Abstract
The GerA nutrient receptor alone triggers germination of Bacillus subtilis spores with l-alanine or l-valine, and these germinations were stimulated by glucose and K+ plus the GerK nutrient receptor. The GerB nutrient receptor alone did not trigger spore germination with any nutrients but required glucose, fructose, and K+ (GFK) (termed cogerminants) plus GerK for triggering of germination with a number of l-amino acids. GerB and GerA also triggered spore germination cooperatively with l-asparagine, fructose, and K+ and either l-alanine or l-valine. Two GerB variants (termed GerB*s) that were previously isolated by their ability to trigger spore germination in response to d-alanine do not respond to d-alanine but respond to the same l-amino acids that stimulate germination via GerB plus GerK and GFK. GerB*s alone triggered spore germination with these l-amino acids, although GerK plus GFK stimulated the rates of these germinations. In contrast to l-alanine germination via GerA, spore germination via l-alanine and GerB or GerB* was not inhibited by d-alanine. These data support the following conclusions. (i) Interaction with GerK, glucose, and K+ somehow stimulates spore germination via GerA. (ii) GerB can bind and respond to l-amino acids, although normally either the binding site is inaccessible or its occupation is not sufficient to trigger spore germination. (iii) Interaction of GerB with GerK and GFK allows GerB to bind or respond to amino acids. (iv) In addition to spore germination due to the interaction between GerA and GerK, and GerB and GerK, GerB can interact with GerA to trigger spore germination in response to appropriate nutrients. (v) The amino acid sequence changes in GerB*s reduce these receptor variants' requirement for GerK and cogerminants in their response to l-amino acids. (vi) GerK binds glucose, GerB interacts with fructose in addition to l-amino acids, and GerA interacts only with l-valine, l-alanine, and its analogs. (vii) The amino acid binding sites in GerA and GerB are different, even though both respond to l-alanine. These new conclusions are integrated into models for the signal transduction pathways that initiate spore germination.
Spores of Bacillus species normally initiate germination in response to specific nutrients (for reviews, see references 17 and 25). The identity of these nutrients varies in a species- and strain-specific manner, although common nutrient germinants are amino acids, sugars, and purine nucleosides. Metabolism of nutrient germinants is not what triggers spore germination. Rather, the binding of nutrients to receptors located in the spore's inner membrane triggers subsequent events including (i) the release of monovalent ions, (ii) the release of the spore core's large depot of divalent cations bound to pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]), and (iii) hydrolysis of the spore's peptidoglycan cortex. The nutrient receptors are encoded by tricistronic operons. Where studied, these operons are expressed only in the developing spore late in sporulation, and loss of any cistron of a particular nutrient receptor operon eliminates the function of that receptor.
B. subtilis spores contain three functional nutrient receptors encoded by the gerA, gerB, and gerK operons, and each receptor or group of receptors responds to different germinants and cogerminants (Fig. 1) (17, 25). The GerA nutrient receptor (GerA) responds to l-alanine and some alanine homologs, and this response is strongly inhibited by d-alanine (17). GerA also responds to l-valine but not to other l-amino acids (17). No single germinant has been identified for the GerB or GerK nutrient receptors (GerB and GerK). However, either of these receptors can respond to a complex mixture of nutrients, although more poorly than GerA (21). GerB and GerK also act cooperatively to trigger germination with l-asparagine plus a mixture of glucose, fructose, and K+ (GFK) and also with l-alanine plus GFK (5, 16, 17, 25, 32, 33). Germination with l-alanine via GerA is also stimulated by glucose, and this stimulation again likely requires cooperation with GerK (14, 16, 24, 34). However, glucose alone does not trigger spore germination and is thus considered a cogerminant, as is GFK. How GerK cooperates with GerA and GerB to trigger germination with combinations of germinants or cogerminants is not known, but cooperative action between different germinant receptors has also been seen with spores of Bacillus cereus and Bacillus anthracis (2, 13).
FIG. 1.
Germinants and cogerminants for different germinant receptors and d-alanine inhibition of these receptors in B. subtilis spores as known at the beginning of this work. Abbreviations used for germinant receptors (boxed letters) are as follows: A, GerA; B, GerB; B*, GerB*; K, GerK. Abbreviations for germinants, cogerminants, and inhibitors are as follows: A, l-alanine; dA, d-alanine; f, d-fructose; g, d-glucose; k, K+; N, l-asparagine; V, l-valine. Germinants are defined as molecules that alone will trigger spore germination. Cogerminants are molecules that stimulate germination with germinants but do not trigger germination alone. The symbol “/” indicates “or,” and there is disagreement in the literature over the identity of the cogerminants in parentheses. Question marks indicate that information is not available. For d-alanine inhibition, y (yes) and n (no) are under the germinant for which inhibition data are available. Note that GFK and l-alanine or l-asparagine are all listed as cogerminants for GerB plus GerK, as none of these compounds alone trigger germination via these two receptors. The designation of glucose as a cogerminant for GerB* and GerK with a question mark adjacent to K is because the GerK requirement for the effect of glucose had not yet been tested (data for this figure are from references 16, 17, 20, and 27).
A number of years ago, our laboratory isolated three point mutations in Bacillus subtilis that allowed spores to germinate slowly with d-alanine (20). The mutations were in the gerB operon, two in gerBA and one in gerBB. The GerB variants, termed GerBA*, GerBB*, and, collectively, GerB*s, also allowed spore germination in l-asparagine (20). The l-asparagine or d-alanine germination of spores with GerB*s required neither GerA nor GerK but was stimulated by glucose (4, 20). We recently returned to the study of GerB*s and were surprised to discover that spores carrying these variants but lacking GerA and GerK germinated well with l-alanine and much better than with d-alanine. This finding prompted an investigation of the specificity of GerB*s for nutrient germinants and the germinant specificity of GerB as well as the cooperation between cogerminants GerK, GerA, GerB, and GerB*s. This communication reports the results of these investigations.
MATERIALS AND METHODS
B. subtilis strains and spore production.
Strains of B. subtilis used in this work are isogenic derivatives of strain PS832, a prototrophic derivative of strain 168, and are listed in Table 1. New strains were made by transformation with chromosomal DNA (1). Mutations of operons encoding nutrient receptors were deletions removing the great majority of the operon's coding sequence (21).
