Abstract
The PmrA/PmrB two-component system encoded by the pmrCAB operon regulates the modification of Salmonella enterica serovar Typhimurium lipopolysaccharide leading to polymyxin B resistance. PmrA and PhoP are the only known activators of pmrCAB. A transposon mutagenesis screen for additional regulators of a pmrC::MudJ fusion led to the identification of a two-component system, termed PreA/PreB (pmrCAB regulators A and B), that controls the transcription of the pmrCAB operon in response to unknown signals. The initial observations indicated that insertions in, or a deletion of, the preB sensor, but not the preA response regulator, caused upregulation of pmrCAB. Interestingly, the expression of pmrCAB was not upregulated in a preAB mutant grown in LB broth, implicating PreA in the increased expression of pmrCAB in the preB strain. This was confirmed by overexpression of preA+ in preAB or preB backgrounds, which resulted in significant upregulation or further upregulation of pmrCAB. No such effect was observed in any tested preB+ backgrounds. Additionally, an ectopic construct expressing a preA[D51A] allele also failed to upregulate pmrC in any of the pre backgrounds tested, which implies that there is a need for phosphorylation in the activation of the target genes. The observed upregulation of pmrCAB occurred independently of the response regulators PmrA and PhoP. Although a preB mutation led to increased transcription of pmrCAB, this did not result in a measurable effect on polymyxin B resistance. Our genetic data support a model of regulation whereby, in response to unknown signals, the PreB sensor activates PreA, which in turn indirectly upregulates pmrCAB transcription.
Bacterial two-component systems (TCS) couple extracellular and intracellular signals to the transcriptional expression of genes or to the posttranslational regulation of molecular motors or enzymes (39). In particular, transcription of virulence genes in microbial pathogens is often tightly regulated and may involve TCS (8). TCS are typically composed of a sensor kinase, frequently membrane bound, and a response regulator. The sensor kinase responds to specific signals resulting in a net increase in the autophosphorylation rate of a conserved histidine residue of its C-terminal transmitter domain. The phosphate moiety is then transferred to a conserved aspartyl residue located in an acidic pocket on the N-terminal receiver domain of the cognate response regulator. The phosphorylated form of the response regulator is the effector of the signal transduction event, activating or repressing transcription or enzyme activities. The signaling is switched off by the unstable nature of the aspartyl phosphate moiety or by cellular phosphatases, including bifunctional sensors (19).
Many Salmonella enterica serovar Typhimurium TCS have been shown to be important for virulence. For instance, SirA/BarA (21, 43) and EnvZ/OmpR (24) are all indirectly involved in regulating SPI-1 invasion genes. Also, the TCS SsrA/SsrB directly controls expression of SPI-2 intramacrophage survival genes (5, 9), while PhoP/PhoQ controls a vast regulon that includes invasion genes, macrophage survival genes, cation transporters, and genes involved in antimicrobial peptide (AP) resistance (10, 30). Often, TCS regulate other TCS at both transcriptional and posttranslational levels. For instance, PhoP has been recently shown to bind and regulate an internal promoter that drives the expression of the SPI-2 regulator ssrB (2), while at the same time, it controls dephosphorylation of the PmrA response regulator via transcriptional regulation of the gene encoding the small protein PmrD (22, 23).
PmrA/PmrB is another TCS both necessary for resistance to polymyxin B (PMB) in vitro and important for oral virulence in the mouse model of enteric fever (14, 15, 36). PmrA/PmrB regulates the expression of a complex regulon that includes pmrCAB, pmrE, pmrG, pmrFHIJKLM, cptA, and several other genes (15, 25, 40, 41). Some of these genes were shown to be involved in modifying the lipopolysaccharide (LPS), altering the surface charge and reducing the binding of cationic APs such as PMB (11, 13, 16). APs are important components of the host innate immune system. They are found at mucosal and skin surfaces and within professional phagocytes (18, 33). APs bind to the LPS in enterobacteria, gaining entry into the cell and most often causing perforation of the cytoplasmic membrane and eventual death (45). Besides being indirectly regulated by PhoP/PhoQ via the mechanism mentioned above, some PmrA-regulated genes such as ugd/pmrE are also transcriptionally controlled by the RcsB/RcsC two-component system (31). The fact that multiple signals/signaling pathways converge to modulate the Salmonella polymyxin resistance regulon suggests its critical importance for the adaptation/survival in both extracellular and intracellular environments. Additionally, previous work from our laboratory suggests that in vivo, regulators other than PmrA might be involved in controlling the expression of the pmrFHIJKLM operon (15).
