Abstract
SecA facilitates protein transport across the eubacterial plasma membrane by its association with cargo proteins and the SecYEG translocon, followed by ATP-driven conformational changes that promote protein translocation in a stepwise manner. Whether SecA functions as a monomer or a dimer during this process has been the subject of considerable controversy. Here we utilize cysteine-directed mutagenesis along with the crystal structure of the SecA dimer to create a cross-linked dimer at its subunit interface, which was normally active for in vitro protein translocation.
The Sec-dependent pathway is the most commonly employed system of protein transport across the eubacterial plasma membrane. It consists of the SecA protein, an ATP-driven motor that associates with both its protein cargo and the SecYEG translocon, the presumed protein-conducting channel (30). ATP-driven conformational cycles of SecA, which in part correspond to SecA membrane insertion and retraction cycles at SecYEG, drive the translocation of proteins in a stepwise fashion (10, 29). There is considerable interest in understanding the mechanistic features of the SecA nanomotor, particularly in light of recent crystal structures for the SecA protein and a SecYEG homolog from Methanococcus jannaschii (12, 21, 25, 28). However, one major obstacle toward this end arises from uncertainty about the oligomeric state of SecA in its translocationally active state.
SecA was originally described as a stable homodimer, but it has a monomer-dimer equilibrium in solution in the micromolar range that is sensitive to both temperature and salt concentration (1, 31). Interaction of SecA with its various ligands, such as anionic phospholipids, ATP, signal peptides, and the SecYEG protein, has been shown to substantially affect this equilibrium, with different results being reported by different investigators (3, 4, 13, 20). Furthermore, there have been reports of both the SecA monomer and dimer associated with an artificially stabilized SecYEG dimer in detergent solutions (9, 27).
It was originally suggested that SecA functions as a dimer based on fluorescence resonance energy transfer experiments probing the stability of differentially labeled SecA heterodimers as well as the poor activity of SecA heterodimers containing an 8-azido-ATP-inactivated subunit (8). However, another study suggested that SecA functions as a monomer based on the inability to detect SecYEG-bound SecA dimers by cross-linking along with the residual translocation activity of a monomer-biased SecA mutant containing six alanine substitutions and lacking the carboxyl-terminal 70 amino acid residues of SecA (20), which are dispensable for SecA function (16). A more recent study by the same group found that a stronger monomer-biased SecA mutant protein lacking both the amino-terminal 11 amino acid residues and carboxyl-terminal 70 amino acid residues (SecAΔ11/N95) appeared to retain significant in vivo and in vitro protein translocation activity, particularly when assayed utilizing a prlA4 strain or inverted membrane vesicles or proteoliposomes derived from this strain (19). SecAΔ11/N95 dimers were not detected in this study. By contrast, a recent study from our laboratory found that the monomer-biased SecAΔ11 mutant protein, while retaining wild-type SecYEG-binding activity, was poorly active for translocation ATPase activity, and it was inactive for in vitro protein translocation (13). This mutant protein was also unable to complement a secA conditional null mutant. Finally, we found that the SecYEG-bound or inserted wild-type SecA dimer could be visualized by cross-linking and that membrane-bound his-tagged SecA could be trapped in vivo into an inactive heterodimer by overproduction of “membrane-stuck” SecA mutant proteins. These findings are inconsistent with membrane-bound SecA acting only as a monomer, and they suggest that SecA functions either solely as a dimer or as part of a monomer-dimer cycle during the protein translocation process.
In order to address the question as to whether the SecA dimer is truly active, we utilized cysteine-directed mutagenesis along with the crystal structure of the Bacillus subtilis SecA dimer, which is highly homologous to Escherichia coli (23, 24), to create a disulfide cross-linked E. coli SecA dimer at its subunit interface. Two different mutant pairs were selected for this purpose: Gly11/Ser661 and Arg637/Gln801 (Fig. 1). We reasoned that since SecA is a conformationally driven nanomotor, we should avoid engineering cysteines into potentially flexible or dynamic regions of the protein that are likely to be important for its mobility function. By contrast, the helical scaffold domain of SecA (amino acid residues 621 to 669 and 727 to 828 for E. coli SecA), which appears to act as a rigid assembly template for the other five domains of SecA and forms much of the interprotomer interface (12), appeared to be a good candidate region to tether the two subunits together. In addition, our recent analysis of the SecAΔ11 mutant protein suggested that the amino terminus might play a role only in stabilizing the SecA dimer (13), and thus, we chose a residue (Gly11) within this region as well. We decided to make both mutations for each pair on a single copy of the secA gene (i.e., Cys11/Cys661 or Cys637/Cys801) in order to create the potential for a doubly disulfide-cross-linked dimer (see Fig. 1). This strategy avoids purifying each single SecA mutant protein and assembling the appropriate mixed dimer in vitro. We also felt that our method should result in a higher proportion of Cys11-Cys661- or Cys637-Cys801-specific cross-links, since if disulfide bonds formed during protein purification by introduction of oxygen into the sample (see below), then the high SecA protein concentrations employed under these conditions should favor SecA dimer stability along with the desired interprotomer cross-links.
