Abstract
A conditional-lethal mutant was isolated as having a flagellar regulatory phenotype at 30°C and being unable to grow at 42°C. Chromosomal mapping localized the mutation to the serT gene, which encodes an essential serine tRNA species (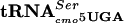 ). DNA sequence analysis revealed the mutation to be a single base change in G:A at position 10 of the serT gene that lies within the D-stem of the essential
). DNA sequence analysis revealed the mutation to be a single base change in G:A at position 10 of the serT gene that lies within the D-stem of the essential 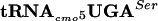 species.
species. 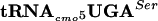 recognizes UCA, UCG, and UCU codons, but UCU is also recognized by
recognizes UCA, UCG, and UCU codons, but UCU is also recognized by 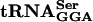 and UCG by
and UCG by 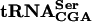 . No other tRNAs are known to read the UCA codon. Thus, the UCA codon is specifically recognized by
. No other tRNAs are known to read the UCA codon. Thus, the UCA codon is specifically recognized by 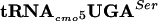 . We show that the anti-σ28 activity of FlgM is defective in the serT mutant strain. The serT allele causes a 10-fold increase in σ28-dependent fliC promoter transcription, indicating a defect in FlgM anti-σ28 activity in the presence of the serT mutation. The flgM gene contains only one UCA codon. Changing the UCA of flgM to ACG reversed the effect of the serT allele. Implications for context effects in regulation of gene expression are discussed.
. We show that the anti-σ28 activity of FlgM is defective in the serT mutant strain. The serT allele causes a 10-fold increase in σ28-dependent fliC promoter transcription, indicating a defect in FlgM anti-σ28 activity in the presence of the serT mutation. The flgM gene contains only one UCA codon. Changing the UCA of flgM to ACG reversed the effect of the serT allele. Implications for context effects in regulation of gene expression are discussed.
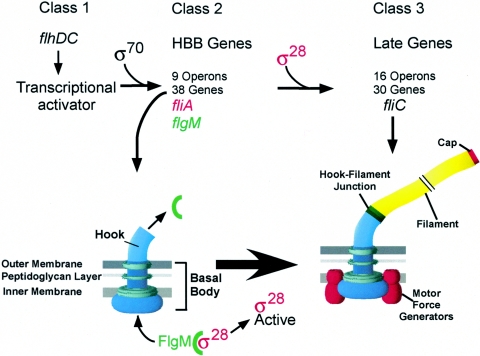
The flagellar regulon of Salmonella enterica serovar Typhimurium includes over 60 genes (reviewed in references 11 and 36). The transcripts of the flagellar regulon are organized into a transcriptional hierarchy based on three promoter classes that are temporally regulated in response to assembly (28) (Fig. 1). At the top of this hierarchy lies the flagellar master operon, flhDC, where the fundamental decision to produce flagella is controlled. The flhDC operon is expressed from what is defined as the class 1 promoter (58). The FlhD and FlhC proteins form a heteromultimeric complex (FlhD2C2) that acts as a transcriptional activator to promote σ70-dependent transcription from the class 2 flagellar promoters (34, 35). The class 2 promoters direct transcription of genes needed for the structure and assembly of the flagellar motor structure, also known as the hook-basal body (HBB). Upon HBB completion, class 3 promoters are transcribed by σ28-RNA polymerase, which is specific for flagellar class 3 promoters. Prior to HBB completion, σ28-RNA polymerase is inhibited by the anti-σ28 factor, FlgM. Upon HBB completion, FlgM is secreted from the cell, presumably through the completed HBB structure, and σ28-dependent transcription ensues. In this way, genes whose products are needed only after HBB formation, such as the flagellin filament genes, are transcribed only when there is a functional motor onto which they may be assembled.
FIG. 1.
The coupling of flagellar gene regulation to flagellum assembly. The gene products of the flhDC operon activate σ70-dependent transcription from flagellar class 2 promoters, which include everything that is needed for the structure and assembly of the HBB, the ion-powered flagellar rotary motor that transverses from the cytoplasm through the membranes and cell wall to the outside of the cell. Also transcribed from a class 2 promoter is the fliA gene, which encodes the alternative σ transcription factor, σ28. Transcription of class 3 promoters by σ28-RNA polymerase produces all the gene products needed after the HBB motor is constructed. The genes expressed from class 3 promoters include everything that is needed late in the assembly process: flagellin, motor force generators, and a brain, known as the chemosensory system, which allows the flagellum to direct movement of the bacterium up a chemical gradient. The anti-σ28 factor FlgM couples class 3 promoter transcription to HBB completion. Upon HBB completion, the secretion specificity of the flagellar type III secretion system changes from hook/rod-type substrates to late substrates. FlgM, a late secretion substrate, is secreted, and σ28 is free, timed to HBB completion, to transcribe the late flagellar genes and complete flagellum assembly and the chemosensory network.
FlgM secretion does not occur in strains defective in HBB formation (21, 22). FlgM secretion also can be prevented in strains with a functional HBB, either by mutation in the FlgM secretion signal (12) or by fusion of FlgM to a bulky protein such as β-galactosidase (LacZ) that retains anti-σ28 activity (25). A fusion of LacZ to the N-terminal region of FlgM is nonmotile, presumably because the fusion fails to be secreted, resulting in constitutive inhibition of σ28 activity (25). Motile revertants in strains expressing the FlgM-LacZ fusion were isolated and found to be specifically defective in FlgM-σ28 interactions (9, 25). We decided to use this positive selection in a screen for conditional-lethal mutants specific to FlgM-σ28 interactions in an attempt to identify any essential genes that might be involved in the regulation of σ28-dependent transcription. Our definition of a conditional-lethal mutant is one that grows at 30°C, but not at 42°C, on rich (LB) medium. This resulted in the isolation in a mutant with a single nucleotide substitution in the 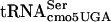 that is defective in FlgM inhibition of σ28-dependent transcription at 30°C and lethal at 42°C.
that is defective in FlgM inhibition of σ28-dependent transcription at 30°C and lethal at 42°C.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains used in this study and their origins are listed in Table 1. Mud vectors were used for lac operon and lacZ gene fusion constructions (24).
TABLE 1.
