Abstract
During B-cell receptor (BCR) signaling, phosphoinositide-3 kinase (PI3K) is thought to function upstream of phospholipase Cγ2 (PLCγ2). PLCγ2 deficiency specifically impedes transitional type 2 (T2) to follicular (FO) mature B-cell transition. Here, we demonstrate that PI3K deficiency specifically impaired T2-to-FO mature B-cell transition and marginal zone B-cell development. Furthermore, we investigated the functional relationship between PI3K and PLCγ2 using PI3K−/−, PLCγ2−/−, and PI3K−/− PLCγ2−/− B cells. Interestingly, PLCγ2 deficiency had no effect on BCR-mediated PI3K activation, whereas PI3K deficiency only partially blocked activation of PLCγ2. Moreover, whereas PI3K−/− PLCγ2−/− double deficiency did not affect hematopoiesis, it resulted in embryonic lethality. PI3K−/− PLCγ2−/− fetal liver cells transplanted into B-cell null JAK3−/− mice failed to restore development of peripheral B cells and failed to progress through early B-cell development at the pro-B- to pre-B-cell transition, a more severe phenotype than was observed with either PI3K or PLCγ2 single-deficiency B cells. Consistent with this finding, BCR signaling was more severely impaired in the absence of both PI3K and PLCγ2 genes than in the absence of either one alone. Taken together, these results demonstrate that whereas PI3K functions upstream of PLCγ2, activation of PLCγ2 can occur independently of PI3K and that PI3K and PLCγ2 also have distinct functions in BCR signal transduction.
B-cell development and maturation are mediated by signals emanating from the pre-B-cell receptor (BCR) and BCR. Signals from the pre-BCR instruct pre-B cells to expand and to undergo rearrangement of immunoglobulin (Ig) light-chain genes, whereas signals transduced by the BCR direct the transition from immature to mature B cells and activation of mature B cells (25, 27, 42, 43). Newly formed immature B cells from the bone marrow emerge into the spleen as transitional B cells of type 1 (T1), which develop into transitional B cells of type 2 (T2). Ultimately, T2 B cells give rise to long-lived mature follicular (FO) and marginal zone (MZ) B cells (42, 43). Elimination of the pre-BCR or BCR arrests B-cell development at the pro-B- to pre-B-cell or at the immature to mature B-cell transitions, respectively (36, 39, 47, 62, 63).
The pre-BCR and BCR have common signal transduction pathway components and both initiate signaling cascades via the two transmembrane subunits Igα and Igβ (29, 34, 68). Engagement of the pre-BCR-BCR first activates the Src family tyrosine kinase Lyn, leading to phosphorylation of immunoreceptor tyrosine-based activation motifs within Igα and Igβ and subsequent recruitment and activation of Syk tyrosine kinase. Activated Syk phosphorylates the adapter protein, B-cell linker protein (BLNK), which, along with transmembrane protein CD19, subsequently facilitates recruitment and activation of the lipid kinase, phosphatidylinositol 3-kinase (PI3K). PI3K phosphorylates membrane lipid phosphatidylinositol-4,5-bisphosphate to produce phosphatidylinositol-3,4,5-trisphosphate (PIP3), which interacts with the pleckstrin homology (PH) domain-containing proteins. In turn, PIP3, together with tyrosine-phosphorylated BLNK, participates in recruitment and activation of Bruton's tyrosine kinase (Btk) and the effector lipid enzyme, phospholipase Cγ2 (PLCγ2), both of which contain PH and SH2 domains (21, 38, 48, 58). Btk in cooperation with Syk further enhances activation of PLCγ2. Subsequently, activated PLCγ2 hydrolyzes phosphatidylinositol 4,5-bisphosphate to generate diacylglycerol and inositol 1,4,5-trisphosphate, two essential second messengers for cellular responses (59, 60).
Current models propose that PI3K functions upstream of PLCγ2 by facilitating recruitment of Btk, one of the PLCγ2-activating kinases, and PLCγ2 itself to the immunological synapse through interactions between Btk and PLCγ2 PH domains and PIP3 (14, 20, 61). The critical roles of both PI3K and PLCγ2 in BCR signaling are underscored by studies of PI3K-deficient and PLCγ2-deficient mice. Mice deficient for all three of the p85α-p55α-p50α regulatory subunits of PI3K exhibit impaired early development of pro-B cells to pre-B cells and a dramatic reduction in the numbers of mature B cells (18, 65), and these mutant mature B cells fail to proliferate in response to BCR ligation (65). Similarly, PLCγ2-deficient mice exhibit impaired late B-cell development and a significant reduction in the numbers of mature B cells (26, 71), and PLCγ2-deficient B cells are unable to respond to antigens (26, 71).
Here, we investigate the functional relationship between PI3K and PLCγ2 in BCR signaling. By employing PI3K−/−, PLCγ2−/−, and PI3K−/− PLCγ2−/− B cells, we demonstrate that PI3K functions upstream of PLCγ2 and that each molecule has a clearly distinct role in BCR signal transduction.
MATERIALS AND METHODS
Mice.
PI3K−/− mice deficient for p85α-p55α-p50α regulatory subunits of PI3K and PLCγ2−/− mice have been previously described (18, 71). PI3K+/− mice were first bred with PLCγ2+/− mice to obtain PI3K+/− PLCγ2+/− double-heterozygous mice, which were intercrossed to generate PI3K−/− PLCγ2−/− double-homozygous mutant mice.
Fetal liver transplantation.
The JAK3−/− recipients were irradiated at a dose of 300 rads and then transplanted, by retroorbital injection, with 2 × 106 nucleated fetal liver cells obtained from wild-type, PI3K−/−, PLCγ2−/−, or PI3K−/− PLCγ2−/− 13- to 14-day-old embryos. Two to 4 months later, the development and functions of bone marrow, splenic, and lymph node B cells from transplanted mice were examined.
Flow cytometry.
Single-cell suspensions of spleen and bone marrow cells were treated with Gey's solution to remove red blood cells and resuspended in phosphate-buffered saline supplemented with 2% bovine serum albumin. The cells were then stained with a combination of fluorescence-conjugated antibodies. Fluorescein isothiocyanate (FITC)-conjugated anti-CD2 (11-0021), biotin-conjugated anti-CD25 (13-0251), and CyChrome-conjugated (15-0452), phycoerythrin (PE)-conjugated (12-0452), and FITC-conjugated (11-0452) anti-B220 were purchased from eBioscience. FITC-conjugated anti-IgD (553439), FITC-conjugated anti-CD21 (553818), biotin-conjugated anti-CD23 (553137), PE-conjugated anti-CD43 (553271), PE-conjugated anti-Thy1.2 (01005B), and CyChrome-conjugated streptavidin (554062) were purchased from BD Biosciences Pharmingen. PE-conjugated anti-CD19 (1575-09) and both FITC-conjugated (1140-02) and PE-conjugated (1140-09) anti-IgM were purchased from Southern Biotechnology. All antibodies were monoclonal. Samples were applied to a flow cytometer (LSRII; Becton Dickinson), and data were collected and analyzed with CellQuest software (Becton Dickinson).
Immunofluorescent histological analysis.
Spleen tissue was embedded in optimal cutting temperature compound (Lab-Tek Products, Naperville, IL) and quickly frozen in liquid nitrogen. The spleen sections (each, 5 μm) were fixed in cold acetone and air dried. Subsequently, the sections were incubated in phosphate-buffered saline containing 1% bovine serum albumin, 10% normal rat serum, and 10% normal goat serum for an hour at room temperature, followed by staining with tetramethyl rhodamine isothiocyanate-conjugated goat anti-mouse IgM (Southern Biotechnology) and FITC-conjugated rat anti-mouse metallophilic macrophage marker MOMA-1 (Serotec, Ltd., Oxford, United Kingdom) at 4°C overnight. After being stained, the sections were washed and mounted with VECTASHIELD mounting medium (Vector Laboratories).
Immunoprecipitation and Western blotting.
B cells were purified from splenocytes by using anti-B220-coated magnetic beads (Miltenyi Biotech). Ninety-five percent of the purified cells were positive for B220. Purified B cells (5 × 106/ml) in RPMI medium with 10% fetal bovine serum were stimulated with anti-IgM (10 μg/ml) (Jackson ImmunoResearch Laboratories) at 37°C for the indicated times. Cells were lysed in lysis buffer and centrifuged to remove debris as previously described (72). For direct Western blotting, cell lysates (20 mg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinyldifluoride (PVDF) membranes, blotted with monoclonal anti-phosphorylated AKT (92715, Cell Signaling) or AKT (sc-1618, Santa Cruz) antibodies, and visualized with ECL chemiluminescent substrates (Amersham). For immunoprecipitation and Western blotting, cell lysates from 5 × 106 cells were incubated with anti-PLCγ2 antibodies (sc-407; Santa Cruz Biotechnology). Immune complexes were precipitated with protein A-Sepharose, subjected to SDS-PAGE, transferred to PVDF membranes, blotted with anti-phosphotyrosine (4G10; Upstate Biotechnology) or anti-PLCγ2 antibodies, and visualized with ECL chemiluminescent substrates.
