Abstract
Interaction of herpes simplex virus (HSV) glycoprotein D (gD) with specific cellular receptors is essential for HSV infection of susceptible cells. Virus mutants that lack gD can bind to the cell surface (attachment) but do not enter, implying that interaction of gD with its receptor(s) initiates the postattachment (entry) phase of HSV infection. In this report, we have studied HSV entry in the presence of the gD-binding variable (V) domain of the common gD receptor nectin-1/HveC to determine whether cell association of the gD receptor is required for HSV infection. In the presence of increasing amounts of the soluble nectin-1 V domain (sNec1123), increasing viral entry into HSV-resistant CHO-K1 cells was observed. At a multiplicity of 3 in the presence of optimal amounts of sNec1123, approximately 90% of the cells were infected. The soluble V domain of nectin-2, a strain-specific HSV entry receptor, promoted entry of the HSV type 1 (HSV-1) Rid-1 mutant strain, but not of wild-type HSV-1. Preincubation and immunofluorescence studies indicated that free or gD-bound sNec1123 did not associate with the cell surface. sNec1123-mediated entry was highly impaired by interference with the cell-binding activities of viral glycoproteins B and C. While gD has at least two functions, virus attachment to the cell and initiation of the virus entry process, our results demonstrate that the attachment function of gD is dispensable for entry provided that other means of attachment are available, such as gB and gC binding to cell surface glycosaminoglycans.
Herpes simplex virus (HSV) enters most cells by pH-independent fusion of the virus envelope with the cell surface membrane through a poorly understood cascade of molecular interactions involving multiple viral glycoproteins and cellular receptors. HSV attachment to the cell involves recognition both of cell surface proteoglycans and one or more specific cellular receptors (see references 70 to 72 for reviews). While binding to proteoglycans is not essential for virus entry, it enhances the efficiency of infection (3, 27, 30, 31, 41). In cell culture, glycoproteins designated gH/gL, gB, and gD have been shown to be essential for entry (5, 15, 21-23, 32, 33, 35, 43, 62). Deletion of one or more of these essential proteins results in enveloped virus particles that are capable of binding to cells but cannot proceed further into the entry cascade (5, 20, 22, 43, 62).
Virus attachment to cell surface glycosaminoglycans (GAGs) is mediated by the virion glycoproteins gC and gB (69, 70, 73) and involves interactions between positively charged sequences on these molecules and negatively charged components of heparan, chondroitin, and dermatan sulfate (3, 27, 66, 73, 80, 81). A second binding event involves recognition by gD of a cognate cell surface receptor. The target cell must express a receptor for gD (25, 51), and gD must bind this receptor to initiate the entry process (23, 25, 33, 43, 51, 79). While it has recently been shown that HSV type 1 (HSV-1) can enter certain cells through endocytosis, productive infection in these instances also requires a gD receptor (48, 55, 56, 58).
Three receptors have been described for HSV-1 gD: (i) herpesvirus entry mediator A (HVEM, or HveA), a member of the tumor necrosis factor alpha receptor family (51), binds gD in vitro (57, 79), and its expression on the surface of nonsusceptible cells is sufficient to mediate viral entry (51, 79). HVEM has a somewhat limited expression pattern in vivo (51). (ii) Nectin-1 or herpesvirus entry mediator C (HveC; also referred to as poliovirus receptor-related protein 1) is a member of the nectin family of adhesion molecules belonging to the immunoglobulin superfamily (25). Nectin-1 is a type I transmembrane glycoprotein (25) with a cytoplasmic C terminus that interacts with the cytoskeleton (64) and an ectodomain comprised of an N-terminal V domain and two C2-like domains. The nectin-1 V domain mediates cell-cell adhesion by homotypic or heterotypic trans interactions with nectin V domains on neighboring cells (63, 75). In addition, nectins can form cis homo-oligomers by interactions involving C2-like domains of adjacent molecules (40, 50). Nectin-1 has three isoforms, nectin-1α (HveC), nectin-1β (HIgR), and nectin-1γ, which are derived from a single primary transcript by alternative splicing (11, 25, 44). The three isoforms share identical V and C domains but differ in their transmembrane and intracellular domains. All three have the ability to bind gD and mediate cellular entry of HSV-1. Nectin-1 has a wide expression pattern in vivo, and its α and β isoforms are probably the major HSV-1 entry receptors in the central and peripheral nervous systems (11, 28, 47). (iii) Heparan sulfate modified by the activity of 3-O-sulfotransferase-3 functions as an entry receptor exclusively for HSV-1 (68), unlike nectin-1 and HVEM, which enable entry of multiple alphaherpesviruses (72).
Epitope mapping and sequence deletion studies have shown that only the V region of the nectin-1 ectodomain is necessary for binding to gD (9, 10, 24, 38, 40). A truncated form of nectin-1, containing just the V domain, binds to gD with the same affinity as the full-length form, although receptor oligomerization is altered by the truncation (40). Functionally, epitopes recognized by monoclonal antibodies to nectin-1 that block HSV infection lie within the V domain (10, 38). Furthermore, a soluble nectin-1 derivative containing the V domain fused to an immunoglobulin Fc domain was found to specifically compete with cell surface nectin-1β for binding to viral gD, resulting in reduced viral entry when the soluble fusion protein was included in the culture medium (10). In addition, it has been shown that a deleted version of nectin-1β consisting of the V domain fused to the transmembrane region was able to confer entry susceptibility to nonpermissive cells, indicating that the C2-like domains are not essential (10). Replacement of the immunoglobulin family poliovirus receptor V domain with the nectin-1 V domain confers new HSV-1 entry-mediating capability to the chimeric poliovirus receptor (10), and similar results have been reported for nectin-1 V domain chimeras with other immunoglobulin-like receptors (24). In combination, these results demonstrated that the V domain of nectin-1 is necessary and sufficient for interaction with gD and that the cell-anchored V domain is sufficient to allow virus entry.
It is generally believed that binding of gD to its cellular receptor(s) provides two functions: (i) stabilization of the HSV-cell interaction initiated by the binding of gC and gB to cell surface GAGs (21, 59); and (ii) initiation of the fusion process between the viral envelope and the cell membrane. Viruses that lack gD can attach to the cell, and cells that lack gD receptors can bind the virus (43, 51, 67), demonstrating that gD interaction with a cell-associated receptor is not required to bring the virus in contact with the cell. It is conceivable, therefore, that soluble gD receptors that lack a transmembrane region and do not associate with the target cells in other ways (44) can mediate entry. Indeed, it has been reported for certain enveloped viruses that soluble receptors, while inhibiting entry into susceptible cells, enable entry into receptor-deficient cells (1, 7, 13, 37, 74).
In this report, we demonstrate that cell association of nectin-1 is not required for nectin-1-dependent HSV-1 infection. We observed substantial virus entry into gD receptor-deficient CHO-K1 cells when the soluble V domain of nectin-1 was present during virus incubation with the cells. This phenomenon was dependent on binding of the soluble molecule to virion gD and interaction of viral gB/gC with cell surface GAGs. No evidence was obtained for cell association of the soluble V domain. These results suggest that gD binding to the cell is not required for virus entry.
MATERIALS AND METHODS
Cells and viruses.
Cricetulus griseus Chinese hamster ovary cells (CHO-K1; ATCC CCL-61) were maintained in Ham's F-12K medium (Gibco-Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Gibco). CHO-K1 cells constitutively expressing full-length human HveC/nectin-1α, referred to here as CHO-Nec1 cells, were kindly provided by Patricia Spear (Northwestern University, Illinois) and grown in Ham's F-12K medium supplemented with 10% FBS and 400 μg/ml G418 (Gibco). pgsA-745 (ATCC CRL-2242) is a CHO cell mutant defective in glycosaminoglycan synthesis (17). pgsD-677 (ATCC CRL-2244) is a CHO cell mutant deficient for heparan sulfate polymerization (42). 293T cells were grown in Dulbecco's modified Eagle's medium (Gibco) with 10% FBS.