In this study, we describe a Salmonella TCS, named PreA/PreB, that was identified in a transposon mutagenesis screen for regulators of pmrCAB. PreA/PreB is similar to the luxS-dependent quorum-sensing regulatory system QseB/QseC in enterohemorrhagic Escherichia coli (37). We demonstrate that PreA activates the transcription of pmrCAB in a PhoP- and PmrA response regulator-independent fashion. The results suggest that sensor kinase PreB inactivates PreA during growth in Luria-Bertani (LB) broth and that PreA is indirectly involved in pmrCAB regulation. Furthermore, the observed increase in pmrCAB transcription does not lead to observable transcriptional activation of most of the PmrA/PmrB regulon or to the alteration of the polymyxin resistance phenotype.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli and Salmonella enterica serovar Typhimurium strains and plasmids used in this study are listed in Table 1. LB broth and agar were used for strain maintenance, cloning, and expression experiments. For gene expression experiments, strains were grown in a rotating drum to mid- to late exponential phase (optical density at 600 nm [OD600] of 0.6 to 0.9). When appropriate, antibiotics were added at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 25 μg/ml; tetracycline, 25 μg/ml; streptomycin, 100 μg/ml.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| BW20339 | F′ 128::Tn10-12(Kan) lacIq ΔlacZM15pro(BA)+/DE3(lac)X74 uidA (ΔMlul)::pir+recA1 ΔphoA532 Δ(phnCΔDEFGHIJKLMNOP)33-30 | 28 |
| DH5α | supE44 Δ(lacZYA-argF) U169 (φ80lacZ ΔM15) hsdR17 recA endA1 gyrA96 thi-1 relA1 | Gibco |
| HB101 | F−thi-1 hsd20 (rB− mB−) supE44 recA13 ara-14 leuB6 proA2 lacY1 rpsL20 (Smr) xyl-5 mtl-1 | 3 |
| SM10λpir | thi-1 thr leu tonA recA supE (RP4-2 Kmrtet::Mu) | 42 |
| Salmonella enterica serovar Typhimurium | ||
| CS019 | ATCC 14028 phoN::Tn10d-kan (Kanr) | 30 |
| JSG210 | ATCC 14208 (CDC6516-60), wild type | ATCC |
| JSG206 | JSG210 phoP::Tn10d-cam (aka CS015) (Camr) | 30 |
| JSG214 | pmrE214::MudJ (Kanr) | This study |
| JSG215 | JSG210 pmrC215::MudJ (Kanr) | 30 |
| JSG420 | pmrA421::Tn10d pmrC215::MudJ (Tetr Kanr) | This study |
| JSG421 | JSG210 pmrA421::Tn10d (Tetr) | 14 |
| JSG542 | CS019 rpsL (Strr) | This study |
| JSG1038 | JSG210 preB1038::Tn10d (Tetr) | This study |
| JSG1039 | JSG210 preB1038::Tn10d pmrC215::MudJ (Tetr Kanr) | This study |
| JSG1040 | ΔpreB2343 pmrE214::MudJ (Kanr) | This study |
| JSG1051 | JSG210 pmrH1923::MudJ (Kanr) | This study |
| JSG1058 | JSG210 preB1038::Tn10d pmr1923::MudJ (Tetr Kanr) | This study |
| JSG1060 | phoP206::Tn10d pmrC215::MudJ (Tetr Kanr) | 30 |
| JSG1527 | preB1038::Tn10d yibD1525::MudJ (Tetr Kanr) | This study |
| JSG1525 | yibD1525::MudJ (Kanr) | 41 |
| JSG1987 | JSG210 preA1987::kan (Kanr) | This study |
| JSG1998 | JSG210 ΔpreA1998 | This study |
| JSG2003 | JSG210 ΔpreA1998 pmrC215::MudJ (Kanr) | This study |
| JSG2115 | ΔpreB2344 pmrC215::MudJ (Kanr) | This study |
| JSG2343 | JSG210 ΔpreB2343 | This study |
| JSG2344 | JSG210 ΔpreB2344::kan (Kanr) | This study |
| JSG2364 | CS019 preB2306(H246G) (Camr) | This study |
| JSG2365 | ΔpreB phoP206::Tn10d pmrC215::MudJ (Tetr Kanr) | This study |
| JSG2366 | ΔpreB pmrA421::Tn10d pmrC215::MudJ (Tetr Kanr) | This study |
| JSG2422 | preB2306[H246G] pmrC215::MudJ (Camr Kanr) | This study |
| JSG2481 | JSG210 ΔpreA1998 pmrA[D51A]2456 pmrC215::MudJ (Kanr) | This study |
| JSG2498 | preB1038::Tn10d pmrA[D51A]2456 pmrC215::MudJ (Tetr Kanr) | This study |
| JSG2499 | pmrA[D51A]2456 pmrC215::MudJ (Kanr) | This study |
| JSG2523 | ΔpreA1998 yibD2420::MudJ (Kanr) | This study |
| JSG2420 | pmrA421::Tn10d ΔpreA1998 yibD2420::MudJ (Tetr Kanr) | This study |
| JSG2527 | ΔpreB2343 pmrA421::Tn10d yibD2420::MudJ (Tetr Kanr) | This study |
| JSG2623 | ΔpreAB::cat (Cmr) | This study |
| JSG2624 | ΔpreAB2626 pmrC215::MudJ (Kanr) | This study |
| JSG2626 | JSG210 ΔpreAB2626 | This study |
| Plasmids | ||
| pWSK29 | pSC101 ori, αlacZ (Apr) | 46 |
| pBAD18 | ColE1 ori, PBAD L(+) Ara inducible (Apr) | 17 |
| pCP20 | FLP recombinase expression plasmid (Cmr Apr) | 6 |
| pKD46 | R101 ori, rep101ts, PBAD expressing λ γ β exo (Apr) | 6 |
| pKD4 | oriR6K, FRT::kan::FRT template plasmid (Apr) | 6 |
| pKD3 | oriR6K, FRT::cat::FRT template plasmid (Apr) | 6 |
| pKAS46 | oriR6K, αlacZ, suicide vector carrying an rpsL locus (Apr Kmr) | 35 |
| pLD55 | oriR6K, αlacZ tetAR (Apr) | 28 |
| pRK2013::Tn7 | ColE1 mob+traRK2 ΔrepRK2repEkan::Tn7 (Tpr Smr Spr) | 7 |
| pQseBC33 | pBAD33 with the E. coli O157:H7 qseBC operon (Apr) | 37 |
| pJSG975 | pWSK29 with a HindIII fragment carrying tetAR from Tn10d and upstream chromosomal DNA from JSG1038 (Tcr Apr) | This study |
| pJSG2381 | pKAS46 with a 1.2-kb insert containing preB[H246G] (Kmr Apr) | This study |
| pJSG2391 | pBluescript with a 1.2-kb fragment containing pmrA[D51A] constructed by SOE-PCR (Apr) | This study |
| pJSG2456 | pLD55 with a 0.7-kb SstI-XhoI PCR fragment containing pmrA[D51A] from pJSG2391 (Tcr Apr) | This study |
| pJSG2558 | pBAD18 with a 0.7-kb fragment containing preA expressed from PBAD (Apr) | This study |
| pJSG2581 | pBAD18 with a 1.5-kb fragment containing preAB expressed from PBAD (Apr) | This study |
| pJSG2700 | pJGS2558 derivative mutagenized to express a preA[D51A] allele (Apr) | This study |
Molecular biology and genetic techniques.