TABLE 1.
B. subtilis strains used
| Strain | Genotype | Source or reference |
|---|---|---|
| FB8 | gerBA* | 20 |
| FB10 | gerBB* | 20 |
| FB20 | ΔgerA::spc | 21 |
| FB22 | ΔgerA::spc gerBA* | 20 |
| FB60 | ΔgerB::cat | 21 |
| FB68 | ΔgerK::ermC | 21 |
| FB87 | ΔgerB::cat ΔgerK::ermC | 21 |
| PS832 | Wild type | Laboratory stock |
| PS3521 | ΔgerA::spc gerBB* | 4 |
| PS3609 | ΔgerA::neo | 11 |
| PS3651 | ΔgerA::neo ΔgerK::ermC | PS3609→FB68a |
| PS3665 | ΔgerA::spc gerBB* ΔgerK::ermC | FB20/FB68→FB10a |
| PS3710 | ΔgerA::spc gerBA* ΔgerK::ermC | FB20/FB68→FB8a |
Strains constructed in this work were prepared by transformation of strains to the right of the arrow with chromosomal DNA from strains to the left of the arrow. When two strains are to the left of the arrow, chromosomal DNA from the strain listed first was used first, and a transformant obtained with this chromosomal DNA was transformed with chromosomal DNA from the strain listed second.
Spores were prepared at 37°C on 2× SG medium agar plates without antibiotics, and spores were harvested, washed, and stored as described previously (18, 19). Spore preparations were free (≥98%) of growing or sporulating cells, cell debris, or germinated spores as seen by phase-contrast microscopy and in some cases by flow cytometry after staining with Syto 16 (Molecular Probes, Eugene, OR) (3, 28).
Spore germination.
Spores at an optical density at 600 nm (OD600) of 7 to 50 were heat shocked (70°C, 30 min) and cooled on ice. The heat-shocked spores were germinated at 37°C and at an OD600 of 0.7 to 1 in 1.5 ml of 25 mM Tris-HCl (pH 8.4). Unless otherwise noted, spore germination was assessed by monitoring the OD600 of the culture, which falls ∼60% upon complete spore germination (4). The fall in OD600 in the absence of germinants was ≤7%/h. This latter fall in OD600 was not due to spore germination, as determined by either flow cytometry (3, 28) or phase-contrast microscopy, but was likely the result of spore aggregation and adsorbtion to test tube walls. The degree of spore germination was also assessed in many experiments by examination of spores by phase-contrast microscopy. In some cases, the percentage of spore germination at various times was assessed by flow cytometry after staining of spores with Syto l6 (3, 28). This assay was particularly useful for assessing very low rates of spore germination (3). In other experiments, the OD270 of the supernatant fluid from germinating spore cultures was measured to assess the percentage of the spore's large depot of DPA that had been released, as described previously (4). The results from the latter two types of analyses were consistent with the results obtained by measurement of the OD600 of germinating cultures. All germination experiments were repeated at least twice, most with two different spore preparations. Rates of spore germination were routinely calculated from measurements of the fall in the OD600 of cultures as described previously (4), taking a 60% fall in the OD600 of as 100% germination. When germination rates were very slow, data from flow cytometry were used to calculate maximum rates of germination as described previously (3). All rates of germination presented are the averages of at least duplicate experiments, each of which gave a rate that was ≤±9% of the average value. The effects of inhibitors on rates of spore germination were measured and calculated as described previously (6), again from results of replicate experiments.
Materials.
l- and d-amino acids (>98% pure) were obtained from Sigma-Aldrich (St. Louis, MO), as was l-alanine dehydrogenase from B. subtilis. For recrystallization, l- and d-amino acids were dissolved in water at ∼70% saturation at ∼75°C and crystallized overnight at 4°C, and the crystals were isolated by filtration, washed briefly with 4°C water, and dried under a vacuum.
For treatment with l-alanine dehydrogenase, 50 mM recrystallized d-alanine was incubated in 1 ml of 250 mM 3-(cyclohexylamino)-1-propanesulfonic acid (pH 10) containing 5 mM NAD. After the addition of an amount of l-alanine dehydrogenase able to oxidize 2 μmol l-alanine per min, the solution was incubated for 4 h at 37°C, and the resultant d-alanine was used at 10 mM for spore germination. When 1.5 mM l-alanine plus 50 mM d-alanine was treated with l-alanine dehydrogenase as described above, ≥85% of the l-alanine was destroyed.
RESULTS
Cooperation between cogerminants GerA, GerB, and GerK in spore germination.
It is well established that GerA alone can give rapid germination of B. subtilis with l-alanine, alanine analogs, and l-valine (Fig. 1) (17). GerK can also stimulate l-alanine germination via GerA when GFK or only glucose plus K+ are added (Fig. 1) (14, 16, 17, 24, 27, 32, 34). However, GFK does not trigger B. subtilis spore germination, and thus, these three components are considered cogerminants. Previous work showing GFK stimulation of GerA-mediated spore germination often used poorly characterized point mutations in operons encoding nutrient receptors. However, we observed this same phenomenon using spores of strains with deletion mutations in these operons. With spores containing only GerA and GerK, glucose and K+ increased the maximum rate of germination with either l-alanine or l-valine by ∼2-fold and decreased the amino acid concentrations giving half-maximal rates of germination by ∼2-fold (Table 2). The magnitudes of the effects of glucose and K+ were similar to those seen in a previous study, as were the concentrations of l-alanine giving half-maximal rates of spore germination (34). Glucose alone had about one-half the effect of both glucose and K+, while K+ alone had no effect (data not shown). The effect with glucose alone may be due to the presence of low levels of K+ in spore preparations or reagents, but we have not studied this further. Another study (16) found that all components of GFK were needed to stimulate l-alanine germination via GerA, with this stimulation being only on the maximum rate of spore germination. However, the effects of glucose and K+ in our work were not altered by the further addition of fructose to 10 mM (data not shown). As expected, with spores containing only GerA, glucose and K+ had no effect on spore germination with l-alanine or l-valine (Table 2), and the addition of fructose also had no effect (data not shown).
TABLE 2.
