Despite many years of groundbreaking research in which studies on the actions of ligand-activatable transcription factors (also known as nuclear steroid hormone receptors and related proteins) have taught us much about control of gene regulation, there is a nagging incongruity in our current understanding of steroid receptor activities. The predominant model of steroid hormone action still does not explain how steroid hormones cause “nongenomic” actions, which occur too rapidly to encompass the multiple macromolecular synthetic events required to allow changes in gene transcription to be manifested as functional changes mediated by the encoded proteins. The efforts of a number of investigators are now newly focused on explanations of important biological phenomena such as steroid-induced electrophysiological effects and their behavioral sequelae, the influence of steroid hormones on secretory release of growth factors and peptide hormones, and their effects on cell proliferation and apoptosis.
Scientists seeking to fill these holes in our understanding of how steroids coordinate both the acute and chronic reproductive or metabolic responses of an entire organism recently convened for an in-depth interactive research conference. Sponsored by the Federation of American Societies for Experimental Biology and entitled “Steroid Receptor Family Members in the Plasma Membrane: The Proteins and their Functions,” the conference (at Copper Mountain, Colorado, from 8 through 13 August 1999) focused on the proteins that mediate various membrane-initiated steroid responses across many cell and tissue types. The meeting, chaired by Cheryl Watson (University of Texas Medical Branch), Ilke Nemere (Utah State University), and Peter Blackmore (Eastern Virginia Medical School), aimed at a comprehensive picture of the mechanisms and protein mediators involved in the rapid actions of steroids at the cell membrane (1). A critical issue was whether the rapid effects of a given steroid are accomplished by a single ligand-activated protein that coordinates a diverse set of mechanisms and responses, or whether multiple steroid-binding molecules operate together to mediate these responses. Also at issue was whether the proteins that trigger these responses are modified forms of the well-known nuclear receptors for steroids or are different proteins.
In the historical keynote address, Clara Segos (University of California, Los Angeles) showed that although she has been retired for several years, she still has her finger on the pulse of the field she initiated. She highlighted early studies from her own and other laboratories that described the presence of steroid binding in membrane fractions, rapid changes in cell architecture upon hormone stimulation, and the signaling at the surface of the cell resulting in functional consequences of steroid action. She emphasized the continuum of effects—starting at the plasma membrane and culminating in nuclear synthetic responses—that make up a coordinated cellular response (2) and many important, but still unresolved, issues such as the relationship of the membrane steroid receptor (mSR) to the nuclear steroid receptor. In the state-of-the-art keynote address, Michelle Lieberherr (Univeristy of Paris) compared signal transduction mechanisms of estrogens, androgens, progestins, and calcitriol in bone, lymphoid, and reproductive tissues. Signaling molecules involved included calcium, inositol phosphates, cAMP and G proteins, and various kinases. However, only subsets of these signals are used by a given cell type responding to a given hormone.
Meeting sessions were organized around the functional consequences of membrane-initiated steroid actions and therefore focused on tissue-related functions. Also discussed were control of the cell cycle, cell death, cell movement, transcellular calcium transport, and membrane fusions (involved in secretion and the acrosome reaction of sperm). Signal summations through various pathways and the diversity of mechanisms employed were demonstrated by several presentations. The variety of systems considered—from fish and newts to rodents and humans—caused attendees to think creatively and cooperatively about the inevitable conflicting information on receptor identity in a field that is still so unresolved.
Most signaling mechanisms initiated by peptide hormone receptors are also activated by membrane actions of steroid hormones (1). The most recent additions to this theme include the regulation of mitogen-activated protein (MAP) kinases and cJun NH2-terminal kinases (JNKs), which transmit signals that can control cell proliferation, at least in part through phosphorylation of transcription factors and their coactivators and corepressors (F. Auricchio, University of Naples; E. Levin, University of California, Irvine; D. Dorsa, University of Washington; N. Weigel, Baylor College of Medicine; and R. Pietras, University of California, Los Angeles). Such membrane-initiated responses can also eventually impinge on transcription. Thus, such activities are most accurately described as membrane-initiated, rather than non-genomic (as they are often called). All these actions have important implications for understanding diseases such as cancer, infertility, neurodegeneration, cardiovascular ailments, developmental anomalies, and osteoporosis. Steroid ligands or their mimetics acting through the membrane-initiated mechanisms may have unique disease-producing, preventive, or therapeutic activities.