Colony assays.
Cells were prepared from livers of day 12 to 13 embryos in Iscove's modified Dulbecco's medium containing 2% fetal bovine serum (StemCell Technologies) and counted in the presence of 3% acetic acid to lyse the erythrocytes. The cells were plated with recombinant cytokines specific for each of the assays, and colonies were scored as previously described (54). For the BFU-E assay, 105 cells/dish were cultured in 3-U/ml recombinant human erythropoietin and 10-ng/ml recombinant murine interleukin 3 (rmIL-3; R&D Systems), and BFU-E colonies were scored at day 8. For the CFU-Meg assay, 5 × 105 cells/dish were cultured in 50-ng/ml recombinant human thrombopoietin (Genzyme), and colonies were scored at day 8. For the CFU-Mix assay, 5 × 104 cells/dish were cultured in 10-ng/ml rmIL-3 and 50-ng/ml recombinant murine SCF (R&D Systems), and colonies were scored at day 12.
Calcium fluorimetry.
Splenocytes (106/ml) from the JAK3−/− recipients were incubated with indo-1AM (Molecular Probes) plus PE-conjugated anti-B220 antibodies (BD Biosciences Pharmingen) at room temperature for 30 min. Cells were then washed and stimulated with anti-IgM antibodies (10 μg/ml). Induction of Ca2+ mobilization was determined in B220+ cells by flow cytometry.
RESULTS
Impaired development of FO and MZ B cells as a consequence of PI3K deficiency is B-cell autonomous.
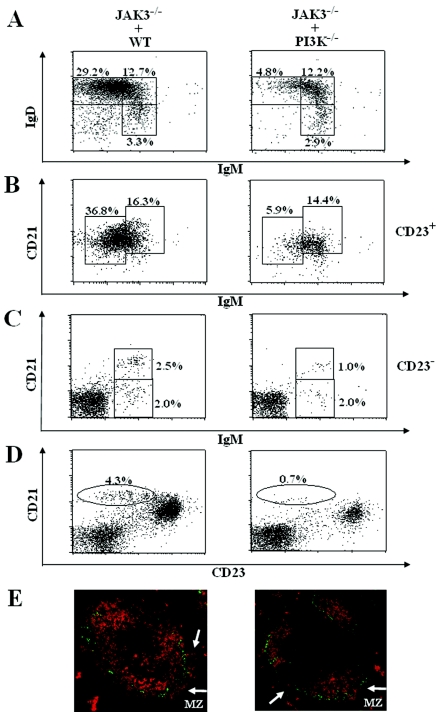
PI3K−/− mice deficient for all three p85α-p55α-p50α regulatory subunits of PI3K are defective in B-cell development and activation; however, these mutant mice exhibit a high degree of perinatal lethality with very few animals surviving beyond 1 week of age, which makes it very difficult to study PI3K-deficient B cells (18). To facilitate further study of PI3K-deficient B cells and to determine whether the defects in B-cell development and function are the result of abnormalities intrinsic to PI3K−/− B cells, we generated chimeric mice by transplanting fetal liver cells from 14- to 16-day-old PI3K−/− embryos into sublethally irradiated B-cell null JAK3-deficient mice (49, 56). The B cells, which were exclusively derived from donor fetal liver cells, that developed in the chimeric mice were analyzed after transplantation. To examine the effect of PI3K deficiency on B-cell maturation, we examined the expression of cell surface markers that distinguish B cells at different stages of maturation. Based on expression of IgD and IgM, splenic B cells can be separated into IgMhi IgD− (T1), IgMhi IgD+ (T2), and IgMlo IgD+ (FO) subsets (42). Fluorescence-activated cell sorter (FACS) analysis of splenic lymphocytes revealed that the transplanted recipients of PI3K−/− fetal liver cells, relative to wild-type fetal liver cells, had a marked decrease in the population of FO mature B cells (IgMlo IgD+) but comparable populations of T1 (IgMhi IgD−) and T2 (IgMhi IgD+) B cells (Fig. 1A).
FIG. 1.
Impairment of the development of PI3K-deficient FO and MZ B cells is B-cell intrinsic. JAK3-deficient mice were reconstituted with wild-type (JAK3−/− + WT) or PI3K−/− (JAK3−/− + PI3K−/−) fetal liver cells. Two to 4 months after the reconstitution, splenocytes from the recipients were stained with a combination of antibodies to IgM, IgD, and B220 or to IgM, CD21, and CD23. (A) FACS analysis with IgM and IgD staining of B220+ gated cells. (B) FACS analysis with CD21 and IgM staining of CD23+ gated cells. (C) FACS analysis with CD21 and IgM staining of CD23− gated cells. (D) FACS analysis with CD21 and CD23 staining of splenocytes. The numbers indicate the percentage of gated cells within the lymphoid populations. The figure shown is representative of six independent analyses. (E) Absence of marginal zone development in the spleens of recipients of PI3K−/− fetal liver cells. Frozen splenic sections derived from JAK3-deficient recipients of wild-type or PI3K−/− fetal liver cells were stained with antibodies to MOMA-1 (green) to detect metallophilic macrophages and anti-IgM (red) to visualize B cells. MZ B-cell layer external to the ring of metallophilic macrophages is indicated by arrows. The data shown are representative of two independent immunostaining experiments.
The striking reduction in the population of FO mature B cells, but normal distribution of T1 and T2 B cells, in spleens derived from recipients of PI3K−/− fetal liver cells was confirmed by staining cells with additional cell surface markers. Splenic B cells can be separated into T1, T2, FO, and MZ subpopulations based on expression of IgM, CD21, and CD23 (44, 52). CD23+ B cells comprise CD21hi IgMhi T2 and CD21int IgMlo FO B cells, whereas CD23− B cells include CD21lo IgMhi T1 and CD21hi IgMhi MZ B cells. In the CD23+-gated splenic lymphocytes, the population of FO mature B cells (CD23+ CD21int IgMlo) was dramatically decreased, whereas the population of T2 B cells (CD23+ CD21hi IgMhi) was similar in recipients of PI3K−/− fetal liver cells, relative to wild-type fetal liver cells (Fig. 1B). Interestingly, in the CD23−-gated splenic lymphocytes, the population of marginal zone B cells (CD23− CD21hi IgMhi) was noticeably reduced, whereas that of T1 B cells (CD23− CD21lo IgMhi) was similar in recipients of PI3K−/−, compared to wild-type, fetal liver cells (Fig. 1C). The effect of PI3K deficiency on the MZ B-cell population was confirmed by additional FACS analysis. MZ B cells can be recognized as CD21hi CD23lo cells. Among splenic lymphocytes, the reduction in the population of MZ B cells (CD21hi CD23lo) in recipients of PI3K−/−, relative to wild-type, fetal liver cells was even more obvious (Fig. 1D).
The reduction of MZ B cells was further confirmed by immunofluorescent staining of frozen tissue sections. Frozen spleen tissue sections from recipients of PI3K−/− or wild-type fetal liver cells were stained with tetramethyl rhodamine isothiocyanate-conjugated goat anti-mouse IgM and FITC-conjugated rat anti-mouse MOMA-1, a specific marker for metallophilic macrophages. The ring of metallophilic macrophages permits visualization of the border between the follicular and marginal zones. In agreement with the flow cytometric results that PI3K deficiency specifically impedes the transition from T2 to FO mature B cells and impairs MZ B-cell development, the B-cell population in the follicular region was severely reduced, and the MZ B cells, which lay external to the ring of metallophilic macrophages, were barely detectable in spleens derived from recipients of PI3K−/− fetal liver cells (Fig. 1E). These results confirm the disappearance of MZ B cells in recipients of PI3K−/− fetal liver cells, despite a normal follicular architecture. Taken together, these results demonstrate that the abnormal development of B cells in PI3K−/− mice is attributable to a B-cell-intrinsic defect. Furthermore, PI3K deficiency specifically impedes the transition of T2 to FO mature B cells and impairs MZ B-cell development.
Upon BCR stimulation, PI3K functions upstream of PLCγ2, but PLCγ2 also can be activated in a PI3K-inpendent manner.
The finding that B-cell development and activation are similarly impaired in PI3K-deficient (Fig. 1) (18, 65) and PLCγ2-deficient (71) mice suggests that there is a functional association between PI3K and PLCγ2 in BCR signaling. To define this functional relationship, we used in vivo genetic methods. Specifically, if PI3K functions upstream of PLCγ2 but not vice versa, activation of PI3K upon BCR stimulation would be predicted to be normal in PLCγ2-deficient B cells, whereas activation of PLCγ2 upon BCR ligation would be expected to be abolished in PI3K-deficient B cells.