Germination of spores of various strains via wild-type germinant receptors and amino acids with or without cogerminantsa
| Strain (receptor[s]) | Maximum rates of spore germination (amino acid concn [mM] for half-maximal rates) with indicated germinant and cogerminantb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
l-Alanine
|
l-Asparagine
|
l-Serine
|
l-Threonine
|
l-Valine
|
||||||
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | |
| FB60 (GerA GerK) | 100c(0.03) | 215 (0.015)d | NDe | NDe | NDe | NDe | NDe | NDe | 93 (1.5) | 197 (0.7)d |
| FB87 (GerA) | 103 (0.03) | 98 (0.03)d | NDe | NDe | NDe | NDe | NDe | NDe | 90 (1.5) | 89 (1.5)d |
| FB20 (GerB GerK) | <1f,g | 38h | <1f | 57h | <1f | 24h | <1f | 12h | NDe | NDe |
| PS3651 (GerB) | <1f,g | <1f,g,h | <1f | <1f,h | <1f | <1f,h | <1f | <1f,h | NDe | NDe |
Spores were germinated with amino acids at concentrations from 0.003 to 33 mM with or without 10 mM glucose and 10 mM KCl (GK) or GFK (with each component at 10 mM), and rates of spore germination were measured and calculated as described in Materials and Methods.
Maximum rates of spore germination are given relative to the value for FB60 spores germinated with l-alanine alone, which was set at 100. This value is equivalent to 100% spore germination in 30 min. “No” and “Yes” refer to the absence and presence, respectively, of GK and/or GFK. Boldface type indicates that glucose and K+ increased the maximum rate of germination with either l-alanine or l-valine by ∼2-fold and decreased the amino acid concentrations giving half-maximal rates of germination by ∼2-fold. Italic font indicates that glucose and K+ had no effect on spore germination with l-alanine or l-valine. Underlining indicates that spores with GerB and GerK (but not GerA) germinated with GFK and l-alanine, l-asparagine, l-serine, or l-threonine (or l-glutamine or l-homoserine [data not shown]) and that all components of GFK were essential.
Value set at 100.
Only GK was added. There was no further effect upon the addition of fructose plus GK.
ND, not determined.
Value determined by flow cytometry.
Similar results were obtained with 200 mM l-alanine.
GFK was added.
In contrast to germination via GerA alone, GerB responds poorly if at all to single germinants (17, 21). However, B. subtilis spores germinate with l-asparagine and GFK via cooperative action between GerB and GerK (17, 33). Again, some mutations used to demonstrate this cooperativity were not well characterized (16, 34). However, this phenomenon was also seen using spores of strains with appropriate deletion mutations (reference 4 and see below). With spores containing only GerB and GerB with GFK added or with both GerB and GerK but without GFK, there was no detectable germination with l-asparagine as well as a number of other l-amino acids (values of <1% with spores of strains FB20 and PS3651) (Table 2 and data not shown). However, spores with GerB and GerK (but not GerA) germinated with GFK and l-alanine, l-glutamine, l-homoserine, l-serine, or l-threonine, and all components of GFK were essential, as was GerB (with spores of strain FB20) (Table 2 and data not shown). The germination of spores with l-alanine plus GFK via GerB and GerK has been shown previously (14, 16, 17, 32, 34), but again, it was generally shown only with poorly characterized gerB and gerK strains. However, the germination with GFK and l-glutamine, l-homoserine, l-serine, or l-threonine via GerB and GerK is a new finding.
In addition to the cooperation between GerB, GerK, l-amino acids, and GFK in stimulating spore germination, we also observed cooperation between GerA, GerB, fructose, and K+. Low concentrations of l-alanine or l-valine (Table 3) alone did not stimulate spore germination with l-asparagine, nor did fructose and K+ stimulate germination with l-alanine, l-valine, or l-asparagine alone (Table 3). However, fructose and K+ in the presence of l-asparagine and low concentrations of l-valine (1 mM) or l-alanine (10 or 33 mM, concentrations too low to trigger germination significantly via GerB [see below]) gave three- to fivefold stimulations in rates of spore germination over those given by l-alanine or l-valine alone (strains PS832 and FB68) (Table 3 and data not shown). This stimulation was GerK independent but required both GerA and GerB (strains FB20 and FB60) (Table 3). Stimulation of spore germination was also seen with GerA, GerB, GFK, and l-asparagine and low concentrations of either l-alanine or l-valine (Table 3). However, with wild-type spores, the degree of this stimulation was less than that seen with fructose and K+ (strains PS832 and FB68) (Table 3), because the triggering of germination of spores by l-asparagine alone via GerB and GerK is stimulated by GFK. However, with spores that have only GerA and GerB (strain FB68), the stimulation of germination by GFK plus l-asparagine and either l-alanine or l-valine was essentially identical to that seen with fructose and K+ plus l-asparagine and either l-alanine or l-valine (strain FB68) (Table 3).
TABLE 3.
Cooperation between GerA and GerB in spore germination with amino acids and cogerminantsa
| Germinant | Relative rates of spore germination with indicated strain, receptors, and cogerminantb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS832 (GerA GerB GerK)
|
FB20 (GerB GerK)
|
FB60 (GerA GerK)
|
FB68 (GerA GerB)
|
|||||||||
| − | FK | GFK | − | FK | GFK | − | FK | GFK | − | FK | GFK | |
| l-Ala | 22 | 26 | 40 | <2 | <2 | <2 | 19 | 21 | 43 | 22 | 21 | 20 |
| l-Val | 11 | 15 | 35 | <2 | <2 | <2 | 10 | 11 | 30 | 13 | 17 | 15 |
| l-Asn | <2 | <2 | 49 | <2 | <2 | 46 | <2 | <2 | <2 | <2 | <2 | <2 |
| l-Asn + l-Ala | 23 | 82 | 100c | <2 | <2 | 50 | 18 | 21 | 42 | 20 | 74 | 78 |
| l-Val + l-Asn | 12 | 76 | 105 | <2 | <2 | 47 | 9 | 11 | 31 | 13 | 97 | 89 |
Spores were germinated with combinations of 33 μM l-alanine, 1 mM l-valine, 2 mM l-asparagine, 10 mM fructose, 10 mM KCl, and 10 mM glucose, and rates of germination were measured and calculated as described in Materials and Methods.