Recombinant virus QOZHG is a previously described, replication-defective derivative of HSV-1 strain KOS (6). Due to deletions and sequence substitutions, this virus does not produce four of the immediate-early (IE) proteins (ICP4, -22, -27, and -47) but expresses reporter genes encoding β-galactosidase and enhanced green fluorescent protein. QOZHG was grown on ICP4/ICP27-complementing Vero-7B cells (45). KHZ.1 is an HSV-1 KOS derivative expressing β-galactosidase from a cytomegalovirus promoter in the thymidine kinase (tk) locus (60). KOS-Rid1/tk12, generously provided by P. Spear, contains the Q27P (rid1) mutant version of gD and expresses the lacZ reporter gene from within the tk locus (77). KHZ.1 and KOS-Rid1/tk12 were grown and titers were determined on Vero cells (ATCC CCL-81).
Antibodies.
Nectin-1 V domain-specific monoclonal antibody (MAb) CK6 and anti-gD polyclonal antibody R7 were kindly provided by G. H. Cohen and R. J. Eisenberg (University of Pennsylvania) (36, 38). Peroxidase-conjugated anti-His tag MAb was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Expression plasmids for soluble proteins. (i) sNec1123ΔHis.
The cDNA sequence encoding the signal peptide and V domain of human nectin-1 (amino acids −30 to +123) was amplified by PCR using primers NecN1 (5′-CCCAAGCTTGCCACCATGGCTCGGATGGGGCTTGCGGGCGCCGC-3′) and NecC153 (5′-CCG CTCGAGCTAGGATCCCTCTATCCAATTGGTGGGTTTGGCCATC-3′). The primer sequences were designed to provide a Kozak translational initiation sequence (GCCACCATG) at the start of the open reading frame and a BamHI restriction site immediately 5′ to the stop codon. Genomic DNA from CHO-Nec1 cells was used as the PCR template. The PCR products were digested with HindIII and XhoI and ligated into the HindIII-XhoI sites of pcDNA3.1(+) (Invitrogen) to generate recombinant plasmid pNec1153Δhis, which was verified by DNA sequencing.
(ii) sNec1123.
Sequences encoding six histidine residues followed by a TAA stop codon were introduced into the BamHI site of pNec1153Δhis using annealed oligonucleotides. The annealed oligonucleotides were generated by mixing equal amounts of primers HIS1 (5′-GATCACACCATCACCATCACCATTAAG-3′) and HIS2 (5′-GATCCTTAATGGTGATGGTGATGGTGT-3′) in annealing buffer (20 mM Tris-HCl, pH 7.5, 50 mM NaCl, and 10 mM MgCl2), heating at 95°C for 5 min, and slowly cooling to room temperature. The final plasmid, pNec1153-his, was verified by DNA sequencing.
(iii) sNec1123TMC.
To generate the expression plasmid for sNec1123TMC, a convenient derivative of pNec1153-his was used. This derivative, pNec1/2/1153-his, contained the coding sequence for amino acids 36 to 70 of mature nectin-2, replacing nectin-1 codons +36 to +67 in pNec1153-his, creating a unique BstEII site at the upstream nectin-1-nectin-2 boundary. The nectin-2 sequence in pNec1/2/1123-his between the BstEII site and a unique EcoRI site flanking the downstream nectin-2-nectin-1 boundary was replaced with a BstEII-EcoRI fragment specifying nectin-1 codons +36 to +67 with the desired mutations at positions +46, +47, and +55 (QN76-77AA,M85F in reference 46). The mutant nectin-1 fragment was generated by annealing of two 3′-complementary oligonucleotides, double-strand formation using the Klenow fragment of Escherichia coli DNA polymerase I, and digestion with BstEII and EcoRI. The sequences of the oligonucleotides were as follows (mutant codons underlined): 5′-ACCCAGGTCACCTGGCAGAAGTCCACCAATGGCTCCAAGGCCGCCGTGGCC ATCTACAACCCA-3′ (TMC+) and 5′-CCGCAGGAATTCCACACGCTCGCGGTAGGGAGCCAGCACGGACACGCCAAAGGATGGGTTGTAGATGGCCAC-3′ (TMC-).
(iv) sNec2135.
The expression construct for the nectin-2 variable domain (pNec2168-his) was kindly provided by S. Wendell (University of Pittsburgh). The construct contained the coding sequence for the signal peptide and V domain (amino acids −33 to +135) of human nectin-2 followed by six histidine codons and a translation termination codon. The tagged nectin-2 sequence was obtained by a two-step PCR procedure on a human testis cDNA library (obtained from P. Robbins, University of Pittsburgh) using overlapping primer pairs. The first primer pair (B5in, 5′-GCCCTCCTGCCGTCGAGATC-3′; B3in, 5′-CTTGGTTCTTGGGCTTGGCTATGACTC-3′) was used for amplification of an internal portion of the coding sequence. The second pair (B5out, 5′-CCCAAGCTTGCCACCATGGCCCGGGCCGCTGCCCTCCTGCCG-3′; B3out, 5′-CCGCTCGAGCTAATGGTGATGGTGATGGTGGGCCTCAGCTTGGTTCTTGGG CTTGGC-3′) was used to complete the sequence by reamplification of the product of the first reaction. The second primer pair provided a HindIII site and a Kozak sequence preceding the nectin-2 translation initiation codon (bold type) in B5out and added six histidine codons, a stop codon (bold type), and an XhoI site after nectin-2 position +135 (underlined) in B3out. The final product was digested with HindIII and XhoI and inserted between the HindIII and XhoI sites of pcDNA3.1(+). Sequence verification was performed for the selected recombinant, pNec2168-his (S. Wendell, personal communication).
(v) sgD287.
The expression plasmid for sgD287 contained the gD coding sequence to amino acid 287 (HindIII-NarI fragment) fused in frame to six histidine codons and a translation termination codon and inserted between the HindIII and EcoRI sites of pcDNA3.1(+) (A. R. Frampton, Jr., personal communication).
Production and purification of soluble proteins.
293T cells were transfected with appropriate expression plasmids using LipofectAMINE-Plus reagent (Invitrogen). Transfected cultures were incubated for 3 days at 37°C, and the culture media were harvested and loaded onto Ni-chelated columns (Clontech, Palo Alto, CA) for purification of the His-tagged proteins according to the manufacturer's protocol. The eluted material was dialyzed at 4°C for 16 h against three exchanges of phosphate-buffered saline (PBS) and concentrated using a Centricon YM-10 centrifugal filter device (Amicon, Bedford, MA). Specific proteins were identified by gel electrophoresis and immunoblotting. The concentration of each purified protein was determined by Bradford assay (Bio-Rad, Hercules, CA).
Recombinant proteins for examination of the effects of the His tag on sNec1123-mediated infection were similarly obtained by transfection of 293T cells. Medium samples were analyzed by Western blotting, and concentrations were calculated from densitometry scans compared to purified sNec1123 standards on the same blot.
Standard infection procedure.
A total of 3 × 105 CHO-K1 cells suspended in cold PBS were preincubated with virus for 30 to 60 min at 4°C on a rocking device in a volume of 280 μl. Soluble recombinant protein was added to 40-μl samples, and the mixtures were incubated for 1 to 2 h at 37°C under constant rocking. The cells were collected by low-speed centrifugation, washed once with PBS, resuspended in 40 μl F-12K-10% FBS medium, and seeded in a single well of a 96-well plate. After incubation for 15 to 17 h in a 5% CO2 incubator at 37°C, the cultures were processed for entry assays. Deviations from this procedure are noted in the text.
Entry assays.