FIG. 1.
Structural location of cysteine cross-links chosen for mutagenesis. The antiparallel physiological B. subtilis SecA homodimer is shown (7, 12), along with the positions of the two mutant pairs chosen for mutagenesis. (A) Asp10/Lys612, corresponding to Gly11/Ser661of E. coli SecA, is shown in yellow/red. (B) Asn588/Arg750, corresponding to Arg637/Gln801of E. coli SecA, is shown in yellow/red.
Mutations were made by the QuikChange procedure as described by the manufacturer (Stratagene), utilizing the SecA-overproducing plasmid pT7secAC4, lacking any cysteine codons, which encodes a functional secA gene (22), and appropriate oligonucleotides (Integrated DNA Technologies), and they were verified by DNA sequence analysis (University of Pennsylvania DNA Sequencing Facility). Analysis of the growth properties of BL21.19 [secA13(Am) supF(Ts) trp(Am) zch::Tn10 recA::CAT clpA::KAN] carrying a chromosomal secA amber allele and a temperature-sensitive amber suppressor along with pT7secA, pT7secA-Cys11/661, or pT7secA-Cys637/801 at 42°C and 30°C showed that these strains had an efficiency of plating (defined as the culture titer determined on LB-ampicillin plates [18] incubated at 42°C divided by that determined at 30°C and performed as described previously [13]) of 1.2, 1.75, and 0.85, respectively, indicating that the mutant pairs did not significantly perturb SecA in vivo function. However, since the E. coli cytoplasm and cis side of the inner membrane constitute a reducing environment, disulfide bonds should not be generally present in intracellular SecA (2).
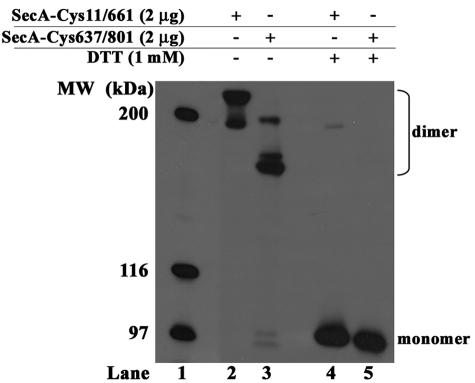
SecA proteins were overproduced and purified as described previously (7) with the exception that dithiothreitol was left out of the final chromatography step. In the absence of a reductant, both the SecA-Cys11/661 and SecA-Cys637/801 proteins existed almost entirely as covalent dimers, since species in the ∼180- to 220-kDa range were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2). Even the slowest-migrating species present in the two protein preparations (Fig. 2, lane 2) was clearly a dimer rather than a trimer or tetramer, since it migrated well below the 251-kDa protein standard (HiMark molecular weight markers; Invitrogen) that was used to estimate its molecular weight (data not shown). The multiple dimer species resolved into the SecA monomer when 1 mM dithiothreitol was included in the buffer. From densitometry of the gel (UN-SCAN-IT; Silk Software), we estimated that the level of SecA monomer within our purified protein preparations was <0.1% and <4% for SecA-Cys11/661 and SecA-Cys637/801, respectively (data not shown). The presence of multiple electrophoretic species for each cross-linked mutant protein is likely to be due to the presence of both single and double disulfide bonds between SecA protomers as well as the presence of some dimers comprised of homologous disulfide bonds (e.g., between Cys801-Cys801). It has been previously noted that SecA-N95-Cys801 can form a dimer after diamide treatment even though both Cys801 residues are not near each other in the SecA dimer crystal structure (12, 19).
FIG. 2.
SDS-PAGE analysis of purified SecA proteins. SDS-PAGE analysis was done according to the method of Laemmli (14) in the absence (−) or presence (+) of 1 mM dithiothreitol (DTT). The elecrophoretic positions of the SecA dimer and monomer are indicated.