DNA purification, molecular cloning, and PCR were performed according to standard procedures (1). Plasmids were mobilized by electroporation. Transposon mutagenesis was performed with Tn10d and MudJ transposons as previously described (12). Marked mutations were transferred between Salmonella enterica serovar Typhimurium strains by P22 HT105 int-102-mediated generalized transduction as described previously (20, 34).
DNA sequencing and bioinformatics.
DNA sequencing was performed using a Big Dye fluorescent terminator and an ABI3770 capillary sequencer at the Nucleic Acid Core Facility, University of Texas Health Science Center at San Antonio, and the Plant Microbe Genomic Facility at The Ohio State University. DNA sequences were analyzed by Blastx at the NCBI (27). Domain structure was analyzed by CDD searches(26). ClustalW was used to produce global progressive alignments (44).
Construction of mutants and plasmids.
Nonpolar deletions of preA and preB were created using λ-Red mutagenesis (6). In particular, primers JG489 and JG490 or JG557 and JG558 (Table 2) were designed to amplify preA′-kan-′preA andpreB′-kan-′preB cassettes, respectively, while primers JG489 and JG558 amplified preA′-cat-′preB using pKD3 or pKD4 as a template. The recombinant antibiotic cassettes were exchanged into the chromosome of Salmonella enterica serovar Typhimurium 14028s(pKD46) to generate strains JSG1987 ΔpreA::kan, JSG2344 ΔpreB::kan, and JSG2626 ΔpreAB::cat (Table 1). The antibiotic cassettes were resolved by introducing pCP20, and the resulting unmarked strains were named JSG1998 ΔpreA, JSG2626 ΔpreAB, and JSG2343 ΔpreB. A missense mutation to glycine was generated at the PreB predicted autophosphorylation site H246. Spliced overlap extension (SOE)-PCR with JG637 and JG634 as flanking primers and JG635 and JG636 as mutagenic primers was used to construct the mutant allele in vitro. The preB[H246G] allele was ligated into the suicide vector pKAS46 as a 1.2-kb EcoRI/NotI fragment to produce plasmid pJSG2381 in E. coli SM10λpir. Allele exchange with pJSG2381 was performed using JSG542, a Salmonella enterica serovar Typhimurium 14028 Strr derivative, as the recipient strain. The correct recombinant was confirmed by sequencing and was labeled JSG2364. The phosphorylation site at residue D51 of pmrA was mutated to alanine by SOE-PCR using primers JG797 and JG798 (flanking primers) with JG795 and JG796 (internal primers). The pmrA[D51A] allele was cloned as an SstI-XhoI fragment into the suicide vector pLD55 (28) to produce plasmid pJSG2456 in E. coli BW20339. Allele exchange into strain JSG2003 preA pmrC::MudJ was performed using Bochner selection, as described previously (28), to produce strain JSG2481. The pmrC::MudJ pmrA[D51A] locus was further mobilized in several genetic backgrounds by P22 transduction selecting for the Kanr marker of MudJ linked to the pmrA mutation. The correct recombinant was verified by PCR and restriction analysis of the pmrA[D51A] allele at the engineered NheI site.
TABLE 2.
Oligonucleotide primers
| Name | 5′ → 3′ sequencea | Purpose |
|---|---|---|
| JG489 | ATGCGAATTTTACTGGTAGAAGATGACACAGTGTAGGCTGGAGCTGCTTCG | λ-Red deletion of preA or preAB |
| JG490 | TCATGCGTCACCCAGGGTGTAGCCGATGCCCATATGAATATCCTCCTTAG | λ-Red deletion of preA |
| JG557 | ATGAAATTGACGCAACGTCTCAGCCTGACAGTGTAGGCTGGAGCTGCTTCG | λ-Red deletion of preB |
| JG558 | TCGGTACGCCTTTGGCGTCGAGCGGCGTTTCATATGAATATCCTCCTTAG | λ-Red deletion of preB or preAB |
| JG634 | ATAAGAATGCGGCCGCAGTTGATGTTGTTCGC | SOE-PCR preB[H246G] |
| JG635 | CCGACGCCGCCGGCGAATTG | SOE-PCR preB[H246G] |
| JG636 | CAATTCGCCGGCGGCGTCGG | SOE-PCR preB[H246G] |
| JG637 | GAATTCTCCGCGGCGTTGCCAAACGA | SOE-PCR preB[H246G] |
| JG708 | GGAATTCATGCGAATTTTACTGG | Amplify preAB |
| JG709 | GGGGTACCTTACCAACTTACTACGGC | Amplify preAB |
| JG795 | CGGGCAGCCCTAAAGCCTGCACCATCAGA | SOE-PCR pmrA[D51A] |
| JG796 | TCTGATGGTGCAGGCTTTAGGGCTGCCCG | SOE-PCR pmrA[D51A] |
| JG797 | CGCGTCGACTATTACAACCGTTATCCACCG | SOE-PCR pmrA[D51A] |
| JG798 | GCTCTAGAGTACTGATTAACTGGAACACC | SOE-PCR pmrA[D51A] |
| JG1055 | CCGGAATTCCGCGAGTTACCGCAAGGAAGAACAGATGCG | Amplify preA |
| JG1056 | CCGGAATTCTCATGCGTCACCCAGGGTGTAGCCGATGC | Amplify preA |
| JG1190 | CTTATGATGCGGTTATTTTAGCGTTGACGCTGCCAGGC | Mutagenize preA[D51] to A |
| JG1191 | TAAAATAACCGCATCATAAGGCGCGCTGTAAAGCG | Mutagenize preA[D51] to A |
Restriction enzyme sites are underlined; P1 and P2 priming sites in pKD3 and pKD4 are in italics.
The entire preAB operon or the preA open reading frame was cloned into pBAD18 for expression from PBAD as a JG708 and JG709 or a JG1055 and JG1056 PCR fragment to produce plasmids pJSG2581 and pJSG2558, respectively. Plasmid pJSG2558 was mutagenized at the conserved D51 residue of preA by oligomutagenesis with primers JG1190 and JG1191 using the Gene Tailor kit (Invitrogen) to generate pJSG2700.
