Abstract
The Ikaros transcription factor is both a key regulator of lymphocyte differentiation and a tumor suppressor in T lymphocytes. Mice carrying a hypomorphic mutation (IkL/L) in the Ikaros gene all develop thymic lymphomas. IkL/L tumors always exhibit strong activation of the Notch pathway, which is required for tumor cell proliferation in vitro. Notch activation occurs early in tumorigenesis and may precede transformation, as ectopic expression of the Notch targets Hes-1 and Deltex-1 is detected in thymocytes from young IkL/L mice with no overt signs of transformation. Notch activation is further amplified by secondary mutations that lead to C-terminal truncations of Notch 1. Strikingly, restoration of Ikaros activity in tumor cells leads to a rapid and specific downregulation of Notch target gene expression and proliferation arrest. Furthermore, Ikaros binds to the Notch-responsive element in the Hes-1 promoter and represses Notch-dependent transcription from this promoter. Thus, Ikaros-mediated repression of Notch target gene expression may play a critical role in defining the tumor suppressor function of this factor.
Acute lymphoblastic leukemia/lymphoma (ALL) is a malignant disease of lymphoid precursor cells arrested at different stages of T- and B-cell development (T-ALL and B-ALL, respectively). ALL affects people of every age and is the most common form of cancer in children. Prognosis is variable, highlighting the heterogeneity of this disease and the potentially different molecular mechanisms involved in tumor initiation and progression. A greater understanding of these multiple mechanisms among ALL subgroups may lead to the development of more effective treatment programs.
The Notch signaling pathway was first implicated in a rare case of T-ALL exhibiting a t(7;9) translocation (15), which resulted in the expression of a constitutively active intracellular Notch 1 protein that acts as a potent oncogene. Recently, activating mutations in the Notch 1 gene have been found in more than 50% of T-ALLs (55), and T-ALL samples frequently express the Notch target genes Hes-1 and pTα (11). Truncated Notch receptors also exhibit transforming activity in vitro and in animal models (5, 9, 20, 23, 42, 46). Collectively, these studies point to the oncogenic potential of the Notch pathway but provide little information as to how or why this pathway is so often activated.
The oncogenic effects of the Notch pathway may be linked to its pleiotropic influence on T lymphocytes (36, 44, 58). Notch receptors are expressed on the cell surface of T cells, and upon binding to Notch ligands expressed on neighboring cells, the intracellular fragment of Notch (NIC) is cleaved by metalloproteases and γ-secretase and translocates to the nucleus. In the nucleus, NIC binds to its downstream transcription factor, CSL/RBP-Jk, transforming CSL/RBP-Jk from repressor to activator and inducing the transcription of Notch-dependent target genes such as Hes-1, Deltex-1, pTα, Meltrin-β, and the Notch receptors themselves (13, 21, 22, 45).
The hematopoietic cell-specific transcription factor Ikaros is also associated with T- and B-ALL (38, 39, 49-51, 53) and has been characterized as a tumor suppressor, as animals expressing targeted mutations in the Ikaros gene all develop T lymphomas (41, 57). Moreover, mice subjected to irradiation or chemical mutagenesis develop thymic tumors that frequently exhibit mutations in the Ikaros locus, resulting in the production of dominant-negative forms of this protein (24, 26, 35, 40, 48). These studies link Ikaros deficiency with tumor development, but the mechanism by which Ikaros functions as a tumor suppressor remains unknown.
Ikaros colocalizes with heterochromatin and associates with NURD complexes in activated T cells, suggesting a function in gene repression (8, 28, 32). Some Ikaros proteins also associate with SWI-SNF complexes, which regulate transcriptional activation (28). These studies suggest that Ikaros represses or activates transcription depending on cell type, stage of differentiation, or target gene (19, 30, 31, 54). Further insight into Ikaros function, however, has been hampered by the relatively few number of target genes identified.
A link between Ikaros deficiency and Notch activation in T lymphomas has been reported by Beverly and Capobianco (7). These authors utilized proviral insertional mutagenesis to characterize mutations that synergized with activated Notch 1 during tumorigenesis and found that 40% of the insertions occurred in the Ikaros gene, leading to the production of dominant-negative Ikaros isoforms. They observed that Ikaros and CSL/RBP-Jk recognized similar DNA sequences containing a core TGGGA motif and hypothesized that Ikaros may antagonize Notch signaling by competing with CSL/RBP-Jk for common target sites, although the physiological relevance of this model was not investigated. Interestingly, the combination of activated Notch and Ikaros inactivation has also been detected in a large proportion of murine thymic lymphomas induced by gamma irradiation (35).
We show here that loss of Ikaros results in the direct and widespread activation of Notch pathway genes in T lymphomas of mice expressing low levels of Ikaros, a process that begins early and amplifies with tumor progression. Ikaros reexpression downregulates Notch target gene expression in tumor cells, and Ikaros represses Notch-induced transcription from the Hes-1 promoter via the CSL/RBP-Jk target sequence. These data further highlight the link between Ikaros and Notch functions during T-cell leukemogenesis and support the concept that Ikaros repression of Notch target gene expression plays a pivotal role in preventing T-cell tumorigenesis.