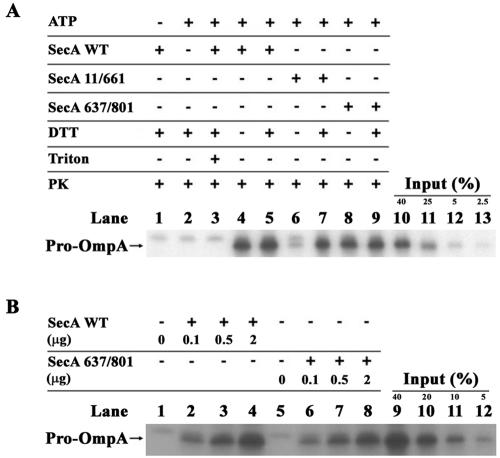
In order to assess the activity of the two SecA mutant proteins, ATPase and in vitro protein translocation assays were performed. The two proteins had very different activity profiles in these assays. Cross-linked SecA-Cys11/661 was severely defective for both its translocation ATPase activity (i.e., SecYEG and preprotein-stimulated [15]) (Fig. 3) and in vitro protein translocation activity (Fig. 4), and these defects were readily reversed by inclusion of 1 mM dithiothreitol in the buffer. By contrast, cross-linked SecA-Cys637/801 was proficient in both activities; its translocation ATPase activity was 73% of that of wild-type SecA and its in vitro protein translocation activity was nearly equivalent to that of the wild-type protein. Curiously both cross-linked proteins displayed lower membrane ATPase activity (i.e., SecYEG stimulated [15]), which was reversed by inclusion of 1 mM dithiothreitol in the buffer, suggestive of suboptimal activation of SecA ATPase activity upon SecYEG binding by the cross-linked dimer. Nonreducing SDS-PAGE analysis of the SecA-Cys637/801 protein before and after incubation in the in vitro protein translocation system indicated that the level of the SecA-Cys637/801 monomer did not increase significantly during this time (i.e., from <4% monomer to <6% monomer; data not shown). The stability of the cross-linked SecA dimer in the in vitro protein translocation system is consistent with the observed inactivity of SecA-Cys11/661 in the absence of dithiothreitol as well as the previously observed absence of significant reducing activity in E. coli inverted membrane vesicles (26). Furthermore, we note that NADH was omitted from our in vitro translocation assays in order to further minimize potential reducing activity. The observed robust in vitro protein translocation activity of SecA-Cys637/801 could not arise solely from the small level of monomer present (estimated at <6%), since our assay system was linearly responsive to the SecA concentration and the observed specific activities of SecA-Cys637/801 and wild-type SecA were nearly comparable (see SecA activity titration in Fig. 4B). By contrast, if the monomer was the only active species present, then the specific activity of SecA-Cys637/801 would have been ∼17-fold lower than that of wild-type SecA. However, we cannot exclude the possibility that the small amount of potential monomer in our preparation may contribute to a particular non-rate-limiting step of the SecA-dependent protein translocation cycle. We conclude that a SecA dimer cross-linked at its subunit interface is sufficient to catalyze efficient in vitro protein translocation, and it also possesses robust translocation ATPase activity. We do note, however, that the particular disulfide bonds contained within SecA-Cys637/801 appeared to interfere somewhat with its conformational activity cycle as judged by the modest reduction in translocation ATPase and in vitro protein translocation activities. By contrast, the disulfide bonds contained within SecA-Cys11/661 were incompatible with the SecA conformational activity cycle as assessed by these experiments.
FIG. 3.
ATPase activities of purified SecA proteins. Endogenous, membrane, and translocation ATPase activity assays for the purified SecA proteins shown are given in the absence (No DTT) or presence (1 mM DTT) of dithiothreitol. WT indicates the wild-type SecA protein. The assays were performed as described previously (32). SecA endogenous (basal), membrane (SecYEG-dependent), and translocation (SecYEG and preprotein-dependent) ATPase activities have been defined previously (15).
FIG. 4.
In vitro protein translocation activity of purified SecA proteins. The assay was performed as described previously (32) except with the omission of NADH. (A) Lanes 1 to 3 show controls indicating that protein translocation was ATP and SecA dependent and that the proteinase K (PK) activity was sufficient to digest untranslocated pro-OmpA. Lanes 10 to 13 calibrate the translocation efficiency by loading different amounts of the complete translocation reaction (identical to lane 5 except without added proteinase K) expressed as a percentage of the total reaction (Input %). The dithiothreitol (DTT) concentration was 1 mM. (B) Titration of SecA-dependent in vitro protein translocation activity in the absence of dithiothreitol.
