Abstract
Vpx protein of human immunodeficiency virus type 2/simian immunodeficiency virus (SIV) has been implicated in the transport of the viral genome into the nuclei of nondividing cells. The mechanism by which Vpx enters the nucleus remains unknown. Here we have identified two distinct noncanonical nuclear localization signals (NLSs) in Vpx of SIVsmPbj1.9 and defined the pathways for its nuclear import. Although nuclear targeting signals identified here are distinct from known nuclear import signals, translocation of Vpx into the nucleus involves the interaction of its N-terminal NLS (amino acids 20 to 40) or C-terminal NLS (amino acids 65 to 75) with importin alpha and, in the latter case, also with importin beta. Collectively, these results suggest that importins interact with Vpx and ensure the effective import of Vpx into the nucleus to support virus replication in nondividing cells.
Primate lentiviruses are able to infect nondividing cells, such as terminally differentiated macrophages (4, 12, 15, 16, 19), a feature that distinguishes them from the onco-retroviruses, which require nuclear membrane dissolution during cell division for successful viral replication (7, 31, 44). Vpx of human immunodeficiency virus type 2 (HIV-2) and simian immunodeficiency virus (SIV) is a 112-amino-acid (aa), 18-kDa protein (20). It is expressed in infected cells in a Rev-dependent manner and is packaged into new virions through its interaction with the p6 region of the p55gag precursor (1, 8, 20, 26). As the Vpx protein is incorporated into the virion (27, 28, 32), it becomes available during early replication events, immediately following entry of the new virion into a target cell even before de novo viral protein synthesis can start (10, 21, 32, 37, 38). Based on such late expression during virus production and early availability during initial infection, it has been proposed that Vpx is involved in the early stages of the viral life cycle, particularly in the efficient import of the viral genome into the nuclear compartment of nonproliferating target cells (10, 32, 37, 39, 40, 42). Vpx was found to participate in the active translocation of the large (Stokes radius, 28 nm) viral preintegration complex (PIC) across the limiting nuclear pore (10). Although Vpx displays evident karyophilic properties, it does not contain a canonical nuclear localization signal (NLS) (32, 37, 39, 40). The mechanism of Vpx-mediated nuclear import appears novel, but how Vpx cooperates with viral and host proteins to transport the viral PIC into the nucleus remains elusive.
Eukaryotic cells possess double-layered nuclear membranes containing multiple nuclear pores that regulate bidirectional transport of macromolecules that are critically required for the maintenance of normal cellular physiology. The nuclear pore complex (NPC) spans the nuclear membrane and creates an aqueous channel with a pore diameter of 9 nm, allowing the theoretical passive diffusion of a protein up to approximately 30 to 40 kDa (5, 9, 17, 18, 35). Translocation across the NPC and into the nucleoplasm and cytoplasm is governed by a protein family known as importins and exportins (11, 17, 33, 34). The importins and exportins engage the appropriate signals of the cargo proteins and mediate their directional transport (33, 34).
The classical NLS consists of either short sequences containing a single stretch of basic amino acid residues like that found in the simian virus 40 (SV40) large T antigen or a bipartite basic NLS with two interdependent basic amino acid clusters with an intervening spacer as found in nucleoplasmin (5, 9, 35). Both of these signals engage a common site on importin alpha, which in turn binds importin beta. The importin beta portion of this newly formed trimeric complex attaches directly to the NPC and targets the cargo inside the nucleus. Delivery is then completed by the binding of nuclear RanGTP to importin beta, thereby inducing dissociation of the complex. While it is not fully understood which factor(s) governs the directionality of transport, it is thought that the steep gradient of RanGTP generated by the GTPase RanGAP in the cytoplasm and the nucleotide exchange factor RCC1 in the nucleus play a central role (5, 33, 34). Importin beta also directly recognizes arginine-rich NLSs and transports the HIV regulatory proteins, such as Tat (43) and Rev (36), to the nucleus without an importin alpha intermediate. A third well-described import signal, termed M9, is present in heterogeneous nuclear ribonucleoprotein A1 (hnRNP-A1) and is similarly dependent on the RanGTP gradient, although it exhibits no sequence homology to the classical NLS (13, 33, 38). The M9 sequence is rich in aromatic amino acids and binds directly to transportin, a member of the karyopherin protein family that binds RanGTP and has 25% homology to importin beta. Unlike with NLSs, the existence of nuclear export signals (NESs) was established relatively recently. Although fewer such sequences have been identified and characterized, many NESs are marked by an abundance of hydrophobic residues.
Despite lacking any known import signal, Vpx is highly nucleophilic. The recent reports from our and other laboratories have revealed that the C-terminal region contributes to the nuclear localization of Vpx (37, 39). In the present investigation, for the first time, we show that two distinct noncanonical nuclear targeting signals reside within SIV Vpx and direct Vpx as well as other heterologous cytoplasmic proteins into the nucleus. Interestingly, we show that Vpx import signals are distinct from the previously known NLSs and demonstrate that Vpx is imported into the nucleus by pathways dependent on and independent of importin alpha. In addition, our data provide evidence for the existence of nuclear export activity in the Vpx protein.