MATERIALS AND METHODS
Mice.
The IkL/L mouse line has been described previously (29). Mice used in this study ranged in their number of backcross generations (5 to 10) onto the C57BL/6 genetic background. All gave similar results.
Antibodies and flow cytometry.
The following reagents were used: anti-CD8α-fluorescein isothiocyanate, anti-CD4-phycoerythrin (both from Caltag), anti-CD3-Cy5 (clone KT3), biotinylated anti-T-cell receptor (TCR) αβ (clone H57-597), and streptavidin Cy5 (Jackson Immunoresearch). Labeled cells were analyzed on a FACSCalibur (BD BioSciences). Results were analyzed using the FlowJo software (TreeStar). Cell sorting was performed on a FACSVantage SE option DiVa (BD BioSciences). The sort purity was >98%.
Cell lines and culture.
Cell lines derived from primary tumors were grown in RPMI 1640, 10% fetal calf serum, 1 mM sodium pyruvate, 2 mM l-glutamine, and 1% antibiotics. Cells were frozen at an early passage (usually after 2 to 3 weeks of amplification) and were thawed and used for a 1-month period for the described experiments. The γ-secretase inhibitor XVIII (compound E; Calbiochem) was used at 1 to 5 μM. For proliferation experiments, cells were labeled with 20 μg/ml CFSE (5,6-carboxyfluorescein diacetate succinimidyl ester; Sigma) at 107 cells/ml and incubated at 37°C for 10 min. After 3 washes, cells (2 × 105 cells/well in 96-well plates) were stimulated with plate-bound anti-CD3 (10 μg/ml, clone 145.2C11; BD Pharmingen) in RPMI 1640, 10% fetal calf serum, 1× nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 5 × 10−5 M 2-mercaptoethanol, and 1% antibiotics. After 72 h, proliferation was visualized by analyzing CFSE loss on gated live cells.
PCR of Dβ2-Jβ2 rearrangements.
Cells (107) were incubated in 500 μl lysis buffer (100 mM Tris-HCl, pH 8.5, 5 mM EDTA, 0.2% sodium dodecyl sulfate, 200 mM NaCl, 100 μg/ml proteinase K) for 4 to 5 h at 37°C. DNA was precipitated by adding 1 volume of isopropanol and resuspended in TE (10 mM Tris-HCl, 0.1 mM EDTA). DNA (1 μl) was used for PCR amplification. The following primers were used: Dβ2.1, 5′-GTAGGCACCTGTGGGGAAGAAACT; Jβ2.7, 5′-TGAGAGCTGTCTCCTACTATCCATT.
Thymectomies.
Four- to 5-week-old mice were anesthetized under sterile conditions, and their thymuses were surgically removed. Thymectomized mice and their control littermates were thereafter maintained in filtered cages.
Microarray analysis.
Experiments were performed using the Affymetrix MG U74Av2 microarray. Total RNA was isolated using the RNeasy kit (QIAGEN). cRNA synthesis and hybridization of the array were performed according to the manufacturer's instructions (Affymetrix). Because of the excellent reproducibility obtained in our Affymetrix facility when separate probes generated from a given RNA sample are hybridized on independent microarrays (http://www-microarrays.u-strasbg.fr), each RNA sample was hybridized on a single chip. Raw microarray data were analyzed using the Affymetrix MAS 5.0 software. We further selected the genes according to the following criteria: a “present call” in at least one of the samples and a coefficient of variation above 0.75 across the 15 samples, yielding a total of 3,521 genes. Hierarchical clustering was performed using the Cluster software (14).
Retrovirus production and transduction.
MigR1 is an murine stem cell virus-based retrovirus with an internal ribosomal entry site and green fluorescent protein (GFP) sequence insert (43). The sequences encoding an intracellular active form of Notch 1 (amino acids 1751 to 2444, NIC1) were obtained by PCR from thymocyte cDNA, sequenced, inserted into the MigR1 vector, and designated Mig-NIC. The Ik1 cDNA was obtained by amplifying the entire coding sequence of Ik1 by PCR from mouse spleen cDNA. Deletion of exon 2-derived sequences was performed by site-directed mutagenesis to obtain Ik1*. The Ik1 and Ik1* cDNAs were cloned into MigR1-GFP and designated Mig-Ik1 and Mig-Ik1*, respectively. The resulting vectors were transfected into Eco-Phoenix packaging cells to produce high-titer retroviral supernatants. IkL/L tumor cell lines were transduced by resuspending cells at 106 cells/ml in retrovirus-containing supernatant (25% total volume) containing 4 μg/ml Polybrene, followed by centrifugation for 90 min at 2,600 rpm. Medium was replaced after an overnight culture. For the kinetics experiments, transduced cells were counted daily and resuspended in fresh medium to a concentration of 106 cells/ml.
Western blots.