Although there are many descriptions of rapid actions of steroid hormones and the molecules to which the initial signal is passed, little is known about the primary signal transducer itself: the controversial mSR form from which the signal cascades emanate. A technical workshop on methods of visualizing and measuring mSRs dealt with techniques to address these important questions. Useful methods include identification of mSRs through steroid analog binding, binding to labeled steroid–bovine serum albumin conjugates (which cannot quickly enter the cell), covalent affinity labeling through photoactive or reactive analogs, immunocytochemistry, and various receptor purification schemes.
It was clear that we would not leave this meeting agreeing about a single protein class of mSRs. Discrepancies between the molecular sizes and pharmacological profiles of intracellular versus putative purified mSRs may or may not be reconciled through explanations such as protein cleavage, modifications, and conformational changes; however, it was evident that antibodies, antisense knockdowns, and cDNA transfection experiments have determined that some mSRs are the same as nuclear receptors (C. Watson; E. Levine; and B. Gametchu, Medical College of Wisconsin), whereas purifications and antibody identifications have pointed to other protein identities. One such alternative identity is an adenosine triphosphatase [V. Ramirez, University of Illinois (3)]. Another example of a membrane receptor distinct from its nuclear receptor is the membrane receptor for calcitriol. It is as yet unidentified, but is recognized by an antibody that doesn’t recognize the nuclear receptor (I. Nemeses) and has a pharmacological profile unlike that of the nuclear receptor (A. Norman, University of California, Riverside). Although some alternative identities might result from the isolation of steroid receptor–associated proteins during purification, we must currently conclude that both the classical nuclear receptors as well as other proteins bind steroids at the cell membrane. Which mechanism prevails may depend on the hormone, cell type, and function to be mediated. Another concept to be considered is that the proteins in the membrane that mediate rapid actions of steroid hormones are likely to be a mixture of proteins that bind steroids, including, in addition to the nuclear receptors, carrier proteins (known as steroid-binding proteins), enzymes, steroid transporters, or other classes of proteins. It is known that other intracellular and extra cellular compartments contain a mixture of such proteins that bind steroids and together contribute to a response.
Nuclear steroid receptors are a class of proteins designed for interactions, and their diversity of functions requires interactions with different subsets of partner proteins known as coactivators and corepressors. It is possible that a host of proteins may also physically interact with steroid receptors in the context of the plasma membrane to initiate their diverse repertoires of signal tranduction cascades. Perhaps the first such example was presented by R. Pietras (University of California, Los Angeles), who described a direct interaction between a membrane estrogen receptor and the HER2/Neu receptor in breast cancer cells. However, others have explained the integration of steroid signals with other signals via direct binding sites for steroids on neurotransmitter receptors, peptide hormone receptors, and channel proteins (4–6). Cross talk between signaling pathways initiated by peptide hormones through their receptors on the cell surface and steroid actions has been demonstrated (7). It remains to be seen whether this cross talk may be partially explained by direct interactions of peptide hormone receptors with membrane-resident steroid receptors. The importance of phosphorylation as a posttranslational regulatory modification to nuclear receptors and comodulators was explored and discussed (N. Weigel) in a new light—that of steroid receptors being possible instigators of signals that result in these phosphorylation events. Integrations of membrane and nuclear effects of steroid hormone receptors were summarized (A. Norman) as an example of how they both contribute to the final complex cellular response to steroids.
Provocative ideas were presented regarding interactions of mSRs with blood-borne steroid-binding proteins such as steroid hormone–binding globulin (W. Rosner, Columbia University School of Medicine; and G. Hammond, University of Western Ontario) and serum albumin (C. Sonnenschein, Tufts University School of Medicine). These carrier proteins may interact at the cell surface through still-unidentified membrane receptors and so functionally encounter mSRs and modulate their responses.