Abbreviations used are as follows: −, no cogerminants added; FK, fructose and KCl; GFK, glucose, fructose, and KCl. Rates of germination are expressed relative to the value for PS832 spores germinating with l-alanine, l-asparagine, and GFK, and this value was set at 100. Boldface type indicates low concentrations of l-alanine, italic type indicates low concentrations of l-valine and underlining indicates low concentrations of l-asparagine. Underlined, boldface type indicates that fructose and K+ in the presence of l-asparagine and low concentrations of l-valine (1 mM) or l-alanine (10 or 33 mM) gave three- to fivefold stimulations in rates of spore germination over those given by l-alanine or l-valine alone. Boldface, italic type indicates that with spores that have only GerA and GerB (strain FB68), the stimulation of germination by GFK plus l-asparagine and either l-alanine or l-valine was essentially identical to that seen with fructose and K+ plus l-asparagine and either l-alanine or l-valine.
Value set at 100.
Response of GerB*s to l-amino acids.
Wild-type GerB alone does not respond to amino acids, as noted above. However, either of several point mutations in the gerB operon, termed gerB* mutations, generates nutrient receptor variants that alone can respond to either d-alanine or l-asparagine, with l-asparagine being more effective (20). Consequently, we were surprised to find that l-alanine also triggered the germination of spores containing GerBA* or GerBB* (Table 4). l-Alanine and l-asparagine also triggered spore germination via GerBA* and GerBB* at much lower concentrations than did d-alanine (Fig. 2 and 3 and data not shown). However, maximum rates of spore germination with l-alanine and GerB*s were only ∼40% of that with GerA and l-alanine (Table 4). GerB*s also required higher concentrations of l-alanine than did GerA to give half-maximal rates of germination (Fig. 2 and Tables 2 and 4). l-Alanine and l-asparagine were not synergistic in stimulating germination via GerB*s. The stimulation of germination of spores of strains PS3521 (which has only GerBB* and GerK) and FB22 (which has only GerBA* and GerK) by mixtures of low concentrations of l-alanine and l-asparagine was the sum of the stimulation given by low concentrations of the individual amino acids (data not shown).
TABLE 4.
Rates of germination of spores of various strains with different amino acidsa
| Germinant(s) | Rates of spore germination with indicated strain (receptor[s])
|
||||
|---|---|---|---|---|---|
| PS832 (GerA GerB GerK) | FB20 (GerB GerK) | PS3651 (GerB) | PS3665 (GerBB*) | PS3710 (GerBA*) | |
| l-Alanine | 100b,c | 41c,e | <0.4d | 42c,f | 35c |
| l-Asparagine | <0.4d | 55c,e | <0.4d | 47c,f | 53c |
| l-Serine | 5c | NDg | <0.2d | 26c,f | 19c |
| d-Alanine | <0.02d | NDg | <0.3d | 16d,f | 5d |
| l-Threonine | <1d | NDg | <0.2d | 18c,f | 3d |
| Glycine | <1d | NDg | 0.8d | 8d,f | <1d |
| l-Valine | 87c | NDg | <0.2d | <0.5d | NDg |
| l-Arginine, l-aspartate, l-glutamate, l-lysine, l-leucine, d-asparagine | <2d | NDg | <0.5d | <0.5d | <0.5d |
Spores were germinated with 10 mM amino acids and the rates of germination were determined as described in Materials and Methods. All germination rates are given relative to the rate of germination of PS832 spores with 10 mM l-alanine, which was set at 100.
This value was set at 100 and is equivalent to 100% spore germination in 18 min.
Value determined by measuring the fall in OD600.
Value determined by flow cytometry.
Determined in the presence of GFK (with each component at 10 mM).
Similar results were obtained when germination was measured by monitoring DPA release.
ND, not determined.
FIG. 2.
Spore germination via GerA, GerBA*, or GerBB* as a function of the concentration of l-alanine. Spores of strains FB87 (has only GerA), PS3710 (has only GerBA*), and PS3665 (has only GerBB*) were germinated with l-alanine, and rates of spore germination were determined as described in Materials and Methods. The symbols for the spores used are as follows: •, FB87 (has only GerA); ▴, PS3710 (has only GerBA*); and ○, PS3665 (has only GerBB*).
FIG. 3.
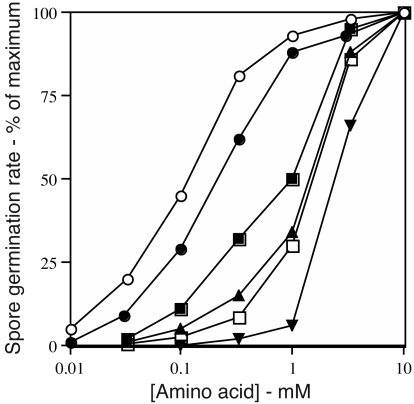
Dependence of the rate of germination of gerBB* spores on the concentrations of various amino acids. Spores of strain PS3665 (has only GerBB*) were heat shocked and germinated with various concentrations of different amino acids, and the rates of spore germination were determined as described in Materials and Methods. Maximum rates of spore germination were taken as those at 10 mM amino acid. The symbols for the amino acids used are as follows: ○, l-alanine; •, l-asparagine; ▪, l-serine; ▴, d-alanine; □, glycine; and ▾, l-threonine.
In addition to l-alanine, l-asparagine, and d-alanine, GerBB* responded to other amino acids including glycine, l-glutamine, l-homoserine, l-serine and l-threonine (Fig. 3, Table 4, and data not shown). GerBA* responded to l-serine but responded poorly if at all to glycine and l-threonine (l-glutamine and l-homoserine were not tested) (Table 4 and data not shown). However, a number of other l-amino acids including l-valine gave no germination of spores with GerB*s, nor did d-asparagine (Table 4). These additional amino acids also did not become effective as germinants when combined with d-glucose using spores of strains FB22 and PS3521 that have GerK as well as GerB*s (data not shown and see below).