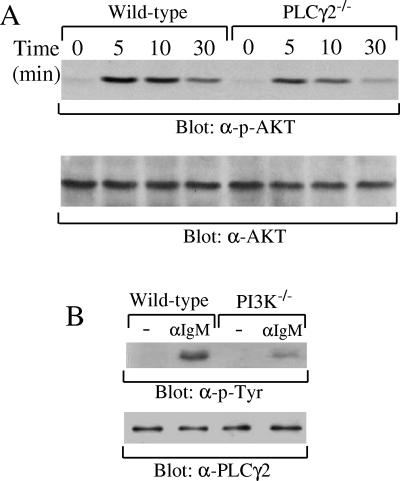
First, we examined whether PI3K could be activated upon BCR engagement in PLCγ2-deficient B cells. Akt translocation to the plasma membrane and activation are established events downstream of PI3K activation in BCR signaling, and Akt activation is a commonly accepted indicator of PI3K activation (12, 15, 23, 28). Splenic B cells were purified from wild-type and PLCγ2-deficient mice and stimulated with anti-IgM antibodies. Activation of Akt was evaluated by immunoblotting with monoclonal antibodies that detect phosphorylation of Ser473 within Akt, which is known to correlate with its kinase activity (1, 6). Upon BCR engagement, the magnitude and kinetics of Akt phosphorylation on Ser473 were comparable in PLCγ2-deficient and wild-type B cells (Fig. 2A). Our finding that PLCγ2 deficiency has no effect on BCR-induced phosphorylation of Akt indicates that PLCγ2 is not required for activation of PI3K following BCR ligation.
FIG. 2.
PLCγ2-independent activation of PI3K and partially PI3K-dependent activation of PLCγ2. (A) Normal activation of AKT in PLCγ2−/− B cells upon BCR ligation. Purified splenic B cells from wild-type or PLCγ2−/− mice were stimulated with anti-IgM antibodies for 0, 5, 10, or 30 min. Cell lysates were subjected to SDS-PAGE and transferred to a PVDF membrane. The membrane was blotted with anti-phosphorylated AKT (α-p-AKT) antibody and subsequently reblotted with anti-AKT (α-AKT) antibody as indicated. (B) Reduced, but not abrogated, activation of PLCγ2 in PI3K−/− B cells upon BCR ligation. Purified splenic B cells from JAK3-deficient recipients of wild-type or PI3K−/− fetal liver cells were stimulated with or without anti-IgM antibodies as indicated. Cell lysates were immunoprecipitated with antibodies to PLCγ2. Precipitated proteins were immunoblotted with anti-phosphorylated tyrosine (α-p-Tyr) or anti-PLCγ2 (α-PLCγ2) antibodies as indicated.
Second, we investigated whether BCR-induced PLCγ2 activation occurred normally in PI3K-deficient B cells. Activation of PLCγ isoforms correlates with an increase in their protein tyrosine phosphorylation (70, 73). BCR-induced tyrosine phosphorylation of PLCγ2 was significantly reduced, but still detectable, in splenic B cells isolated from JAK3-deficient recipients of PI3K−/−, relative to wild-type, fetal liver cells (Fig. 2B). Therefore, activation of PLCγ2 depends largely, but not totally, on PI3K. Taken together, these results demonstrate that PI3K functions upstream of PLCγ2 but that activation of PLCγ2, albeit to a limited extent, can occur independently of PI3K downstream of BCR ligation.
Deletion of both PI3K and PLCγ2 genes results in an embryonic lethality.
To further define the relationship between PI3K and PLCγ2 during BCR signaling, we sought to determine the effect of PI3K−/− PLCγ2−/− double deficiency on B-cell development and activation and compared the phenotypes of the double-deficiency mice to those of PI3K or PLCγ2 single-deficiency mice. If the role of PI3K is simply to provide PIP3 to the PH domain of PLCγ2, the effect of PI3K−/− PLCγ2−/− double deficiency on B-cell development and activation would be identical to that of PI3K or PLCγ2 single deficiency alone.
PI3K−/− mice die perinatally (18), and PLCγ2−/− mice are sterile (71). To establish a line from which PI3K−/− PLCγ2−/− mice could be obtained, PI3K+/− mice were first bred with PLCγ2+/− mice to obtain PI3K+/− PLCγ2+/− double-heterozygous mutant mice, which were phenotypically normal. PI3K+/− PLCγ2+/− mice were then intercrossed. Genotyping of the newborn offspring revealed no double-homozygous mutant mice, with one exception: a PI3K−/− PLCγ2−/− pup was born runted and died within 1 day (Table 1). By contrast, PI3K single-deficiency (PI3K−/− PLCγ2+/+), as well as PI3K-deficient and PLCγ2-heterozygous-deficient (PI3K−/− PLCγ2+/−), newborns were present, albeit at a lower frequency than expected (Table 1), consistent with the previous report that PI3K−/− mice die at the perinatal stage (18). Moreover, genotyping of embryos at various stages of gestation revealed that PI3K−/− PLCγ2−/− embryos were detected with the expected frequencies at 12 to 16 days of gestation, but none was found at 17 to 19 days (Table 1). By contrast, PI3K−/− PLCγ2+/+ and PI3K−/− PLCγ2+/− embryos were detected at the expected frequency throughout embryonic development (Table 1). Therefore, homozygous deletion of both PI3K and PLCγ2 genes results in embryonic lethality at day 17, which represents a more severe phenotype than is exhibited by single deficiency of either gene alone.
TABLE 1.
Genotypic distribution of embryos and newborn mice from PI3K+/− PLCγ2+/− intercrossesa
| Age | Total no. | Genotype (% expected frequency)
|
|||
|---|---|---|---|---|---|
| PI3K+/+ PLCγ2+/+ (6.25) | PI3K−/− PLCγ2+/+ (6.25) | PI3K−/− PLCγ2+/− (12.5) | PI3K−/− PLCγ2−/− (6.25) | ||
| E12-13.5 | 122 | 4 (3.3) | 9 (7.4) | 18 (14.8) | 6 (4.9) |
| E14-15 | 140 | 11 (7.9) | 5 (3.5) | 13 (9.3) | 3 (2.1) |
| E16 | 98 | 8 (8.2) | 4 (4.1) | 16 (16.3) | 6 (6.1) |
| E17-19 | 65 | 5 (7.7) | 4 (6.2) | 4 (6.2) | 0 (0) |
| Newborn (<4 days) | 221 | 17 (7.7) | 6 (4.2) | 16 (7.2) | 1 (0.5) |
Embryos and newborn mice from PI3K+/− PLCγ2+/− females crossed to PI3K+/− PLCγ2+/− males were genotyped by PCR. The embryos were collected between days 12 and 19 of pregnancy; the newborn mice were <4 days old. The expected frequency of each genotype is indicated under the genotype. E, embryonic day.
The catalytic subunits for PI3K are p110α, p110β, and p110δ, which are either ubiquitously expressed (p110α and p110β) (69) or predominantly expressed (p110d) (9, 69) in hematopoietic cells. PCLγ2 is mainly expressed in hematopoietic cell lineages (5, 11). Thus, because both PI3K and PLCγ2 are expressed in hematopoietic cells, we examined a number of hematopoietic parameters in PI3K−/− and PI3K−/− PLCγ2−/− embryos. No obvious effects of PI3K−/− deficiency or PI3K−/− PLCγ2−/− double deficiency on embryonic hematopoiesis were detected, as fetal livers from both mutant embryos had a normal red appearance, indicating the presence of red blood cells (data not shown). In colony assays of fetal liver hematopoietic progenitors, there were no detectable differences between wild-type, PI3K−/−, and PI3K−/− PLCγ2−/− mice in the number of erythroid (BFU-E) or megakaryocyte (CFU-Meg) progenitors (Table 2). There were also no differences in the frequency of the mixed colonies that developed in response to treatment with SCF and IL-3 (Table 2). We conclude from these findings that the embryonic lethality exhibited by PI3K−/− PLCγ2−/− mice is not due to impaired hematopoiesis.
TABLE 2.
Colony formation ability of hematopoietic progenitors in liver cells from wild-type, PI3K−/−, and PI3K−/− PLCγ2−/− embryos in response to various cytokinesa
| Cytokine | Colony type | Genotype
|
||
|---|---|---|---|---|
| WT | PI3K−/− | PI3K−/− PLCγ2−/− | ||
| Epo | BFU-E | 12 ± 2 | 7 ± 3 | 8 ± 4 |
| TPO | CFU-Meg | 16 ± 2 | 19 ± 1 | 14 ± 2 |
| SCF/IL-3 | CFU-Mix | 134 ± 17 | 136 ± 9 | 125 ± 26 |
Fetal liver cells were obtained from wild-type, PI3K−/−, or PI3K−/− PLCγ2−/− embryos at 12 to 13 days of gestation and plated in colony assays. The results indicate means ± standard deviation of number of colonies per 105 cells plated. The results were from two independent analyses. Epo, erythropoietin.