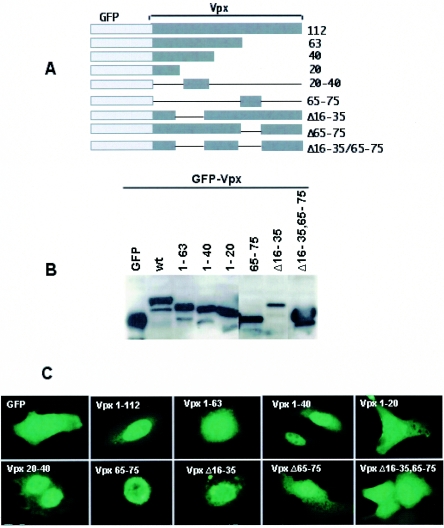
Evidence for the presence of two noncanonical transferable nuclear localization signals in Vpx.
The goal of this study was to define the signals and pathway(s) of nuclear import used by the Vpx protein of HIV-2/SIV. Recently we reported that the exchange of conserved residues in both the N and the C terminus impaired Vpx nuclear import (39, 40). For example, replacement of H39 at the N terminus and T67, R70, L74, and I75 at the C terminus resulted in impairment of Vpx nuclear transport (39, 40), suggesting the presence of two different nuclear targeting signals in Vpx, as in HIV-1 Vpr (24, 25). To determine the subcellular localization of Vpx, Vero cells were transfected with Green fluorescent protein (GFP)-Vpx fusion proteins (Fig. 1A) using the T7 RNA polymerase expression vaccinia virus system (14), and the chamber slides were viewed with an upright Nikon E800 microscope and photographed with a DXM1200 camera using Image Pro-plus software (Media Cybernetics). GFP was selected as a fusion partner to identify the nuclear targeting signal as it localizes to the nucleus when fused to Vpx and can directly be visualized in living cells. Epifluorescence microscopy of the transfected cells revealed that GFP fusion proteins containing wild-type Vpx were imported into the nuclear interior (Fig. 1C). This confirmed the presence of a functional nuclear targeting signal within Vpx. In contrast, GFP alone when tested was found to be distributed throughout the cell, consistent with its passive diffusion (Fig. 1C).
FIG.1.
Evidence for the presence of two functional noncanonical NLSs in SIV Vpx. (A) Schematic diagram of GFP-Vpx fusion proteins. (B) Cos-7 cells were transfected with various GFP-Vpx deletion constructs, and cell lysates were prepared 16 h after transfection and resolved by SDS-12% PAGE. Western blot analysis was performed using monoclonal anti-GFP antibody at a 1:1,000 dilution followed by horseradish peroxidase-conjugated specific secondary antibodies (1:2,000 dilution) and developed using the Enhanced Chemiluminescence Plus detection system (Amersham Pharmacia). (C) Subcellular localization of GFP-Vpx fusion proteins. Vero cells were infected with vaccinia TF7-3 and transfected with various GFP-Vpx expression plasmids, and the localization of GFP fusion proteins was visualized directly after fixing of the transfected cells with 3% paraformaldehyde. Immunofluorescence analysis of these constructs suggests that GFP-Vpx1-40 and -Vpx20-40 translocate into the nucleus like full-length Vpx, which provides evidence for the presence of additional nuclear targeting signal in the amino terminus of Vpx. (D) Serial laser sections through a representative cell that expressed wild-type Vpx protein. N-terminal (Vpx1-40) and C-terminal (Vpx65-75) NLSs were obtained by confocal laser scanning microscopy. Photomicrographs were numbered from 1, which corresponds to the nuclear periphery, through 5, which corresponds to the region through which the cell was adsorbed to the slide. (E) Sequence comparison of various types of NLSs, such as the classical lysine-rich type (SV40 Tag NLS), the arginine-rich HIV-1 Rev NLS, and the M9 signal of hnRNP-A1 with Vpx nuclear targeting signals. Comparative analysis suggests that Vpx NLSs are distinct from previously known NLSs. (F) Sequence-specific nuclear import of Vpx in digitonin-permeabilized HeLa cells. In vitro nuclear import assays (2) were performed using fusions of wild-type Vpx (His tag-Vpx) and its amino-terminal NLS (aa 1 to 40) and a mutant variant of Vpx1-40 (Vpx1-40 D26F) to the carboxy terminus of GST as substrates. Rabbit reticulocyte lysate (Promega) containing an energy-regenerating system (1 mM ATP, 0.5 mM GTP, 10 mM creatine phosphate, and 0.4 μm creatine phosphokinase) was used as a source of cytosolic factors. Transport reactions were performed using His-Vpx, GST-Vpx1-40, and Vpx1-40 D26F fusion proteins at a concentration of 1 to 5 μg, while GST was used as a negative control. Transport reaction mixtures were set up in ice and then allowed to proceed for 60 min at 30°C. Samples were analyzed by indirect immunofluorescence using anti-Vpx or anti-GST antibody followed by Alexa fluor 488-conjugated secondary antibody and epifluorescence. wt, wild type.
To further identify the presence of the NLS in the N terminus of Vpx, we used a series of plasmids that encode variants of Vpx with deletions in different regions of the protein, as shown schematically in Fig. 1A. GFP-Vpx fusion proteins with internal deletions in Vpx were generated by PCR using appropriate primers (see supplement 1 in the supplemental material). All GFP-Vpx fusion proteins were found to express equally, as determined by Western blot analysis using monoclonal GFP antibody (Fig. 1B). Analysis of their subcellular localization showed that GFP fusion proteins with Vpx containing aa 1 to 63 (Vpx1-63) and Vpx1-20 were localized in the cytoplasm (Fig. 1C). In contrast, nuclear localization was observed for GFP-Vpx1-40, similar to what occurred with full-length Vpx (Fig. 1C), indicating the presence of a nuclear targeting signal in the N terminus of Vpx. Interestingly, the fusion protein GFP-Vpx20-40 transported efficiently to the nucleus (Fig. 1C). Taken together, these results provide evidence for the presence of a functional NLS in the N-terminal domain (aa 20 to 40) of Vpx, in addition to the recently identified NLS in the C terminus of Vpx (37, 39). Vpx with the N-terminal NLS (Vpx20-40) is sufficient to target a heterologous protein to the nucleus. Surprisingly, GFP-Vpx1-63 expressed in either Cos-7 or Vero cells localized in the cytoplasmic compartment, in contrast to the nuclear localization of GFP-Vpx20-40. Possible shuttling of the fusion protein GFP-Vpx1-63 into and out of the nucleus with a longer dwell time in cytoplasmic compartment explains this paradox. Collectively, these data suggest that Vpx contains two nuclear targeting signals for the efficient nuclear localization of Vpx.