Virus entry was determined by staining of infected cell monolayers for β-galactosidase activity (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside [X-Gal] staining) or by quantitative colorimetric assay (o-nitrophenyl-β-d-galactopyranoside [ONPG]), as previously described (54). Briefly, cultures were fixed with 0.25% glutaraldehyde (Sigma, St. Louis, MO) for 1 min at room temperature, washed twice with PBS (pH 7.4), and stained with 0.2 mg/ml X-Gal (Sigma). Stained cultures were photographed under a Nikon Diaphot microscope (Nikon, Melville, NY), and computer-enlarged images were used to count the total number of cells and the number of blue cells in defined fields. For ONPG assays, the cells were lysed in 150 μl of 1% NP-40, 1 mM MgCl2, 50 mM β-mercaptoethanol, and 4 mg/ml ONPG (Sigma). The lysates were incubated at 37°C until a light yellow color developed, and the reactions were terminated by the addition of an equal volume of 1 M Na2CO3. Enzyme activity was determined by reading the absorbance at 405 nm.
Electrophoresis and Western blotting.
Gel electrophoresis and immunoblotting of purified His-tagged proteins were performed as previously described (54). For analysis of unpurified, secreted recombinant proteins produced by transfected 293T cells, 40-μl medium samples were electrophoresed. Different amounts of purified sNec1123 protein were included as standards. These blots were reacted with MAb CK6 (38) (1:5,000 dilution) for 12 h at 4°C, washed, and incubated with horseradish peroxidase-conjugated goat anti-mouse antibody (1:15,000 dilution; Sigma) for 45 min at room temperature. Duplicate blots were reacted with peroxidase-conjugated anti-His tag MAb (Santa Cruz). Peroxidase activity was detected using an ECL kit (Amersham-Pharmacia, Piscataway, NJ).
sNec1123/sgD287 prebinding assay.
A total of 3 × 105 CHO-K1 cells were suspended in 0 to 500 nM sNec1123, sgD287, or both in 280 μl PBS and incubated for 1.5 h at 4°C under constant agitation. The cells were collected, washed twice with cold PBS, and resuspended in 280 μl cold QOZHG virus dilution (multiplicity of infection [MOI] of 1) in PBS. After incubation of the mixture for 1 h at 4°C under continued agitation, fresh sNec1123 protein (500 nM) was added and the samples were incubated for 1 h at 37°C with agitation. Aliquots (40 μl/well) were seeded in a 96-well plate and incubated with 100 μl fresh F-12K-10% FBS for 15 to 17 h in a 5% CO2 environment at 37°C prior to processing for ONPG assay.
Heparin competition assay.
Various dilutions of QOZHG in PBS were preincubated with 25 μg/ml heparin for 1 h at 4°C under agitation. A total of 3 × 105 CHO-K1 cells were resuspended in 280 μl preincubation mix and incubated for 30 min at 4°C. sNec1123 was then added to a final concentration of 450 nM, and the cells were incubated for 1 h at 37°C under continued agitation. Aliquots of 40 μl were seeded with 100 μl F-12K-10% FBS in a 96-well plate and left overnight in a 37°C-5% CO2 incubator. Samples were then processed for ONPG assay. Heparin inhibition of QOZG infection of CHO-Nec1 cells was tested by the same protocol without the addition of sNec1123.
RESULTS
Preparation of soluble nectin-1 V domain.
Soluble, histidine (His6)-tagged nectin-1 V domain, comprised of residues 1 to 123 of mature human nectin-1 (sNec1123), was produced by transfection of human 293T cells with an appropriate expression construct (pNec1153-his). Culture media were collected after 72 h and enriched by passage over a Ni2+ affinity column. sNec1123 was identified by immunoblotting using His6-specific and nectin-1 V domain-specific antibodies (54) (data not shown). As a quality control, the enriched preparation was found to inhibit HSV-1 infection of CHO cells expressing a transfected nectin-1 cDNA construct (CHO-Nec1 cells [25]) in a dose-dependent manner (data not shown), consistent with previous reports (10, 25, 40, 44).
sNec1123 promotes HSV-1 entry into gD receptor-deficient CHO-K1 cells.
To measure HSV entry into cells, we used a replication-defective recombinant virus, QOZHG, that carries an expression cassette for β-galactosidase (6). This virus was chosen to prevent viral spread and limit virus-mediated cytotoxicity. Since spread and cytotoxicity are peripheral events that may influence viral reporter gene expression upon infection, this approach should enable a more accurate assessment of virus entry efficiency compared to the use of replicating, cytotoxic viruses that resemble wild-type (wt) HSV more closely. Nonetheless, as referred to in our account below, many of the results reported here have been confirmed with a replicating virus.
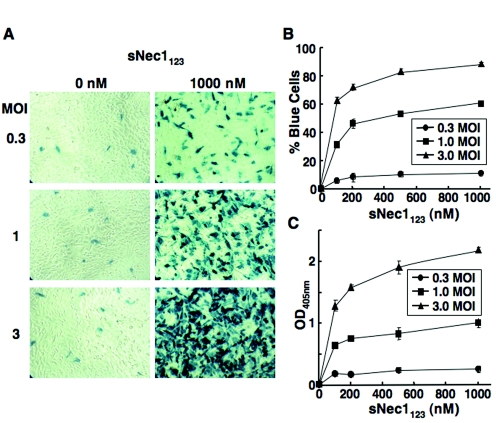
Using QOZHG, we determined whether sNec1123 could promote HSV-1 infection of HSV-resistant CHO-K1 cells. The virus and cells were mixed and incubated in suspension for 1 h at 4°C before addition of sNec1123 and further incubation at 37°C for 90 min. The cells were then plated, and β-galactosidase activity was visualized 16 h later by X-Gal staining. Representative results are shown in Fig. 1A. While few blue cells were observed after infection in the absence of sNec1123, modest to dramatic increases in the number of blue cells were evident at increasing MOIs when the infections were carried out in the presence of 1 μM soluble receptor. As illustrated in Fig. 1B, the percentage of blue cells in triplicate cultures increased with both the MOI and the sNec1123 dose. At the highest MOI and dose, nearly 90% of the cells were reproducibly infected. Quantitative measurement of cumulative β-galactosidase expression in triplicate cultures of infected cells by ONPG assay showed a similar profile of dose and MOI dependence (Fig. 1C). These results, along with qualitatively similar observations using a replication-competent reporter virus (data not shown, but see Fig. 3C, below), demonstrated that sNec1123 could mediate HSV-1 entry into gD receptor-deficient CHO-K1 cells, resulting in expression of a viral reporter gene.
FIG. 1.
Soluble Nec1123-mediated HSV-1 infection of CHO-K1 cells. CHO-K1 cells in suspension were incubated with reporter virus QOZHG at three different MOIs for 1 h at 4°C and infected for 1.5 h at 37°C in the presence of increasing concentrations of sNec1123 protein. The cells were then collected and plated. (A) Monolayers stained with X-Gal 16 h after plating. (B) Enlarged images of stained monolayers were used to count the number of blue and total cells in defined fields, and the results were computed as the percentage of blue cells in each well. (C) Separate monolayers were collected for quantitative measurement of β-galactosidase expression by ONPG assay. Results in panels B and C represent averages of triplicate determinations.
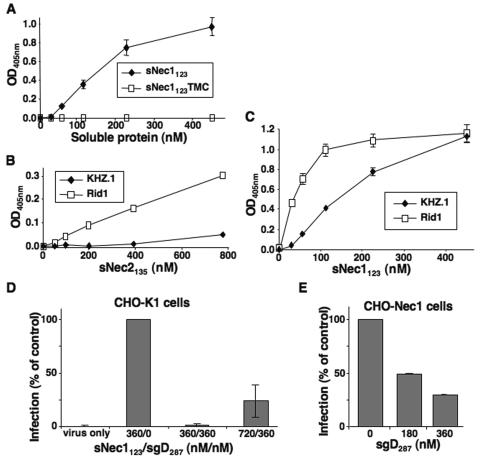
FIG. 3.