Nuclear extracts were prepared according to the method of Andrews and Faller (2), separated on a 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, transferred to nitrocellulose, and blotted overnight with antibodies specific to Notch 1 product(s) cleaved at Val1744 (catalog no. 2421; Cell Signaling). Blots were washed and incubated for 30 min with secondary horseradish peroxidase-conjugated donkey anti-rabbit antibodies (Jackson Immunoresearch). After washing, proteins were revealed by incubation with an enhanced chemiluminescence Supersignal chemiluminescent substrate (Pierce) and exposed to film. Total protein contents on the membranes were evaluated by staining with amido black.
RT-PCR.
Total RNA was isolated using the RNeasy kit (QIAGEN). Reverse transcription (RT)-PCR was performed as previously described (4) using the following primers: β-actin, 5′-GTGACGAGGCCCAGAGCAAGAG, 5′-AGGGGCCGGACTCATCGTACTC; Meltrin-β, 5′-AGTCCCAGGGATGCCAAGTGTGG, 5′-ATCACGGGACCCACACTCTTAGG; Ifi-204, 5′-AGGCAACCAAAGTTAGTGTG, 5′-GTTCTCCCGACTGAGTCTGG; Notch 1, 5′-CGGTGTGAGGGTGATGTCAATG, 5′-GAATGTCCGGGCCAGCGCCACC; Notch 3, 5′-GACCACTTTGCAGATGGCCG, 5′-GCGAGATGATCCAGCAGCAG; Deltex-1, 5′-CACTGGCCCTGTCCACCCAGCCTTGGCAGG, 5′-ATGCGAATTCGGGAAGGCGGGCAACTCAGG; Hes-1, 5′-ATGCCAGCTGATATAATGGAG, 5′-ACGCTCGGGTCTGTGCTGAGC; pTα, 5′-CACACTGCTGGTAGATGGAAGGC, 5′-GTCAGGAGCACATCGAGCAGAAG; cyclin D1: 5′-GCGTGCAGAAGGAGATTGTG, 5′-CCGGATAGAGTTGTCAGTGT; TdT, 5′-GAAGATGGGAACAACTCGAAGAG, 5′-CAGGTGCTGGAACATTCTGGGAG; CD3ɛ, 5′-CCAGTGCTGGGACATTGCTG, 5′-GGGAGGAGGTATGGGGTGTG; Delta-like 1, 5′-CTGAGGTGTAAGATGGAAGCG, 5′-CAACTGTCCATAGTGCAATGG; Delta-like 3, 5′-ACTCTTGGTCATCCACGTT, 5′-CAAAGAGTCTCCAGTCGGT; Delta-like 4, 5′-CCGTCGATTCGCTCGAGTAGGAGCCTACTCAGACAC, 5′-CTCCTCTCTGCTTTCTCATT; Jagged 1, 5′-GGATGATGGGAACCCTGTCAAG, 5′-TGTTTATTTGTCCAGTTCGGGTGT; Jagged 2, 5′-GTCCTTCCCACATGGGAGTT, 5′-GTTTCCACCTTGACCTCGGT. The following oligonucleotides were used for internal probes: Notch 1, 5′-CCCCTGTGACCCACGTGGCACCCAGAACTG; Notch 3, 5′-ATGCTGGCCTCCTTCTGTGG; Hes-1, 5′-GACGGCCTCTGAGCACAGAA; Deltex-1, 5′-GCGGCTGGTCACAGCATCTGGCTATGAGGG; pTα, 5′-CAGCTCTCCTTGCCTTCTGAAGAGCT; Meltrin-β, 5′-CATGCTGGACCCAGGGCTGGTGATGA; Ifi-204, 5′-CTGTGTCTCTGATGTGAACG. mRNA levels were normalized to those of β-actin.
Real-time RT-PCR.
Real-time RT-PCR was performed using TaqMan assays, run on an ABI Prism 7000 system (Applied Biosystems). The expression of GAPDH, Hes-1, and Deltex-1 were analyzed using predefined assays (Mm99999915_g1, Mm00468601_m1, Mm00492297_m1 for GAPDH, Hes-1, Deltex-1, respectively). Hes-1 and Deltex-1 results were normalized relative to the level of GAPDH using the 2−ΔΔCT method (34).
Sequencing of Notch 1 PEST mutations.
Total RNA from primary IkL/L tumors was isolated using the RNeasy kit. RT-PCR was performed as described above. The PCR primers used were as follows: for the NOD domain, 5′-AGTGCAACCCCCTGTATGAC, 5′-CGGCCTCAATCTTGTAAGGA; for the PEST region (a region rich in proline, glutamic acid, serine, and threonine), 5′-CAGGTGCAGCCACAGAACT, 5′-TTAAAAGGCTCCTTTGGTCG; for the C-terminal end of NIC-1, 5′-TGAGACTGCCCAAAGTGTTGC, 5′-ACTGAGGTGTGGCTGTGATG. PCR products were purified and sequenced using the same primers or cloned using the Topo TA cloning kit (Invitrogen). Ten subclones were sequenced for each sample; mutations were considered real when at least 3 subclones showed the same mutated sequence. Analysis and alignment were performed using BLAST and BioEdit (http://www.mbio.ncsu.edu/BioEdit). The Notch 1 reference sequence was obtained with GenBank accession number NM_008714.