Serial sections through positively immunostained cells showed that a GFP fusion protein containing wild-type Vpx or fragments of Vpx (with either aa 1 to 40 or aa 65 to 75) was localized predominantly in the nuclei and nuclear envelopes of transfected cells (Fig. 1D). Interestingly, Vpx65-75 accumulated primarily in the nuclear membrane and Vpx1-40 was localized throughout the nucleus. The discrepancy between the localization of Vpx1-40 in the previous report (39) and that in the present investigation may be due to the difference in the import and/or export kinetics of the fusion proteins used. In the absence of homology between these two Vpx NLS sequences and with other known signals, like classical or M9 import signal sequences (Fig. 1E), it appears that Vpx may be imported to the nucleus by more than one novel import pathways. To further understand the mechanism of Vpx nuclear import, we first tested whether Vpx-mediated nuclear import could be reconstituted in vitro using digitonin-permeabilized HeLa cells (2) (see supplement 2 in the supplemental material). Vpx1-40 and Vpx1-40-D26F were PCR amplified and ligated to pET41b at BamHI and XhoI sites as a fusion with glutathione S-transferase (GST). The fusion proteins were purified by the standard protocol described elsewhere (22, 40). Consistent with the in vivo observations, Vpx was effectively imported into the nuclei of digitonin-permeabilized HeLa cells but not into the nucleoli (Fig. 1F). The reporter cytoplasmic protein GST was found exclusively in the cytoplasm, and when it was fused with Vpx1-40 but not its mutant (Vpx1-40-D26F), it was efficiently imported into the nucleus (Fig. 1F). These results indicate that Vpx import into the nucleus through the NPC is mediated by transferable and autonomous signals.
Vpx nuclear transport is a signal-mediated process and is sensitive to wheat germ agglutinin (WGA).
Entry based on purely facilitated diffusion would be expected to yield a localization pattern consistent with equilibration of the protein between the nuclear and cytoplasmic compartments. To distinguish between the facilitated diffusion and actual nuclear accumulation, the retention of fusion proteins in the nuclear compartment by binding to cellular factors after their entry into the nucleus was examined. To eliminate nonanchored chimeric proteins after transfection, Vero cells were treated with digitonin at 55 μg/ml before fixation in 3% paraformaldehyde. The cells on coverslips were then examined by direct immunofluorescence. The validity of this assay was confirmed by expression vectors that encode GFP, GFP-simian virus 40 (SV40) NLS, and GFP-Rev NLS fusion proteins. For generating HIV-1 Rev NLS and SV40 NLS GFP fusion proteins, the respective forward and reverse primers (see supplement 1 in the supplemental material) were annealed before being ligated at EcoRI and XhoI sites in the pcDNA3 vector as GFP fusion proteins. As expected, the GFP was distributed throughout the transiently transfected cells and was completely eliminated on prior treatment of the cells with digitonin (Fig. 2A). By contrast, GFP-SV40 NLS and GFP-Rev NLS fusion proteins were localized in the nuclei of both untreated and digitonin-treated cells (Fig. 2A). Thus, it appears that pretreatment of the cells with digitonin abolishes the diffusion of GFP but does not affect the signal-mediated nuclear transport process of fusion proteins. Interestingly, treatment with digitonin eliminated much of the diffused cytoplasmic staining but not the nuclear staining of GFP fusion proteins containing full-length Vpx, Vpx65-75, and Vpx1-40 (Fig. 2A). Thus, it appeared that both NLSs of Vpx might be anchored in the nuclear compartment by binding to cellular factors. Collectively, these observations suggest that both N- and C-terminal NLSs are important for binding to transport receptors for efficient Vpx nuclear import. Since these signals are distinct from the known NLSs, the nuclear targeting of Vpx mediated by these domains may involve a novel mechanism(s).
FIG. 2.
Vpx is imported into the nucleus by a signal-mediated pathway. (A) Photomicrographs of transfected HeLa cells that express the indicated fusion constructs. HeLa cells were transfected with plasmids that encode GFP-Vpx, Vpx1-40, Vpx65-75, the HIV-1 Rev NLS, and the SV40 NLS or the control protein, GFP. At 12 h after transfection, cells were treated (+) with digitonin at 55 μg/ml or were not treated (−) with digitonin and then fixed in 3% paraformaldehyde. The fusion proteins were directly visualized by epifluorescence microscopy. (B) Vpx nuclear import is sensitive to wheat germ agglutinin. Vpx, Rev NLS, and SV40 NLS expression plasmids were transfected into Cos-7 cells and observed for nuclear import in the presence of 0.5 mg of WGA/ml (+WGA) or in the absence of WGA (−WGA) All samples were analyzed by fluorescence microscopy as described in the legend for Fig. 1. DAPI, 4′,6′-diamidino-2-phenylindole.