Specificity of sNec1123-mediated entry. Results are from ONPG assays 16 h after plating. (A) Comparison of QOZHG entry mediated by sNec1123 and the gD binding-defective mutant derivative sNec1123TMC. CHO-K1 cells were infected at an MOI of 1 in the presence of increasing amounts of sNec1123 or sNec1123TMC protein. (B) Virus entry mediated by soluble nectin-2 V domain (sNec2135). CHO-K1 cells were infected with replication-competent, lacZ-expressing viruses containing wild-type gD (KHZ.1) (60) (MOI = 12) or rid1 mutant gD (KOS-Rid1/tk12) (77) (MOI = 6) in the presence of increasing amounts of sNec2135 protein. (C) Entry of KHZ.1 (MOI = 6) and KOS-Rid1/tk12 (MOI = 3) in the presence of sNec1123. (D) Inhibition of the entry-promoting activity of sNec1123 by preincubation of the soluble receptor with soluble gD ectodomain, sgD287. Soluble receptor and soluble gD were mixed in the indicated molar proportions (nM/nM) and incubated for 15 min at 4°C. QOZHG was then added, and the mixture was incubated for another 15 min at 4°C. CHO-K1 cells in suspension were then infected at 37°C for 50 min (MOI = 3) and plated with fresh medium. The numbers under the bars show the concentrations of the two soluble molecules during infection. Results are expressed as percent infection relative to the positive (360/0) control. (E) Inhibition of infection of receptor-bearing cells by soluble gD. CHO-Nec1 cells in suspension were incubated with sgD287 at the indicated concentrations for 30 min at 4°C prior to infection with QOZHG (MOI = 3) for 50 min at 37°C. Results are expressed as percent infection relative to the control without sgD. All results represent averages of triplicate determinations, and each experiment was performed at least twice.
Efficiency of sNec1123-mediated infection of receptor-deficient cells.
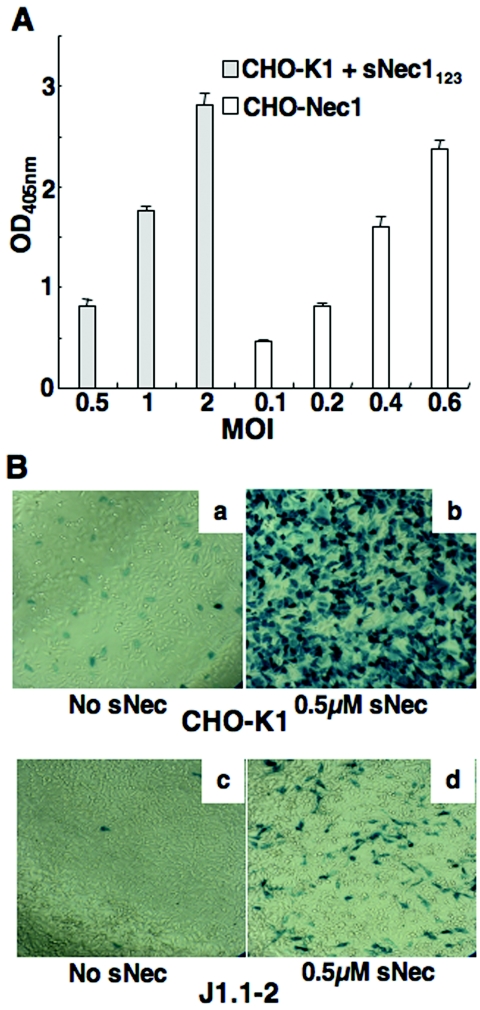
To obtain an estimate of the relative efficiency of sNec1123-mediated infection, we compared QOZHG entry into CHO-K1 cells in the presence of sNec1123 with entry of the same virus into CHO-Nec1 cells in the absence of soluble receptor. Infections were performed following virus preincubation with the cells, as above, and entry was determined by ONPG assay. The results indicated that sNec1123-mediated entry was approximately 2.5-fold less efficient than entry mediated by cell surface nectin-1 (Fig. 2A).
FIG. 2.
Soluble Nec1123-mediated HSV-1 infection of gD receptor-deficient cells. (A) Comparison of virus entry mediated by soluble nectin-1 V domain and cellular nectin-1. CHO-K1 and CHO-Nec1 cells were infected in suspension with QOZHG at different MOIs in the presence (CHO-K1) or absence (CHO-Nec1) of 450 nM sNec1123. The cells were plated, and β-galactosidase expression in triplicate cultures was measured by ONPG assay. (B) Comparison of sNec1123-mediated infection of CHO-K1 and J1.1-2 cells. QOZHG and sNec1123 (500 nM) were mixed and incubated for 1 h at 4°C prior to infection of CHO-K1 or J1.1-2 cells in suspension at an MOI of 3 for 1 h at 37°C. The cells were then plated, and virus entry was visualized by X-Gal staining 15 h later. Both experiments in this figure (A and B) were repeated twice with similar results.
We were curious to determine whether sNec1123 could mediate HSV infection of other receptor-deficient cell lines. As an example, we chose J1.1-2 cells. It has been reported that receptor-bearing derivatives of these cells are infected by fusion of the HSV envelope with the plasma membrane (26), unlike receptor-bearing derivatives of CHO cells, which are infected by virus endocytosis (56, 58). In the experiment shown in Fig. 2B, we preincubated QOZHG with sNec1123 at 4°C prior to infection of J1.1-2 or CHO-K1 cells in suspension. It can be seen that infection of CHO-K1 cells (panel b) yielded vastly more blue cells than infection of J1.1-2 cells (panel d) at the same MOI and sNec1123 concentration. Infection by our standard protocol involving preincubation of the virus and cells at 4°C followed by infection in the presence of soluble receptor yielded essentially the same results (data not shown). While these experiments demonstrated that sNec1123-mediated infection of receptor-deficient cells is not limited to CHO-K1 cells, the apparent difference in efficiency remains to be carefully explored.
Infection requires sNec1123 interaction with viral gD.
We generated a mutant sNec1123 protein, denoted sNec1123TMC, to examine whether the entry-promoting activity of the wt molecule involved interaction with gD. sNec1123TMC contained a combination of mutations previously shown to abolish gD binding (QN76-77AA,M85F [46]). Although recognized on a Western blot by both nectin-1- and His tag-specific antibodies, sNec1123TMC failed to inhibit HSV infection of CHO-Nec1 cells at concentrations that produced 50% inhibition using wt sNec1123 (data not shown). This result confirmed that the mutant protein could not compete with cellular nectin-1 for binding to gD. As shown in Fig. 3A, the TMC version of sNec1123 also failed to enable QOZHG entry into CHO-K1 cells, unlike wt sNec1123.
To strengthen the suggestion from these results that soluble receptor-mediated virus entry into CHO-K1 cells requires specific interaction of the mediator with gD in the viral envelope, the related V domain of nectin-2 was tested. Nectin-2 (HveB) functions as an entry receptor for HSV-1 strains that have specific amino acid changes in gD, such as the rid1 (Q27P) mutation, but not for wt HSV-1 (77). As illustrated in Fig. 3B, soluble nectin-2 V domain (sNec2135) promoted entry of the replication-competent rid1 mutant virus KOS-Rid1/tk12 (77) into CHO-K1 cells, whereas little entry was observed with a replicating wt gD virus, KHZ.1 (60). At the same time, both viruses were capable of entry in the presence of sNec1123 (Fig. 3C), consistent with the abilities of nectin-1 to bind both wt and rid1 mutant gD (39) and to serve as an entry receptor for the corresponding viruses (25). Entry of the Rid1 virus saturated at a lower sNec1123 concentration than entry of KHZ.1, perhaps due to the reportedly increased affinity of nectin-1 for gD(rid1) compared to gD(wt) (39, 40). KOS-Rid1/tk12 entry mediated by sNec2135 appeared less efficient than entry of the same virus mediated by sNec1123, as anticipated from observations that nectin-2/HveB may be a less efficient entry receptor for HSV(rid1) strains than nectin-1/HveC (24).