The l-alanine germination of spores with GerB*s was surprising, since the GerB* strains were characterized as giving spores that germinated with d-alanine (20). The germination of spores containing GerB*s by l-alanine and other l-amino acids suggested that GerB*s might actually respond to contaminating l-amino acids in d-alanine. Indeed, the d-alanine concentrations giving half-maximal rates of germination of spores with GerB*s were much higher than needed for the same response to l-alanine (Fig. 2 and 3 and data not shown). Consequently, we recrystallized the d-alanine and also treated the recrystallized material with l-alanine dehydrogenase. Strikingly, with the recrystallized and recrystallized/enzyme-treated material, respectively, the spore germination rates at 10 mM amino acid decreased by 30 and 90% relative to those with the starting d-alanine for spores of both PS3665 (which contains only GerBB*) and PS3710 (which contains only GerBA*). The amino acid concentrations giving half-maximum rates of germination of spores of PS3665 and PS3710, respectively, also increased from 1.6 and 2.5 mM with the starting d-alanine to >5 mM for the recrystallized and recrystallized/enzyme-treated material.
Since germination of spores with GerB*s by d-alanine was likely due to contaminants, this might explain the germination of these spores by amino acids other than l-alanine and l-asparagine. Indeed, higher concentrations of other amino acids were needed for half-maximal rates of spore germination than those of l-alanine and l-asparagine (Fig. 2). However, recrystallization did not alter the effectiveness of l-serine and l-threonine in germinating spores with GerBB*, as neither the maximum rate of germination nor the amino acid concentrations giving half-maximum germination rates were changed by recrystallization (data not shown). Recrystallization also did not decrease the effectiveness of l-serine in triggering spore germination via GerBA* and had no effect on l-alanine or l-asparagine germination via GerB*s (data not shown). In contrast, recrystallization decreased the maximum rate of spore germination with glycine by 60% and increased the glycine concentration giving half-maximal rates of germination via GerBB* by ∼4-fold. This suggests that contaminants were responsible for the germination of spores with GerBB* by glycine.
Effect of d-glucose on spore germination via GerB*s.
Previous work has shown that glucose stimulates the germination of spores via GerB*s with d-alanine by ∼2-fold (20). This was also true for germination via GerB*s with l-alanine, l-asparagine, l-serine, and l-threonine (Table 5). The effect of glucose was not dependent on GerA (data not shown) but required GerK (Table 5). However, glucose alone did not germinate spores with GerB*s (data not shown). In addition to stimulating the maximum rate of germination of spores with GerB*s by amino acids, glucose decreased the amino acid concentrations giving half-maximal rates of spore germination by ∼2-fold (Table 6), but this effect was seen only with spores that contained GerK (Table 6). Fructose and K+ did not increase the stimulation of l-amino acid germination of spores with GerB*s and GerK over that obtained with glucose (data not shown).
TABLE 5.
Rates of germination of gerB* spores with amino acids with or without glucosea
| Strain (receptor[s] present) | Rates of spore germination with indicated germinant and glucoseb
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
l-Alanine
|
l-Asparagine
|
l-Serine
|
l-Threonine
|
|||||
| No | Yes | No | Yes | No | Yes | No | Yes | |
| FB22 (GerBA* GerK) | 31 | 74 | 45 | 100c | 26 | 48 | 4 | 12 |
| PS3710 (GerBA*) | 29 | 28 | 36 | 35 | 28 | 30 | 4 | 3 |
| PS3521 (GerBB* GerK) | 32 | 76 | 40 | 82 | 27 | 52 | 12 | 27 |
| PS3665 (GerBB*) | 36 | 36 | 39 | 40 | 25 | 23 | 12 | 11 |
Spores of various strains were germinated with amino acids (10 mM l-alanine, 6 mM l-asparagine, 30 mM l-serine, or 30 mM l-threonine) with or without 10 mM glucose, and the rates of spore germination were measured and calculated as described in Materials and Methods.
Rates of spore germination are given relative to the value for FB22 spores germinated with l-asparagine plus glucose, which was set at 100. This value is equivalent to 100% spore germination in 22 min. “No” and “Yes” refer to the absence and presence, respectively, of glucose. Boldface type indicates that glucose stimulates the germination of spores via GerB*s with l-alanine, l-asparagine, l-serine, and l-threonine by ∼2-fold. Italic type indicates that the effect of glucose was not dependent on GerA but required GerK.
Value set at 100.
TABLE 6.
Amino acid concentrations needed for half-maximal rates of germination of spores of various strainsa
| Strain (receptor[s] present) | Concn of amino acid for half-maximal rates of spore germination (mM) with indicated germinant and cogerminantb
|
|||||
|---|---|---|---|---|---|---|
|
l-Alanine
|
l-Asparagine
|
l-Serine
|
||||
| No | Yes | No | Yes | No | Yes | |
| FB20 (GerB GerK) | − | 0.15c | − | 0.15c | − | 1c |
| FB22 (GerBA* GerK) | 0.5 | 0.3d | 0.35 | 0.25d | 1.8 | 0.9 |
| PS3710 (GerBA*) | 0.5 | 0.5d | 0.25 | 0.27d | 2 | 2.1 |
| PS3521 (GerBB* GerK) | 0.3 | 0.1d | 0.2 | 0.1d | 1.3 | 0.7 |
| PS3665 (GerBB*) | 0.13 | 0.12d | 0.15 | 0.15d | 1 | 1.1 |
Spores were germinated with different amino acids (0.033 to 10 mM l-alanine, 0.02 to 6 mM l-asparagine, or 0.1 to 30 mM l-serine) with or without 10 mM glucose or GFK (with each component at 10 mM). Spore germination was measured, germination rates were calculated as described in Materials and Methods, and concentrations of amino acids needed for half-maximal rates of germination were determined from plots of germination rate versus the log of amino acid concentrations.
“No” and “Yes” refer to the absence and presence, respectively, of glucose and/or GFK. Boldface type indicates that glucose decreased the amino acid concentrations giving half-maximal rates of spore germination by ∼2-fold, and italic type indicates that this effect was seen only with spores that contained GerK. −, does not germinate.
GFK added.
Glucose only added.
Germination of spores with l-amino acids via GerB.
As noted above, spores lacking GerA but containing GerK and GerB only germinate with l-alanine if GFK is present (strain FB20) (16, 17, 32, 33) (Table 6), even when 200 mM l-alanine was used (Table 2). Germination of spores lacking GerA with l-threonine or l-serine also required GFK (strain FB20) (Table 6), and all three constituents of GFK were needed (Table 6 and data not shown). As expected, the ability of GFK plus l-amino acids to trigger spore germination via GerB required GerK (strain PS3651) (Table 2).