Wheat germ agglutinin, a lectin, binds to N-acetyl-d-glucosamine residues present on many of the nucleoporins and blocks NLS-mediated import both in vivo and in vitro without restricting passive diffusion of small molecules. To evaluate the effects of WGA on Vpx import, WGA (0.5 mg/ml) was added to the cells transfected with GFP-Vpx, GFP-SV40 NLS, and GFP-Rev NLS. All three NLSs were imported with similar efficiencies in the absence of lectin (Fig. 2B). In contrast, WGA impairs Vpx-, Rev NLS-, and SV40 NLS-mediated nuclear import (Fig. 2B). These results suggest that Vpx interacts with the NPC like the HIV Rev and SV40 NLSs for its nuclear import.
Vpx nuclear import pathways are saturable.
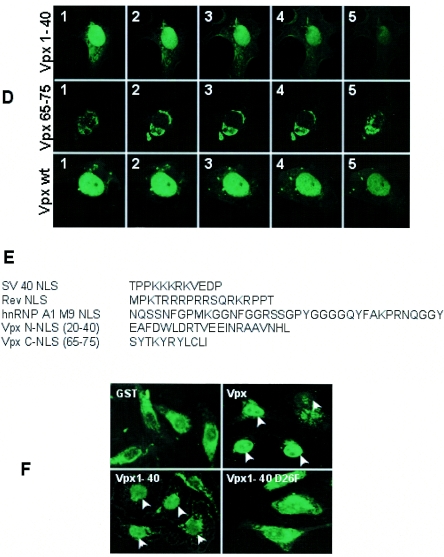
The nuclear import of Vpx in the presence of cognate competitors was next studied to confirm whether Vpx is transported into the nucleus via classical import pathways and whether importins are involved in nuclear translocation. One of the defining features of signal-mediated nuclear transport is that it is saturable by the presence of excess cognate competitor. Recent studies suggest that overexpression of the SV40 NLS domain potently inhibits importin alpha-mediated import but that the HIV-1 Rev NLS may saturate importin beta-mediated nuclear import (36). To investigate this aspect of Vpx NLS function, an immunofluorescence assay was performed using GFP-Vpx, GFP-Vpx65-75, GFP-Vpx1-40, GFP-SV40 NLS, GFP-Rev NLS, and nuclear-import-defective Vpx mutants as substrates. Results in Fig. 3A suggest that expression of Vpx altered both SV40 NLS- and Rev NLS-mediated nuclear import. Interestingly, expression of the SV40 NLS and Rev NLS resulted in a pattern of interference that was the same as that seen with Vpx as the competitor in the import of Rev and SV40 NLSs. Furthermore, the nuclear-import-defective Vpx mutants did not alter the import of both the SV40 and Rev NLSs, whereas the Vpx mutant (Vpx W49S/53S/56S) that retains wild-type nuclear transport altered SV40 NLS- and Rev NLS-mediated nuclear import (data not shown). These results suggest that the Vpx-mediated interference of SV40 and Rev NLS nuclear import is specific. The most likely explanation for this observation is that the Vpx NLS may interact with overlapping common sites on importin alpha and importin beta. In addition, expression of the Vpx65-75 protein inhibited the nuclear import of full-length Vpx (Fig. 3B) and Vpx1-40 (data not shown), whereas expression of Vpx1-40 did not prevent the nuclear entry of Vpx65-75 (data not shown) or full-length Vpx (Fig. 3B), suggesting that the nuclear import of Vpx65-75 occurs through a receptor positioned downstream in the same transport pathway utilized by Vpx1-40.
FIG. 3.
Vpx blocks HIV-1 Rev NLS- and SV40 NLS-mediated nuclear import. (A) Import of the SV40 NLS and HIV-1 Rev NLS were impaired in the presence of Vpx expression in HeLa cells. Similarly, Vpx translocation was inhibited by the expression of the SV40 NLS as well as the HIV-1 Rev NLS, which suggests that Vpx may utilize the same pathway that is utilized by the SV40 NLS and Rev NLS, as indicated by its competitive inhibition. Vpx expression plasmids were cotransfected with either the SV40 NLS or the HIV-1 Rev NLS in Cos-7 cells, and the cellular localization of GFP fusion proteins was visualized directly after fixing of the transfected cells with 3% paraformaldehyde; Vpx was visualized by indirect immunofluorescence using anti-Vpx monoclonal antibody followed by an Alexa fluor 488-conjugated secondary antibody and epifluorescence. (B) Overexpression of the C-terminal Vpx65-75 NLS interfered with the nuclear import of both the full-length and N-terminal NLS of Vpx.
Vpx interacts with receptors in the classical nuclear import pathway.