Taken with our recently published work showing the relative inactivity of a SecA monomer-biased mutant (13), our combined studies strongly argue that SecA can function solely as a dimer. This conclusion is consistent with the early biochemical study of Driessen (8) as well as the genetic observation from Kumamoto's group that duplication of the secA gene that resulted in a head-to-tail covalent dimer was functional in vivo (17). While we were writing up our work, a study similar to ours was published (6). While the approach and conclusion of that study were similar to ours, there are important differences between the two studies. de Keyzer et al. utilized the naturally occurring carboxyl-terminal cysteine residues of SecA to cross-link the enzyme. Thus, as pointed out by these authors, their study had the limitation that suboptimal SecB-independent OmpA translocation was analyzed, since these cysteine residues are important for SecB binding by SecA (11). More importantly, their study did not completely resolve the issue of whether SecA truly functioned as a dimer or as a tethered monomer, since the highly mobile, extreme carboxyl-terminal region (5, 12) was used for cross-linking. By contrast, our study utilized the physiological subunit interface and the rigid helical scaffold domain within the core of SecA for cross-linking, and thus, it is far less likely that SecA could function as a tethered monomer in the latter case. Additional studies are now feasible utilizing an engineered SecA-Cys637/801 dimer to address the question of whether each protomer functions in a more independent fashion or by a subunit switching mechanism whereby each protomer obligately alternates its conformational cycle with its partner in order to produce the stepwise pattern of protein translocation that has been reported previously (29).
Acknowledgments
We thank Christopher R. Zito for performing the in vitro protein translocation reactions.
This work was supported by grant GM42033 from NIGMS.
REFERENCES
- 1.Akita, M., A. Shinkai, S. Matsuyama, and S. Mizushima. 1991. SecA, an essential component of the secretory machinery of Escherichia coli, exists as homodimer. Biochem. Biophys. Res. Commun. 174:211-216. [DOI] [PubMed] [Google Scholar]
- 2.Aslund, F., and J. Beckwith. 1999. Bridge over troubled waters: sensing stress by disulfide bond formation. Cell 96:751-753. [DOI] [PubMed] [Google Scholar]
- 3.Benach, J., Y.-T. Chou, J. J. Fak, A. Itkin, D. D. Nicolae, P. C. Smith, G. Wittrock, D. L. Floyd, C. M. Golsaz, L. M. Gierasch, and J. F. Hunt. 2003. Phospholipid-induced monomerization and signal-peptide-induced oligomerization of SecA. J. Biol. Chem. 278:3628-3638. [DOI] [PubMed] [Google Scholar]
- 4.Bu, Z., L. Wang, and D. Kendall. 2003. Nucleotide binding induces changes in the oligomeric state and conformation of SecA in a lipid environment: a small-angle neutron-scattering study. J. Mol. Biol. 332:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou, Y.-T., J. Swain, and L. Gierasch. 2002. Functionally significant mobile regions of Escherichia coli SecA ATPase identified by NMR. J. Biol. Chem. 277:50985-50990. [DOI] [PubMed] [Google Scholar]
- 6.de Keyzer, J., E. van der Sluis, R. Spelbrink, N. Nijstad, B. de Kruijff, N. Nouwen, C. van der Does, and A. Driessen. 2005. Covalently dimerized SecA is functional in protein translocation. J. Biol. Chem. 280:35255-35260. [DOI] [PubMed] [Google Scholar]
- 7.Ding, H., J. F. Hunt, I. Mukerji, and D. Oliver. 2003. B. subtilis SecA ATPase exists as an antiparallel dimer in solution. Biochemistry 42:8729-8738. [DOI] [PubMed] [Google Scholar]
- 8.Driessen, A. 1993. SecA, the peripheral subunit of the Escherichia coli precursor protein translocase, is functional as a dimer. Biochemistry 32:13190-13197. [DOI] [PubMed] [Google Scholar]
- 9.Duong, F. 2003. Binding, activation, and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase. EMBO J. 22:4375-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Economou, A., J. A. Pogliano, J. Beckwith, D. B. Oliver, and W. Wickner. 1995. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell 83:1171-1181. [DOI] [PubMed] [Google Scholar]