Western blot analysis of His6-PreA proteins.
Whole-cell lysates of Salmonella strains were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto HyBond nitrocellulose (Amersham). Proteins were detected using a primary polyclonal antibody raised in rabbit against recombinant His6-PreA (1:2,000 dilution), a donkey anti-rabbit immunoglobulin G secondary antibody conjugated to alkaline phosphatase (1:4,000 dilution) (Amersham), and the chemiluminescent substrate CPD-Star (Roche) according to a standard Western blot protocol (1).
Enzyme assays.
β-Galactosidase assays were carried out using either a spectrophotometric method with ortho-nitrophenyl-β-d-galactopyranoside (ONPG) as a substrate or a modified fluorometric method with 4-methyl-umbelliferyl-β-galactopyranoside (MUGal) (29). Assays were performed in triplicate. Specific enzyme activities are reported in Miller units (ONPG assays) or in picomoles of released 4-methyl-umbelliferone per second per OD unit per milliliter of culture (MUGal assays).
MIC assays.
Assays were carried out as previously described (38). Briefly, strains were grown in Mueller-Hinton broth overnight, and 104 cells per well were added to polypropylene microtiter plates. Each strain was tested for growth and pellet accumulation against serial dilutions of PMB in 0.2% bovine serum albumin-0.01% acetic acid for 16 h.
RESULTS
Identification and sequence analysis of the preAB operon.
Inan effort to identify additional putative regulators of the pmrCAB locus, a Tn10d mutant pool constructed in Salmonella enterica serovar Typhimurium 14028s was transduced into the reporter strain JSG215 (pmrC::MudJ). Out of 30,000 transductants, a mutant colony producing a strong blue color on LB plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-thiogalactopyranoside) was isolated. The mutant phenotype was named Pre (for pmrCAB regulator), while the mutant strain was named JSG1038. The mutation was transduced back into the parent strain to rule out second-site mutations (creating strain JSG1039), and the phenotype was confirmed. A HindIII fragment containing the region upstream of the transposon, as well as the transposon tetracycline resistance cassette, was shotgun cloned into pWSK29, producing pJSG975. DNA sequencing from the transposon 5′ end and sequence analyses revealed that the Tn10d insertion mapped to a gene corresponding to the Salmonella enterica serovar Typhimurium LT2 locus ygiY (STM3178), which has homology to orthodox bacterial sensor kinases (Fig. 1), most notably to E. coli K-12 and O157:H7 QseC (87 to 88% similarity; E = 0.0). YgiY is predicted to be a 51-kDa protein with two transmembrane segments between residues 8 and 56 and residues 149 and 199. CDD searches and alignments with known sensor kinases predicted a putative autophosphorylation site at residue H246. Upstream of ygiY is ygiX (STM3177), which encodes a 24-kDa putative response regulator of the OmpR subfamily with high similarity to E. coli O157:H7 QseB (93% similarity; E = 1 × 10−107), Pectobacterium carotovorum PmrA (64% similarity; E=8 × 10−49), E. coli CFT073 PmrA (63% similarity; E = 1×10−47), and Salmonella enterica serovar Typhimurium PmrA (58% similarity; E = 7 × 10−43). The new TCS locus was renamed preAB. These two genes form an apparent operon, with the stop codon of ygiX overlapping the start codon of ygiY, an arrangement suggestive of translational coupling. The high similarity and identity to E. coli QseB/QseC, which controls motility in response to AI-2 quorum-sensing signals in E. coli strains (37), and the almost-identical map position (Fig. 1) directly upstream of mdaB, a putative NADH-dependent oxidoreductase, and ygiN, a putative quinol monooxygenase, and downstream of STM3175, an AraC-like regulator, and ygiW suggested that these two TCS may be potential orthologs.
FIG. 1.