We also varied sporulation and germination conditions in attempts to render l-alanine (10 mM) germination via GerB independent of GFK and GerK. With FB20 (which contains only GerB and GerK) spores, changes included (i) sporulation at 23 to 45°C or with 1 M NaCl; (ii) germination with 0.1 to 1 M NaCl, LiCl, KCl, or (NH4)2SO4, with 0.1 to 3 M urea, or with 1 to 30% dimethyl sulfoxide; and (iii) germination at 23 to 45°C or at pHs from 5 to 10. None of these changes eliminated the GFK and GerK requirement for spore germination with l-alanine via GerB (data not shown).
Effect of inhibitors on spore germination.
The data given above suggested that GerB*s respond not to d-alanine but to l-amino acids, including l-alanine. The response of GerA to l-alanine is strongly inhibited by d-alanine (17). With 10 mM d-alanine, 0.1 mM l-alanine gave no germination via GerA, although 0.1 mM l-alanine alone gave almost the maximum germination rate (Fig. 1 and Tables 2 to 7). Triggering of spore germination with l-valine via GerA was also inhibited by d-alanine (Table 7). These observations as well as the selection of spores with GerB*s by their germination with d-alanine suggest that d-alanine does not inhibit the response of GerB*s to l-alanine, and this was what was found (Table 7). Germination via GerB, l-alanine, and GFK was also not inhibited by d-alanine, and germination of spores with l-asparagine via GerB or GerB* was not inhibited by d-asparagine (Table 7).
TABLE 7.
Effect of inhibitors on germination of spores of different strains by amino acidsa
| Strain (receptor[s] present; germinant[s]) | % Inhibition of the rate of spore germination with indicated inhibitor
|
||||
|---|---|---|---|---|---|
| 10 mM d-alanine | 10 mM d-asparagine | 0.13% butanol | 0.6 mM chlorocresol | 2.7% ethanol | |
| PS832 (GerA GerB GerK; l-Ala) | >95 (95)b | NDc | 95 (95)b | >95 (95)b | >95 (95)b |
| PS832 (GerA GerB GerK; l-Val) | >90 | NDc | >95 | >95 | >95 |
| FB20 (GerB GerK; l-Ala & GFK) | <5 | <5 | 10 | <5 | 80 |
| FB20 (GerB GerK; l-Asn & GFK) | NDc | <10 | <5 (<5)b | <5 (<5)b | 80 (50)b |
| PS3710 (GerBA*; l-Ala) | <5 | <10 | 45 | 50 | 75 |
| PS3710 (GerBA*; l-Asn) | NDc | <10 | 50 | 50 | 85 |
| PS3665 (GerBB*; l-Ala) | <5 | <10 | 45 | 30 | 50 |
| PS3665 (GerBB*; l-Asn) | NDc | <10 | 25 | 20 (10)b | 45 (15)b |
Spores were germinated with 10 mM l-alanine or 6 mM l-asparagine with or without GFK (with each component at 10 mM) and with or without various inhibitors. Germination was measured, and the percent inhibition of the rate of spore germination was determined as described in Materials and Methods.
Values in parentheses are from reference 6.
ND, not done.
There were also differences in the response of GerB*s and GerA to other inhibitors (Table 7). Triggering of spore germination by GerA was inhibited by low concentrations of butanol, chlorocresol, or ethanol (Table 7), as found previously (6). However, GerB and GerB*s were less sensitive to these inhibitors when l-alanine or l-asparagine was used to trigger germination (Table 7).
DISCUSSION
Some experiments in this work have used spores of strains with defined deletions in gerA, gerB, or gerK to confirm conclusions made previously using spores of strains with point mutations (14, 16, 17, 33). These conclusions include that (i) glucose and K+ plus GerK stimulate spore germination with l-alanine via GerA and (ii) GFK plus GerK are essential for spore germination with l-alanine or l-asparagine via GerB. New findings made in this work are as follows. (i) Only glucose, K+, and GerK are needed for maximum stimulation of spore germination with l-alanine via GerA. The involvement of K+ was not established in previous work (14, 32). A previous study suggested that fructose is also essential for the glucose-plus-K+ stimulation of spore germination with l-alanine via GerA (16). However, this was not found in a previous study (34). We also found no effect of fructose, and the glucose used in our work had not been autoclaved. (ii) Glucose and K+ plus GerK stimulate spore germination with l-valine via GerA. (iii) GerB and GerK plus GFK respond not only to l-asparagine or l-alanine but also to a number of other l-amino acids. (iv) Fructose, K+, and l-asparagine plus GerB stimulate spore germination via GerA and l-alanine or l-valine. (v) GerB* variants do not respond to d-alanine but respond to the same general spectrum of l-amino acids to which GerB responds when GerK and GFK are present. (vi) The amino acid changes in GerB*s have rendered these variants independent of fructose and K+ and largely but not totally independent of GerK plus glucose. However, stimulation of GerB* function by glucose requires GerK. (vii) The response of GerB and GerB* to l-alanine and l-asparagine is not inhibited by the corresponding d-amino acids, while GerA function with l-valine is inhibited by d-alanine. There are also differences in the responses of GerA, GerB, and GerB*s to other inhibitors, some of which have been seen previously (6).
A notable new conclusion listed above is that GerB*s respond not to d-alanine but to a variety of l-amino acids. The gerB* mutations were previously reported to allow B. subtilis spore germination with d-alanine alone (20). The isolation of these mutant strains appears to have been due to a combination of fortunate circumstances, which are as follows: (i) the d-alanine used for mutant selection likely contained some l-amino acids, probably l-alanine; (ii) l-alanine triggering of spore germination via GerA is strongly inhibited by d-alanine, such that this receptor's action is blocked by a high ratio of d- to l-alanine (17); and (iii) GerB's action with l-alanine, while requiring GFK plus GerK, is not inhibited by d-alanine. The action of alanine racemase, an enzyme in surface layers of spores of B. subtilis and other Bacillus species (23), also may have generated l-alanine from the d-alanine used as a germinant in the original mutant isolation. The point mutations that give the gerB* phenotype appear to have made GerB* action independent of GFK plus GerK, although some stimulation by glucose plus GerK remains.