Electrophoretic mobility shift assay (EMSA).
Ik1 and Ik1* proteins were produced in transfected Cos cells, using expression vectors containing Ik1 or Ik1* cDNA. Nuclear extracts were prepared by resuspending 107 cells in 500 μl lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT]). After 10 min, cells were vortexed, and nuclei were pelleted and resuspended in 50 μl of the following buffer: 20 mM HEPES, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, protease inhibitor cocktail. Lysates were kept on ice for 20 min and vortexed thoroughly. After centrifugation, supernatants (nuclear extracts) were quantified using the Bradford colorimetric assay. Three micrograms of nuclear extract was used for each sample. Samples were incubated for 25 min at room temperature with 2 μg poly(dI-dC), 1 μg bovine serum albumin, and 10 μM ZnCl2 in 19 μl of HGDE buffer (20 mM HEPES, pH 7.9, 0.2 mM EDTA, 20% glycerol, 100 mM KCl, 1 mM DTT). End-labeled, double-stranded probe (50 × 103 cpm) was then added to each reaction mixture, and the mixture was incubated for 25 min on ice. Protein-DNA complexes were resolved on a 5% polyacrylamide gel, dried, and analyzed by autoradiography. The following probes were used: Hes-1, 5′-TGAAAGTTACTGTGGGAAAGAAAGTTTGGGAAGTTCACACGAG (22); IkBS4, 5′-TCAGCTTTTGGGAATGTATTCCCTGTCA (37).
Luciferase reporter gene assay.
The Hes-1 luc and the Hes-1ΔAB luc luciferase reporter plasmids (22) were used to analyze Ikaros repression of NIC-dependent transcriptional activity. For luciferase activity, 2.5 × 105 HeLa cells were seeded in six-well plates and transfected with 0.5 μg Hes-1 luc or Hes-1ΔAB luc, 1 μg cytomegalovirus-phosphoglycerate kinase-NIC, increasing concentrations (1 to 2 μg) of Ik1, and 10 ng Renilla luciferase control plasmid (Promega) with 6 μl of Lipofectamine in a total volume of 2 ml OptiMEM (Invitrogen). At 48 h posttransfection, cells were lysed, and luciferase and Renilla light units were measured in a Lumac Biocounter M2500 luminometer according to the manufacturer's suggestions for the dual-luciferase reporter assay system (Promega). Luciferase values were corrected for transfection efficiency by dividing luciferase light units by Renilla light units (expressed as relative luciferase units).
RESULTS
Loss of Ikaros leads to early and aggressive T-lymphoma development.
We previously described a mouse line bearing a hypomorphic mutation (IkL/L) at the Ikaros locus; these mice express low levels of Ikaros in their hematopoietic cells (29). Strikingly, all homozygote IkL/L mice develop thymic lymphomas between 3 to 4 months of age and rapidly die from an enlarged thymus or general organ failure due to metastasis in the bone marrow, spleen, kidneys, or liver (Fig. 1A; also data not shown). “Early” thymic tumors, usually detected by 10 to 12 weeks of age, were defined as a slightly enlarged thymus, exhibiting a heterogeneous CD4, CD8, and TCR αβ expression profile and no detectable metastasis (Fig. 1). “Late” thymic tumors, found in animals after 20 weeks of age, exhibited a varied phenotype: most were CD4lo CD8+, several were predominantly CD4+ CD8+, others were CD4lo CD8lo, and almost all expressed intermediate to high levels of TCR. Collectively, these tumors resembled those described in other mouse lines exhibiting loss-of-function mutations in the Ikaros locus (41, 57). Most tumors appeared to be monoclonal, although some showed two or more TCR β rearrangements (Fig. 1B). IkL/L tumors were highly aggressive, as sublethally irradiated wild-type (WT) mice transplanted with primary tumor cells (106 IkL/L tumor cells) succumbed to leukemia 10 to 20 days after transplantation (data not shown).
FIG. 1.
Phenotype and thymic requirement of IkL/L tumors. (A) Snapshots of a WT thymus (4-week-old mouse), an early IkL/L tumor (13-week-old mouse) and a late IkL/L tumor (22-week-old mouse) with metastases in the spleen and kidneys. Th, thymus; L, lung; H, heart; Li, liver. (B) Left panels: flow cytometry analysis of CD4, CD8, and TCR αβ expression on thymocytes from WT and nontransformed IkL/L thymuses (3-week-old mice) and from early and late IkL/L tumors. Histograms show the TCR αβ expression on total thymocytes. Results are shown for a representative of >20 samples studied for each case. Right panels: PCR analysis of Dβ2-Jβ2 rearrangements from DNA of WT, premalignant IkL/L thymocytes, and distinct IkL/L tumors. Results are representative of 10 samples.
Tumor initiation is strictly dependent on the thymus.