Homozygous deletion of both PI3K and PLCγ2 genes completely blocks B-cell development in the periphery.
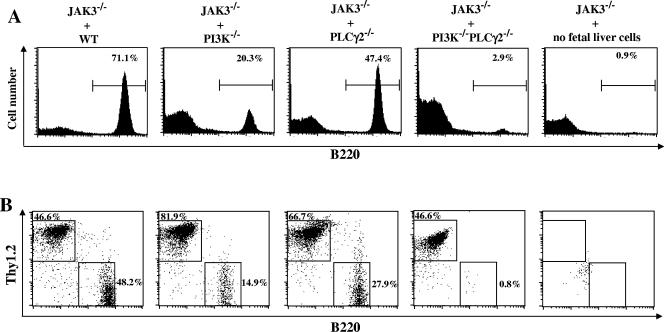
To overcome embryonic lethality of PI3K−/− PLCγ2−/− double-deficiency mice, we examined B-cell development in JAK3-deficient mice that were transplanted with PI3K−/− PLCγ2−/− double-deficiency fetal liver cells. Interestingly, B cells were barely detectable in spleens obtained from recipients of PI3K−/− PLCγ2−/− fetal liver cells, as was the case for negative control animals, which received no donor fetal liver cells at all (Fig. 3A). By contrast, spleens derived from recipients of PI3K or PLCγ2 single-deficiency fetal liver cells had sizeable B-cell populations, albeit at levels lower than recipients of wild-type fetal liver cells (Fig. 3A). These results are consistent with previous findings that PI3K-deficient or PLCγ2-deficient mice have reduced numbers of mature B cells (18, 26, 65, 71). Similarly, B cells were also absent from lymph nodes derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells but present, although at levels lower than recipients of wild-type fetal liver cells, in lymph nodes derived from recipients of PI3K−/− or PLCγ2−/− fetal liver cells (Fig. 3B). These data demonstrate that development of peripheral B cells is completely abrogated by deletion of both PI3K and PLCγ2 genes, which is a more severe phenotype than is observed with either PI3K or PLCγ2 single-deficiency mice.
FIG. 3.
PI3K and PLCγ2 double deficiency completely blocks B-cell development in the periphery. JAK3-deficient mice were transplanted with wild-type (JAK3−/− + WT), PI3K−/− (JAK3−/− + PI3K−/−), PLCγ2−/− (JAK3−/− + PLCγ2−/−), or PI3K−/− PLCγ2−/− (JAK3−/− + PI3K−/− PLCγ2−/−) fetal liver cells or without fetal liver cells (JAK3−/− + no fetal liver cells). Two to 4 months after the reconstitution, splenocytes from the recipients were analyzed. (A) Absence of B cells in spleens derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells. Splenocytes from the recipients were FACS analyzed with B220 staining. The percentages of B220+ cells in the gated lymphoid population are indicated. (B) Absence of B cells but normal presence of T cells in lymph nodes derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells. Lymph node cells from the recipients were FACS analyzed with B220 and Thy1.2 staining. The percentages of cells in the gated lymphoid population are indicated. The figure shown is representative of five independent analyses.
The fact that PI3K−/− PLCγ2−/− B cells failed to develop in the periphery could simply be due to inabilities of hematopoietic progenitors in PI3K−/− PLCγ2−/− fetal liver to reconstitute recipient mice. To investigate this possibility, we examined T-cell development in JAK3-deficient recipients of PI3K−/− PLCγ2−/− fetal liver cells. JAK3-deficient mice have a thymic rudiment and significant numbers of splenic T cells (49, 56); therefore, development of T cells in recipients was examined in lymph nodes. Whereas few detectable Thy1.2+ T cells were present in lymph nodes derived from recipients transplanted with no donor cells, the population of lymph node T cells was similar in recipients of PI3K−/− PLCγ2−/−, relative to wild-type, fetal liver cells (Fig. 3B). In agreement with previous studies that PI3K or PLCγ2 deficiency has no effect on T-cell development, normal T-cell development was also observed for lymph nodes derived from recipients of PI3K−/− or PLCγ2−/− fetal liver cells (Fig. 3B). Therefore, although PI3K and PLCγ2 double deficiency completely blocks B-cell development in the periphery, it has no effect on T-cell development.
Deletion of both PI3K and PLCγ2 genes impedes the pro- to pre-B-cell transition.
To further determine the stage at which B-cell development is impaired by PI3K and PLCγ2 double deficiency, we examined B-cell subpopulations in bone marrow derived from JAK3-deficient recipients of wild-type, PI3K−/−, PLCγ2−/−, PI3K−/− PLCγ2−/− recipients of fetal liver cells or animals that did not receive any fetal liver cells. Consistent with the finding that PI3K or PLCγ2 single deficiency severely diminishes the population of mature B cells in the periphery (Fig. 3) (18, 26, 65, 71), the B220+ B-cell population in bone marrow derived from recipients of PI3K−/− or PLCγ2−/− fetal liver cells was markedly or slightly reduced, respectively, relative to bone marrow derived from recipients of wild-type fetal liver cells (Fig. 4A). Interestingly, although there were almost no detectable peripheral B cells in recipients of PI3K−/− PLCγ2−/− fetal liver cells (Fig. 3A), bone marrow from these recipients contained a proportion of B cells comparable to that observed with bone marrow from recipients of PI3K single-deficiency fetal liver cells (Fig. 4A). By contrast, there were very few B220+ B cells in bone marrow derived from animals that received no fetal liver cells (Fig. 4A).
FIG.4.
PI3K and PLCγ2 double-deficiency impedes pro-B- to pre-B-cell transition. JAK3-deficient mice were reconstituted with wild-type (JAK3−/− + WT), PI3K−/− (JAK3−/− + PI3K−/−), PLCγ2−/− (JAK3−/− + PLCγ2−/−), or PI3K−/− PLCγ2−/− (JAK3−/− + PI3K−/− PLCγ2−/−) fetal liver cells or without fetal liver cells (JAK3−/− + no fetal liver cells). Two to 4 months after the reconstitution, bone marrow cells from the recipients were analyzed. (A) Reduced B cells in bone marrow derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells. Bone marrow cells from the recipients were FACS analyzed by B220 staining. Histograms show the percentage of B220+ cells within the lymphoid cell gate. (B) Absence of mature B cells and reduced immature B cells in bone marrow derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells. Bone marrow cells from the recipients were FACS analyzed with B220 and IgM staining. The percentages of cells in the gated lymphoid population are indicated. (C) Increased pro-B cells and decreased pre-B cells in bone marrow derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells. Bone marrow cells from the recipients were FACS analyzed with CD43 and IgM staining of B220+ gated cells. Percentages indicate cells in the gated lymphoid populations. (D) Increased pro-B cells in bone marrow derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells. Bone marrow cells from the recipients were FACS analyzed with B220 and CD43 staining. Percentages indicate cells in the B220+ gated cells. *, percentage of B220+ CD43+ cells in the B220+ gate of lymphocytes in bone marrow derived from recipients that received no fetal liver cells is artificially high because of the low overall numbers of B220+ cells. (E) Decreased CD25+ pre-B cells in bone marrow derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells. Bone marrow cells from the recipients were FACS analyzed by B220, IgM, and CD25 staining. Percentages indicate CD25+ cells in the B220+ IgM− gated cells. (F) Decreased CD19+ CD2+ later developmental B cells in recipients of PI3K−/− PLCγ2−/− fetal liver cells. Bone marrow cells from the recipients were FACS analyzed by CD19 and CD2 staining. Percentages indicate cells in the lymphoid gated cells. (G) Increased apoptosis of mature B cells in bone marrow derived from recipients of PI3K−/− PLCγ2−/− fetal liver cells. Bone marrow cells from the recipients were stained with B220 and IgM. B220high IgM+ mature B cells were analyzed for apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assays. The figure shown is representative of two (E to G) to five (A to D) independent analyses.