Additional evidence that sNec1123-mediated virus entry involves specific interaction of the mediator with viral gD was provided by the following observations. (i) Preincubation of the soluble nectin-1 V domain with soluble gD ectodomain (sgD287) reduced the entry-promoting activity of sNec1123 in a manner dependent on the molar ratio of sNec1123 to sgD287 (Fig. 3D). The ectodomain of gD, truncated with a C-terminal His6 tag after position 287, was expressed in 293T cells and purified from the supernatant, essentially as described for sNec1123. Preincubation of sNec1123 with an equimolar amount of soluble gD for 15 min at 4°C abolished sNec1123-mediated QOZHG infection of CHO-K1 cells, while low but measurable infection was observed when the molar amount of sNec1123 was twice that of sgD287. As a quality control for our soluble gD preparation, we confirmed that preincubation of the protein with CHO-Nec1 cells inhibited virus infection (Fig. 3E), consistent with previous reports (10, 12). (ii) sNec1123-mediated infection of CHO-K1 cells was inhibited by increasing amounts of anti-gD antibodies in the infection mixture (data not shown). Together, these results showed that HSV-1 infection of receptor-deficient CHO-K1 cells in the presence of soluble nectin V-domain required viral gD and specific interaction of gD with the V domain.
Soluble Nec1123 mediates viral entry without binding to the cell.
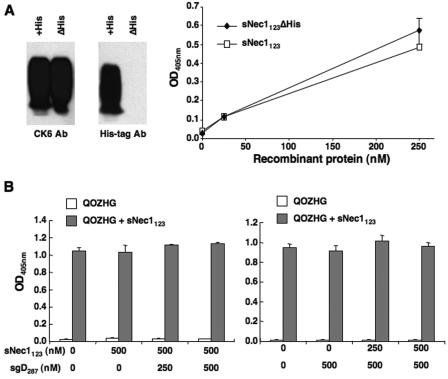
As a potential explanation for the apparent ability of sNec1123 to mediate viral entry into CHO-K1 cells, we considered the possibility that the soluble protein associates with the cell surface. One mechanism could involve binding of the positively charged histidine tag to negatively charged cell surface determinants, such as heparan sulfate. To examine this possibility, we used a sNec1123 expression construct that lacked the C-terminal histidine codons (pNec1153Δhis) to produce soluble protein by standard transfection of 293T cells and incubation of the cells with serum-free medium. For comparison, His6-containing sNec1123 was produced by the same procedure. The media were collected, and the yields of both products examined by immunoblotting using nectin-1-specific antibody CK6 (38). As illustrated in Fig. 4A, similar amounts of CK6-reactive proteins were observed in the two samples. Using an anti-His tag antibody, the same series of bands were detected in medium from pNec1153-his-transfected cells (Fig. 4A, +His), but not in medium from pNec1153Δhis-transfected cells (ΔHis), confirming the identities of the products. Increasing amounts of the two unpurified protein preparations were used in KHZ.1 entry assays into CHO-K1 cells. The results demonstrated that both preparations were active in mediating virus entry and that their specific activities were comparable (Fig. 4A). Thus, a critical role for the histidine tag in sNec1123-mediated infection of CHO-K1 cells was excluded.
FIG. 4.
Examination of sNec1123 association with the target cells. (A) Effect of the His6 tag on sNec1123-mediated entry. Left, soluble nectin-1 V domain with (+His) or without (ΔHis) His tag was produced by transfection of 293T cells and identified in the culture medium by immunoblotting using nectin-1 (CK6) and His tag-specific antibodies. Concentrations of the recombinant proteins were determined by densitometry comparison to purified sNec1123 standards (data not shown). Right, different amounts of the collected 293T medium (expressed as nM recombinant protein) were included in KHZ.1 infections of CHO-K1 cells at an MOI of 3. Entry was determined by ONPG assay. (B) Effects of preincubation of CHO-K1 cells with sNec1123 and/or soluble gD ectodomain (sgD287) on QOZHG infection (MOI = 1) in the presence (gray bars) or absence (open bars) of 500 nM sNec1123. Concentrations of soluble proteins during the preincubation period are indicated at the bottom. Following the preincubation, cells were washed and incubated with virus for 1 h at 4°C prior to infection at 37°C for 1 h in the presence or absence of freshly added sNec1123. The cells were collected and plated with fresh medium, and entry was determined by ONPG assay the following day. The two panels show independent experiments, each performed in triplicate.
By immunofluorescence analysis, no binding of sNec1123 to the surface of CHO-K1 cells was observed, although binding to gD-expressing cells was readily detected (data not shown). A functional assay was used to further determine whether sNec1123 binding to the cells, alone or complexed with gD, could be a condition for sNec1123-mediated virus entry. We preincubated CHO-K1 cells for 1.5 h at 4°C with sNec1123, soluble gD ectodomain (sgD287), or different ratios of the two soluble proteins. The cells were then washed and exposed to QOZHG virus in the presence or absence of sNec1123. The results can be summarized as follows (Fig. 4B): (i) preincubation with sNec1123 failed to render the cells susceptible to HSV-1 infection in the absence of sNec1123 added at the time of infection; (ii) preincubation of the cells with sNec1123 did not inhibit entry in the presence of sNec1123; and (iii) preincubation of the cells with premixed sNec1123 and sgD287 or sgD287 alone did not alter virus infection in the presence or absence of sNec1123. These results indicated that sNec1123 alone did not attach to the cell surface to either function as a virus receptor or block potential binding sites for sNec1123-coated virus. Furthermore, they argued against gD-dependent binding of sNec1123 to critical structures on the cell surface and vice versa, against sNec1123-dependent gD binding to potential coreceptors. We conclude that sNec1123-mediated infection of gD receptor-deficient CHO-K1 cells occurred without specific binding of the soluble mediator to the cells.
Efficient sNec1123-mediated entry of HSV-1 into CHO-K1 cells depends on interaction of gB and/or gC with cell surface GAGs. The ability of sNec1123 to stimulate viral entry without itself binding to the cell surface implied that its involvement was in triggering virus penetration into the cytoplasm rather than causing adsorption of the virus to the cell surface. To determine whether adsorption was at all necessary for sNec1123-mediated virus entry into CHO-K1 cells, we tested the contributions of gB, gC, and GAGs to the process.
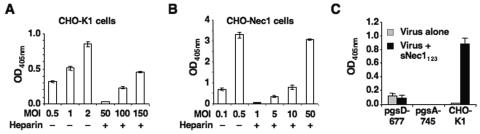
First, we examined whether blocking the GAG binding sites of gB and gC by heparin would diminish sNec1123-mediated virus entry into CHO-K1 cells. As illustrated in Fig. 5A, preincubation of QOZHG with 25 μg/ml heparin reduced sNec1123-mediated infection approximately 200-fold. For comparison, infection of CHO-Nec1 cells was reduced approximately 100-fold (Fig. 5B). Thus, sNec1123-mediated virus entry was sensitive to heparin, but not much more than unassisted infection of CHO-Nec1 cells. These results were consistent with previous reports that envelope gD binding to cognate cell surface receptors does not contribute significantly to initial virus attachment (21, 43, 51). In addition, they indicated that virus attachment to gD receptor-deficient cells was not completely blocked by heparin. This likely reflects additional interactions between the virus and cells, such as the recently described heparin-resistant binding of HSV gB to an unknown receptor (4).
FIG. 5.
Requirement for GAG binding in sNec1123-mediated infection. (A) Heparin inhibition of sNec1123-mediated virus entry. Different dilutions of QOZHG were preincubated with or without 25 μg/ml heparin for 1 h at 4°C and incubated with CHO-K1 cells in suspension for 30 min at 4°C prior to the addition of 450 nM sNec1123 and infection for 1 h at 37°C. The cells were plated with fresh medium, and entry was measured by ONPG assay the next day. Data represent the averages of triplicate determinations. (B) Heparin inhibition of infection via cellular nectin-1. QOZHG was preincubated with heparin as above and adsorbed to CHO-Nec1 cells. Infection was as described above without added sNec1123 protein. The cells were processed for ONPG assay as above. (C) Infection of GAG-deficient CHO cells. CHO-K1 and GAG-deficient pgsD-677 and pgsA-745 cells were infected with QOZHG at an MOI of 3 in the presence (black bars) or absence (gray bars) of 450 nM sNec1123 protein. Virus entry was determined by ONPG assay. Values are averages from triplicate infections.