List of bacterial strains
| Salmonella serovar Typhimurium strain | Genotype | Sourcea |
|---|---|---|
| E12A2 | pipB::Tc-lux | B. Finlay |
| LT2 | Wild type | J. Roth |
| TH2430 | ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH2893 | ΔflgG-L2157 fliC5050::MudJ fliA5059::Tn10dTc fljBenx vh2 | |
| TH3933 | motA5461::MudJ | |
| TH3227 | ataA::[P22 sieA44 Kn-9 PfliC(−435→+6)-cat 9+] | |
| TH3228 | ataA::[P22 sieA44 Kn-9 PfliC(−435→+6)-cat 9+] flgM5208::MudB | |
| TH3238 | flu-5398(serT) ataA::[P22 sieA44 Kn-9 PfliC(−435→+6)-cat 9+] flgM5208::MudB | |
| TH6652 | hpaB::Tn10dTc | |
| TH6654 | STM1081::Tn10dCm | |
| TH7012 | hpaB::Tn10dTc fla-5398(serT) | |
| TH7368 | yccW::Tn10dTc | |
| TH7953 | pKD46/flgM6085::tetRA(start) | V. Rosu |
| TH8191 | pKD46/flgM6101::tetRA(stop) | V. Rosu |
| TH9390 | flgM6421(S7, UCA:ACG) | |
| TH9392 | flgN6423(S5, UCA:ACG) | |
| TH9515 | flgM6421(S7, UCA:ACG) flgN6430(S5, UCA:ACG) | |
| TH9524 | flgM6421 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9525 | flgM6423 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9526 | flgM6421 flgN6430 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9623 | hpaB::Tn10dTc fla-5398(serT) flgM6421 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9624 | hpaB::Tn10dTc fla-5398(serT) flgN6423 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9625 | hpaB::Tn10dTc fla-5398(serT) flgM6421 flgN6430 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9626 | hpaB::Tn10dTc flgM6421 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9627 | hpaB::Tn10dTc flgN6423 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9628 | hpaB::Tn10dTc flgM6421 flgN6430 ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9784 | hpaB::Tn10dTc ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9785 | hpaB::Tn10dTc fla-5398(serT) ΔflgG-L2157 fliC5050::MudJ fljBenx vh2 | |
| TH9786 | motA5461::MudJ flgM6421 | |
| TH9787 | motA5461::MudJ flgN6423 | |
| TH9788 | motA5461::MudJ flgM6421 flgN6430 | |
| TT10423 | ΔproAB47/F′ pro+ lac+ zzf-1831::Tn10dTc | J. Roth |
| TT10604 | pNK972 (Apr Tn10 transposase)/LT2 | J. Roth |
| TT10427 | ΔproAB47/F′ pro+ lac+ zzf-1836::Tn10dCm | J. Roth |
Unless indicated otherwise, all strains were constructed during the course of this work.
Media and standard genetic manipulations.
Media, growth conditions, transductional methods, and motility assays were as described previously (21, 22). The generalized transducing phage of Salmonella serovar Typhimurium, P22 HT105/1 int-201, was used in all transductional crosses (48).
Mapping regulatory mutants to flgM and fliA.
Mutants that allowed PfliC-cat expression in the presence of an FlgM-LacZ fusion (flgM5208::MudB) were isolated by their ability to grow on L medium with 12.5 μg/ml chloramphenicol (Cm) (see Results). Phage P22 was grown on the Cmr revertants of strain TH3228 (FlgM-LacZ PfliC-cat) and used to check for linkage of the mutation to the flgM-lacZ fusion or the σ28 structural gene fliA. For linkage to the flgM region, the phage lysates were used to transduce strain TH3227 that carries the PfliC-cat construct to MudB-encoded Apr and screened for resistance or sensitivity to chloramphenicol. Any mutation in the flgM-lacZ fusion will show 100% linkage to Cmr. For linkage to the fliA region, phage lysates were used to transduce strain TH2893 (ΔflgG-L2157 [HBB−], fliC5050::MudJ [PfliC-lac], fliA5059::Tn10dTc [σ28 defective]) to Lac+. Since TH2893 carries an insertion in the fliA gene, the fliC-lac transcriptional fusion (fliC5050::MudJ) cannot be expressed. However, transduction to fliA+ will not result in a Lac+ phenotype because the strain carries an HBB mutation (ΔflgG-L2157), so FlgM is not secreted. However, if the donor lysate carries an FlgM bypass mutation in fliA, then this lysate will transduce TH2893 to growth on minimal lactose at high efficiency (9, 32).
Isolation of Tn10dTc and Tn10dCm insertions near the serT gene.
P22 was grown separately on strains TT10423 and TT10604, which carry the Tn10dTc and Tn10dCm insertions, respectively, in the F plasmid and used to transduce strain TT10427 to either tetracycline (Tc) or chloramphenicol resistance, respectively. TT10427 carries plasmid pNK972, which constitutively expresses Tn10 transposase. Because there is no F plasmid in strain TT10427, it is not possible to inherit the Th10dTc and Tn10dCm transposons by homologous recombination. The Tcr and Cmr transductants are inherited by transposition into the recipient chromosome. More than 50,000 Tcr or Cmr transductants were pooled together, and P22 lysates were prepared on the pooled cells carrying insertions located at positions throughout the chromosome. In order to isolate Tn10dTc and Tn10dCm insertions in the serT region, the pooled Tn10dTc lysate was used to transduce TH3238 (serT [42°C-lethal]) to Tcr on L-Tc plates, and the colonies were screened for growth at 42°C (serT+). Two Tn10dTc insertions that were 50% (hpaB) and 82% (yccW) linked to the serT temperature-sensitive (ts) phenotype were isolated. A P22 lysate grown on the pooled Tn10dCm insertions was used to transduce the strain carrying the 82%-linked (yccW) Tn10dTc insertion to Cmr, which was followed by screening for loss of the Tn10dTc allele (Tcs). By chance, one Tn10dCm insertion (STM1081) was found to be 90% linked to this Tn10. PCR amplification of a strain carrying both closely linked insertions with a primer that would recognize the ends of Tn10dTc and Tn10dCm gave a product which, after DNA sequence analysis, revealed the Tn10dTc insertion to be in the yccW locus and the Tn10dCm insertion to be in the STM1081 locus. The other Tn10dTc insertion was subject to semirandom PCR (42), and the sequenced product revealed it to reside in the hpaB gene. The STM1081::Tn10dCm insertion was 78% linked to serT and 35% linked to hpaB by P22 transduction.
Site-directed mutagenesis.
The UCA codons in flgM and flgN were changed to AGC codons by site-directed mutagenesis using the bacteriophage lambda recombinase system (λ-Red) of Datsenko and Wanner (15). Insertion of the tetA and tetR genes (tetRA) from transposon Tn10 were previously placed at the 5′ (TH7953) and 3′ (TH8191) ends of the flgM gene (V. Rosu and K. T. Hughes, unpublished). Large primers (Integrated DNA Technologies) that contained the TCA-to-AGC substitutions in flgM and flgN were purchased. For FlgM, primer FlgM-AGC7mut (5′-AGCTGGCCGCTACAACGTAACCCTCGATGAGGATAAATAAATGAGCATTGACCGTACCAGCCCTTTGAAACCCGTTAGCACTGTCCAGACGCGCGAAACCA-3′) was made double stranded by hybridization with primer FlgM-AGC7Rev (5′-TGGTTTCGCGCGTCTGGA-3′), which was followed by fill-in reaction with ThermalAce (Invitrogen) according to the manufacturer's instructions and purification by ethanol precipitation. For FlgN, primer FlgN-AGC5mut (5′-CTCGCTCATTCGCGAGGCGCAGAGCTACTTACAGAGTAAATAAGCGTATGACTCGTTTGAGCGAAATACTTGACCAGATGACCACCGTCCTGAATGACCTGA-3′) was made double stranded by hybridization with primer FlgN-AGC5mutRev (5′-TTCAGGTCA TTCAGGACG-3′), which was followed by fill-in reaction with ThermalAce (Invitrogen) according to the manufacturer's instructions and purification by ethanol precipitation. For each recombination reaction, 1 μg of filled-in FlgM and FlgN DNA products were electroporated into electrocompetent TH7953 and TH8191, respectively, that were grown in L medium containing ampicillin (Ap) and arabinose (added 1 h prior to harvesting). Selection was made on Tcs plates (37). Mutational changes were verified by DNA sequence analysis.
β-Galactosidase assays.