Map of preAB genes, mutations, and plasmids. Boxes represent open reading frames. The solid circle indicates a Tn10d insertion. Locations of the preA and preB deletions are noted below the gene map, and a solid line delimiting the region cloned into the expression plasmids pJSG2581 and pJSG2558 is shown.
Both PreB and PreA affect pmrCAB transcription.
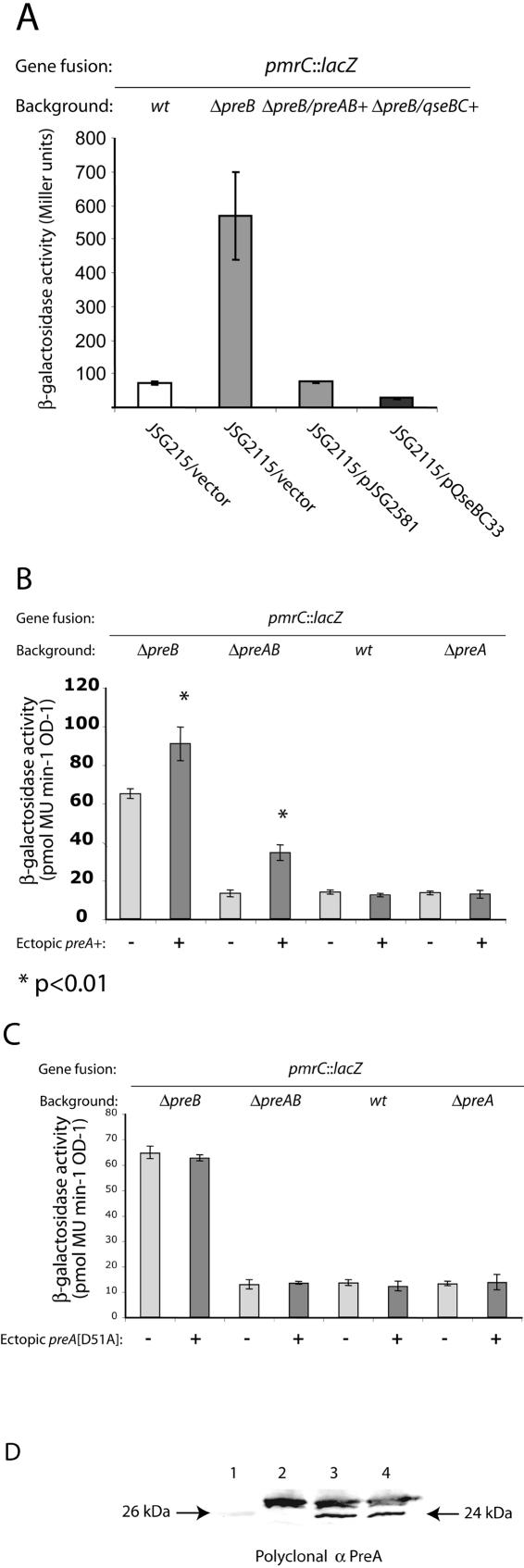
The regulatory effect of the preB::Tn10d insertion on pmrCAB operon expression was quantified by β-galactosidase enzyme assays upon growth of the bacteria in LB medium to exponential-early stationary phase. Reporter gene activity was measured in a pmrC::MudJ background. The results showed an eightfold increase in pmrCAB transcription upon introduction of the preB polar insertion (strain JSG1039) (Fig. 2). To rule out possible polar effects of the Tn10d insertion on the downstream genes mdaB and ygiN (Fig. 1), we created an unmarked deletion in preB (strain JSG2115) using λ-Red mutagenesis. This mutation caused the expected upregulation of pmrCAB at levels comparable to those observed in strain JSG1039 (Fig. 2), and its effects were complemented by preB in trans (Fig. 3). We were also able to complement the regulatory effect of the ΔpreB mutation with plasmid pQseBC33 (Fig. 3), which carries the homologous E. coli operon qseBC, implying functional homology between the two regulatory systems. In general, null mutations in the sensor kinase inactivate a two-component system, but cross talk with the response regulator by alternative kinase or by acetyl phosphate may lead to sensor-independent expression of the two-component system regulon. To test whether the effect caused by preB was replicated by a preA mutation, we introduced a deletion in the response regulator by λ-Red mutagenesis (creating strains JSG1998 and JSG2003). The resulting mutants did not produce the 26-kDa polypeptide after Western blot analysis using a polyclonal antibody against PreA (Fig. 3D, lane 2). To our surprise, a preA strain did not show upregulation of pmrC::MudJ (Fig. 2). This result was also repeated after reengineering the mutants using independently constructed alleles. Similar regulatory patterns were obtained by using other plasmid-borne and chromosomal gene fusions to the pmrCAB promoter (4), ruling out specific effects on the MudJ operon fusion in pmrC.
FIG. 2.
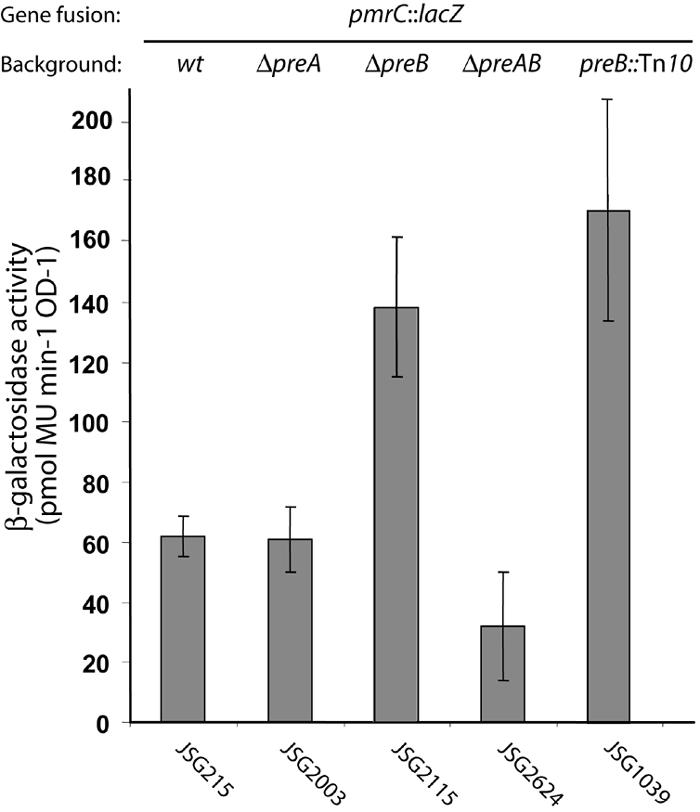
Effect of preA or preB mutations on pmrCAB transcription. Salmonella strains were grown in LB medium to an OD600 of 0.6 before β-galactosidase assays were performed. Activities are expressed in picomoles of 4-methylumbelliferone (MU) per minute per OD unit. Error bars indicate the standard deviations. The following strains were used: JSG215 (pmrC-lacZ), JSG1039 (preB::Tn10d pmrC-lacZ), JSG2003 (ΔpreA pmrC-lacZ), JSG2115 (ΔpreB pmrC-lacZ), and JSG2624 (ΔpreAB pmrC-lacZ). wt, wildtype.
FIG. 3.
Ectopic expression of complementing and suppressing regulatory genes. (A) Complementation of preB mutant. (B) Ectopic expression of preA+. (C) Ectopic expression of preA[D51A]. (D) Western blot analysis of Salmonella whole-cell lysates using polyclonal anti-His6-PreA (α PreA). Lane 1, molecular mass marker; lane 2, JSG1998/pBAD18; lane 3, JSG1998/pJSG2558; lane 4, JSG1998/pJSG2700. Salmonella strains were grown in LB medium with l-arabinose (0.2%) to induce expression of the complementing/suppressing genes. β-Galactosidase assays were performed as described in Materials and Methods. Activities are expressed in Miller units (colorimetric protocol) or picomoles of 4-methylumbelliferone (MU) per minute per OD (fluorometric protocol). Error bars indicate the standard deviations. The following strains were used: JSG215 (pmrC-lacZ), JSG2115 (ΔpreB pmrC-lacZ), JSG2624 (ΔpreAB pmrC-lacZ), JSG2003 (ΔpreA pmrC-lacZ), JSG2115 (ΔpreB pmrC-lacZ), and JSG1998 (ΔpreA). Plasmid pJSG2581 is preAB expressed from PBAD, pQseBC is qseBC expressed from PBAD (pBAD18 vector), plasmid pJSG2558 is preA expressed from pBAD18, and plasmid pJSG2700 expresses preA[D51A]. wt, wild type.