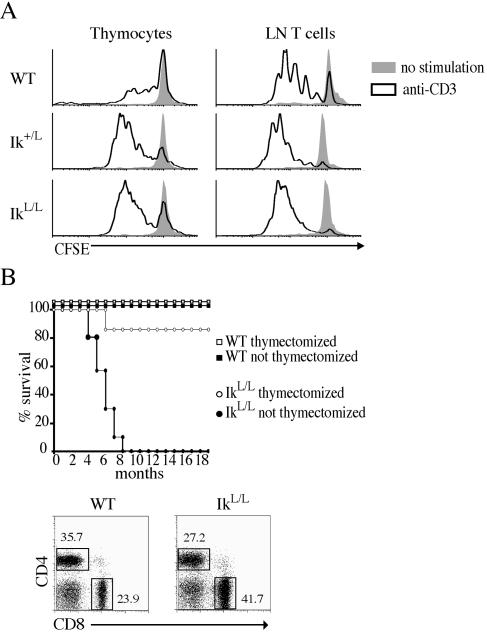
It has been postulated that Ikaros-deficient T cells could transform to a malignant state in vivo because of their lower activation threshold to stimulation (3, 56). We have also found that thymocytes and peripheral T cells of healthy IkL/L and Ik+/L mice hyperproliferate to anti-CD3 stimulation in vitro (Fig. 2A). However, all IkL/L tumors appeared to start in the thymus, suggesting a strong dependence on signals received in this organ for leukemogenesis. To determine the contribution of the thymic environment to tumor development, we compared the survival rate of thymectomized or sham-treated cohorts of adult IkL/L mice and WT littermates (n = 10 for each genotype) at 5 weeks of age. All nonthymectomized IkL/L mice died of thymic lymphoma by 8 months, but thymectomized IkL/L animals remained healthy and tumor free (Fig. 2B). Importantly, the thymectomized mutants retained a significant pool of mature T cells in the periphery even after 1.5 years. These cells displayed CD4/CD8 ratios similar to those of young IkL/L mice, with a frequently elevated CD8+ T-cell population (Fig. 2B).
FIG. 2.
Hyperproliferation of Ik+/L and IkL/L thymocytes and T cells to anti-CD3 stimulation and requirement of the thymus for tumorigenesis. (A) Cells were labeled with CFSE and stimulated with plate-bound anti-CD3 for 3 days. Proliferation was visualized by analyzing CFSE loss on gated live cells. Results are representative of 4 similar experiments. (B) Top panel: WT and IkL/L mice were thymectomized at 5 weeks of age (n = 10 for each group). The graph shows the survival curve of mice monitored over 1.5 years following surgery. One thymectomized IkL/L mouse died of non-cancer-related causes during this period. All nonthymectomized IkL/L mice died of thymic lymphoma. Bottom panels: CD4/CD8 expression of lymph node T cells from thymectomized WT and IkL/L mice 1.5 years after surgery. Numbers indicate the percentages of cells in each gate. Results are representative of 4 experiments.
Collectively, our data demonstrate that mature IkL/L T lymphocytes do not undergo transformation and that IkL/L tumors arise from an immature stage of thymocyte differentiation and/or require a thymic environment.
IkL/L thymic lymphoma cells exhibit activation of the Notch pathway.
To gain insight into the molecular changes involved in Ikaros-dependent tumor development, we compared the global gene expression profiles of leukemic versus nontransformed thymocytes, using Affymetrix U74Av2 oligonucleotide arrays. Fifteen RNA samples were studied: 6 from late IkL/L thymic tumors, 4 from thymic tumors of TEL-JAK2 transgenic mice which closely resemble IkL/L tumors in phenotype (10), 5 control samples from whole-thymocyte populations of 3-week-old WT and 2 premalignant IkL/L mice (defined as nontransformed by virtue of their CD4/CD8 profile and TCR Vα and Vβ chain usage) (data not shown).
Clustering analysis revealed a strong similarity in the transcriptional profiles within each sample group. WT and premalignant IkL/L thymocytes segregated together, while the TEL-JAK2 tumors formed their own distinct profile. Of the IkL/L tumors, 5 clustered together while one (T90) appeared more related to nontransformed thymocytes. Nonetheless, all of the IkL/L tumors (including T90) consistently and specifically overexpressed a group of 14 genes (Fig. 3A and Table 1), suggesting that these genes may comprise a common molecular signature among IkL/L tumors. Strikingly, they included Notch 1 and the Notch targets Deltex-1, Hes-1, and pTα (the others were known genes with unrelated functions or unknown expressed sequence tags). Activation of the Notch pathway was confirmed by RT-PCR in all IkL/L tumors, including many tumors not used for the transcriptome analysis (>30 tumors analyzed) (not shown). In addition, other Notch target genes such as Meltrin-β and Ifi-204, as well as Notch 3, were also overexpressed in IkL/L tumors (data not shown and Fig. 3B). These data indicate that an activated Notch pathway is a prominent feature in IkL/L tumors.
FIG. 3.