β-Galactosidase assays were performed as described by Maloy (37). Cells were grown at 37°C until mid-log phase. Assays were performed in triplicate, and the values are reported as activity units in nmol/min/optical density at 650 nm/ml.
RESULTS
Isolation of mutants defective in FlgM-LacZ inhibition of fliC promoter transcription.
A P22 phage that constitutively expresses kanamycin resistance (Kmr) and carries the chloramphenicol acetyltransferase (cat) gene expressed from the fliC promoter (PfliC-cat) was constructed. When lysogenized into the wild-type Salmonella serovar Typhimurium strain LT2 by selection for Kmr, the cells become Cmr, due to σ28-dependent transcription of PfliC-cat. We have previously described the isolation of lacZ translational fusions to the flgM gene using a Mud-lac translational fusion vector that result in the expression of FlgM-LacZ fusion proteins (23). One allele, flgM5208::MudB, results in a fusion of the first 86 amino acids of the 97-amino-acid FlgM protein to LacZ and retains the anti-σ28 activity of FlgM (25). When the flgM5208::MudB insertion is introduced into wild-type strain LT2 or in a strain that retains a wild-type copy of the flgM gene, the cells become nonmotile, presumably because the FlgM-LacZ fusion cannot be secreted. Thus, the dominant anti-σ28 activity of the FlgM-LacZ fusion is not coupled to HBB completion, and inhibition of σ28 by FlgM-LacZ is constitutive, resulting in loss of motility. Another possibility was that the FlgM-LacZ fusion blocks flagellar secretion by itself, getting stuck in the flagellar secretion machinery during the secretion process. This was shown not to be the case (see below).
Motile revertants of strains carrying the FlgM-LacZ fusion that remained LacZ+ were isolated (25). Two of these were mutants with single-amino-acid-substitutions in FlgM, I58L and L66S. These were later shown to be specifically defective in interactions with σ28 (9, 10, 16). Other motile revertants in strain LT2 expressing the FlgM-LacZ fusion were mutants with single amino acid substitutions in σ28 that were defective in interactions with FlgM (9, 10, 16). The fact that mutants specific to FlgM-σ28 interactions resulted in motility in the presence of the FlgM-LacZ fusion and that intact FlgM with these substitutions is secreted normally argues that the FlgM-LacZ fusion does not block the flagellar secretion apparatus.
We decided to exhaustively search for mutants defective in FlgM-σ28 interactions in an attempt to uncover any essential genes that might be involved in this flagellar regulatory process. Strain TH3228 was constructed for this purpose. It harbors a bacteriophage P22 lysogen with a PfliC-cat construct and the dominant FlgM-LacZ fusion (flgM5208::MudB). Strain TH3228 is Cms due to FlgM-LacZ inhibition of σ28-dependent transcription of PfliC-cat. If either the I58L or the L66S substitution is introduced into the FlgM-LacZ fusion construct, the cells express PfliC-cat and are Cmr. One hundred fifty independent cultures of TH3228 were plated on LB medium containing chloramphenicol (12.5 μg/ml) and incubated at 30°C. After overnight incubation, Cmr colonies appeared, and the plates were replica printed to two LB-Cm plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 80 μg/ml) and incubated separately at 30°C and 42°C. From each of the initial 20 independent cultures, three Cmr colonies were chosen; one Lac+ (X-Gal; blue), one Lac+/− (X-Gal; light blue), and one Lac− (X-Gal; white) colony were isolated and checked for linkage to the flgM and fliA loci (see Materials and Methods). The 40 light-blue and white Cmr mutants were all linked to the flgM locus and are presumed defective in the expression of the FlgM-LacZ fusion. Of the 20 independent Lac+ (X-Gal; blue) Cmr mutants isolated, 8 were linked to the σ28 structural gene (fliA), 10 were linked to flgM, and 2 carried mutations in both the flgM and fliA loci. For the remaining 130 independent cultures tested, only Lac+ colonies were screened for linkage; 62 were linked to flgM, 46 were linked to fliA, and 22 were linked to both flgM and fliA. From the 150 independent cultures screened, only 12 yielded mutants that grew on Cm at 30°C but not at 42°C. One Cmr ts mutant mapped to flgM, and one mapped to fliA. The remaining 10 were not linked to flgM or fliA. Five mapped to the flg operon downstream of flgM, presumably in HBB structural genes or the flg-linked RNase E gene (rne), but were not further characterized. We tested if any were temperature sensitive on LB medium in the absence of chloramphenicol as potential mutants with changes in essential genes, and only one, the fla-5398 mutant, was found to be unable to grow on LB medium at 42°C and was characterized further.
Mapping the fla-5398 temperature-sensitive lethal mutation.
A Tn10dTc transposon insertion that was 50% linked by P22 transduction to the fla-5398 ts-lethal phenotype was isolated (see Materials and Methods). DNA sequence analysis revealed the Tn10dTc insertion to be in the hpaB gene (see Materials and Methods). We checked the linkage of the fla-5398 ts-lethal phenotype to other insertions in the vicinity of hpaB. A putA::MudCm allele was 1% linked to fla-5398 by P22 cotransduction; a pipB::Tc insertion was 89% linked, suggesting that the fla-5398 allele was close to the pipB locus. In order to facilitate mapping, an insertion of Tn10dCm that was 78% linked to the fla-5398 allele and 35% linked to the hpaB::Tn10dTc insertion by P22 cotransduction was isolated. DNA sequence analysis revealed this insertion to be in the STM1081 gene. Three-factor cross mapping (not shown) between the Tn10dCm insertion in STM1081, the hpaB::Tn10dTc insertion, and the fla-5398 allele revealed the fla-5398 allele to be located between STM1081 and hpaB.
The fla-5398 allele is a mutation in a serine tRNA gene, serT.
The precise location of the fla-5398 allele was determined by estimation of the distance of fla-5398 from the hpaB::Tn10dTc and STM1081::Tn10dCm insertions using the modified formula of Wu (46, 55). Examination of the annotated sequence of the Salmonella serovar Typhimurium LT2 genome did not reveal an obvious gene that when mutated might account for the phenotype of inhibition of FlgM function at 30°C and lethality at 42°C. It was not until we looked at the actual DNA sequence of the region that we found that a potential essential gene, serT, coding for 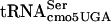 , was located where the fla-5398 allele mapped. We sequenced the serT locus and identified a single nucleotide change where G10 is replaced by A10 in the D-stem. Thus, a base substitution mutation in the serine tRNA,
, was located where the fla-5398 allele mapped. We sequenced the serT locus and identified a single nucleotide change where G10 is replaced by A10 in the D-stem. Thus, a base substitution mutation in the serine tRNA, 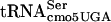 (G10:A10), resulted in a defect in the FlgM-LacZ inhibition of σ28-dependent PfliC-cat transcription at 30°C and lethality at 42°C.
(G10:A10), resulted in a defect in the FlgM-LacZ inhibition of σ28-dependent PfliC-cat transcription at 30°C and lethality at 42°C.
Effect of the 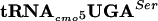 -specific UCA codon on flgM and flgN gene expression.
-specific UCA codon on flgM and flgN gene expression.