- 11.Fekkes, P., J. de Wit, A. Boorsma, H. Friesen, and A. Driessen. 1999. Zinc stabilizes the SecB binding site of SecA. Biochemistry 38:5111-5116. [DOI] [PubMed] [Google Scholar]
- 12.Hunt, J. F., S. Weinkauf, L. Henry, J. J. Fak, P. McNicholas, D. B. Oliver, and J. Deisenhofer. 2002. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science 297:2018-2026. [DOI] [PubMed] [Google Scholar]
- 13.Jilaveanu, L. B., C. R. Zito, and D. Oliver. 2005. Dimeric SecA is essential for protein translocation. Proc. Natl. Acad. Sci. USA 102:7511-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Lill, R., K. Cunningham, L. A. Brundage, K. Ito, D. Oliver, and W. Wickner. 1989. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 8:961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuyama, S., E. Kimura, and S. Mizushima. 1990. Complementation of two overlapping fragments of SecA, a protein translocation ATPase of Escherichia coli, allows ATP binding to its amino-terminal region. J. Biol. Chem. 265:8760-8765. [PubMed] [Google Scholar]
- 17.McFarland, L., O. Francetic, and C. Kumamoto. 1993. A mutation of Escherichia coli SecA protein that partially compensates for the absence of SecB. J. Bacteriol. 175:2255-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Or, E., D. Boyd, S. Gon, J. Beckwith, and T. A. Rapoport. 2004. The bacterial ATPase SecA functions as a monomer in protein translocation. J. Biol. Chem. 280:9097-9105. [DOI] [PubMed] [Google Scholar]
- 20.Or, E., A. Navon, and T. Rapoport. 2002. Dissociation of the dimeric SecA ATPase during protein translocation across the bacterial membrane. EMBO J. 21:4470-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne, A. R., W. M. Clemons, and T. A. Rapoport. 2004. A large conformational change of the translocation ATPase SecA. Proc. Natl. Acad. Sci. USA 101:10937-10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramamurthy, V., and D. Oliver. 1997. Topology of the integral-membrane form of Escherichia coli SecA protein. J. Biol. Chem. 272:23239-23246. [DOI] [PubMed] [Google Scholar]
- 23.Sadaie, Y., H. Takamatsu, K. Nakamura, and K. Yamane. 1991. Sequencing reveals similiarity of the wild-type div+ gene of Bacillus subtilis to the Escherichia coli secA gene. Gene 98:101-105. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt, M. G., E. E. Rollo, J. Grodberg, and D. B. Oliver. 1988. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J. Bacteriol. 170:3404-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma, V., A. Arockiasamy, D. R. Ronning, C. G. Savva, A. Holzenburg, M. Braunstein, W. R. Jacobs, and J. C. Sacchettini. 2003. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc. Natl. Acad. Sci. USA 100:2243-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tani, K., H. Tokuda, and S. Mizushima. 1990. Translocation of proOmpA possessing an intramolecular disulfide bridge into membrane vesicles of Escherichia coli. J. Biol. Chem. 265:17341-17347. [PubMed] [Google Scholar]
- 27.Tziatzios, C., D. Schubert, M. Lotz, D. Gundogan, H. Betz, H. Schagger, W. Hasse, F. Duong, and I. Collinson. 2004. The bacterial protein-translocation complex: SecYEG dimers associate with one or two SecA molecules. J. Mol. Biol. 340:513-524. [DOI] [PubMed] [Google Scholar]
- 28.van den Berg, B., W. M. Clemons, I. Collinson, Y. Modls, E. Hartmann, S. C. Harrison, and T. A. Rapoport. 2003. X-ray structure of a protein-conducting channel. Nature 427:36-44. [DOI] [PubMed] [Google Scholar]
- 29.van der Wolk, J. P. W., J. G. de Wit, and A. J. M. Driessen. 1997. The catalytic cycle of the Escherichia coli SecA ATPase comprises two distinct preprotein translocation events. EMBO J. 16:7297-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veenendaal, A., C. van der Does, and A. Driessen. 2004. The protein-conducting channel SecYEG. Biochim. Biophys. Acta 1694:81-95. [DOI] [PubMed] [Google Scholar]
- 31.Woodbury, R. L., S. Hardy, and L. Randall. 2002. Complex behavior in solution of homodimeric SecA. Protein Sci. 11:875-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zito, C. R., and D. Oliver. 2003. Two-stage binding of SecA to the bacterial translocon regulates ribosome-translocon interaction. J. Biol. Chem. 278:40640-40646. [DOI] [PubMed] [Google Scholar]