Activation of the Notch pathway in IkL/L tumors. (A) A region of the hierarchical clustering of the microarray data, showing the 14 genes which are significantly overexpressed in all IkL/L tumors. Sample nomenclature: T, IkL/L tumor; Tel-Jak, tumor from a TEL-JAK2 transgenic mouse; WT, unfractionated WT thymocytes (3-week-old mice); IkL/L, unfractionated IkL/L premalignant thymocytes (3-week-old mice). Red indicates upregulated genes; green indicates downregulated genes. (B) RT-PCR of Notch pathway genes in purified subsets of WT thymocytes and early IkL/L tumors. DN, CD4− CD8− CD3−; DP, CD4+ CD8+ CD3lo; CD4 SP, CD4+ CD8− CD3hi; CD8 SP, CD4− CD8+ CD3hi. Sorting gates are shown in the accompanying dot blots. The PCRs for individual genes were run on the same gels but separated in the figure to better distinguish the samples. Representative data were obtained from 6 different early IkL/L tumors. (C) RT-PCR of Notch pathway genes in purified subsets of WT and premalignant IkL/L thymocytes (4-week-old mice). PCR products were blotted and hybridized with specific internal oligonucleotide probes for each gene. Results are representative of three similar experiments. (D) Real-time RT-PCR of DP thymocytes for Deltex-1 and Hes-1, using samples from 4 WT and 4 IkL/L mice (4 weeks old), including those used for panel C. *, P < 0.01.
TABLE 1.
Genes significantly over- or underexpressed in IkL/L tumorsa
| Gene type and accession no. | Name | IkL/L tumors | TEL-JAK2 tumors | Control thymocytes |
|---|---|---|---|---|
| Overexpressed genes | ||||
| Z11886 | Notch 1 | 4,191 | 1,154 | 1,112 |
| U38252 | Deltex-1 | 3,298 | 647 | 375 |
| D16464 | Hes-1 | 642 | 126 | 139 |
| U16958 | pTα | 1,188 | 219 | 330 |
| X04367 | Platelet-derived growth factor receptor beta | 339 | 46 | 35 |
| AA930526 | Myotubulin-related protein 13 | 415 | 18 | 55 |
| AF073881 | Myotubularin homologous protein | 855 | 87 | 115 |
| AI846517 | Cytochrome b561 | 287 | 32 | 54 |
| AI838470 | Proline-rich polypeptide 6 | 429 | 50 | 96 |
| AI464596 | Expressed sequence tag | 1,333 | 128 | 305 |
| U92477 | Abl SH3 binding protein | 465 | 141 | 121 |
| X61453 | NCK-associated protein 1 | 352 | 85 | 93 |
| AI850569 | Pyrrolone-5-carboxylate reductase family member 2 | 1,304 | 241 | 375 |
| U21906 | Mus musculus major histocompatibility complex class Ib gene | 3,423 | 745 | 1,099 |
| Underexpressed genes | ||||
| D49691 | Leucocyte-specific protein 1 | 450 | 4,037 | 2,921 |
| M14044 | Calpactin I heavy chain | 1,461 | 6,522 | 5,417 |
| AW123880 | Mus musculus X-box binding protein 1 | 128 | 471 | 381 |
| 019597 | cdk4 and cdk6 inhibitor p19 protein | 524 | 1,304 | 1,813 |
Genes overexpressed in IkL/L tumors were selected according to the following criteria: (i) a coefficient of variation of >0.85 when their expression values were compared with those of a fictive gene with expressions of 1 in the IkL/L tumors (6 samples) and 0 in the control thymocytes (5 samples) and TEL-JAK2 tumors (4 samples); (ii) a >3-fold difference between the average expression in IkL/L tumors and that of all other samples; (iii) an average expression value in IkL/L tumors of >200 (thus excluding genes with very weak expression levels). Genes underexpressed in IkL/L tumors were selected using similar criteria. Notch pathway genes are shown in boldface type. Values correspond to averages of normalized expression values of all samples within the indicated group.
To determine if Notch activation occurs early in tumorigenesis, we analyzed the expression of Notch pathway genes in early IkL/L tumors. As a baseline reference, WT thymocytes were sorted into DN (CD4− CD8− CD3−), DP (CD4+ CD8+ CD3lo), CD4 SP (CD4+ CD8− CD3hi), and CD8 SP (CD4− CD8+ CD3hi) populations. Since early IkL/L tumors were heterogeneous in CD4/CD8 expression, they were also purified into phenotypically similar, though not necessarily developmentally identical, DP and CD8 SP subsets (Fig. 3B). WT thymocytes showed a dynamic and temporally restricted pattern of Notch target gene expression during maturation from DN to DP and finally to the CD4 and CD8 SP subsets, with sharp on-off transitions between populations, consistent with previous data (12, 13, 17, 25). None of the Notch target genes tested were simultaneously expressed by individual WT subpopulations. In striking contrast, every one of these genes was expressed in the IkL/L tumor cells, in both the “DP” and “CD8 SP” subsets. These data indicate that Notch activation is an early feature of IkL/L tumor development.