The 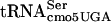 species (SerT) recognizes the UCA, UCG, and UCU codons. However, two of these codons, UCG and UCU, are recognized by alternative serine tRNA species,
species (SerT) recognizes the UCA, UCG, and UCU codons. However, two of these codons, UCG and UCU, are recognized by alternative serine tRNA species, 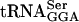 and
and 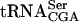 species, respectively. No other tRNA is known to read the UCA codon. Thus, the UCA codon is specifically recognized by
species, respectively. No other tRNA is known to read the UCA codon. Thus, the UCA codon is specifically recognized by 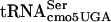 . In FlgM, only one serine residue at amino acid 7 (S7) is coded for by UCA, while there are eight UCA codons in lacZ. The presence of 9 UCA codons in the flgM-lacZ fusion is likely to make the translation of the fusion less efficient in a serT allele background (see Discussion). We moved the fla-5398 (serT) allele into an flgM+ strain defective in HBB formation (ΔflgG-L) that also carries the lac operon expressed from the σ28-dependent fliC promoter (PfliC-lac [fliC5050::MudJ]). In an HBB− (serT+) strain, FlgM is not secreted, and fliC-lac expression is inhibited 100-fold compared to an isogenic HBB+ strain (21). However, if we introduce the serT (fla-5398) allele into the HBB− strain, β-galactosidase activity increases 10-fold at the permissive temperature (30°C) (Table 2; compare TH9784 to TH9785). Thus, even though only one UCA codon is present in flgM without the lacZ fusion, the serT allele results in a defect in FlgM anti-σ28 activity. The fact that the reporter is lacZ and sensitive to the serT mutation (40) suggests that the effect might be even larger if we used a reporter that did not contain UCA codons (see Discussion). The flgM S7 UCA codon was changed to AGC by site-directed mutagenesis (see Materials and Methods). When the S7 UCA codon was changed to the alternative serine codon AGC, the serT (fla-5398) allele no longer prevented FlgM inhibition of σ28-dependent fliC-lac transcription (Table 2; compare TH9623 to TH9785 and TH9784). The change of UCA to AGC did not affect fliC-lac transcription in a serT+ background (Table 2; compare TH9784 to TH9626). In the absence of the serT allele, the AGC codon had no effect on FlgM activity: the strains had the same motility as the wild type, and there was no effect on σ28-dependent transcription (data not shown).
. In FlgM, only one serine residue at amino acid 7 (S7) is coded for by UCA, while there are eight UCA codons in lacZ. The presence of 9 UCA codons in the flgM-lacZ fusion is likely to make the translation of the fusion less efficient in a serT allele background (see Discussion). We moved the fla-5398 (serT) allele into an flgM+ strain defective in HBB formation (ΔflgG-L) that also carries the lac operon expressed from the σ28-dependent fliC promoter (PfliC-lac [fliC5050::MudJ]). In an HBB− (serT+) strain, FlgM is not secreted, and fliC-lac expression is inhibited 100-fold compared to an isogenic HBB+ strain (21). However, if we introduce the serT (fla-5398) allele into the HBB− strain, β-galactosidase activity increases 10-fold at the permissive temperature (30°C) (Table 2; compare TH9784 to TH9785). Thus, even though only one UCA codon is present in flgM without the lacZ fusion, the serT allele results in a defect in FlgM anti-σ28 activity. The fact that the reporter is lacZ and sensitive to the serT mutation (40) suggests that the effect might be even larger if we used a reporter that did not contain UCA codons (see Discussion). The flgM S7 UCA codon was changed to AGC by site-directed mutagenesis (see Materials and Methods). When the S7 UCA codon was changed to the alternative serine codon AGC, the serT (fla-5398) allele no longer prevented FlgM inhibition of σ28-dependent fliC-lac transcription (Table 2; compare TH9623 to TH9785 and TH9784). The change of UCA to AGC did not affect fliC-lac transcription in a serT+ background (Table 2; compare TH9784 to TH9626). In the absence of the serT allele, the AGC codon had no effect on FlgM activity: the strains had the same motility as the wild type, and there was no effect on σ28-dependent transcription (data not shown).
TABLE 2.
Effect of mutant G10:A10-tRNAcmo5UGASer on FlgM anti-σ28 activity
| Strain | Relative genotype or phenotype | β-Galactosidase activitya |
|---|---|---|
| TH9784 | HBB−serT+ | 2.0 ± 0.23 |
| TH9626 | HBB−serT+ FlgM(ACG) | 1.7 ± 0.26 |
| TH9627 | HBB−serT+ FlgN(ACG) | 4.1 ± 0.01 |
| TH9628 | HBB−serT+ FlgM(ACG) FlgN(ACG) | 1.8 ± 0.12 |
| TH9785 | HBB−serT | 23.6 ± 0.91 |
| TH9623 | HBB−serT FlgM(ACG) | 1.3 ± 0.10 |
| TH9624 | HBB−serT FlgN(ACG) | 6.8 ± 0.21 |
| TH9625 | HBB−serT FlgM(ACG) FlgN(ACG) | 1.2 ± 0.11 |
In nmol/min/optical density at 650 nm/ml.
We also examined the effect of the serT (fla-5398) allele on flgN gene activity. Loss of FlgN results in reduced FlgM levels in the cell, leading to a derepression of fliC-lac expression in HBB− strains (27). The flgN gene also has a single UCA codon that codes for serine at amino acid 5 of FlgN (33). We changed this codon to AGC and found that it had a slight effect (twofold) on fliC-lac transcription in a serT+ background (Table 2; compare TH9784 to TH9627) but resulted in restoration of inhibition of fliC-lac transcription, although not to the same degree as the change from UCA to AGC in flgM (Table 2; compare TH9624 to TH9623 and TH9785). The double changes from UCA to AGC in both flgM and flgN had the same effect as just the single change in flgM (Table 2; compare TH9625 to TH9785, TH9623, and TH9624). Thus, the serT (fla-5398) allele can affect both flgM and flgN gene translation.
DISCUSSION
An extensive search was performed with 150 independent cultures in order to look for novel regulatory mutants affecting FlgM inhibition of σ28-dependent transcription of the fliC promoter. The strain in which the selection was set up had an FlgM-LacZ fusion that would inhibit σ28-dependent transcription even in strains with a functional HBB structure, because the fusion of LacZ to FlgM prevents secretion through the HBB structure (25). The selection utilized a fusion of the fliC promoter (PfliC) to the cat gene (PfliC-cat), so that when PfliC was transcribed, the cells became Cmr. The selection was done at 30°C and then coupled to a screen for inability to grow at 42°C. In this way, we hoped to identify previously uncharacterized essential genes that might play a role in FlgM inhibition of σ28-dependent transcription.
The selection yielded some unexpected results. All but one of the Cmr revertants (non-ts) carry mutations that mapped to either the flgM or the fliA region. We expected mutants defective in the clpXP protease genes. It had been shown that loss of ClpPX protease results in a more stable σ28 protein that can overcome FlgM inhibition of σ28-dependent transcription in HBB mutant strains (3, 53). We propose that the addition of LacZ to the C terminus of FlgM enhances the stability of FlgM, resulting in a selection that is specific for mutations that are specific to FlgM-σ28 interactions, such that loss of ClpXP protease does not stabilize σ28 enough to overcome the excess inhibition by the FlgM-LacZ fusion. The fact that 22 of the 150 cultures yielded double mutants carrying mutations that mapped to both the flgM and fliA regions suggests that the selection used (Cmr by PfliC-cat in the presence of FlgM-LacZ) required a significant loss in the anti-σ28 activity of the FlgM-LacZ fusion. However, the mutants were not screened for the presence of mutations in clpXP protease, so it is possible that many of the mutants that had either an flgM-linked or an fliA-linked allele also carried a second mutation in clpXP.