Our deliberations shed light on the themes of receptor identities, partners, mechanistic pathways, and functions (see Fig. 1). Definitive identification of the membrane proteins that initiate responses to some steroid hormones is still in progress. Nevertheless, strong evidence that membrane receptors can be both modified versions of nuclear steroid receptors and also proteins that are unrelated to nuclear receptors indicates that both camps will continue to provide new insights into the integration of membrane-initiated and nuclear effects of steroid hormones.
Fig. 1.
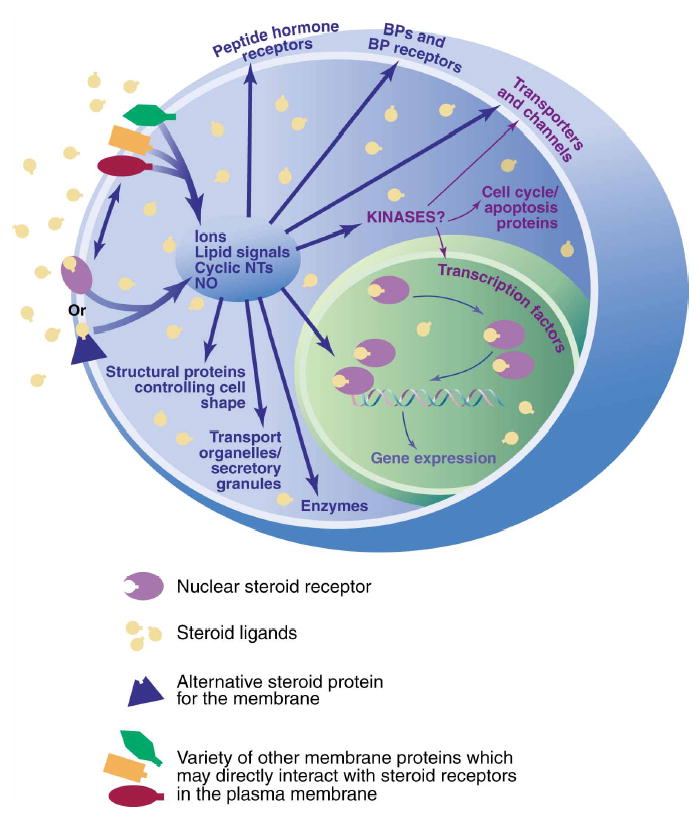
Steroids acting through membrane receptors can initiate many different forms of cellular signaling with both genomic and nongenomic consequences. Membrane receptor forms can be modified versions of their nuclear receptor counterparts or can be unrelated proteins. Proteins may interact with these receptors in the cell membrane to bring about diverse biological consequences, depending on the hormonal and cellular contexts. BP, binding protein.
References
- 1.Watson CS, Gametchu B. Membrane-initiated steroid actions and the proteins that mediate them. Proc Soc Exp Biol Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 2.Szego CM. Cytostructural correlates of hormone action: new common ground in receptor-mediated signal propagation for steroid and peptide agonists. Endocrine. 1994;2:1079–1093. [Google Scholar]
- 3.Zheng J, Ramirez VD. Purification and identification of an estrogen binding protein from rat brain: oligomycin sensitivity-conferring protein (OSCP), a subunit of mitochondrial F0F1-ATP synthase/ATPase. J Steroid Biochem Mol Biol. 1999;68:65–75. doi: 10.1016/s0960-0760(98)00161-7. [DOI] [PubMed] [Google Scholar]
- 4.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1936. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 5.Zingg HH, Grazzini E, Breton C, Larcher A, Rozen F, Russo C, Guillon G, Mouillac B. Geonomic and nongeonomic mechanisms of oxytocin receptor regulation. Adv Exp Med Biol. 1998;449:287–295. doi: 10.1007/978-1-4615-4871-3_36. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald RL, Olsen RW. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 7.Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, Korach KS. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci USA. 1992;89:4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]