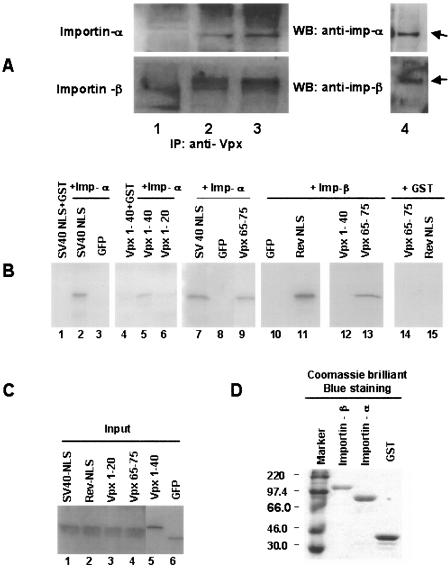
To test the above-mentioned model of Vpx nuclear import, we performed immunoprecipitation experiments in which the interaction between Vpx and the nuclear transport receptors importin alpha and importin beta was tested in vivo. Specifically, cell lysates from Cos-7 cells cotransfected with Vpx alone or Vpx and importin alpha were immunoprecipitated with Vpx monoclonal antibody, followed by Western blotting using anti-importin alpha antibody. Interestingly, results in Fig. 4A suggest that Vpx interacts with and coprecipitates both endogenous (upper panel, lane 3) and ectopically expressed (lane 2) importin alpha. Surprisingly, we observed a similar pattern of interaction of Vpx with the importin beta (Fig. 4A, lower panel, lanes 2 and 3). These results are consistent with the observed inhibition of HIV-1 Rev NLS and SV40 NLS nuclear import by Vpx expression and suggest that Vpx NLSs bind to both importin alpha and importin beta and are imported into the nucleus.
FIG. 4.
Vpx interacts with both importin alpha and importin beta in vivo as well as in vitro. (A) Vpx expression plasmids alone or cotransfected with importin alpha or importin beta expression plasmids in Cos-7 cells. After 16 h of transfection, cell lysates were subjected to immunoprecipitation with monoclonal anti-Vpx antibody, followed by Western blotting with anti-importin alpha (upper panel) or anti-importin beta (lower panel) antibodies. Lane 1, untransfected cell lysates; lane 2, cell lysates from Vpx and importin alpha- or importin beta-cotransfected cells; lane 3, Vpx-transfected cell lysates; lane 4, endogenous importin alpha or importin beta detected by Western blotting with respective antibodies without being subjected to immunoprecipitation. (B) [35S]methionine-labeled Vpx1-40, Vpx1-20, and Vpx65-75 were incubated with GST, GST-importin , and GST-importin beta, which had been immobilized on glutathione-Sepharose. The 35S-labeled HIV-1 Rev NLS, SV40 NLS, alpha, and GFP were used as controls. (C) The input (20%) of the indicated [35S]methionine-labeled proteins was resolved by SDS-15% PAGE and analyzed by autoradiography. (D) Importin alpha and importin beta were expressed and purified as GST fusion proteins as described elsewhere (22). Purified fusion proteins were dialyzed and rebound with glutathione-Sepharose beads, and equal amounts of GST, GST-importin alpha, and GST-importin beta were used in all the in vitro pull-down assays, as indicated by Coomassie blue staining. All the purified fusion proteins were resolved by SDS-10% PAGE. Molecular weights (in thousands) are noted at the left. Imp, importin.
Nuclear targeting signals of Vpx interact with both importin alpha and importin beta.
To further characterize the nuclear transport receptor(s) involved in Vpx nuclear import, we performed a series of in vitro GST pull-down assays (see supplement 3 in the supplemental material). To determine which part of the Vpx NLS interacts with importin alpha and importin beta, GFP-Vpx1-40, GFP-Vpx65-75, and GFP were radiolabeled with [35S]methionine using the TNT reticulocyte lysate system and mixed with glutathione-Sepharose beads that had been prebound with either GST or GST fused to importin alpha or importin beta, and bound proteins were resolved by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis (SDS-15% PAGE), followed by autoradiography. Results in Fig. 4B clearly indicate that the C-terminal NLS (aa 65 to 75) of Vpx interacts with importin alpha (lane 9) as well as importin beta (lane 13) but that N-terminal NLS of Vpx1-40 interacts with importin alpha (lane 5) but not with importin beta (lane 12). Interestingly, Vpx1-20 did not interact with importin alpha (lane 6) and provides evidence for the presence of an NLS within aa 20 to 40 of Vpx. This was further supported by the nuclear accumulation of Vpx20-40 (Fig. 1C). To verify the integrity of in vitro GST pull-down assay, radiolabeled importin alpha with glutathione-Sepharose-bound importin beta was used as the positive control (data not shown). These data provide evidence that Vpx interacts with both importin alpha and importin beta through its two signals for efficient nuclear import.
Vpx65-75 interaction with importin beta was further supported by the nuclear membrane localization of GFP-Vpx65-75 in the transfected cells (Fig. 1D). Equal amounts of radiolabeled proteins (Fig. 4C) as well as GST, GST-importin alpha, and GST-importin beta bound with glutathione-Sepharose beads (Fig. 4D) were used in all in vitro pull-down assays. We used the NLSs of SV40 and HIV-1 Rev as add-on positive controls (Fig. 4B, lanes 2 and 11). As a negative control, we used GST and observed no interaction of Vpx NLSs (Fig. 4B, lanes 4 and 14), SV40 (lane 1), or HIV-1 Rev (lane 15) with GST. Importantly, the inability of GST to interact with Vpx NLSs, as well as the lack of interaction between GFP and importin alpha or importin beta (lanes 3, 8, and 10), served to illustrate the specificity of the interaction between import receptors (importin alpha and importin beta) and Vpx. It is likely that Vpx's interaction with importin alpha and importin beta in these pull-down experiments is a consequence of direct interaction. It is possible that Vpx interacts with different sites on importin alpha and importin beta and provides evidence that the noncanonical NLS-containing proteins can be imported to the nucleus by receptors in the classical import pathway. Interaction of Vpx NLSs with importin alpha suggests two possible mechanisms for Vpx import: (i) that the Vpx NLS interacts with importin alpha, which in turn interacts with importin beta before being imported into the nucleus, and (ii) that importin alpha may interact with Vpx and transport into the nucleus independently of importin beta, similar to the import of Ca2+/calmodulin-dependent protein kinase type IV (CaMKIV) (29). Recent report suggests that CaMKIV interacts with importin alpha and is transported into the nucleus without forming a complex with importin beta despite there being no identifiable NLS in CaMKIV. These results suggest that importin alpha itself is capable of functioning as the transport receptor for the noncanonical NLS-containing cargo proteins into the nucleus. How importin alpha and importin beta recognize apparently distinct motifs and whether they can simultaneously bind to the same Vpx molecule remains to be determined.