Second, we investigated virus entry into two CHO-K1 subclones that lack some or all of the GAGs involved in HSV attachment. The pgsD-677 cell line is deficient for heparan sulfate polymerization, and pgsA-745 cells are defective in glycosaminoglycan synthesis (17, 42). Following incubation with QOZHG, little or no viral gene expression was observed in CHO-K1 cells or either of the mutant lines in the absence of sNec1123. In the presence of sNec1123, increased reporter gene activity was observed in CHO-K1 cells, but not in the mutant lines (Fig. 5C). Similar results were obtained using the replicating virus KHZ.1 (data not shown). In addition, we observed that the level of sNec1123-mediated entry into CHO-K1 cells of a replication-competent mutant virus that lacked gC and the GAG-binding polylysine (pK) region of gB (KgC−gBpK− [41]) was more than 15-fold lower than that of KHZ.1 (data not shown). Together, these observations indicated that virus interaction with glycosaminoglycans plays a major role in efficient sNec1123-dependent infection.
DISCUSSION
We have shown that soluble, truncated forms of the HSV entry receptors nectin-1/HveC and nectin-2/HveB are able to stimulate entry of HSV-1 into receptor-deficient CHO-K1 cells. Entry was dependent on adherence of the soluble receptor to viral envelope gD and on gB/gC-mediated virus attachment to cell surface glycosaminoglycans, but it did not appear to involve association of the soluble receptor with the cell surface. Together, these results support the notion that the principal function of HSV entry receptors is to promote virus entry rather than cell attachment.
Studies in the past 2 years have demonstrated that HSV-1 infects gD receptor-bearing CHO cells mainly by receptor-independent virus uptake into endocytic vesicles, followed by receptor- and low-pH-dependent virus release into the cytoplasm (56, 58). In contrast, infection of Vero cells occurs by pH-independent virus envelope fusion with the cytoplasmic membrane and requires prior interaction with a gD receptor. Both pathways result in genome delivery to the nucleus, as evidenced by viral and/or reporter gene expression, but differences in entry efficiencies may exist. Based on evidence that HSV infects receptor-bearing J1.1-2 cells, derived from receptor-deficient J1.1-2 BHK cells, by envelope fusion with the cell membrane, a recent report suggested that the HSV entry pathway is determined by intrinsic differences between cell lines (26). We have tested receptor-deficient J1.1-2 cells and observed that these cells are susceptible to sNec1123-mediated infection, although less dramatically than CHO-K1 cells. Whether the lower rate of J1.1-2 infection signifies differences in entry pathways remains to be conclusively shown, but the observation indicates that our results are not peculiar to a single cell line.
Our observations relate to a previous study demonstrating that intracellular expression of the secreted γ isoform of nectin-1 rendered HSV-resistant J1.1-2 cells susceptible to HSV infection (44). A significant difference between that study and ours is that we used an exogenous mediator, purified sNec1123 protein, while the effector molecule employed in the earlier study was produced by the target cells. The endogenous production of nectin-1γ raised the possibility that incomplete release of the molecule from the cell membrane would provide cell-associated binding sites for gD which could function as bona fide entry receptors. Indeed, nectin-1γ was detected on the surface of these producer cells, providing a satisfactory explanation for the acquired susceptibility of these cells to HSV infection (44).
Nectin-1 and -2 are members of a larger family of cell adhesion molecules that can interact with each other in various ways (cis or trans) and combinations (homo- or heterotypic) (63). These properties raised the concern that our sNec1123 could form functional cell surface receptors for gD by associating with known or unknown nectins on CHO or J1.1-2 cells. For example, it has been demonstrated that J1.1-2 cells possess nectin-3 (8), which can interact in trans with nectin-1 (18). The following considerations argue against such mechanisms. First, evidence has been reported that cis dimerization of nectins requires the membrane-proximal C2-like domain (40, 49), suggesting that the V domain alone will not dimerize in cis with heterotypic nectins on the cell surface. Second, the ability of nectin-1 to interact in trans with other nectins is mutually exclusive with its ability to bind to HSV-1 gD (61, 65), arguing that sNec1123 bound to nectin-3 or other nectins on the cell surface will not simultaneously interact with viral gD to trigger entry. Experimentally, we observed that preincubation of CHO-K1 cells with sNec1123 followed by removal of unbound protein failed to render the cells susceptible to HSV and failed to inhibit infection in the presence of sNec1123. Likewise, preincubation of the cells with sNec1123 complexed with soluble gD followed by removal of unbound material did not inhibit sNec1123-mediated infection, indicating that there was no gD-dependent binding of sNec1123 to the cells that could block subsequent binding of free or virus-complexed sNec1123. Together, these considerations favor the interpretation that sNec1123 did not associate with any known or unknown CHO-K1 or J1.1-2 cell surface structures to form functional gD receptors on these cells, thus supporting the conclusion that sNec1123 was capable of triggering HSV entry into resistant cells without prior binding to the cell surface.
Soluble receptor-mediated infection has been reported previously for other viruses, most notably subgroup A avian sarcoma and leukosis virus (ASLV-A) (13) and ASLV-B (37), but also certain human immunodeficiency virus type 2 and simian immunodeficiency virus strains (1, 7, 78), as well as mouse hepatitis virus (MHV) (16, 74). In the human immunodeficiency virus type 2 and simian immunodeficiency virus systems, enhanced infection mediated by soluble CD4 may be due to conformational changes in soluble CD4-bound gp120 that promote interaction of gp120 with essential coreceptors (82). HSV binding to GAGs is reportedly stabilized by binding of gD to cognate receptors (22, 59), but this is likely an indirect effect caused by the increased number of contacts between the virus and cell. We did not see inhibition of sNec1123-mediated infection by prior incubation of the cells with premixed sNec1123 and soluble gD ectodomain, arguing against a scenario in which nectin-1 binding to gD enables gD interaction with an unknown coreceptor. However, given the very recent demonstration of GAG-independent HSV gB binding to cell surfaces (4), it is conceivable that this newly described interaction is enhanced by sNec1123 binding to virion gD. In the MHV study, soluble receptor was shown to mediate or enhance viral spread to receptor-deficient cells by spike protein-dependent cell fusion, but attempts at direct MHV infection of receptor-deficient cells were unsuccessful, even under conditions designed to bring the virions in close proximity to the cell surface (74). Compared to ASLV, GAG binding by HSV appears to represent the missing attachment function provided by extraneous manipulation of the infection conditions in the ASLV studies (13, 37). In both the HSV and ASLV systems, the soluble receptor appears to enable virus penetration rather than attachment.
Soluble nectin-1, like soluble HVEM and soluble receptors for other viruses, can be used to inhibit infection of susceptible cells (2, 10, 14, 19, 25, 34, 40, 44, 51, 76, 79). Traditionally, this effect has been interpreted as evidence of competition between the soluble and cell-associated receptors for virus binding. However, it has also been recognized that soluble receptors may cause virus inactivation (2, 29, 52, 53). We have examined different conditions for sNec1123-mediated infection and observed the most consistently high levels of infection when the soluble molecule was added after virus attachment to the cells at 4°C. We imagine that soluble receptor binding to virion gD at 37°C triggers the entry machinery, leading to infection if the virus is localized at the cell surface at the time of sNec1123 binding. In the absence of prior virus attachment to the cells, we speculate that sNec1123 binding to the virus at 37°C results in inactivation of the viral entry machinery. We further speculate that soluble receptor-mediated inhibition of HSV infection of receptor-bearing cells may be mediated in part by the same mechanism of virus inactivation. In three studies reporting soluble nectin-1-mediated inhibition of HSV infection of nectin-1-bearing cells, the soluble receptor was preincubated with the virus at 37°C prior to infection (10, 25, 44). A fourth study reported inhibition after preincubation of the virus and soluble receptors at 4°C (40), which could reflect rapid virus inactivation at the infection temperature prior to adequate attachment of the receptor-coated virus particles to the cells. While these hypotheses require experimental validation, they provide a tentative rationale for the seemingly contradictory observations that soluble gD receptor inhibits HSV entry into receptor-bearing cells but promotes entry into receptor-negative cells. In this regard, it has been reported that HSV infection of HVEM-bearing cells is inhibited by soluble nectin-1, and vice versa, that infection of nectin-1-bearing cells is inhibited by soluble HVEM (25). These observations were interpreted as supporting evidence that the HVEM- and nectin-1-binding domains of gD overlap. Our hypothesis offers the alternative explanation that the conditions of these experiments—virus preincubation with soluble receptor at 37°C—may have inactivated the virus entry machinery irrespective of the nature of the HSV entry receptor present on the target cells.