In the screen for the identification of essential genes involved in FlgM regulation of PfliC transcription, one ts-lethal mutant from the 150 independent cultures that prevented FlgM-LacZ inhibition of PfliC-cat transcription at the permissive temperature and failed to grow on LB medium at 42°C was isolated. This mutant had a G10:A10 substitution mutation in the D-stem of an essential tRNA gene, serT (4), which encodes 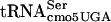 (5). The serT gene is essential, because only
(5). The serT gene is essential, because only 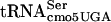 will recognize the UCA codon (26, 49).
will recognize the UCA codon (26, 49).
It was fortuitous that we used PfliC-cat and not PfliC-lac as our reporter with some other nonsecreted FlgM fusion. If we compare strains TH9784 (hpaB::Tn10dTc ΔflgG-L2157 fliC5050::MudJ fljBenx vh2) and TH9785 (TH9784 serT), we find that they are isogenic serT+ and serT strains, respectively. The β-galactosidase assays shown in Table 2 show that TH9784 has 10-fold-higher levels of activity and that the cells are darker blue on X-Gal (not shown). This all fits with the serT mutants having reduced FlgM levels corresponding to increased transcription of PfliC-lac. However, if we compare the strains on MacConkey lactose indicator plates, we find that TH9784 is pink in color after overnight incubation, indicating low levels of lactose utilization, while TH9785 is completely white, indicating no lactose fermentation (not shown). How can this be if TH9785 has 10-fold-higher levels of β-galactosidase? It takes many more genes than just the β-galactosidase gene to ferment lactose to achieve the Lac+ phenotype. It is likely that many genes required to ferment lactose are defective in the presence of the SerT-G10:A10 tRNA, resulting in the Lac− phenotype, and that the expression of only one gene is required for the LacZ+ phenotype. Because lacZ mRNA has 8 UCA codons, the 10-fold increase in β-galactosidase in TH9785 probably represents a lower limit of increased transcription of PfliC-lac due to reduced translation of lacZ mRNA in the presence of the SerT-G10:A10 tRNA.
The textbook picture of a tRNA is the secondary cloverleaf structure: an amino acid acceptor stem on top; an anticodon stem and loop on the bottom; a left arm known as the D-stem and D-loop, due to the presence of many dihydrouridine modifications in the D-loop; and a right arm known as the TψC stem and TψC loop, due to the presence of a conserved pseudouridine (ψ) residue (5). When folded into the tertiary structure, the D-stem and D-loop become fused with the anticodon stem and loop (29, 43). The base substitution of A for G would result in the loss of a G:C base pair in the D-stem that might allow a conformational change in the tRNA tertiary structure at 42°C. Such a conformational change probably does not result in a nonfunctional tRNA, because several proteins containing UCA codon products are normally expressed in an Escherichia coli serT mutant strain with the identical temperature-sensitive lethal G10:A10 allele (1).
The identical (G10:A10) substitution mutation in the serT gene had been isolated previously in Escherichia coli and named divE42 because it affected cell cycle-dependent enzymes (39). When shifted to 42°C, the cells grew until they doubled in volume, but when shifted down to low temperature, they began to divide synchronously. The mutant also exhibited low β-galactosidase levels at the nonpermissive temperature (42°C) (1, 40). Synthesis of a number of cell cycle-dependent proteins was decreased significantly in the divE42 mutant at 42°C (47). DNA sequence analysis revealed divE42 to be a mutation in 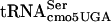 , and it was renamed serT (50). The divE42 allele could be complemented in trans by a wild-type copy of the serT+ gene expressed from an F′ plasmid, indicating that it was a recessive allele (47). Remarkably, a pBR322-derived plasmid expressing the divE42 allele could also complement the chromosomal divE42 allele for growth at 42°C, implying that stability or a concentration-dependent interaction required for translation of essential genes is lost at 42°C (57). Furthermore, the E. coli divE42 allele is more labile at 44°C than at 42°C, suggesting that the mutant phenotype shows a gradient effect with temperature (1). Presumably, the translation of essential genes is reduced at elevated temperatures to the degree that the cell can no longer grow. It was fortuitous that the Salmonella serT mutant was defective in flgM translation at 30°C, since this enabled it to be picked up in our mutant screen.
, and it was renamed serT (50). The divE42 allele could be complemented in trans by a wild-type copy of the serT+ gene expressed from an F′ plasmid, indicating that it was a recessive allele (47). Remarkably, a pBR322-derived plasmid expressing the divE42 allele could also complement the chromosomal divE42 allele for growth at 42°C, implying that stability or a concentration-dependent interaction required for translation of essential genes is lost at 42°C (57). Furthermore, the E. coli divE42 allele is more labile at 44°C than at 42°C, suggesting that the mutant phenotype shows a gradient effect with temperature (1). Presumably, the translation of essential genes is reduced at elevated temperatures to the degree that the cell can no longer grow. It was fortuitous that the Salmonella serT mutant was defective in flgM translation at 30°C, since this enabled it to be picked up in our mutant screen.
In Salmonella serovar Typhimurium, serT was identified by DNA sequence analysis as flanking Salmonella pathogenicity island 5 (54). The serT gene is located basically at the same positions in both the E. coli and Salmonella serovar Typhimurium chromosomes. Further studies with the divE42 allele from E. coli suggested that many proteins with serine residues expressed from UCA codons are made at normal levels in the presence of the divE42 allele, suggesting that the context of translation may be critical to the effect of divE42 on protein expression. The temperature-sensitive phenotype of the divE42 mutant could be suppressed by a double mutation in rne (RNase E) and pnp (polynucleotide phosphorylase) (31), members of the mRNA degradosome (8). The lacZ mRNA, containing eight UCA codons, is unstable in the divE42 mutant, but stability is restored in a strain that also carries the rne-1 and pnp-7 mutations (1). This suggests that a defect in translation of UCA by the G10:A10 substitution in 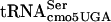 could lead to mRNA instability.
could lead to mRNA instability.
Other suppressors of the temperature-sensitive growth of the E. coli divE42 allele were isolated. Intragenic suppressors include C25:U25, which can now form a base pair with A10, and G67:A67, which forms a base pair with the acceptor stem (41). One unusual mutant, resulting from a single amino acid substitution of amino acid 275 of trigger factor (TF), actually reversed the phenotype: the tig divE42 double mutant grew at 42°C but not at 30°C, and the growth defect at 30°C could not be complemented by divE+ but could be complemented by tig+ (38). TF is a ribosome-associated chaperone that associates with the DnaK protein-folding system to catalyze proper folding of newly synthesized cytosolic proteins. TF is nonessential except in dnaK mutants (17, 52). TF consists of an N-terminal domain that mediates ribosome binding and by itself is sufficient to complement for chaperone activity (30). A central domain with peptidyl-prolyl cis/trans isomerase activity, substrate binding activity, and a C-terminal domain contributes to the chaperone activity (30). Amino acid 275 is located at the beginning of the C-terminal domain (amino acids 248 to 432) (30). In their model, Nakano et al. propose that reduced TF at 42°C compensates for the reduction of an essential cell protein (X) produced at low levels in the divE42 mutant at 42°C. They propose that the ratio of TF to X determines lethality. In divE42 tig+ cells, the TF+:X ratio is too high at 42°C, and cell division is inhibited. In the divE42 tig double mutant at 42°C, low TF activity resulting from the tig mutation and low X levels resulting from the divE42 mutation result in normal TF:X ratios, and the cells grow. At 30°C, X levels are wild type in the divE42 mutant, but TF is low, so the TF:X ratios are too low.