Evidence for the presence of nuclear export activity in Vpx.
Mutagenesis experiments were performed in the context of the full-length Vpx protein and were potentially confounded by the presence of the import signal within residues 61 to 80. To our surprise, the GFP-Vpx1-63 fusion protein, expressed in either HeLa or Cos-7 cells, localized in the cytoplasmic compartment despite the nuclear localization of GFP-Vpx20-40 (Fig. 1C). We next considered the possibility that these fusion proteins were shuttling into and out of the nucleus, but they appeared cytoplasmic due to a longer dwell time in that cellular compartment. Nuclear export mediated by CRM1 is inhibited in the presence of leptomycin B (LMB) due to a covalent modification in the central conserved domain (30, 45). Accordingly, we studied the subcellular localization of GFP-Vpx in the presence of a nuclear export inhibitor, LMB (20 ng/ml), with and without a translational inhibitor, cycloheximide (50 μg/ml), and observed the effects on the nuclear accumulation of GFP-Vpx fusion proteins in transfected cells (Fig. 5A). We observed the cytoplasmic localization of GFP-Vpx in the presence of cycloheximide alone, while nuclear accumulation in the presence of both cycloheximide and LMB suggests the presence of a nuclear export signal in Vpx (Fig. 5A). Interestingly, we noticed that cycloheximide (with or without LMB) did not alter the nuclear accumulation of Vpx Y66,69,71S, and the cytoplasmic accumulation of the Vpx H82S mutant (Fig. 5A) suggests that tyrosine residues play an important role in Vpx nuclear export. As an added control, we examined the effect of the same doses of cycloheximide and LMB on the shuttling of a GFP-HIV-1 Rev fusion protein and found it to inhibit the protein's nuclear export (Fig. 5A). In addition, we used the nuclear export-defective Rev protein to further check the integrity of the assay and observed no change in the localization (nuclear) of GFP-mutant Rev in the presence of cycloheximide alone or along with LMB (Fig. 5A). Taken together, these data suggest that Vpx possesses both an NLS and an NES. Our results are in contrast with those in a previous report by Belshan and Ratner (3) for HIV-2 Vpx. The discrepancy between these two findings may be due to the different assay systems as well as the viral stains used. The presence of a functional NES may thus serve to ensure an adequate cytoplasmic supply of the karyophilic Vpx protein for incorporation into virions via its interaction with the Gag precursor. To test whether Vpx incorporation into virus particles is dependent on nuclear export, 293T cells were transfected with SIVsmPBj1.9 wild-type Vpx as well as Vpx mutant (Vpx H82S as well as Vpx Y66,69,71S) proviral clones. At 48 h, virions were pelleted from culture supernatants and analyzed by Western blotting for the presence of Vpx and Gag proteins in the virions. As seen in Fig. 5B, wild-type Vpx and the Vpx H82S mutant were incorporated into virions with comparable efficiencies (lanes 1 and 4). In contrast, the Y66,69,71S mutations impaired Vpx incorporation into virus particles (lane 3) despite the fact that equal amounts of protein expression were noticed in the cell lysates (data not shown). Together, these results suggest that tyrosine residues play a critical role in Vpx nuclear export and influence the availability of Vpx protein in the cytoplasm for its incorporation into virus particles through its interaction with the structural protein pr55gag. In summary, we have identified two novel import signals within SIV Vpx and established the existence of an NES. The presence of these import and export signals probably ensures representation of Vpx in the two different cellular compartments, where it performs critical functions in the viral life cycle.
FIG. 5.
Assessment of the nuclear export property of Vpx. (A) Cos-7 cells were transfected with GFP-Vpx, Vpx Y66,69,71S, Vpx H82A, GFP-HIV-1 wild-type Rev (Revwt), and GFP-HIV-1 mutant Rev (Revm) expression plasmids and treated with the translational inhibitor cycloheximide (Cyclo; 5 μg/ml) alone or in combination with 20 ng/ml of LMB. LMB inhibits exportin-1/CRM1-mediated nuclear export of proteins. The localization of GFP fusion proteins was visualized directly after fixing of the transfected cells with 3% paraformaldehyde. Localizations of Vpx mutant proteins were determined by indirect immunofluorescence analysis using Vpx-specific monoclonal antibody followed by Alexa fluor 488 as described elsewhere (40). Immunofluorescence analysis suggested that Vpx is a nuclear export protein and that its export is sensitive to LMB, similar to what occurs with HIV-1 Rev. Revm is the nuclear export-defective mutant (the conserved Leu residues in the NES were exchanged with Ala), and the pattern of this mutant protein's localization was not changed in the presence or absence of the indicated drugs. (B) The nuclear export of Vpx is required for its packaging into virus particles. 293T cells were transfected with wild-type Vpx and the indicated Vpx mutant SIVsmPBJ1.9 proviral clones. At 48 h, viral supernatants were clarified by centrifugation at 5,000 × g for 15 min, and virions were pelleted through a 20% sucrose cushion by ultracentrifugation at 100,000 × g for 2 h. The pelleted virions were resuspended in loading buffer and resolved by SDS-12% PAGE. Vpx and Gag proteins were detected by Western blot analysis using Vpx- and Gag-specific monoclonal antibodies, respectively, followed by enhanced chemiluminescence. Wt, wild-type Vpx; ΔVpx, a control construct lacking a functional vpx open reading frame; DAPI, 4′,6′-diamidino-2-phenylindole.