This differential regulatory phenotype implies that the effect on pmrCAB was sensor dependent but response regulator independent, possibly due to cross talk of the kinase to unknown regulators. Another interpretation of this result is that under the growth conditions employed, the proper signal activating PreB kinase activity was absent; therefore, a preA response regulator mutant would not show differential regulation compared to the wild-type. However, for this to be true, one has to assume that the loss of PreB exposes the PreA protein to nonphysiological kinase cross talk that leads to its constitutive activation. To test these models, a ΔpreAB::cat pmrC::MudJ double-mutant strain was created and pmrC transcription was analyzed upon growth in LB medium. The loss of preA in this double mutant reversed the phenotype of pmrC upregulation observed in the preB mutant (Fig. 2), confirming that the effect of deleting preB was mediated by PreA and not by PreB cross talk to unknown regulators. In an additional attempt to disprove the cross talk hypothesis, the PreB conserved histidine at residue 246, the putative autophosphorylation site identified by sequence alignments to other transmitter domains of TCS kinases, was mutated to a glycine residue. Expression of pmrC::MudJ was measured in wild-type, preB, and preB[H246G] backgrounds (Fig. 4). Transcriptional activity of the fusion was not significantly increased in the preB[H246G] background compared to that of wild type (ca. twofold). This regulatory phenotype is identical to that of a preA or a preAB mutation. These data suggest a model in which (i) both PreA and PreB affect pmrCAB transcription, (ii) the PreB sensor is acting as a phosphatase rather than an activating kinase when bacteria are grown in LB medium, (iii) the PreB[H246G] protein retains its phosphatase activity, and (iv) PreA is phosphorylated by cross talk in the absence of PreB.
FIG. 4.
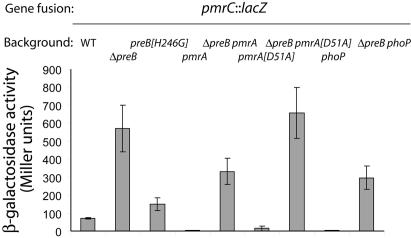
Effects of pmrA, phoP, and the PreB putative autophosphorylation site on PreB-mediated regulation of pmrCAB. Salmonella strains were grown in LB medium, and β-galactosidase assays were performed as described in Materials and Methods. Activities are expressed in Miller units. Error bars indicate the standard deviations. The following strains were used: JSG215 (pmrC-lacZ), JSG1039 (preB pmrC-lacZ), JSG2422 (preB[H246G] pmrC-lacZ), JSG420 (pmrA pmrC-lacZ), JSG2366 (ΔpreB pmrA::Tn10d pmrC-lacZ), JSG2499 (pmrA[D51A] pmrC-lacZ), JSG2498 (preB pmrA[D51A] pmrC-lacZ), JSG1060 (phoP pmrC-lacZ), and JSG2365 (ΔpreB phoP pmrC-lacZ). WT, wild type.
As additional confirmation of the involvement of PreA in pmrC regulation, we analyzed the ΔpreAB::cat pmrC::MudJ strain with an inducible plasmid-borne preA. Ectopic expression of preA led to a more-than-twofold increase of β-galactosidase levels, demonstrating that PreA can activate, directly or indirectly, the pmrCAB operon (Fig. 3B). The increase of activity by ectopic expression of preA was also statistically significant in the ΔpreB background (P = 0.008) but less than twofold in magnitude, likely because the chromosomal copy of preA+ was also expressed (Fig. 3B). In support of the general paradigm that PreA requires phosphorylation for activating its target genes, a preA[D51A] allele ectopically expressed in pBAD18 failed to activate pmrCAB in all the backgrounds tested, in spite of the production of a polypeptide of the expected molecular weight detected using a polyclonal antibody raised against a His6-PreA protein (Fig. 3C). Consistent with the hypothesis that PreB is acting as a phosphatase during growth in LB medium, upregulation of pmrCAB by ectopic preA was not observed in preB+ backgrounds (wild type and the preA mutant) (Fig. 3B). Gel shift assays using unphosphorylated or chemically phosphorylated PreA failed to show binding of this protein to the pmrAB regulatory region (4), implying indirect regulation of pmrCAB by PreA.
Regulation of pmrCAB transcription is independent of PmrA and PhoP.
PmrA is the only known direct activator of pmrCAB transcription. PmrA is directly activated by the PmrB kinase and indirectly activated by PhoP/PhoQ via the small protein PmrD, which stabilizes the phosphorylated form of PmrA (22). A model that explains the apparent repressing effect of PreB on pmrCAB transcription might have involved interference of the PreB sensor with PhoP/PhoQ activity. When phoP was disrupted, transcription of pmrCAB was greatly decreased (Fig. 4) as expected due to the decreased concentration of cellular PmrD. However, when the phoP null mutation was introduced into a preB background, the transcriptional activity of pmrCAB still increased by 7-fold compared to strain JSG215 pmrC::MudJ (Fig. 4) and by ca. 100-fold compared to strain JSG1060 phoP pmrC::MudJ. This result indicates that the effect of a PreB deletion on pmrCAB transcription was independent of the PhoQ/PhoP/PmrD proteins.
An alternative model of PreB-mediated regulation of pmrCAB could involve PmrA/PmrB, known direct regulators of pmrCAB. To address this, we introduced a missense substitution to alanine in the putative phosphorylation site of PmrA at residue D51, and the resulting allele was exchanged into a pmrC::MudJ reporter strain. Under pmrCAB-inducing conditions, the pmrA[D51A] allele resulted in decreased pmrCAB expression, but the addition of a null mutation in preB again strongly upregulated the pmrC::MudJ reporter fusion (JSG2499 versus JSG2498) (Fig. 4). A similar result was obtained by using a pmrA null mutation (JSG420 versus JSG2366) (Fig. 4). Collectively, these results demonstrate that the PreA/PreB-mediated regulation of pmrCAB is independent of PmrA and PhoP.