The serine codon usages in Salmonella serovar Typhimurium per thousand are 8.4 (UCU), 10.6 (UCC), 8.0 (UCA), 9.5 (UCG), 8.6 (AGU), and 17.7 (AGC), and in E. coli are 8.4 (UCU), 8.6 (UCC), 7.1 (UCA), 8.9 (UCG), 8.8 (AGU), and 16.0 (AGC). While UCA usage is the lowest, UCA is far from rare. Suppression of the G10:A10 substitution mutation in 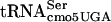 by mutants in the mRNA degradosome (rne-1 and pnp-7 mutations) suggests that stalling plays a critical role in the phenotype. The temperature-sensitive nature of the mutant is as follows: defective at 30°C (evidenced by reduction in flgM translation), more defective at 42°C (evidenced by inhibition of cell division and significant reduction in levels of some proteins), and even more defective at 44°C (evidenced by further reduction in affected proteins at 44°C). Self-complementation of divE42 by the divE42 allele present in high copy numbers is consistent with the loss of a concentration-dependent interaction with G10:A10-
by mutants in the mRNA degradosome (rne-1 and pnp-7 mutations) suggests that stalling plays a critical role in the phenotype. The temperature-sensitive nature of the mutant is as follows: defective at 30°C (evidenced by reduction in flgM translation), more defective at 42°C (evidenced by inhibition of cell division and significant reduction in levels of some proteins), and even more defective at 44°C (evidenced by further reduction in affected proteins at 44°C). Self-complementation of divE42 by the divE42 allele present in high copy numbers is consistent with the loss of a concentration-dependent interaction with G10:A10-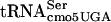 that is dependent on the G10:A10-
that is dependent on the G10:A10-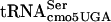 tertiary structure.
tertiary structure.
We rule out the possibility that the UCA codon early (amino acid codon 7) in the flgM gene accounts for sensitivity to the serT mutation. It was possible that G10:A10-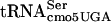 - dependent stalling of translation early in the flgM gene transcript resulted in enhanced flgM mRNA degradation. We examined the 12 flg-linked flagellar hook-basal body structural genes for numbers and locations of UCA codons and obtained the following results (with gene, number of amino acids, number of serine residues, and number of UCA codons listed in that order and followed by the UCA codon position[s], if any, in parentheses): flgA, 219, 13, 1 (49); flgB, 138, 8, 0; flgC, 134, 9, 1 (107); flgD, 232, 15, 0; flgE, 400, 34, 3 (18, 173, 180); flgF, 250, 15, 2 (30, 198); flgG, 260, 20, 2 (4, 64); flgH, 232, 20, 0; flgI, 365, 28, 3 (37, 109, 281); flgJ, 316, 26, 0; flgK, 553, 46, 4 (61, 105, 440, 490); and flgL, 317, 31, 1 (261). We observed 17 UCA codons/265 serine codons (0.64%) that were located throughout the genes. One gene, flgG, had a UCA at the position corresponding to amino acid 4, a position similar to that in the flgM gene. Given that the serT mutant shows normal motility at 30°C, it seems likely that the flagellar genes are translated at normal efficiencies.
- dependent stalling of translation early in the flgM gene transcript resulted in enhanced flgM mRNA degradation. We examined the 12 flg-linked flagellar hook-basal body structural genes for numbers and locations of UCA codons and obtained the following results (with gene, number of amino acids, number of serine residues, and number of UCA codons listed in that order and followed by the UCA codon position[s], if any, in parentheses): flgA, 219, 13, 1 (49); flgB, 138, 8, 0; flgC, 134, 9, 1 (107); flgD, 232, 15, 0; flgE, 400, 34, 3 (18, 173, 180); flgF, 250, 15, 2 (30, 198); flgG, 260, 20, 2 (4, 64); flgH, 232, 20, 0; flgI, 365, 28, 3 (37, 109, 281); flgJ, 316, 26, 0; flgK, 553, 46, 4 (61, 105, 440, 490); and flgL, 317, 31, 1 (261). We observed 17 UCA codons/265 serine codons (0.64%) that were located throughout the genes. One gene, flgG, had a UCA at the position corresponding to amino acid 4, a position similar to that in the flgM gene. Given that the serT mutant shows normal motility at 30°C, it seems likely that the flagellar genes are translated at normal efficiencies.
As mentioned above, certain proteins with serine residues encoded by UCA are present at lower levels in the divE42 mutant strain, and the number of proteins affected increases with increasing temperature. One interpretation of the observed effect of the serT mutant tRNA is that there is less-efficient charging of it. This should give a lower concentration of the ternary complex and thereby stalling of the ribosome and less-efficient translation. This could significantly affect mRNA instability of some transcripts but not that of others.
Another interpretation is that the context of the UCA codon in the mRNA is critical for translation by G10:A10-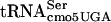 . Such a context effect could be due to an adjacent base influencing the ability to read the UCA codon or the fitting of an adjacent tRNA to the UCA codon. The binding efficiency of tRNAfMet for the initiation codon, AUG, of the Qβ coat gene was shown to increase more than threefold when the G residue adjacent to the 3′ side of the AUG was replaced by an A residue (51). Structures of tRNA species can be altered to read four codons, consistent with the possibility that even a given triplet-specific tRNA could be influenced by adjacent bases (44). There are several lines of evidence to suggest that efficient translation of UCA codons by G10:A10-
. Such a context effect could be due to an adjacent base influencing the ability to read the UCA codon or the fitting of an adjacent tRNA to the UCA codon. The binding efficiency of tRNAfMet for the initiation codon, AUG, of the Qβ coat gene was shown to increase more than threefold when the G residue adjacent to the 3′ side of the AUG was replaced by an A residue (51). Structures of tRNA species can be altered to read four codons, consistent with the possibility that even a given triplet-specific tRNA could be influenced by adjacent bases (44). There are several lines of evidence to suggest that efficient translation of UCA codons by G10:A10-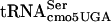 might be dependent on which tRNAs read adjacent codons. The efficiency of the supE amber suppressor in translating the UAG codon varies over an order of magnitude depending on the nucleotide adjacent to the 3′ side of the codon (7). The frameshift suppressor tRNA SufJ will read four codons where the first three are ACC and the fourth base can be anything, suggesting that the SufJ tRNA sterically prevents another tRNA from reading the fourth codon (6). The influence of reading context on the efficiency of nonsense suppression has been observed by a number of labs (2, 13, 14, 18-20, 45, 56). Thus, context is critical for translational efficiency, and it is likely that the fact that some UCA-containing mRNAs are translated more efficiently than others represents an example of context effects. If the G10:A10 substitution in