In this report, we have provided evidence that both importin alpha and importin beta serve as nuclear transport receptors for Vpx and suggest that Vpx engages one or more transport receptors to enter the nucleus. Similarly, ribosomal proteins are preferentially transported by one receptor but will associate with a second receptor of lower affinity when the primary receptor is absent (23). In addition, signal recognition particle protein 19 is one of many proteins imported into the nucleus by more than one member of the importin receptor family (6). Our results do not distinguish a principal import receptor between importin alpha and importin beta for nuclear import of Vpx in vivo. The data on subcellular localization and in vivo and in vitro interactions favor importin alpha as the primary import receptor. By analogy to ribosomal proteins, importin beta may constitute a backup pathway for Vpx import. Lack of homology between two NLSs of Vpx does not necessarily mean that these two signals bind different regions of importin alpha. For example, four receptors that bind rpL23a share <15% sequence identity, yet all four interact with the same segment of rpL23a (41). It will be interesting to determine whether the novel nuclear targeting domains identified in Vpx interact with importins directly or by a piggyback mechanism. In addition, it will also be interesting to determine the role that importin beta plays in this interaction—whether it simply stabilizes the importin alpha and Vpx65-75 interaction or whether it can bind to Vpx65-75 directly. In sum, these results suggest that the two nuclear targeting signals present in Vpx may operate effectively to enhance viral replication and spread in nondividing cells by facilitating the nuclear uptake of the PIC, perhaps by directly engaging the NPC and by influencing the import kinetics. A detailed understanding of the molecular mechanism governing the nuclear transport of HIV/SIV is expected to provide insights for developing new antiviral molecules that may effectively inhibit the virus' replication in nondividing cells.
Supplementary Material
Acknowledgments
This work was supported by grants from the Department of Biotechnology (BT/PB2448/BRB/15/228/2001 and BT/PR4154/BRB/10/336/2003), Government of India, to S.M. Support was also received from the Centre for DNA Fingerprinting and Diagnostics and by graduate fellowships from the Council of Scientific and Industrial Research (CSIR), Government of India, to P.K.S., P.R.K., and M.R.K.S.R.
We are indebted to B. H. Hahn (University of Alabama at Birmingham, Birmingham, Alabama) for providing Vpx mutants, T. Sekimoto (Osaka University, Osaka, Japan) for importin alpha and importin beta expression plasmids, and Minoru Yoshida (University of Tokyo, Tokyo, Japan) for providing LMB. We thank T. Ramasarma for critically reading the manuscript.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Accola, M. A., A. A. Bukovsky, M. S. Jones, and H. G. Göttlinger. 1999. A conserved dileucine-containing motif in p6gag governs the particle association of Vpx and Vpr of simian immunodeficiency viruses SIVmac and SIVagm. J. Virol. 73:9992-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam, S. A., R. S. Marr, and L. Gerace. 1990. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 111:807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belshan, M., and L. Ratner. 2003. Identification of the nuclear localization signal of human immunodeficiency virus type 2 Vpx. Virology 311:7-15. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett, A. H., and P. A. Silver. 1997. Nucleocytoplasmic transport of macromolecules. Microbiol. Mol. Biol. Rev. 61:193-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean, K. A., O. von Ahsen, D. Gorlich, and H. M. Fried. 2001. Signal recognition particle protein 19 is imported into the nucleus by importin 8 (RanBP8) and transportin. J. Cell Sci. 114:3479-3485. [DOI] [PubMed] [Google Scholar]
- 7.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzio, P., S. Choe, M. Ebright, R. Knoblauch, and N. R. Landau. 1995. Mutational analysis of cell cycle arrest, nuclear localization, and virion packaging of human immunodeficiency virus type 1 Vpr. J. Virol. 69:7909-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingwall, C., and R. A. Laskey. 1991. Nuclear targeting sequences a consensus? Trends Biochem. Sci. 16:478-481. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher, T. M., B. Brichacek, N. Sharova, M. A. Newman, G. Stivahtis, P. M. Sharp, M. Emerman, B. H. Hahn, and M. Stevenson. 1996. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM). EMBO J. 15:6155-6165. [PMC free article] [PubMed] [Google Scholar]
- 11.Fouchier, R. A., B. E. Meyer, J. H. Simon, U. Fischer, A. V. Albright, F. Gonzalez-Scarano, and M. H. Malim. 1998. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J. Virol. 72:6004-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouchier, R. A., B. E. Meyer, J. H. Simon, U. Fischer, and M. H. Malim. 1997. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 16:4531-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridell, R. A., R. Truant, L. Thorne, R. E. Benson, and B. R. Cullen. 1997. Nuclear import of hnRNP A1 is mediated by a novel cellular cofactor related to karyopherin-beta. J. Cell Sci. 110:1325-1331. [DOI] [PubMed] [Google Scholar]
- 14.Fuerst, T. R., P. L. Earl, and B. Moss. 1987. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol. Cell. Biol. 7:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallay, P., S. Swingler, C. Aiken, and D. Trono. 1995. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell 80:379-388. [DOI] [PubMed] [Google Scholar]