To investigate if Notch activation occurs before overt transformation, we evaluated purified subpopulations of thymocytes from healthy 4-week-old IkL/L mice by RT-PCR. IkL/L thymocytes showed clear activation of some Notch target genes when analyzed at the subpopulation level (Fig. 3C). Deltex-1 expression was detected in IkL/L DP cells but not in WT DP cells, Hes-1 was expressed at higher levels in IkL/L DP and SP thymocytes, and pTα was upregulated in CD4 SP cells (in one mouse shown). The significant overexpression of Deltex-1 and Hes-1 in the IkL/L DP population was confirmed by real-time RT-PCR (Fig. 3D). These results suggest that some Notch target genes are dysregulated early in IkL/L thymocytes, prior to the onset of tumorigenesis. That other genes were unaffected indicates that Ikaros deficiency selectively allows the activation of a few Notch pathway genes in IkL/L cells.
IkL/L tumor cells express cleaved Notch 1 proteins of various sizes.
To determine if the upregulation of Notch target gene expression is correlated with Notch signaling, IkL/L tumor cells were analyzed at the protein level for the presence of cleaved Notch. Cleaved Notch 1 (NIC-1) was readily detectable in the nuclear extracts of cells from all IkL/L tumors tested (n = 25) (Fig. 4A). Interestingly, the NIC-1 proteins were often, though not always, smaller than the expected 120-kDa polypeptide (47) and ranged from 60 to 120 kDa. Since the antibody we used recognizes the N-terminal part of NIC-1 and abnormal splicing was not detected in the 3′ region of the Notch 1 transcripts (not shown), we hypothesized that these smaller forms were truncated at their C termini. As activating mutations deleting the negative regulatory C-terminal PEST domain of Notch 1 have recently been found in a significant fraction of T-ALL patients (55), we cloned and sequenced the 3′ region of the Notch 1 transcripts (nucleotides [nt] 6614 to 7675) as well as the more 5′ sequence encoding the heterodimerization domain which consists of the noncovalently associated extracellular and transmembrane subunits (NOD domain; nt 4659 to 5210) of 10 primary IkL/L tumors (Fig. 4B). All tumors were normal in the NOD domain. Remarkably, 7 tumors showed frameshift mutations leading to PEST domain deletions. These predicted truncations were consistent with the size of the NIC-1 polypeptides detected in each tumor by Western blotting (not shown). Normal Notch 1 cDNA clones were also identified in these tumors, although it was unclear from these bulk assays whether individual cells possessed one WT and one mutant allele or if the tumors contained a mix of cells possessing either WT or mutant NIC-1. Three tumors which had WT size NIC-1 polypeptides showed normal Notch 1 cDNA clones (not shown). These studies suggest that Notch 1 is a frequent target of secondary mutations in IkL/L tumors.
FIG. 4.
Overexpression of Notch 1 in primary IkL/L tumors and presence of PEST mutations. (A) Nuclear extracts (3 μg) from WT and late IkL/L tumors were analyzed by Western blotting with an anti-cleaved Notch1 antibody. Note the presence and the variability in size of cleaved NIC-1 proteins. (B) Top panel: comparison of Notch 1 cDNAs. The top line represents the Notch 1 cDNA. NIC-1 corresponds to nt 5259 to 9193. The cDNA sequenced (nt 6614 to 7675) corresponds to the region surrounding the PEST domain. The black box represents the PEST coding region (nt 7495 to 7564). Below, the bold downward arrows correspond to mutation sites. The lower lines correspond to the RNA sequence alignment of the IkL/L tumors, where the upward pointing arrows denote insertions, the boxes denote combined deletion-insertions, and the dots represent deletions. The ages (in weeks) of the mice were as follows: T102, 22; T72, 24; T99, 22; T103, 18; T172, 17; T157, 19; T81, 23. The T81 tumor contained 2 different mutations as well as the WT sequence. All mice had well-developed tumors and expressed a smaller sized NIC-1. Five subclones from a WT mouse were also sequenced and cloned; no mutations were found (not shown). Bottom panel: alignment and prediction of the protein sequences for the above NIC-1 region. The top line represents WT NIC-1. The asterisks denote the presence of a predicted stop codon in the tumor samples.
Notch signaling regulates tumor cell proliferation.
Our results indicate that the upregulation of Notch target gene expression in IkL/L tumors results from enhanced Notch signaling in these cells. To determine if Notch signaling and subsequent activation play a functional role in controlling the transformed phenotype of IkL/L tumor cells, we targeted this pathway in loss-of-function studies using two cell lines established from distinct IkL/L tumors. The IkL/L lines grew rapidly in the absence of exogenous cytokines and were phenotypically similar to primary tumors (Fig. 5A). We restricted our analyses to early passage cells to mimic the primary tumor phenotype as closely as possible and avoid the complications of ex vivo mutations. Similar to primary IkL/L tumor cells, these cell lines expressed high mRNA levels for Notch 1, Notch 3, the Notch target genes (Hes-1, Deltex-1, Ifi-204), and transcripts for Notch ligands (mostly Delta-like 1, Delta-like 3, and Jagged 2) which could mediate paracrine Notch activation (Fig. 5B). They also expressed high levels of cleaved NIC-1 protein (Fig. 5C).