Detailed FACS analysis of bone marrow cells further revealed remarkable differences in early B-cell development among recipients of PI3K−/−, PLCγ2−/−, or PI3K−/− PLCγ2−/−, relative to wild-type, fetal liver cells. Recipients of PI3K−/− fetal liver cells had a dramatically decreased population of mature B cells (B220hi IgM+) and, a slightly decreased population of immature B cells (B220+ IgM+) (Fig. 4B), whereas the population of pre-B cells (B220+ CD43− IgM−) was markedly decreased, and the population of pro-B cells (B220+ CD43+ IgM−) was slightly increased (Fig. 4C). Recipients of PLCγ2−/− fetal liver cells had a decrease in the population of mature B cells, relatively normal populations of immature and pre-B cells, and a slightly increased population of pro-B cells (Fig. 4B and 4C). By contrast, mature B cells were barely detectable in recipients of PI3K−/− PLCγ2−/− fetal liver cells, which exhibited decreased populations of both immature and pre-B cells, and a dramatic increase in the population of pro-B cells (Fig. 4B and C). Within the B220+ population, the increase in the proportion of pro-B cells (B220+ CD43+) in recipients of PI3K−/− PLCγ2−/−, relative to PI3K−/−, PLCγ2−/−, or wild-type fetal liver cells, was even more obvious (Fig. 4D). In fact, the majority of B cells in recipients of PI3K−/− PLCγ2−/− fetal liver cells were pro-B cells (Fig. 4D). Moreover, B-cell precursors down-regulated CD43 and up-regulated CD25 and CD2 during the progression from the pro- and large pre-B cells to small pre-B cells. Within the B220+ IgM− pro-B-cell to pre-B-cell fraction, the CD25+ small pre-B-cell population was decreased in recipients of PI3K−/− or PLCγ2−/−, relative to wild-type, fetal liver cells (Fig. 4E). Interestingly, this CD25+ small pre-B-cell population was further decreased in recipients of PI3K−/− PLCγ2−/−, relative to PI3K−/− or PLCγ2−/−, fetal liver cells (Fig. 4E). Consistent with the increased proportion of pro-B cells and a decreased pre-B-cell population, the population of B cells at later developmental stages (CD19+ CD2+) was dramatically decreased in recipients of PI3K−/− PLCγ2−/− fetal liver cells, relative to PI3K−/−, PLCγ2−/−, or wild-type fetal liver cells (Fig. 4F). As expected, the animals that received no donor fetal liver cells had only a few pro-B and pre-B cells (Fig. 4B to F). Taken together, our results indicate that absence of both PI3K and PLCγ2 genes results in obstruction of early B-cell development at the pro-B-cell stage immediately prior to the pre-BCR checkpoint, which is a developmental block not observed with B cells in the absence of PI3K or PLCγ2 alone.
The severe impairment of PI3K−/− PLCγ2−/− B-cell development prompted us to examine effect of PI3K−/− PLCγ2−/− double deficiency on B-cell survival. Deletion of both PI3K and PLCγ2 genes results in absence of peripheral B cells. Thus, bone marrow cells derived from recipients of wild-type, PI3K−/−, PLCγ2−/−, or PI3K−/− PLCγ2−/− fetal liver cells were stained with B220 and IgM, and subsequently pro- and pre- (B220+ IgM−), immature (B220+ IgM+), and mature (B220high IgM+) B cells from the recipients were compared for apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay. No significant alteration in apoptosis was detected in pro-B and pre-B or immature B cells from all recipients (data not shown). By contrast, the mature B-cell population in bone marrow derived from recipients of PI3K−/− fetal liver cells, relative to wild-type or PLCγ2-deficient fetal liver cells had an increased proportion of apoptotic cells (Fig. 4G). Interestingly, the residual mature B cells from bone marrow of recipients of PI3K−/− PLCγ2−/− fetal liver cells, compared to PI3K−/− fetal liver cells, exhibited a further increase in the percent of apoptotic cells (Fig. 4G). Thus, PI3K−/− PLCγ2−/− double deficiency dramatically increases apoptosis of mature B cells in bone marrow.
Deletion of both PI3K and PLCγ2 genes completely impairs BCR signaling.
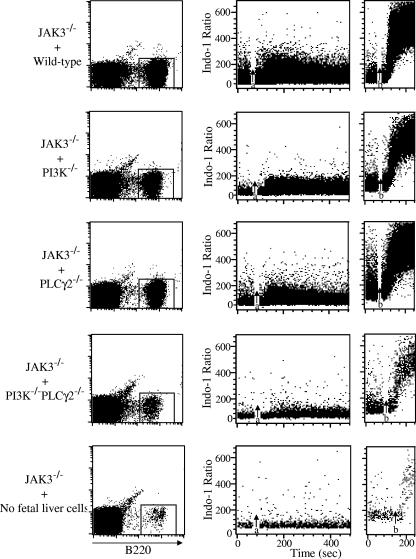
The finding that double deficiency of PI3K and PLCγ2 impairs B-cell development much more severely than does deficiency of either PI3K or PLCγ2 alone prompted us to compare BCR signaling in wild-type, PI3K−/−, PLCγ2−/−, and PI3K−/− PLCγ2−/− B cells. Splenocytes from recipients of wild-type, PI3K−/−, PLCγ2−/−, PI3K−/− PLCγ2−/− fetal liver cells or animals that did not receive any fetal liver cells were stained with B220, and BCR-induced Ca2+ flux in B220+ B cells was examined by FACS analysis. BCR-induced Ca2+ flux in B220+ cells obtained from recipients of PI3K−/− or PLCγ2−/− fetal liver cells was considerably reduced but still apparent compared to that observed with B220+ cells obtained from recipients of wild-type fetal liver cells (Fig. 5). By contrast, BCR-induced Ca2+ flux was barely detectable in residual B220+ cells obtained from a large amount of splenocytes derived from recipients of PI3K−/− PLCγ2−/− double-deficiency fetal liver cells, similar to that observed for the few remaining splenic JAK3−/− B220+ cells obtained from a large amount of splenocytes derived from animals that received no fetal liver cells (Fig. 5). These data demonstrate the existence of a PI3K-independent pathway for BCR-mediated activation of PLCγ2, as well as a PI3K-dependent but PLCγ2-independent pathway for BCR-induced Ca2+ flux. Disruption of both PI3K and PLCγ2 genes resulted in more severely impaired BCR signaling than was observed in the absence of either PI3K or PLCγ2 alone.
FIG. 5.
PI3K and PLCγ2 double deficiency completely impairs BCR signaling. JAK3-deficient mice were reconstituted with wild-type (JAK3−/− + WT), PI3K−/− (JAK3−/− + PI3K−/−), PLCγ2−/− (JAK3−/− + PLCγ2−/−), or PI3K−/− PLCγ2−/− (JAK3−/− + PI3K−/− PLCγ2−/−) fetal liver cells or without fetal liver cells (JAK3−/− + no fetal liver cells). Two to 4 months after the reconstitution, splenocytes from the recipients were collected and then incubated with indo-1AM and PE-conjugated anti-B220 antibody. Cells were then washed and stimulated with anti-IgM antibodies. Induction of Ca2+ mobilization was determined with B220-positive cells by flow cytometry. Anti-IgM antibodies were added at the time indicated by arrow a. Ionomycin was added at the time indicated by arrow b.
DISCUSSION
Activation of PI3K by BCR engagement leads to production of PIP3, which in turn participates in recruitment and activation of PH domain-containing Btk, the upstream tyrosine kinase for PLCγ2, and of PLCγ2 itself (20, 36, 46, 56). Direct evidence for the importance of the interaction between PIP3 and the PH domain of Btk in BCR signaling has been provided by studies that demonstrated that a point mutation within the PH domain of Btk results in defective activation of Btk and impaired activation of PLCγ2 upon BCR ligation (32, 65). Previous studies have also shown that a mutated version of the PH domain of PLCγ1 prevents growth factor-induced PLCγ1 membrane targeting and activation (13), which provides indirect evidence that an interaction of PIP3 with the PH domain of PLCγ2 may also be important in BCR signaling. The purpose of the present study was to use in vivo genetic methods to directly test the hypothesis that PI3K functions upstream of PLCγ2 in BCR signal transduction and to evaluate the interplay between these enzymes in B-cell development. We found that whereas PLCγ2 deficiency had no effect on activation of PI3K, PI3K deficiency impaired but did not abolish activation of PLCγ2, indicating that PLCγ2 is activated downstream of BCR ligation in both a PI3K-dependent and a PI3K-independent manner. We also found that PI3K-PLCγ2 double deficiency, which resulted in embryonic lethality, more severely impaired BCR signaling and B-cell development than did single deficiency of either PI3K or PLCγ2 alone, indicating that PI3K and PLCγ2 each play distinct and nonredundant roles in BCR signal transduction and B-cell development.