Our observation of soluble receptor-mediated HSV infection of receptor-deficient cells agrees with previous studies indicating that the normal subsurface interaction of nectin-1 with the cytoskeleton is not directly involved in HSV entry (24, 64). Instead, our results suggest that the essential consequence of gD's binding to its receptor is activation of the viral fusion machinery, be it at the cytoplasmic or endosomal membrane, and that this outcome requires only the gD-binding V domain of nectin-1. Accordingly, our observations are consistent with current models proposing a conformational change in gD upon receptor binding which leads to activation of the fusion functions of other HSV envelope glycoproteins, most likely gB and/or gH/gL (reviewed by Spear et al. [71, 72]). For certain cell types, activation by this mechanism may not be sufficient, and exposure to an acidic environment may be required (58), thus delaying the fusion step until the virus appears in endosomes. In this instance, conformational changes initiated by extracellular contact between gD and its receptor must be remarkably stable to persist through the process of endocytic internalization to the stage of endosomal release. It remains to be determined how this scenario can be reconciled with our proposal that premature activation of the fusion machinery results in virus inactivation.
Acknowledgments
We thank Roselyn J. Eisenberg and Gary H. Cohen (University of Pennsylvania) for antibodies, Patricia G. Spear (Northwestern University) for CHO-nectin1 cells, and Paul Robbins (University of Pittsburgh) for the human testis cDNA library. We gratefully acknowledge the contributions of Steve Wendell (pNec2168-his) and Art Frampton, Jr. (psgD287) in our lab.
This work was supported by “Program in Excellence in Gene Therapy” grant HL66949-01 from the NIH NHLBI and grants GM34534-18 from the NIH NIGMS and DK44935-08 from the NIH NIDDK (all to J.C.G.). H.K. and H.B. were supported in part by a grant from the National Nuclear R&D Program, Korea Ministry of Science and Technology.
REFERENCES
- 1.Allan, J. S., J. Strauss, and D. W. Buck. 1990. Enhancement of SIV infection with soluble receptor molecules. Science 247:1084-1088. [DOI] [PubMed] [Google Scholar]
- 2.Balliet, J. W., J. Berson, C. M. D'Cruz, J. Huang, J. Crane, J. M. Gilbert, and P. Bates. 1999. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 73:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banfield, B. W., Y. Leduc, L. Esford, K. Schubert, and F. Tufaro. 1995. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J. Virol. 69:3290-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, W., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, X., J. Li, M. Mata, J. Goss, D. Wolfe, J. C. Glorioso, and D. J. Fink. 2000. Herpes simplex virus type 1 ICP0 protein does not accumulate in the nucleus of primary neurons in culture. J. Virol. 74:10132-10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clapham, P. R., A. McKnight, and R. A. Weiss. 1992. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J. Virol. 66:3531-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocchi, F., D. Fusco, L. Menotti, T. Gianni, R. J. Eisenberg, G. H. Cohen, and G. Campadelli-Fiume. 2004. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 101:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocchi, F., M. Lopez, P. Dubreuil, G. Campadelli Fiume, and L. Menotti. 2001. Chimeric nectin1-poliovirus receptor molecules identify a nectin1 region functional in herpes simplex virus entry. J. Virol. 75:7987-7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocchi, F., M. Lopez, L. Menotti, M. Aoubala, P. Dubreuil, and G. Campadelli-Fiume. 1998. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc. Natl. Acad. Sci. USA 95:15700-15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly, S. A., J. J. Whitbeck, A. H. Rux, C. Krummenacher, S. van Drunen Littel-van den Hurk, G. H. Cohen, and R. J. Eisenberg. 2001. Glycoprotein D homologs in herpes simplex virus type 1, pseudorabies virus, and bovine herpes virus type 1 bind directly to human HveC(nectin-1) with different affinities. Virology 280:7-18. [DOI] [PubMed] [Google Scholar]
- 13.Damico, R., and P. Bates. 2000. Soluble receptor-induced retroviral infection of receptor-deficient cells. J. Virol. 74:6469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deen, K. C., J. S. McDougal, R. Inacker, G. Folena-Wasserman, J. Arthos, J. Rosenberg, P. J. Maddon, R. Axel, and R. W. Sweet. 1988. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature 331:82-84. [DOI] [PubMed] [Google Scholar]
- 15.Desai, P., P. Schaffer, and A. Minson. 1988. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes-simplex virus type 1: evidence that gH is essential for virion infectivity. J. Gen. Virol. 69:1147-1156. [DOI] [PubMed] [Google Scholar]
- 16.Dveksler, G. S., S. E. Gagneten, C. A. Scanga, C. B. Cardellichio, and K. V. Holmes. 1996. Expression of the recombinant anchorless N-terminal domain of mouse hepatitis virus (MHV) receptor makes hamster of human cells susceptible to MHV infection. J. Virol. 70:4142-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabre, S., N. Reymond, F. Cocchi, L. Menotti, P. Dubreuil, G. Campadelli-Fiume, and M. Lopez. 2002. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C′-C"-D beta-strands of the nectin1 V domain. J. Biol. Chem. 277:27006-27013. [DOI] [PubMed] [Google Scholar]
- 19.Fisher, R. A., J. M. Bertonis, W. Meier, V. A. Johnson, D. S. Costopoulos, T. Liu, R. Tizard, B. D. Walker, M. S. Hirsch, R. T. Schooley, et al. 1988. HIV infection is blocked in vitro by recombinant soluble CD4. Nature 331:76-78. [DOI] [PubMed] [Google Scholar]
- 20.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller, A. O., and W. C. Lee. 1992. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J. Virol. 66:5002-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuller, A. O., R. E. Santos, and P. G. Spear. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J. Virol. 63:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller, A. O., and P. G. Spear. 1987. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc. Natl. Acad. Sci. USA 84:5454-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geraghty, R. J., A. Fridberg, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 2001. Use of chimeric nectin-1(HveC)-related receptors to demonstrate that ability to bind alphaherpesvirus gD is not necessarily sufficient for viral entry. Virology 285:366-375. [DOI] [PubMed] [Google Scholar]
- 25.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 26.Gianni, T., G. Campadelli-Fiume, and L. Menotti. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 78:12268-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruenheid, S., L. Gatzke, H. Meadows, and F. Tufaro. 1993. Herpes simplex virus infection and propagation in a mouse L cell mutant lacking heparan sulfate proteoglycans. J. Virol. 67:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haarr, L., D. Shukla, E. Rodahl, M. C. Dal Canto, and P. G. Spear. 2001. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology 287:301-309. [DOI] [PubMed] [Google Scholar]
- 29.Hart, T. K., R. Kirsh, H. Ellens, R. W. Sweet, D. M. Lambert, S. R. Petteway, Jr., J. Leary, and P. J. Bugelski. 1991. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 88:2189-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herold, B., R. Visalli, N. Susmarski, C. Brandt, and P. Spear. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulfate and glycoprotein B. J. Gen. Virol. 75:1211-1222. [DOI] [PubMed] [Google Scholar]
- 31.Herold, B. C., S. I. Gerber, T. Polonsky, B. J. Belval, P. N. Shaklee, and K. Holme. 1995. Identification of structural features of heparin required for inhibition of herpes simplex virus type 1 binding. Virology 206:1108-1116. [DOI] [PubMed] [Google Scholar]
