [DOI] [PubMed] [Google Scholar]
- 15.Polack, F. P., and R. A. Karron. 2004. The future of respiratory syncytial virus vaccine development. Pediatr. Infect. Dis. J. 23:S65-S73. [DOI] [PubMed] [Google Scholar]
- 16.Rueda, P., B. Garcia-Barreno, and J. A. Melero. 1994. Loss of conserved cysteine residues in the attachment (G) glycoprotein of two human respiratory syncytial virus escape mutants that contain multiple A-G substitutions (hypermutations). Virology 198:653-662. [DOI] [PubMed] [Google Scholar]
- 17.Schwarze, J., D. R. O'Donnell, A. Rohwedder, and P. J. Openshaw. 2004. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am. J. Respir. Crit. Care Med. 169:801-805. [DOI] [PubMed] [Google Scholar]
- 18.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected]. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripp, R. A. 2004. Pathogenesis of respiratory syncytial virus infection. Viral Immunol. 17:165-181. [DOI] [PubMed] [Google Scholar]
- 20.Tripp, R. A., L. P. Jones, L. M. Haynes, H. Zheng, P. M. Murphy, and L. J. Anderson. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2:732-738. [DOI] [PubMed] [Google Scholar]
- 21.Tripp, R. A., D. Moore, L. Jones, W. Sullender, J. Winter, and L. J. Anderson. 1999. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J. Virol. 73:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripp, R. A., D. Moore, J. Winter, and L. J. Anderson. 2000. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. J. Virol. 74:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valarcher, J. F., H. Bourhy, A. Lavenu, N. Bourges-Abella, M. Roth, O. Andreoletti, P. Ave, and F. Schelcher. 2001. Persistent infection of B lymphocytes by bovine respiratory syncytial virus. Virology 291:55-67. [DOI] [PubMed] [Google Scholar]