Our finding that deficiency of the p85α-p55α-p50α regulatory subunits of PI3K impairs BCR-mediated activation of PLCγ2 is consistent with recent studies that have shown that BCR-mediated PLCγ2 activation, as measured by increases in [Ca2+]int (10, 32, 50) or inositol 1,4,5-trisphosphate generation (10), is impaired in mice that are deficient for (10, 32) or express a catalytically inactive form of (50) the p110δ catalytic subunit of PI3K. However, the precise role that PI3K plays in activating PLCγ2 has yet to be defined. The currently favored model proposes that PIP3 moieties, generated on the inner leaflet of the plasma membrane by the activity of PI3K, provide binding sites for the PH domains of both PLCγ2 and Btk (14, 20, 61), thereby facilitating Btk-mediated phosphorylation and activation of PLCγ2. However, the extent to which PI3K is required for phosphorylation of Btk and for facilitating Btk-mediated phosphorylation of PLCγ2 is somewhat controversial. Thus, mice deficient for the catalytic subunit of PI3K exhibited impairments in both BCR-mediated tyrosine phosphorylation of two specific tyrosine residues in Btk (Y551 and Y223) and activation of PLCγ2 in one study (10), but in another study, they exhibited impairment only in BCR-induced Ca2+ flux, with no effect on global tyrosine phosphorylation of either Btk or PLCγ2 (32). In yet a third study, deficiency of the p85α regulatory subunit of PI3K was also found to have no effect on global tyrosine phosphorylation of Btk; however, PLCγ2 activity was not evaluated (64). Thus, to the extent that increases in the global phosphotyrosine levels on Btk and PLCγ2 reflect increases in enzyme activity, these findings suggest that interactions between PIP3 and the PH domains of Btk and PLCγ2 may not be required for activation of Btk and PLCγ2 per se. An alternative possibility is that binding of the PH domains of PLCγ2 and Btk to PIP3 moieties on the inner leaflet of the plasma membrane serves only to bring Btk and PLCγ2 into proximity with their relevant substrates. Therefore, although it is understood that PI3K functions upstream of PLCγ2, further studies will be necessary for fully understanding the mechanism by which PI3K activates PLCγ2.
The ability of BCR ligation to induce Ca2+ flux, albeit reduced, in the absence of PI3K indicates that PI3K participates in but is not absolutely required for the activation of PLCγ2. The PI3K-independent pathway for activation of PLCγ2 could involve the adapter protein, BLNK, which is phosphorylated by Syk tyrosine kinase after BCR activation. Phosphorylated tyrosine residues of BLNK provide docking sites for the SH2-containing signaling molecules (19, 75). Previous studies have shown that an interaction between tyrosine-phosphorylated BLNK and the SH2 domains of PLCγ2 is essential for full activation of PLCγ2 (19, 30) and that lack of BLNK or mutation of the PLCγ2 SH2 domains impairs BCR-mediated activation of PLCγ2 (31, 33, 45, 53). Together, these results and those of the present study suggest that interactions between tyrosine-phosphorylated BLNK and the SH2 domains of PLCγ2, as well as between PIP3 and the PH domain of PLCγ2, may cooperate to optimize the activation of PLCγ2 upon BCR engagement. Examination of the ability of PLCγ2 to be activated in the absence of both BLNK and PI3K is required to test this hypothesis.
We observed in the present study that PI3K and PLCγ2 double deficiency induced embryonic lethality, which was a more severe phenotype than was observed with mice that were deficient for either PI3K or PLCγ2 alone. The catalytic subunits for the regulatory subunits, p85α-p55α-p50α, are p110α, p110β, and p110δ, which belong to class IA PI3K enzymes (16, 37, 51). The p110α and p110β subunits are ubiquitously expressed (69), whereas the p110δ subunit is predominantly expressed in hematopoietic cells (9, 69). Class IA PI3Ks are activated by multiple tyrosine kinase-associated receptors, including antigen and cytokine receptors (37, 51). Most of PI3K−/− mice deficient for p85α-p55α-p50α regulatory subunits die at birth, whereas some mutant animals survive for 3 to 7 weeks (17, 18). Although the cause of the perinatal lethality of these mutant mice is not fully understood, necrosis in hepatocyte and brown fat, calcification of cardiac tissue, and hypoglycemia have been observed with the mutant mice (17). PCLγ2, which is activated by a variety of receptors (3-5, 13, 35, 41, 46, 55, 57, 66, 76), is not thought to play a role in embryonic development because the majority of PLCγ2−/− mice survive into adulthood (71). Nevertheless, the finding that PI3K and PLCγ2 double deficiency, but not deficiency of either gene alone, results in an embryonic lethality supports the notion that each of these molecules plays a unique role in signaling; both roles are required for embryonic development to proceed normally. Interestingly, despite the fact that PLCγ2 is predominantly expressed in hematopoietic cells (5, 11), embryonic lethality of the double-deficiency mice is not due to impaired hematopoiesis. The cause of the embryonic lethality induced by PI3K and PLCγ2 double deficiency remains to be determined.
In the process of B-cell maturation, signals from the BCR play an important role in driving B-cell precursors to differentiate into one of three subsets of long-lived mature B cells, including FO, MZ, and B1 B cells. However, the exact mechanism by which BCR-mediated signal transduction controls the fate of maturing B cells has not been defined. It has been suggested that BCR signal strength might influence B-cell maturation, such that strong BCR signals favor B1 B-cell development, intermediate BCR signals promote FO B-cell development, and weak BCR signals support MZ B-cell development (7). This hypothesis has been supported by the observations that complete absence of BCR signals results in the loss of all mature B-cell populations (39). Severely weakened BCR signals, for example, due to the deficiency of Btk (8) or PLCγ2 (71), lead to loss of FO and B1 but not MZ B cells; slightly weakened BCR signals, for example, as a result of deficiency of protein kinase Cβ, cause loss of B1 but not FO and MZ B cells (40). However, our finding that PI3K and PLCγ2 deficiencies similarly affected BCR-mediated signal transduction but differentially affected FO and MZ B-cell maturation is inconsistent with this hypothesis. Specifically, we found in the present and previous (18, 65) studies that even though PI3K and PLCγ2 deficiency weakened BCR-induced Ca2+ flux to the same extent, deficiency of the p85α-p55α-p50α regulatory subunits of PI3K impaired FO, MZ, and B1 B-cell development, whereas we showed in previous studies (71, 74) that PLCγ2 deficiency impaired FO and B1 but not MZ B-cell development. Furthermore, our finding that both BCR signaling and B-cell development were more severely impaired in PI3K−/− PLCγ2−/− double-deficiency mice than in either PI3K or PLCγ2 single-deficiency mice, supports the concept that PI3K and PLCγ2 have distinct functions in BCR-mediated B-cell maturation. Thus, our present studies of the contributions of PI3K and PLCγ2 to BCR-mediated B-cell maturation imply that qualitative differences in the BCR signal transduction pathway, as well as differences in the strength of the BCR signal, determine the fate of maturing B cells. Moreover, PI3K quite possibly participates in other signaling cascades (for example, derived from chemokines), which might also regulate the maturation of peripheral B cells (2). The tyrosine kinase Pyk2 and the Rho GTP exchange factor Lsc are potentially involved in chemokine signaling (22, 24). Deficiency of Pyk2 (24) or Lsc (22) in mice severely impairs MZ B-cell development. It is possible that PI3K participates in the signaling of an as-yet-undefined chemokine receptor to contribute to B-cell maturation.
PI3K and PLCγ2 double deficiency results in a complete absence of B cells in the periphery. The impairment of the transition from pro-B cells to pre-B cells caused by the double deficiency certainly contributes to this peripheral B-cell defect. However, signals from BCR mediate not only B-cell maturation but also emigration of newly generated immature B cells from the bone marrow to the periphery (67). The mutant immature B cells that lack most of the cytoplasmic tail of Igα, a signaling component of BCR, exhibit an impairment of emigration from the bone marrow to the periphery (67). Thus, PI3K and PLCγ2-double deficiency could block emigration of newly formed immature B cells from the bone marrow, which might also contribute to the paucity of peripheral B cells. In addition, PI3K−/− PLCγ2−/− mature B cells in the bone marrow have markedly increased apoptosis relative to either single-deficiency or wild-type cells, which might also play a role in the disappearance of peripheral B cells.
The qualitatively unique contribution made by PI3K to the BCR signal transduction pathway has yet to be identified. One pathway that may be unique to PI3K is activation of Akt. PI3K activity contributes to activation of Akt during BCR signaling (12, 15, 23, 28) and PI3K deficiency completely blocks BCR-mediated activation of Akt (10, 50, 64), whereas, as shown in the present studies, PLCγ2 deficiency has no effect on Akt activation. Akt plays a crucial role in protection against apoptotis (12, 28), and interestingly, rates of apoptosis are similarly increased in PI3K−/− single-deficiency and PI3K−/− PLCγ2−/− double-deficiency cells, relative to wild-type B cells (18, 64; data not shown). We propose, therefore, that impaired activation of Akt might contribute to the more severe impairment in B-cell development observed with PI3K−/− PLCγ2−/− mice, relative to PLCγ2−/− mice. Further studies to define the functions of PI3K, apart from its role in activation of PLCγ2, that contribute to the development of B cells may be warranted.