PreB does not affect PmrA-regulated genes involved in LPS modifications.
Increases in the transcription levels of pmrCAB could be expected to upregulate PmrA-dependent loci. The PmrA-regulated loci pmrE and pmrHFIJKLM encode proteins necessary for LPS modifications leading to PMB resistance. Toexamine if these loci were affected by the loss of preB, β-galactosidase activities in JSG1040 (preB pmrE::MudJ) and JSG1058 (preB pmrI::MudJ) were measured and compared to those of reporter strains with a wild-type regulatory background (JSG214 and JSG1051). The activity of both fusions was unaffected by the loss of preB (Fig. 5). Consistent with the transcriptional data, MIC assays for PMB resistance indicated that the preB mutant showed equal levels of resistance (2 μg/ml) to the wild-type strain. Interestingly, when we examined the effect of preB on another PmrA-regulated gene of unknown function, yibD (strain JSG1525 versus strain JSG1527) (Fig. 5), we observed a ninefold upregulation of its expression in a preB background, similar to what was seen with pmrCAB. Also, as observed with pmrCAB, the upregulation of yibD in a preB mutant was largely independent of PmrA, and the loss of preA had little impact on yibD expression (Fig. 5). Although not all PmrA-regulated genes have been examined, PreB has only been shown to affect yibD and pmrCAB transcription thus far.
FIG. 5.
Effect of preB mutations on the expression of pmrA regulon promoters. Salmonella strains were grown in LB medium, and β-galactosidase assays were performed as described in Materials and Methods. Activities are expressed in Miller units. Error bars indicate the standard deviations. Data are from a representative experiment with two replicates. The following strains were used: JSG1051 (pmrHFI-lacZ), JSG1058 (preB pmrI-lacZ), JSG214 (pmrE-lacZ), JSG1040 (preB pmrE-lacZ), JSG1525 (yibD-lacZ), JSG1527 (preB yibD-lacZ), JSG2527 (ΔpreB pmrA yibD::lacZ), JSG2420 (ΔpreA yibD-lacZ), and JSG2523 (ΔpreA pmrA yibD-lacZ).
DISCUSSION
In this study, we describe an uncharacterized TCS of Salmonella enterica serovar Typhimurium, termed PreA/PreB, that activates transcription of the pmrCAB operon and of another PmrA-regulated gene, yibD. The pmrAB TCS is critical for the activation of a large regulon involved in polymyxin B resistance. In a Tn10d transposon screen for genes having an effect on pmrC transcription, we isolated an insertion in the PreB sensor kinase. Based on sequence similarity and map position, the PreA/PreB TCS is homologous to the previously identified E. coli QseB/QseC TCS (37). This E. coli TCS was shown to be responsive to luxS-dependent quorum-sensing signals for regulating flagellar gene expression. PreA/PreB and QseB/QseC behave as potential orthologs based on the successful complementation of preB null mutants by the E. coli TCS. In spite of functional complementation, in experiments not reported in this paper, we show that their regulons are not overlapping and that the biological role of this TCS in the two organisms may be different. The transcriptional regulation of preAB, its genome-wide regulatory effects, and its role in virulence will be described in a separate manuscript.
Initially, the regulation of pmrCAB by PreA/PreB appeared to be unusual in that the sensor kinase, rather than the response regulator, seemed responsible for repressing its transcription during growth in LB medium. Typically, upon phosphorylation, the response regulator activates or represses gene transcription in response to signals perceived by its cognate sensor kinase. Therefore, null mutations in the response regulator usually have the same phenotype of mutations in the sensor kinase. This is not the case for preA and preB. There are a few examples of TCS pairs cross talking with each other and leading to a bifurcation of regulatory pathways. For instance, the Salmonella enterica serovar Typhimurium CpxA sensor was shown to indirectly activate hilA, an AraC-like transcription factor regulating SPI-1, at the transcriptional level in a CpxR response regulator-independent manner (32). Another example of cross talk between response regulators and kinases of different cognate pairs is found in E. coli, where the CreC sensor kinase regulates the PhoB-PhoR system (47). Subsequent experiments using preAB double mutants and ectopic expression of preA led us to formulate a different model whereby PreA acts as an activator of pmrCAB, likely in an indirect fashion. Furthermore, the observation that neither a preA nor a preAB mutation affects pmrCAB expression, while preB mutations do, suggests that under the growth conditions used, the PreA/PreB system is not perceiving a signal able to stimulate the kinase activity of the sensor. Concurrently, we must hypothesize that the absence of the cognate sensor kinase leads to a constitutive activation of PreA by cross talk. Based on the data collected with the ectopic expression of preA[D51A], the activation of wild-type PreA occurs by phosphorylation at residue D51, following a common TCS paradigm. Point mutations abolishing only the putative phosphorylation site of PreB showed phenotypes similar to those of preA and preAB mutations, presumably because its phosphatase activity is unaffected, and/or the PreB[H246G] protein complexes PreA, protecting it from cross talk.
Because the only two known activators of pmrCAB are PmrA, by autoregulation (14, 36), and PhoP, via the PmrD small protein (23), we tested both regulatory systems for their genetic interactions with the preB mutation. We found that functional null mutations in pmrA (both deletions and missense mutations) or phoP did not affect the observed upregulation of pmrC in the preB background. This suggests that PreA/PreB does not act through the two known regulatory pathways controlling pmrCAB.
Given that preB null mutations lead to the upregulation of pmrCAB transcription, one would expect that the concomitant increased levels of PmrA and PmrB proteins may result in the upregulation of genes in the PmrA regulon. This was not the case, because of several genes tested, only pmrCAB and yibD, an open reading frame of unknown function regulated by PmrA (41), were upregulated in a preB background, and the polymyxin resistance phenotype was not altered compared to that of the wild type. Perhaps PreA/PreB potentiates the PmrA/PmrB system by increasing the levels of these proteins to allow a quicker response upon encountering PmrA/PmrB-activating conditions. It is not clear why yibD is the only PmrA-regulated gene to also be regulated by PreA/PreB.