To explain the results noted above, we suggest that GerB contains a relatively nonspecific binding site for l-amino acids. However, either this binding site is masked in the absence of GFK plus GerK or the binding of l-amino acids to GerB does not generate a signal sufficient to trigger spore germination without the participation of GFK plus GerK. Presumably, the amino acid changes in GerB*s have caused slight structural changes that mimic some change in GerB caused by the action of GFK plus GerK. These slight structural changes either unmask the l-amino acid binding site in GerB*s or allow this type of receptor variant alone to trigger spore germination when amino acids are bound, or both. The changes in gerBA and gerBB giving rise to the gerB* mutations studied in this work are point mutations that change amino acids in relatively hydrophobic regions that are fairly well conserved in GerA and GerK (Fig. 4). The change is nonconservative in GerBA* (proline to serine) but is relatively conservative in GerBB* (phenylalanine to isoleucine) (Fig. 4). Unfortunately, the structures of the nutrient receptors are not known, although the three proteins encoded by the operons encoding each nutrient receptor physically interact (11, 12, 17, 20). Proteins of one receptor can also physically interact with those of a different nutrient receptor, although the degree of this interaction is not clear (12, 30). If the gerB* mutations do cause a structural change that makes GerB*s independent of GFK plus GerK, it might be possible to cause this structural change in GerB with chemicals that cause structural changes in proteins. However, we were unable to make GerB independent of GFK plus GerK despite changing sporulation and germination conditions, including the addition of chemicals that cause structural changes in proteins.
FIG. 4.
Amino acid sequences in regions of GerA, GerB, and GerK in which amino acid changes give GerB* variants. The amino acid sequence changes in B. subtilis GerBA* and GerBB* were described previously (20). Residues that are identical in the GerA, GerB, and GerK receptors are shown in boldface type.
The GerK requirement for glucose to stimulate spore germination via GerA, GerB, or GerB* indicates that glucose binds to GerK, as has been suggested previously (14, 34). The lack of effect of fructose on spore germination via GerA with l-alanine, glucose, and K+ plus GerK indicates that GerK does not bind fructose. This is consistent with the lack of an effect of fructose on the stimulation of spore germination via GerB*s with amino acids and glucose plus GerK. These findings suggest that the fructose-responsive component is GerB. This is consistent with the fructose-plus-K+ stimulation of spore germination by GerA, GerB, and l-asparagine plus either l-alanine or l-valine. Presumably, the amino acid changes in GerB*s have eliminated the fructose requirement for the function of these variants. While K+ was required for GerA to function cooperatively with GerK or GerB, it is difficult to ascribe K+ binding to one nutrient receptor, and K+ could interact with all three receptors. However, the amino acid changes in GerB*s have eliminated the K+ requirement for cooperative action between GerB* and GerK.
When processing information to arrive at an appropriate germination response, the spore has a number of concerns. It cannot be too responsive to very low levels of nutrients, as it might germinate under conditions that are unfavorable for growth. It also must integrate signals from different nutrients, as low levels of different nutrients may indicate a more favorable environment than do high levels of one nutrient. While we have learned much about spore germination in recent years, how spores process information that can affect rates of germination is still unclear. Outstanding questions on this topic include the following: (i) what is the signal output upon binding of germinants to their cognate receptors, (ii) how do cogerminants such as glucose stimulate spore germination, and (iii) what is the mechanism whereby two different nutrient receptors act cooperatively or synergistically? We propose alternative but not mutually exclusive working models to explain the signal processing that must take place in spore germination (Fig. 5). Note, however, that neither model incorporates a role for the GerD protein that also influences spore germination via GerA, GerB, or GerK (17, 25). In one model (model A) (Fig. 5A), individual nutrient receptors are present in the spore's inner membrane either alone or in receptor complexes. Each nutrient receptor or receptor complex can generate an output that determines the rate of spore germination, although the nature of this output is not known. In the second model (model B) (Fig. 5B), the individual nutrient receptors interact with a hypothetical signal integrator that integrates input and generates an output that determines the rate of spore germination. Both models have attractive and unattractive features. For model A, the concept of receptor complexes is attractive because it mirrors what has been established in signal processing by receptors in bacterial chemotaxis (9, 15, 31). There are also data suggesting that different germinant receptors physically interact (12, 30). Model A needs no additional (and at present hypothetical) components to explain signal processing in germination, as it requires only the formation of receptor complexes. However, there must be high affinity between receptors for significant complex formation between different nutrient receptors, since levels of nutrient receptors in the spore's inner membrane are low (22). It also seems unlikely that stimulation by a germinant or cogerminants would drive receptor complex formation. Since lipid probes in the inner spore membrane are largely immobile (7), it seems likely that protein diffusion in this membrane will also be extremely slow. Thus, receptor complex formation would likely have to take place only late in sporulation, when the inner spore membrane is still fluid.
FIG. 5.
Alternative models for signal processing in B. subtilis spore germination based on both previous and current work. (A) The receptors can operate either alone or when complexed with other receptors to give a particular rate of spore germination. While only complexes of two receptors are shown, all three receptors could be in a complex in this model. The short arrows beneath GerB and GerK indicate that these receptors alone give little if any germination. (B) The signals from individual receptors, which may be very different in intensity, are fed into a hypothetical signal integrator that integrates and/or sums all signals to generate an output that is a particular rate of spore germination. The specific role of K+ is not shown in either A or B, since K+ may interact with all three receptors. Abbreviations used for receptors are in boxes and are as follows: A, GerA; B, GerB; B*, GerB*; K, GerK. Abbreviations used for germinants or cogerminants are as follows: A, l-alanine; AA, l-alanine, l-asparagine, l-serine, or l-threonine; f, fructose; g, glucose; V, l-valine. The symbol “/” indicates “or.” Cogerminants are shown in parentheses above the affected receptor; germinants are also shown above the affected receptor.