Acknowledgments
This work was supported in part by NIH grants RO1 AI52327 (R.W.) and R01 HL073284 (D.W.) and by American Cancer Society grant RSG CCG-106204 (D.W.).
We thank David A. Fruman and Lewis C. Cantley for PI3K−/− mice. We thank Debra K. Newman for critical review of the manuscript and for helpful discussions. We gratefully acknowledge the technical support of Shoua Yang and Laura Savatski.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Ansel, K. M., and J. G. Cyster. 2001. Chemokines in lymphopoiesis and lymphoid organ development. Curr. Opin. Immunol. 13:172-179. [DOI] [PubMed] [Google Scholar]
- 3.Blake, R. A., G. L. Schieven, and S. P. Watson. 1994. Collagen stimulates tyrosine phosphorylation of phospholipase C-gamma 2 but not phospholipase C-gamma 1 in human platelets. FEBS Lett. 353:212-216. [DOI] [PubMed] [Google Scholar]
- 4.Bonilla, F. A., R. M. Fujita, V. I. Pivniouk, A. C. Chan, and R. S. Geha. 2000. Adapter proteins SLP-76 and BLNK both are expressed by murine macrophages and are linked to signaling via Fcγ receptors I and II/III. Proc. Natl. Acad. Sci. USA 97:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourette, R. P., G. M. Myles, J. L. Choi, and L. R. Rohrschneider. 1997. Sequential activation of phoshatidylinositol 3-kinase and phospholipase C-γ2 by the M-CSF receptor is necessary for differentiation signaling. EMBO J. 16:5880-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 7.Cariappa, A., and S. Pillai. 2002. Antigen-dependent B-cell development. Curr. Opin. Immunol. 14:241-249. [DOI] [PubMed] [Google Scholar]
- 8.Cariappa, A., M. Tang, C. Parng, E. Nebelitskiy, M. Carroll, K. Georgopoulos, and S. Pillai. 2001. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity 14:603-615. [DOI] [PubMed] [Google Scholar]
- 9.Chantry, D., A. Vojtek, A. Kashishian, D. A. Holtzman, C. Wood, P. W. Gray, J. A. Cooper, and M. F. Hoekstra. 1997. p110δ, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J. Biol. Chem. 272:19236-19241. [DOI] [PubMed] [Google Scholar]
- 10.Clayton, E., G. Bardi, S. E. Bell, D. Chantry, C. P. Downes, A. Gray, L. A. Humphries, D. Rawlings, H. Reynolds, E. Vigorito, and M. Turner. 2002. A crucial role for the p110δ subunit of phosphatidylinositol 3-kinase in B cell development and activation. J. Exp. Med. 196:753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggeshall, K. M., J. C. McHugh, and A. Altman. 1992. Predominant expression and activation-induced tyrosine phosphorylation of phospholipase C-γ2 in B lymphocytes. Proc. Natl. Acad. Sci. USA 89:5660-5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 13.Dusi, S., M. Donini, V. Della Bianca, and F. Rossi. 1994. Tyrosine phosphorylation of phospholipase C-γ2 is involved in the activation of phosphoinositide hydrolysis by Fc receptors in human neutrophils. Biochem. Biophys. Res. Commun. 201:1100-1108. [DOI] [PubMed] [Google Scholar]
- 14.Falasca, M., S. K. Logan, V. P. Lehto, G. Baccante, M. A. Lemmon, and J. Schlessinger. 1998. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 17:414-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke, T. F., D. R. Kaplan, and L. C. Cantley. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88:435-437. [DOI] [PubMed] [Google Scholar]
- 16.Fruman, D. A. 2004. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr. Opin. Immunol. 16:314-320. [DOI] [PubMed] [Google Scholar]
- 17.Fruman, D. A., F. Mauvais-Jarvis, D. A. Pollard, C. M. Yballe, D. Brazil, R. T. Bronson, C. R. Kahn, and L. C. Cantley. 2000. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat. Genet. 26:379-382. [DOI] [PubMed] [Google Scholar]
- 18.Fruman, D. A., S. B. Snapper, C. M. Yballe, L. Davidson, J. Y. Yu, F. W. Alt, and L. C. Cantley. 1999. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science 283:393-397. [DOI] [PubMed] [Google Scholar]
- 19.Fu, C., C. W. Turck, T. Kurosaki, and A. C. Chan. 1998. BLNK: a central linker protein in B cell activation. Immunity 9:93-103. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda, M., T. Kojima, H. Kabayama, and K. Mikoshiba. 1996. Mutation of the pleckstrin homology domain of Bruton's tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5-tetrakisphosphate binding capacity. J. Biol. Chem. 271:30303-30306. [DOI] [PubMed] [Google Scholar]
- 21.Gauld, S. B., J. M. Dal Porto, and J. C. Cambier. 2002. B cell antigen receptor signaling: roles in cell development and disease. Science 296:1641-1642. [DOI] [PubMed] [Google Scholar]
- 22.Girkontaite, I., K. Missy, V. Sakk, A. Harenberg, K. Tedford, T. Potzel, K. Pfeffer, and K. D. Fischer. 2001. Lsc is required for marginal zone B-cells, regulation of lymphocyte motility and immune responses. Nat. Immunol. 2:855-862. [DOI] [PubMed] [Google Scholar]
- 23.Gold, M. R., M. P. Scheid, L. Santos, M. Dang-Lawson, R. A. Roth, L. Matsuuchi, V. Duronio, and D. L. Krebs. 1894. The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J. Immunol. 163:1894-1905. [PubMed] [Google Scholar]
- 24.Guinamard, R., M. Okigaki, J. Schlessinger, and J. V. Ravetch. 2000. Absence of marginal zone B-cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31-36. [DOI] [PubMed] [Google Scholar]
- 25.Hardy, R. R., and K. Hayakawa. 2001. B-cell development pathways. Annu. Rev. Immunol. 19:595-621. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto, A., K. Takeda, M. Inaba, M. Sekimata, T. Kaisho, S. Ikehara, Y. Homma, S. Akira, and T. Kurosaki. 2000. Cutting edge: essential role of phospholipase C-γ 2 in B cell development and function. J. Immunol. 165:1738-1742. [DOI] [PubMed] [Google Scholar]
- 27.Healy, J. I., and C. C. Goodnow. 1998. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 16:645-670. [DOI] [PubMed] [Google Scholar]
- 28.Hemmings, B. A. 1997. Akt signaling: linking membrane events to life and death decisions. Science 275:628-630. [DOI] [PubMed] [Google Scholar]
- 29.Hombach, J., T. Tsubata, L. Leclercq, H. Stappert, and M. Reth. 1990. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature 343:760-762. [DOI] [PubMed] [Google Scholar]
- 30.Ishiai, M., M. Kurosaki, R. Pappu, K. Okawa, I. Ronko, C. Fu, M. Shibata, A. Iwamatsu, A. C. Chan, and T. Kurosaki. 1999. BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells. Immunity 10:117-125. [DOI] [PubMed] [Google Scholar]
- 31.Ishiai, M., H. Sugawara, M. Kurosaki, and T. Kurosaki. 1999. Cutting edge: association of phospholipase C-γ2 Src homology 2 domains with BLNK is critical for B cell antigen receptor signaling. J. Immunol. 163:1746-1749. [PubMed] [Google Scholar]
- 32.Jou, S. T., N. Carpino, Y. Takahashi, R. Piekorz, J. R. Chao, D. Wang, and J. N. Ihle. 2002. Essential, nonredundant role for the phosphoinositide 3-kinase p110δ in signaling by the B-cell receptor complex. Mol. Cell. Biol. 22:8580-8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jumaa, H., B. Wollscheid, M. Mitterer, J. Wienands, M. Reth, and P. J. Nielsen. 1999. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity 11:547-554. [DOI] [PubMed] [Google Scholar]
- 34.Karasuyama, H., A. Kudo, and F. Melchers. 1990. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J. Exp. Med. 172:969-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiener, P. A., B. M. Rankin, A. L. Burkhardt, G. L. Schieven, L. K. Gilliland, R. B. Rowley, J. B. Bolen, and J. A. Ledbetter. 1993. Cross-linking of Fcγ receptor I (FcγRI) and receptor II (FcγRII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J. Biol. Chem. 268:24442-24448. [PubMed] [Google Scholar]
- 36.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B-cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 37.Koyasu, S. 2003. The role of PI3K in immune cells. Nat. Immunol. 4:313-319. [DOI] [PubMed] [Google Scholar]
- 38.Kurosaki, T. 1999. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17:555-592. [DOI] [PubMed] [Google Scholar]
- 39.Lam, K. P., R. Kuhn, and K. Rajewsky. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90:1073-1083. [DOI] [PubMed] [Google Scholar]