- 16.Gallay, P., T. Hope, D. Chin, and D. Trono. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. USA 94:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorlich, D. 1997. Nuclear protein import. Curr. Opin. Cell Biol. 9:412-419. [DOI] [PubMed] [Google Scholar]
- 18.Gorlich, D., and I. W. Mattaj. 1996. Nucleocytoplasmic transport. Science 271:1513-1518. [DOI] [PubMed] [Google Scholar]
- 19.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson, L. E., R. C. Sowder, T. D. Copeland, R. E. Benveniste, and S. Oroszlan. 1988. Isolation and characterization of a novel protein (X-ORF product) from SIV and HIV-2. Science 241:199-202. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch, V. M., M. E. Sharkey, R. Byrum, W. R. Elkins, B. H. Hahn, J. D. Lifson, and M. Stevenson. 1998. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat. Med. 4:1401-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imamoto, N., T. Shimamoto, T. Takao, T. Tachibana, S. Kose, M. Matsubae, T. Sekimoto, Y. Shimonishi, and Y. Yoneda. 1995. In vivo evidence for involvement of a 58 kDa component of nuclear pore-targeting complex in nuclear protein import. EMBO J. 14:3617-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakel, S., and D. Gorlich. 1998. Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 17:4491-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins, Y., M. McEntee, K. Weis, and W. C. Greene. 1998. Characterization of HIV-1 vpr nuclear import: analysis of signals and pathways. J. Cell Biol. 143:875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamata, M., and Y. Aida. 2000. Two putative alpha-helical domains of human immunodeficiency virus type 1 Vpr mediate nuclear localization by at least two mechanisms. J. Virol. 74:7179-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kappes, J. C., C. D. Morrow, S. W. Lee, B. A. Jameson, S. B. H. Kent, L. E., Hood, G. M. Shaw, and B. H. Hahn. 1989. Identification of a novel retroviral gene unique to human immunodeficiency virus type 2 and simian immunodeficiency virus SIVMAC. J. Virol. 62:3501-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kappes, J. C., J. S. Parkin, J. A. Conway, J. Kim, C. G. Brouillette, G. M. Shaw, and B. H. Hahn. 1993. Intracellular transport and virion incorporation of vpx requires interaction with other virus type-specific components. Virology 193:222-233. [DOI] [PubMed] [Google Scholar]
- 28.Kewalramani, V. N., and M. Emerman. 1996. Vpx association with mature core structures of HIV-2. Virology 218:159-168. [DOI] [PubMed] [Google Scholar]
- 29.Kotera, I., T. Sekimoto, Y. Miyamoto, T. Saiwaki, E. Nagoshi, H. Sakagami, H. Kondo, and Y. Yoneda. 2005. Importin alpha transports CaMKIV to the nucleus without utilizing importin beta. EMBO J. 24:942-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96:9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahalingam, S., B. Van Tine, M. L. Santiago, F. Gao, G. M. Shaw, and B. H. Hahn. 2001. Functional analysis of the simian immunodeficiency virus Vpx protein: identification of packaging determinants and a novel nuclear targeting domain. J. Virol. 75:362-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakielny, S., and G. Dreyfuss. 1999. Transport of proteins and RNAs in and out of the nucleus. Cell 23:677-690. [DOI] [PubMed] [Google Scholar]
- 34.Nigg, E. A. 1997. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature 386:779-787. [DOI] [PubMed] [Google Scholar]
- 35.Ohno, M., M. Fornerod, and I. W. Mattaj. 1998. Nucleocytoplasmic transport: the last 200 nanometers. Cell 92:327-336. [DOI] [PubMed] [Google Scholar]
- 36.Palmeri, D., and M. H. Malim. 1999. Importin beta can mediate the nuclear import of an arginine-rich nuclear localization signal in the absence of importin alpha. Mol. Cell. Biol. 19:1218-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pancio, H. A., N. Vander Heyden, and L. Ratner. 2000. The C-terminal proline-rich tail of human immunodeficiency virus type 2 vpx is necessary for nuclear localization of the viral preintegration complex in nondividing cells. J. Virol. 74:6162-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard, V. W., W. M. Michael, S. Nakielny, M. C. Siomi, F. Wang, and G. Dreyfuss. 1996. A novel receptor-mediated nuclear protein import pathway. Cell 86:985-994. [DOI] [PubMed] [Google Scholar]
- 39.Rajendra Kumar, P., P. K. Singhal, S. S. Vinod, and S. Mahalingam. 2003. A non-canonical transferable signal mediates nuclear import of simian immunodeficiency virus Vpx protein. J. Mol. Biol. 331:1141-1156. [DOI] [PubMed] [Google Scholar]
- 40.Rajendra Kumar, P., P. K. Singhal, M. R. Subba Rao, and S. Mahalingam. 2005. Phosphorylation by MAPK regulates simian immunodeficiency virus Vpx protein nuclear import and virus infectivity. J. Biol. Chem. 280:8553-8563. [DOI] [PubMed] [Google Scholar]
- 41.Sherman, M. P., C. M. de Noronha, M. I. Heusch, S. Greene, and W. C. Greene. 2001. Nucleocytoplasmic shuttling by human immunodeficiency virus type 1 Vpr. J. Virol. 75:1522-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trono, D. 1998. When accessories turn out to be essential. Nat. Med. 4:1368-1369. [DOI] [PubMed] [Google Scholar]
- 43.Truant, R., and B. R. Cullen. 1999. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol. Cell. Biol. 19:1210-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff, B., J. J. Sanglier, and Y. Wang. 1997. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 4:139-147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.