might be dependent on which tRNAs read adjacent codons. The efficiency of the supE amber suppressor in translating the UAG codon varies over an order of magnitude depending on the nucleotide adjacent to the 3′ side of the codon (7). The frameshift suppressor tRNA SufJ will read four codons where the first three are ACC and the fourth base can be anything, suggesting that the SufJ tRNA sterically prevents another tRNA from reading the fourth codon (6). The influence of reading context on the efficiency of nonsense suppression has been observed by a number of labs (2, 13, 14, 18-20, 45, 56). Thus, context is critical for translational efficiency, and it is likely that the fact that some UCA-containing mRNAs are translated more efficiently than others represents an example of context effects. If the G10:A10 substitution in 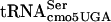 affects translation in a context-dependent manner, the suppression of divE42 by itself in high copy numbers would be consistent with an effect on the 5′ side. This would mean that the ability of G10:A10-
affects translation in a context-dependent manner, the suppression of divE42 by itself in high copy numbers would be consistent with an effect on the 5′ side. This would mean that the ability of G10:A10-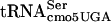 to interact with the tRNA in the P site of the ribosome is more likely to be sensitive to G10:A10-
to interact with the tRNA in the P site of the ribosome is more likely to be sensitive to G10:A10-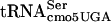 concentration than the ability of a tRNA to enter the A site with G10:A10-
concentration than the ability of a tRNA to enter the A site with G10:A10-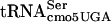 present in the P site. It remains to be determined if the ability of G10:A10-
present in the P site. It remains to be determined if the ability of G10:A10-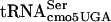 to read the UCA codon is affected by context. To our knowledge, the involvement of position 10 in the D-stem of tRNA in tRNA-tRNA interactions during translation has not been demonstrated. However, tRNA-tRNA interactions during translation have not been characterized to any significant extent. The finding that some proteins with UCA codons are translated normally, while others are not, argues against a simple loss-of-function type of mutant. It is possible that G10:A10-
to read the UCA codon is affected by context. To our knowledge, the involvement of position 10 in the D-stem of tRNA in tRNA-tRNA interactions during translation has not been demonstrated. However, tRNA-tRNA interactions during translation have not been characterized to any significant extent. The finding that some proteins with UCA codons are translated normally, while others are not, argues against a simple loss-of-function type of mutant. It is possible that G10:A10-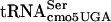 results in a conformational change that affects its ability to sterically fit adjacent to specific tRNA species in the ribosome during translation. The results presented here remind us that context effects can be critical for gene expression and could play an important role in gene regulation by determining the level at which a given gene is expressed in the cell and also in gene evolution.
results in a conformational change that affects its ability to sterically fit adjacent to specific tRNA species in the ribosome during translation. The results presented here remind us that context effects can be critical for gene expression and could play an important role in gene regulation by determining the level at which a given gene is expressed in the cell and also in gene evolution.
Acknowledgments
This work was supported by Public Health Service grant GM056141 from the National Institutes of Health (to K.T.H.).
We thank Brett Finlay for his gift of strain E12A2.
REFERENCES
- 1.Aiso, T., and R. Ohki. 1998. An rne-1 pnp-7 double mutation suppresses the temperature-sensitive defect of lacZ gene expression in a divE mutant. J. Bacteriol. 180:1389-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaboshi, E., M. Inouye, and A. Tsugita. 1976. Effect of neighboring nucleotide sequences on suppression efficiency in amber mutants of T4 phage lysozyme. Mol. Gen. Genet. 149:1-4. [DOI] [PubMed] [Google Scholar]
- 3.Aldridge, P., J. Karlinsey, and K. T. Hughes. 2003. The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol. Microbiol. 49:1333-1345. [DOI] [PubMed] [Google Scholar]
- 4.Berlyn, M. K. B., K. B. Low, and K. E. Rudd. 1996. Linkage map of Escherichia coli K-12, edition 9, p. 1715-1902. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 5.Björk, G. R. 1996. Stable RNA modification, p. 861-886. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 6.Bossi, L., and J. R. Roth. 1981. Four-base codons ACCA, ACCU and ACCC are recognized by frameshift suppressor sufJ. Cell 25:489-496. [DOI] [PubMed] [Google Scholar]
- 7.Bossi, L., and J. R. Roth. 1980. The influence of codon context on genetic code translation. Nature 286:123-127. [DOI] [PubMed] [Google Scholar]
- 8.Carpousis, A. J. 2002. The Escherichia coli RNA degradosome: structure, function and relationship in other ribonucleolytic multienzyme complexes. Biochem. Soc. Trans. 30:150-155. [PubMed] [Google Scholar]
- 9.Chadsey, M. S., and K. T. Hughes. 2000. A multipartite interaction between Salmonella transcription factor, s28, and its anti-sigma factor FlgM: implications for s28 holoenzyme destabilization through stepwise binding. J. Mol. Biol. 306:915-929. [DOI] [PubMed] [Google Scholar]
- 10.Chadsey, M. S., J. E. Karlinsey, and K. T. Hughes. 1998. The flagellar anti-sigma factor FlgM actively dissociates Salmonella typhimurium s28 RNA polymerase holoenzyme. Genes Dev. 12:3123-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chilcott, G. S., and K. T. Hughes. 2000. The coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chilcott, G. S., and K. T. Hughes. 1998. The type III secretion determinants of the flagellar anti-transcription factor, FlgM, extend from the amino-terminus into the anti-s28 domain. Mol. Microbiol. 30:1029-1040. [DOI] [PubMed] [Google Scholar]
- 13.Colby, D. S., P. Schedl, and C. Guthrie. 1976. A functional requirement for modification of the wobble nucleotide in the anticodon of a T4 suppressor tRNA. Cell 9:449-463. [DOI] [PubMed] [Google Scholar]
- 14.Comer, M., C. Guthrie, and W. H. McClain. 1974. An ochre suppressor of bacteriophage T4 that is associated with a transfer RNA. J. Mol. Biol. 90:665-676. [DOI] [PubMed] [Google Scholar]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daughdrill, G. W., M. S. Chadsey, J. E. Karlinsey, K. T. Hughes, and F. W. Dahlquist. 1997. The C-terminal half of the anti-sigma factor, FlgM, becomes structured when bound to its target s28. Nat. Struct. Biol. 4:285-291. [DOI] [PubMed] [Google Scholar]
- 17.Deuerling, E., A. Schulze-Specking, T. Tomoyasu, A. Mogk, and B. Bukau. 1999. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 400:693-696. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein, S. I., and S. Altman. 1977. Coding properties of an ochre-suppressing derivative of Escherichia coli tRNAITyr. J. Mol. Biol. 112:453-470. [DOI] [PubMed] [Google Scholar]
- 19.Feinstein, S. I., and S. Altman. 1978. Context effects on nonsense suppression in Escherichia coli. Genetics 88:201-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fluck, M. M., W. Salser, and R. H. Epstein. 1977. The influence of the reading context upon the suppression of nonsense codons. Mol. Gen. Genet. 151:137-149. [DOI] [PubMed] [Google Scholar]