Model B, on the other hand, requires a new component needed for signal processing in spore germination, the signal integrator. This component may be present in large amounts ensuring association with the low levels of the various nutrient receptors, even if the affinity between these components is not extremely strong. This signal integrator would need a threshold signal level in order to cause spore germination, and presumably, glucose or GFK plus GerK cannot generate such a signal. However, this signal could be synergistic with the signal from l-amino acids plus GerB. As noted above, synergism between different germinant receptors in triggering spore germination has also been seen with spores of B. anthracis and B. cereus (2, 13). The major unattractive feature of model B is the need for significant levels of a new spore component, the signal integrator, as this component has not been identified. An early event triggered by the binding of germinants to nutrient receptors is DPA release, and there is significant evidence that the proteins encoded by the spoVA operon are involved in this DPA release (8, 26, 28). At least one SpoVA protein, SpoVAD, is located in the spore's inner membrane (29), the location of the nutrient receptors (10, 22). Consequently, it is reasonable to assume that all SpoVA proteins are in the inner membrane, perhaps as a protein complex. The level of SpoVAD is also ∼100-fold higher than those of individual nutrient receptors (29). Thus, there is more than enough SpoVA protein in spores for some complex of these proteins to play the role of the signal integrator. In addition, preliminary results indicate that at least GerA and GerB proteins interact with SpoVAC (30). The possibility that SpoVA proteins might interact with nutrient receptors is also suggested by the fact that SpoVAF has significant amino acid sequence homology to GerAA, GerBA, and GerKA (8). While we cannot yet decide between models A and B, we hope that the presentation of these models will suggest future experiments that may provide further insight into the mechanism of spore germination.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (grant GM19698) to P.S.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlass, P. J., C. W. Houston, M. O. Clements, and A. Moir. 2002. Germination of Bacillus cereus spores in response to L-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148:2089-2095. [DOI] [PubMed] [Google Scholar]
- 3.Black, E. P., K. Koziol-Dube, D. Guan, J. Wei, B. Setlow, D. E. Cortezzo, D. G. Hoover, and P. Setlow. 2005. Factors influencing the germination of Bacillus subtilis spores via activation of nutrient receptors by high pressure. Appl. Environ. Microbiol. 71:5879-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrera-Martinez, R.-M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corfe, B. M., R. L. Sammons, D. A. Smith, and A. Moir. 1994. The gerB region of the Bacillus subtilis 168 chromosome encodes a homologue of the gerA spore germination operon. Microbiology 140:471-478. [DOI] [PubMed] [Google Scholar]
- 6.Cortezzo, D. E., B. Setlow, and P. Setlow. 2004. Analysis of the action of compounds that inhibit the germination of spores of Bacillus species. J. Appl. Microbiol. 96:725-741. [DOI] [PubMed] [Google Scholar]
- 7.Cowan, A. E., E. M. Olivastro, D. E. Koppel, C. A. Loshon, B. Setlow, and P. Setlow. 2004. Lipids in the inner membrane of spores of Bacillus subtilis are largely immobile. Proc. Natl. Acad. Sci. USA 101:7733-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis, N. R., P. M. Wollman, J. B. Stock, D. J. Derosier, and D. R. Thomas. 2004. Three-dimensional structure and organization of a receptor/signaling complex. Proc. Natl. Acad. Sci. USA 101:17480-17485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudson, K. D., B. M. Corfe, E. H. Kemp, I. M. Feavers, P. J. Coote, and A. Moir. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J. Bacteriol. 183:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi, T., and P. Setlow. 2004. Effects of a gerF mutation on the germination of spores of Bacillus subtilis. J. Bacteriol. 186:2984-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi, T., and P. Setlow. 2005. Interaction between individual protein components of the GerA and GerB nutrient receptors that trigger germination of Bacillus subtilis spores. J. Bacteriol. 187:2513-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ireland, J. A., and P. C. Hanna. 2002. Amino acid- and purine riboside-induced germination of Bacillus anthracis ΔSterne endospores: gerS mediates responses to aromatic amino acids. J. Bacteriol. 184:1296-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irie, R., T. Okamoto, and Y. Fujita. 1982. A germination mutant of Bacillus subtilis deficient in response to glucose. J. Gen. Appl. Microbiol. 28:345-354. [Google Scholar]
- 15.Li, M., and G. L. Hazelbauer. 2005. Adaptational assistance in clusters of bacterial chemoreceptors. Mol. Microbiol. 56:1617-1626. [DOI] [PubMed] [Google Scholar]
- 16.McCann, K. P., C. Robinson, R. L. Sammons, D. A. Smith, and B. M. Corfe. 1996. Alanine germinant receptors of Bacillus subtilis. Lett. Appl. Bacteriol. 23:290-294. [DOI] [PubMed] [Google Scholar]
- 17.Moir, A. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44:531-553. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson, W. L., and P. Setlow. 1990. Spore germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, England.
- 19.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 182:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redmond, C., L. W. Baille, S. Hibbs, A. J. Moir, and A. Moir. 2004. Identification of proteins in the exosporium of Bacillus anthracis. Microbiology 150:355-363. [DOI] [PubMed] [Google Scholar]
- 24.Sammons, R. L., A. Moir, and D. A. Smith. 1981. Isolation and properties of spore germination mutants of Bacillus subtilis 168 deficient in the initiation of germination. J. Gen. Microbiol. 124:229-241. [Google Scholar]
- 25.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 26.Tovar-Rojo, F., M. Chander, B. Setlow, and P. Setlow. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatasubramanian, P., and K. Johnstone. 1993. Biochemical analysis of germination mutants to characterize germination receptors of Bacillus subtilis 1604 spores. J. Gen. Microbiol. 139:1921-1926. [DOI] [PubMed] [Google Scholar]
- 28.Vepachedu, V. R., and P. Setlow. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 239:71-77. [DOI] [PubMed] [Google Scholar]
- 29.Vepachedu, V. R., and P. Setlow. 2005. Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J. Bacteriol. 187:5677-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vepachedu, V. R., and P. Setlow. 2005. Unpublished results.
- 31.Wadhams, G. H., and J. P. Armitage. 2004. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5:1024-1037. [DOI] [PubMed] [Google Scholar]
- 32.Wax, R., E. Freese, and M. Cashel. 1967. Separation of two functional roles of l-alanine in the initiation of Bacillus subtilis spore germination. J. Bacteriol. 94:522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wax, R., and E. Freese. 1968. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of l-alanine. J. Bacteriol. 95:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasuda, Y., and K. Tochikubo. 1984. Relation between D-glucose and L- and D-alanine in the initiation of germination of Bacillus subtilis spores. Microbiol. Immunol. 28:197-207. [DOI] [PubMed] [Google Scholar]