Our data imply that the signal activating PreA/PreB, not yet identified, is absent during growth in LB medium to exponential-early stationary phase (Fig. 6). The analysis of the genome-wide regulon controlled by this novel TCS may help to build a new hypothesis concerning its function in vivo and the signals modulating its activation in various niches.
FIG. 6.
Working model for PreA/PreB regulation of pmrCAB. (A) The genes pmrCAB and yibD are part of the PmrA regulon. Extracellular signals that affect the PreB sensor are unknown, but growth in LB medium activates its phosphatase activity, maintaining the PreA response regulator in the unphosphorylated conformation. In a PreB[H246G] background, PreA is also inactivated by the phosphatase activity of the mutant protein. (B) Under PreA/PreB-inducing conditions not yet identified, PreA indirectly regulates pmrCAB and yibD. (C) Ectopic PreA, but not PreA[D51A], can activate pmrCAB in a ΔpreB mutant even when grown in LB medium, presumably because of signal-independent cellular cross talk, which may become unmasked and more important due to the absence of the cognate sensor protein.
Acknowledgments
This work was funded by a grant from the National Institutes of Health to J.S.G. (grant AI43521).
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology, vol. 1. John Wiley & Sons, New York, N.Y.
- 2.Bijlsma, J. J., and E. A. Groisman. 2005. The PhoP/PhoQ system controls the intramacrophage type three secretion system of Salmonella enterica. Mol. Microbiol. 57:85-96. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 4.Carroll-Portillo, A. 2004. Ph.D. thesis. University of Texas Health Science Center at San Antonio, San Antonio, Tex.
- 5.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garmendia, J., C. R. Beuzon, J. Ruiz-Albert, and D. W. Holden. 2003. The roles of SsrA-SsrB and OmpR-EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology 149:2385-2396. [DOI] [PubMed] [Google Scholar]
- 10.Groisman, E. A., E. Chiao, C. J. Lipps, and F. Heffron. 1989. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. USA 86:7077-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groisman, E. A., J. Kayser, and F. C. Soncini. 1997. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J. Bacteriol. 179:7040-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunn, J. S., W. J. Belden, and S. I. Miller. 1998. Identification of PhoP-PhoQ activated genes within a duplicated region of the Salmonella typhimurium chromosome. Microb. Pathog. 25:77-90. [DOI] [PubMed] [Google Scholar]
- 13.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 14.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 17.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harwig, S. S., T. Ganz, and R. I. Lehrer. 1994. Neutrophil defensins: purification, characterization, and antimicrobial testing. Methods Enzymol. 236:160-172. [DOI] [PubMed] [Google Scholar]
- 19.Hoch, J., and T. Silhavy. 1995. Two-component signal transduction. ASM Press, Washington, D.C.
- 20.Hughes, K. T., and J. R. Roth. 1985. Directed formation of deletions and duplications using Mud(Ap, lac). Genetics 109:263-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston, C., D. A. Pegues, C. J. Hueck, A. Lee, and S. I. Miller. 1996. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol. Microbiol. 22:715-727. [DOI] [PubMed] [Google Scholar]
- 22.Kato, A., and E. A. Groisman. 2004. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 18:2302-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kox, L. F., M. M. Wosten, and E. A. Groisman. 2000. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 19:1861-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:1872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchal, K., S. De Keersmaecker, P. Monsieurs, N. van Boxel, K. Lemmens, G. Thijs, J. Vanderleyden, and B. De Moor. 2004. In silico identification and experimental validation of PmrAB targets in Salmonella typhimurium by regulatory motif detection. Genome Biol. 5:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchler-Bauer, A., and S. H. Bryant. 2004. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 32:W327-W331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGinnis, S., and T. L. Madden. 2004. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 32:W20-W25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZa for cloning, mutagenesis and allele replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 29.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouslim, C., and E. A. Groisman. 2003. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol. Microbiol. 47:335-344. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama, S., A. Kushiro, T. Asahara, R. Tanaka, L. Hu, D. J. Kopecko, and H. Watanabe. 2003. Activation of hilA expression at low pH requires the signal sensor CpxA, but not the cognate response regulator CpxR, in Salmonella enterica serovar Typhimurium. Microbiology 149:2809-2817. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberger, C. M., R. L. Gallo, and B. B. Finlay. 2004. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 101:2422-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid, M. B., and J. R. Roth. 1983. Genetic methods for analysis and manipulation of inversion mutations in bacteria. Genetics 105:517-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skorupski, K., and R. K. Taylor. 1996. Positive selection vectors for allelic exchange. Gene 169:47-52. [DOI] [PubMed] [Google Scholar]
- 36.Soncini, F. C., and E. A. Groisman. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796-6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 40.Tamayo, R., A. M. Prouty, and J. S. Gunn. 2005. Identification and functional analysis of Salmonella enterica serovar Typhimurium PmrA-regulated genes. FEMS Immunol. Med. Microbiol. 43:249-258. [DOI] [PubMed] [Google Scholar]
- 41.Tamayo, R., S. S. Ryan, A. J. McCoy, and J. S. Gunn. 2002. Identification and genetic characterization of PmrA-regulated genes and genes involved in polymyxin B resistance in Salmonella enterica serovar Typhimurium. Infect. Immun. 70:6770-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor, R. K., C. Manoil, and J. J. Mekalanos. 1989. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J. Bacteriol. 171:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teplitski, M., R. I. Goodier, and B. M. Ahmer. 2003. Pathways leading from BarA/SirA to motility and virulence gene expression in Salmonella. J. Bacteriol. 185:7257-7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 47.Wanner, B. L. 1992. Is cross regulation by phosphorylation of two-component response regulator proteins important in bacteria? J. Bacteriol. 174:2053-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]