- 21.Gillen, K. L., and K. T. Hughes. 1991. Molecular characterization of flgM, a gene encoding a negative regulator of flagellin synthesis in Salmonella typhimurium. J. Bacteriol. 173:6453-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillen, K. L., and K. T. Hughes. 1991. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J. Bacteriol. 173:2301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillen, K. L., and K. T. Hughes. 1993. Transcription from two promoters and autoregulation contribute to the control of expression of the Salmonella typhimurium flagellar regulatory gene flgM. J. Bacteriol. 175:7006-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groisman, E. A. 1991. In vivo genetic engineering with bacteriophage Mu. Methods Enzymol. 204:180-212. [DOI] [PubMed] [Google Scholar]
- 25.Hughes, K. T., K. L. Gillen, M. J. Semon, and J. E. Karlinsey. 1993. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science 262:1277-1280. [DOI] [PubMed] [Google Scholar]
- 26.Ishikura, H., Y. Yamada, and S. Nishimura. 1971. Structure of serine tRNA from Escherichia coli. I. Purification of serine tRNA's with different codon responses. Biochim. Biophys. Acta 228:471-481. [DOI] [PubMed] [Google Scholar]
- 27.Karlinsey, J. E., J. Lonner, K. L. Brown, and K. T. Hughes. 2000. Translation/secretion coupling by type III secretion systems. Cell 102:487-497. [DOI] [PubMed] [Google Scholar]
- 28.Karlinsey, J. E., S. Shugo Tanaka, V. Bettenworth, S. Yamaguchi, W. Boos, S. I. Aizawa, and K. T. Hughes. 2000. Completion of the hook-basal body of the Salmonella typhimurium flagellum is coupled to FlgM secretion and fliC transcription. Mol. Microbiol. 37:1220-1231. [DOI] [PubMed] [Google Scholar]
- 29.Kim, S. H., F. L. Suddath, G. J. Quigley, A. McPherson, J. L. Sussman, A. H. Wang, N. C. Seeman, and A. Rich. 1974. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science 185:435-440. [DOI] [PubMed] [Google Scholar]
- 30.Kramer, G., A. Rutkowska, R. D. Wegrzyn, H. Patzelt, T. A. Kurz, F. Merz, T. Rauch, S. Vorderwulbecke, E. Deuerling, and B. Bukau. 2004. Functional dissection of Escherichia coli trigger factor: unraveling the function of individual domains. J. Bacteriol. 186:3777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubo, T., T. Aiso, and R. Ohki. 2000. Eight UCA codons differentially affect the expression of the lacZ gene in the divE42 mutant of Escherichia coli. Can. J. Microbiol. 46:577-583. [PubMed] [Google Scholar]
- 32.Kutsukake, K., and T. Iino. 1994. Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar regulon and flagellar formation in Salmonella typhimurium. J. Bacteriol. 176:3598-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutsukake, K., T. Okada, T. Yokoseki, and T. Iino. 1994. Sequence analysis of the flgA gene and its adjacent region in Salmonella typhimurium, and identification of another flagellar gene, flgN. Gene 143:49-54. [DOI] [PubMed] [Google Scholar]
- 34.Liu, X., and P. Matsumura. 1996. Differential regulation of multiple overlapping promoters in flagellar class II operons in Escherichia coli. Mol. Microbiol. 21:613-620. [DOI] [PubMed] [Google Scholar]
- 35.Liu, X., and P. Matsumura. 1994. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J. Bacteriol. 176:7345-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 37.Maloy, S. R. 1990. Experimental techniques in bacterial genetics. Jones and Bartlett, Boston, Mass.
- 38.Nakano, H., Y. Yamada, H. Ishikura, and H. Inokuchi. 1998. A mutation in the gene for trigger factor suppresses the defect in cell division in the divE42 mutant of Escherichia coli K12. Mol. Gen. Genet. 260:75-80. [DOI] [PubMed] [Google Scholar]
- 39.Ohki, M., and H. Mitsui. 1974. Defective membrane synthesis in an E. coli mutant. Nature 252:64-66. [DOI] [PubMed] [Google Scholar]
- 40.Ohki, M., and S. Sato. 1975. Regulation of expression of lac operon by a novel function essential for cell growth. Nature 253:654-656. [DOI] [PubMed] [Google Scholar]
- 41.Ohki, R., and S. Kawamata. 1992. The function of divE (tRNA1Ser). Cell Sci. 8:704-713. [Google Scholar]
- 42.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 43.Robertus, J. D., J. E. Ladner, J. T. Finch, D. Rhodes, R. S. Brown, B. F. Clark, and A. Klug. 1974. Structure of yeast phenylalanine tRNA at 3 Å resolution. Nature 250:546-551. [DOI] [PubMed] [Google Scholar]
- 44.Roth, J. R. 1974. Frameshift mutations. Annu. Rev. Genet. 8:319-346. [DOI] [PubMed] [Google Scholar]
- 45.Salsar, W., M. Fluck, and R. Epstein. 1969. The influence of the reading context upon the suppression of nonsense codons. Mol. Gen. Genet. 105:125-130. [DOI] [PubMed] [Google Scholar]
- 46.Sanderson, K. E., and J. R. Roth. 1988. Linkage map of Salmonella typhimurium, edition VII. Microbiol. Rev. 52:485-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato, T., M. Ohki, T. Yura, and K. Ito. 1979. Genetic studies of an Escherichia coli K-12 temperature-sensitive mutant defective in membrane protein synthesis. J. Bacteriol. 138:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmieger, H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119:75-88. [DOI] [PubMed] [Google Scholar]
- 49.Steege, D. A., and J. I. Horabin. 1983. Temperature-inducible amber suppressor: construction of plasmids containing the Escherichia coli serU− (supD−) gene under control of the bacteriophage lambda pL promoter. J. Bacteriol. 155:1417-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamura, F., S. Nishimura, and M. Ohki. 1984. The E. coli divE mutation, which differentially inhibits synthesis of certain proteins, is in tRNASer1. EMBO J. 3:1103-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taniguchhi, T., and C. Weissmann. 1978. Site-directed mutations in the initiator region of the bacteriophage Qβ coat cistron and their effect on ribosome binding. J. Mol. Biol. 118:533-565. [Google Scholar]
- 52.Teter, S. A., W. A. Houry, D. Ang, T. Tradler, D. Rockabrand, G. Fischer, P. Blum, C. Georgopoulos, and F. U. Hartl. 1999. Polypeptide flux through bacterial Hsp70: DnaK cooperates with trigger factor in chaperoning nascent chains. Cell 97:755-765. [DOI] [PubMed] [Google Scholar]
- 53.Tomoyasu, T., T. Ohkishi, Y. Ukyo, A. Tokumitsu, A. Takaya, M. Suzuki, K. Sekiya, H. Matsui, K. Kutsukake, and T. Yamamoto. 2002. The ClpXP ATP-dependent protease regulates flagellum synthesis in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood, M. W., M. A. Jones, P. R. Watson, S. Hedges, T. S. Wallis, and E. E. Galyov. 1998. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol. Microbiol. 29:883-891. [DOI] [PubMed] [Google Scholar]
- 55.Wu, T. T. 1966. A model for three-point analysis of random general transduction. Genetics 54:405-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yahata, H., T. Ocada, and A. Tsugita. 1970. Adjacent effect on suppression efficiency. Mol. Gen. Genet. 106:208-212. [DOI] [PubMed] [Google Scholar]
- 57.Yamada, Y., and H. Ishikura. 1994. Suppression of the serT42 mutation with modified tRNA(1Ser) and tRNA(5Ser) genes. Nucleic Acids Res. 22:3124-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanagihara, S., S. Iyoda, K. Ohnishi, T. Iino, and K. Kutsukake. 1999. Structure and transcriptional control of the flagellar master operon of Salmonella typhimurium. Genes Genet. Syst. 74:105-111. [DOI] [PubMed] [Google Scholar]