- 32.Highlander, S. L., W. H. Cai, S. Person, M. Levine, and J. C. Glorioso. 1988. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J. Virol. 62:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Highlander, S. L., S. L. Sutherland, P. J. Gage, D. C. Johnson, M. Levine, and J. C. Glorioso. 1987. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J. Virol. 61:3356-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussey, R. E., N. E. Richardson, M. Kowalski, N. R. Brown, H. C. Chang, R. F. Siliciano, T. Dorfman, B. Walker, J. Sodroski, and E. L. Reinherz. 1988. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature 331:78-81. [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. Minson, and D. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isola, V. J., R. J. Eisenberg, G. R. Siebert, C. J. Heilman, W. C. Wilcox, and G. H. Cohen. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63:2325-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knauss, D. J., and J. A. Young. 2002. A fifteen-amino-acid TVB peptide serves as a minimal soluble receptor for subgroup B avian leukosis and sarcoma viruses. J. Virol. 76:5404-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krummenacher, C., I. Baribaud, M. Ponce de Leon, J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J. Virol. 74:10863-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krummenacher, C., A. H. Rux, J. C. Whitbeck, M. Ponce-de-Leon, H. Lou, I. Baribaud, W. Hou, C. Zou, R. J. Geraghty, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1999. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J. Virol. 73:8127-8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laquerre, S., R. Argnani, D. B. Anderson, S. Zucchini, R. Manservigi, and J. C. Glorioso. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72:6119-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lidholt, K., J. L. Weinke, C. S. Kiser, F. N. Lugemwa, K. J. Bame, S. Cheifetz, J. Massague, U. Lindahl, and J. D. Esko. 1992. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. USA 89:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ligas, M., and D. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez, M., F. Cocchi, E. Avitabile, A. Leclerc, J. Adelaide, G. Campadelli-Fiume, and P. Dubreuil. 2001. Novel, soluble isoform of the herpes simplex virus (HSV) receptor nectin1 (or PRR1-HIgR-HveC) modulates positively and negatively susceptibility to HSV infection. J. Virol. 75:5684-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marconi, P., D. Krisky, T. Oligino, P. L. Poliani, R. Ramakrishnan, W. F. Goins, D. J. Fink, and J. C. Glorioso. 1996. Replication-defective HSV vectors for gene transfer in vivo. Proc. Natl. Acad. Sci. USA 93:11319-11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez, W. M., and P. G. Spear. 2002. Amino acid substitutions in the V domain of nectin-1 (HveC) that impair entry activity for herpes simplex virus types 1 and 2 but not for pseudorabies virus or bovine herpesvirus 1. J. Virol. 76:7255-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mata, M., M. Zhang, X. Hu, and D. J. Fink. 2001. HveC (nectin-1) is expressed at high levels in sensory neurons, but not in motor neurons, of the rat peripheral nervous system. J. Neurovirol. 7:476-480. [DOI] [PubMed] [Google Scholar]
- 48.Milne, R. S., A. V. Nicola, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyahara, M., H. Nakanishi, K. Takahashi, K. Satoh-Horikawa, K. Tachibana, and Y. Takai. 2000. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J. Biol. Chem. 275:613-618. [DOI] [PubMed] [Google Scholar]
- 50.Momose, Y., T. Honda, M. Inagaki, K. Shimizu, K. Irie, H. Nakanishi, and Y. Takai. 2002. Role of the second immunoglobulin-like loop of nectin in cell-cell adhesion. Biochem. Biophys. Res. Commun. 293:45-49. [DOI] [PubMed] [Google Scholar]
- 51.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus 1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 52.Moore, J. P., J. A. McKeating, W. A. Norton, and Q. J. Sattentau. 1991. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J. Virol. 65:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 54.Nakano, K., R. Asano, K. Tsumoto, H. Kwon, W. F. Goins, I. Kumagai, J. B. Cohen, and J. C. Glorioso. 2004. Herpes simplex virus targeting to the EGF receptor by a gD-specific soluble bridging molecule. Mol. Ther. 11:617-626. [DOI] [PubMed] [Google Scholar]
- 55.Nicola, A. V., J. Hou, E. O. Major, and S. E. Straus. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 79:7609-7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicola, A. V., M. Ponce de Leon, R. Xu, W. Hou, J. C. Whitbeck, C. Krummenacher, R. I. Montgomery, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 72:3595-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez, A., and A. O. Fuller. 1998. Stable attachment for herpes simplex virus penetration into human cells requires glycoprotein D in the virion and cell receptors that are missing for entry-defective porcine cells. Virus Res. 58:21-34. [DOI] [PubMed] [Google Scholar]
- 60.Rasty, S., W. F. Goins, and J. C. Glorioso. 1995. Site-specific integration of multigenic shuttle plasmids into the herpes simplex virus type 1 (HSV-1) genome using a cell-free Cre-lox recombination system. Methods Mol. Genet. 7:114-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reymond, N., S. Fabre, E. Lecocq, J. Adelaide, P. Dubreuil, and M. Lopez. 2001. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 276:43205-43215. [DOI] [PubMed] [Google Scholar]
- 62.Roop, C., L. Hutchinson, and D. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakisaka, T., and Y. Takai. 2004. Biology and pathology of nectins and nectin-like molecules. Curr. Opin. Cell Biol. 16:513-521. [DOI] [PubMed] [Google Scholar]
- 64.Sakisaka, T., T. Taniguchi, H. Nakanishi, K. Takahashi, M. Miyahara, W. Ikeda, S. Yokoyama, Y. F. Peng, K. Yamanishi, and Y. Takai. 2001. Requirement of interaction of nectin-1α/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J. Virol. 75:4734-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Satoh-Horikawa, K., H. Nakanishi, K. Takahashi, M. Miyahara, M. Nishimura, K. Tachibana, A. Mizoguchi, and Y. Takai. 2000. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 275:10291-10299. [DOI] [PubMed] [Google Scholar]
- 66.Shieh, M.-T., and P. Spear. 1994. Herpes virus-induced cell fusion that is dependent on cell surface heparan sulfate on soluble heparin. J. Virol. 68:1224-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shieh, M. T., D. WuDunn, R. I. Montgomery, J. D. Esko, and P. G. Spear. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 69.Spear, P. 1993. Membrane fusion induced by herpes simplex virus, p. 201-232. In J. Bentz (ed.), Viral fusion mechanisms. CRC Press, Boca Raton, Fla.
- 70.Spear, P. G. 1993. Entry of alphaherpesviruses into cells. Semin. Virol. 4:167-180. [Google Scholar]
- 71.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 72.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 73.Spear, P. G., M. T. Shieh, B. C. Herold, D. WuDunn, and T. I. Koshy. 1992. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv. Exp. Med. Biol. 313:341-353. [DOI] [PubMed] [Google Scholar]
- 74.Taguchi, F., and S. Matsuyama. 2002. Soluble receptor potentiates receptor-independent infection by murine coronavirus. J. Virol. 76:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takai, Y., and H. Nakanishi. 2003. Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116:17-27. [DOI] [PubMed] [Google Scholar]
- 76.Traunecker, A., W. Luke, and K. Karjalainen. 1988. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature 331:84-86. [DOI] [PubMed] [Google Scholar]
- 77.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 78.Werner, A., G. Winskowsky, and R. Kurth. 1990. Soluble CD4 enhances simian immunodeficiency virus SIVagm infection. J. Virol. 64:6252-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whitbeck, J. C., C. Peng, H. Lou, R. Xu, S. H. Willis, M. Ponce de Leon, T. Peng, A. V. Nicola, R. I. Montgomery, M. S. Warner, A. M. Soulika, L. A. Spruce, W. T. Moore, J. D. Lambris, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1997. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a mediator of HSV entry. J. Virol. 71:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams, R. K., and S. E. Straus. 1997. Specificity and affinity of binding of herpes simplex virus type 2 glycoprotein B to glycosaminoglycans. J. Virol. 71:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wudunn, D., and P. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]