- 40.Leitges, M., C. Schmedt, R. Guinamard, J. Davoust, S. Schaal, S. Stabel, and A. Tarakhovsky. 1996. Immunodeficiency in protein kinase cβ-deficient mice. Science 273:788-791. [DOI] [PubMed] [Google Scholar]
- 41.Liao, F., H. S. Shin, and S. G. Rhee. 1993. Cross-linking of Fc gamma RIIIA on natural killer cells results in tyrosine phosphorylation of PLC-gamma 1 and PLC-gamma 2. J. Immunol. 150:2668-2674. [PubMed] [Google Scholar]
- 42.Loder, F., B. Mutschler, R. J. Ray, C. J. Paige, P. Sideras, R. Torres, M. C. Lamers, and R. Carsetti. 1999. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J. Exp. Med. 190:75-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin, F., and J. F. Kearney. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195-201. [DOI] [PubMed] [Google Scholar]
- 44.Martin, F., and J. F. Kearney. 2000. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12:39-49. [DOI] [PubMed] [Google Scholar]
- 45.Minegishi, Y., J. Rohrer, E. Coustan-Smith, H. M. Lederman, R. Pappu, D. Campana, A. C. Chan, and M. E. Conley. 1999. An essential role for BLNK in human B cell development. Science 286:1954-1957. [DOI] [PubMed] [Google Scholar]
- 46.Miura, O., N. Nakamura, J. N. Ihle, and N. Aoki. 1994. Erythropoietin-dependent association of phosphatidylinositol 3-kinase with tyrosine-phosphorylated erythropoietin receptor. J. Biol. Chem. 269:614-620. [PubMed] [Google Scholar]
- 47.Mombaerts, P., J. Iacomini, R. S. Johnson, K. Herrup, S. Tonegawa, and V. E. Papaioannou. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869-877. [DOI] [PubMed] [Google Scholar]
- 48.Niiro, H., and E. A. Clark. 2002. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2:945-956. [DOI] [PubMed] [Google Scholar]
- 49.Nosaka, T., J. M. van Deursen, R. A. Tripp, W. E. Thierfelder, B. A. Witthuhn, A. P. McMickle, P. C. Doherty, G. C. Grosveld, and J. N. Ihle. 1995. Defective lymphoid development in mice lacking Jak3. Science 270:800-802. [DOI] [PubMed] [Google Scholar]
- 50.Okkenhaug, K., A. Bilancio, G. Farjot, H. Priddle, S. Sancho, E. Peskett, W. Pearce, S. E. Meek, A. Salpekar, M. D. Waterfield, A. J. Smith, and B. Vanhaesebroeck. 2002. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science 297:1031-1034. [DOI] [PubMed] [Google Scholar]
- 51.Okkenhaug, K., and B. Vanhaesebroeck. 2003. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 3:317-330. [DOI] [PubMed] [Google Scholar]
- 52.Oliver, A. M., F. Martin, and J. F. Kearney. 1999. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 162:7198-7207. [PubMed] [Google Scholar]
- 53.Pappu, R., A. M. Cheng, B. Li, Q. Gong, C. Chiu, N. Griffin, M. White, B. P. Sleckman, and A. C. Chan. 1999. Requirement for B cell linker protein (BLNK) in B cell development. Science 286:1949-1954. [DOI] [PubMed] [Google Scholar]
- 54.Parganas, E., D. Wang, D. Stravopodis, D. J. Topham, J. C. Marine, S. Teglund, E. F. Vanin, S. Bodner, O. R. Colamonici, J. M. van Deursen, G. Grosveld, and J. N. Ihle. 1998. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93:385-395. [DOI] [PubMed] [Google Scholar]
- 55.Park, D. J., H. K. Min, and S. G. Rhee. 1992. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J. Biol. Chem. 267:1496-1501. [PubMed] [Google Scholar]
- 56.Park, S. Y., K. Saijo, T. Takahashi, M. Osawa, H. Arase, N. Hirayama, K. Miyake, H. Nakauchi, T. Shirasawa, and T. Saito. 1995. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity 3:771-782. [DOI] [PubMed] [Google Scholar]
- 57.Ren, C. L., T. Morio, S. M. Fu, and R. S. Geha. 1994. Signal transduction via CD40 involves activation of lyn kinase and phosphatidylinositol-3-kinase, and phosphorylation of phospholipase Cγ2. J. Exp. Med. 179:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reth, M., J. Wienands, and W. W. Schamel. 2000. An unsolved problem of the clonal selection theory and the model of an oligomeric B-cell antigen receptor. Immunol. Rev. 176:10-18. [DOI] [PubMed] [Google Scholar]
- 59.Rhee, S. G., and Y. S. Bae. 1997. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 272:15045-15048. [DOI] [PubMed] [Google Scholar]
- 60.Rhee, S. G., and K. D. Choi. 1992. Regulation of inositol phospholipid-specific phospholipase C isozymes. J. Biol. Chem. 267:12393-12396. [PubMed] [Google Scholar]
- 61.Salim, K., M. J. Bottomley, E. Querfurth, M. J. Zvelebil, I. Gout, R. Scaife, R. L. Margolis, R. Gigg, C. I. Smith, P. C. Driscoll, M. D. Waterfield, and G. Panayotou. 1996. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 15:6241-6250. [PMC free article] [PubMed] [Google Scholar]
- 62.Schuler, W., I. J. Weiler, A. Schuler, R. A. Phillips, N. Rosenberg, T. W. Mak, J. F. Kearney, R. P. Perry, and M. J. Bosma. 1986. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell 46:963-972. [DOI] [PubMed] [Google Scholar]
- 63.Shinkai, Y., G. Rathbun, K. P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A. M. Stall, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855-867. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki, H., S. Matsuda, Y. Terauchi, M. Fujiwara, T. Ohteki, T. Asano, T. W. Behrens, T. Kouro, K. Takatsu, T. Kadowaki, and S. Koyasu. 2003. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat. Immunol. 4:280-286. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki, H., Y. Terauchi, M. Fujiwara, S. Aizawa, Y. Yazaki, T. Kadowaki, and S. Koyasu. 1999. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science 283:390-392. [DOI] [PubMed] [Google Scholar]
- 66.Ting, A. T., C. J. Dick, R. A. Schoon, L. M. Karnitz, R. T. Abraham, and P. J. Leibson. 1995. Interaction between lck and syk family tyrosine kinases in Fc gamma receptor-initiated activation of natural killer cells. J. Biol. Chem. 270:16415-16421. [DOI] [PubMed] [Google Scholar]
- 67.Torres, R. M., H. Flaswinkel, M. Reth, and K. Rajewsky. 1996. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science 272:1804-1808. [DOI] [PubMed] [Google Scholar]
- 68.Tsubata, T., and M. Reth. 1990. The products of pre-B-cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J. Exp. Med. 172:973-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanhaesebroeck, B., M. J. Welham, K. Kotani, R. Stein, P. H. Warne, M. J. Zvelebil, K. Higashi, S. Volinia, J. Downward, and M. D. Waterfield. 1997. P110δ, a novel phosphoinositide 3-kinase in leukocytes. Proc. Natl. Acad. Sci. USA 94:4330-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wahl, M. I., T. O. Daniel, and G. Carpenter. 1988. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science 241:968-970. [DOI] [PubMed] [Google Scholar]
- 71.Wang, D., J. Feng, R. Wen, J. C. Marine, M. Y. Sangster, E. Parganas, A. Hoffmeyer, C. W. Jackson, J. L. Cleveland, P. J. Murray, and J. N. Ihle. 2000. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity 13:25-35. [DOI] [PubMed] [Google Scholar]
- 72.Wang, D., D. Stravopodis, S. Teglund, J. Kitazawa, and J. N. Ihle. 1996. Naturally occurring dominant negative variants of Stat5. Mol. Cell. Biol. 16:6141-6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiss, A., G. Koretzky, R. C. Schatzman, and T. Kadlecek. 1991. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-γ1. Proc. Natl. Acad. Sci. USA 88:5484-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen, R., Y. Chen, L. Xue, J. Schuman, S. Yang, S. W. Morris, and D. Wang. 2003. Phospholipase Cγ2 provides survival signals via Bcl2 and A1 in different subpopulations of B cells. J. Biol. Chem. 278:43654-43662. [DOI] [PubMed] [Google Scholar]
- 75.Wienands, J., J. Schweikert, B. Wollscheid, H. Jumaa, P. J. Nielsen, and M. Reth. 1998. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J. Exp. Med. 188:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu, X., and A. S. Chong. 1996. Vav in natural killer cells is tyrosine phosphorylated upon cross-linking of Fc gamma RIIIA and is constitutively associated with a serine/threonine kinase. Biochem. J. 318:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]