FIG. 5.
IkL/L tumors require Notch activation for their proliferation. (A) Phenotype of IkL/L tumor cell lines. CD4/CD8 expression of the T64 and T29 cell lines derived from distinct primary IkL/L tumors. Two other lines (T153 and T68) were also established and gave similar results (not shown). (B) RT-PCR analysis of Notch pathway genes in T64 and T29 cells compared with WT and pretransformed IkL/L thymocytes. (C) Western blot of cleaved NIC-1 protein from nuclear extracts of T64 and T29 cells and a primary IkL/L tumor (5 μg each). (D) The T29 cell line was transduced with the MigR1 or Mig-NIC retroviral vectors at day 0 and cultured in the presence or absence of 5 mM GSI, added at day 3. Transduced cells were distinguished from nontransduced cells by their expression of the GFP reporter. Cumulative GFP+ cell numbers are shown over a period of 6 days. Similar results were obtained using 1 μM GSI. (E) RNA was extracted 48 h after addition (+) or not (−) of GSI (day 5 of culture). Expression of β-actin, Deltex-1, and Hes-1 was analyzed by RT-PCR. Results are representative of 4 experiments. Similar results were obtained with other IkL/L tumor cell lines.
To prevent Notch signaling, we cultured these cell lines in the presence of a γ-secretase inhibitor (GSI), which blocks the cleavage of surface Notch and subsequent translocation of NIC to the nucleus (1, 55). The specificity of this inhibitor for Notch was controlled for by ectopically expressing NIC-1 in the same cells via retroviral transduction, which bypasses the effects of the cleavage block. Transduced cells were distinguished from nontransduced cells by their expression of the GFP reporter. As shown in Fig. 5D, GSI treatment alone stopped the proliferation of IkL/L tumor cells. Importantly, proliferation was restored when NIC-1 was concomitantly introduced in these cells (Fig. 5D). Furthermore, Deltex-1 and Hes-1 expression were abrogated in GSI-treated cells but restored in cells expressing NIC-1, regardless of GSI treatment (Fig. 5E). Collectively, these results demonstrate that IkL/L tumor cell proliferation is dependent on Notch activation.
Ikaros represses Notch pathway genes and inhibits tumor cell proliferation.
To determine if Ikaros directly influences the expression of Notch target genes, we reexpressed Ikaros in the IkL/L cell lines by retroviral transduction. Retroviral vectors containing either Ikaros1 (Ik1), the predominant Ikaros isoform detected in the nucleus of lymphocytes, or the Ikaros1* (Ik1*) isoform, which lacks exon 2 and is expressed at low levels in IkL/L hematopoietic cells (29), were used in these assays. Transduced cells were distinguished from nontransduced cells by virtue of the GFP reporter. Forty-eight hours after transduction, we compared the expression of Notch 1, Notch 3, and Notch target genes in sorted GFPhi and GFP− cells (Fig. 6A). The expression of all Notch pathway genes was significantly downregulated in the GFPhi population in the IkL/L lines transduced with MigR1-Ik1 or MigR1-Ik1* (Fig. 6B). The expression of Ifi-204 and Meltrin-β was nearly abrogated when either Ik1 or Ik1* was reexpressed. Deltex-1 and Hes-1 mRNA levels were also severely diminished when measured by real-time RT-PCR (Fig. 6C). Since the expression of genes not known to be associated with the Notch pathway (i.e., TdT, CD3e, β-actin) was unaffected in these experiments, our observations suggest that Ikaros selectively represses the expression of Notch target genes.
FIG. 6.
Repression of Notch pathway gene expression in IkL/L tumor cell lines expressing Ik1 or Ik1*. (A) T29 cells were transduced with the indicated retroviruses and sorted 48 h after transduction into GFPhi and GFP− populations. (B) Expression of the indicated genes was analyzed by RT-PCR. Results are representative of 5 experiments. +, present; −, absent. (C) Real-time RT-PCR data were obtained from both T29 and T64 cells transduced as above in two independent experiments, different from those for which results are shown in panel b. (D) The T29 cell line was transduced with MigR1, Mig-Ik1, or Mig-IK1* at day 0 and cultured over 5 days. Cumulative GFPhi cells are shown. Similar results were obtained with other IkL/L tumor cell lines. Results are representative of 5 experiments.
Ikaros expression also led to a progressive loss of GFPhi cells in the IkL/L tumor cell lines. Quantification of GFPhi cells from MigR1-, Mig-Ik1-, and Mig-Ik1*-transduced cultures indicated that Ikaros-expressing cells expanded poorly over time (Fig. 6D), similar to cells treated with GSI (Fig. 5D). When the same cultures were analyzed for apoptosis using annexin V staining, no differences were detected between the GFPhi and GFP− cells (not shown). Together, these data show that when Ikaros function is restored in IkL/L tumor cells, both proliferation and Notch target gene expression are inhibited.
Ikaros binds to the CSL/RBP-Jk binding site in the Hes-1 promoter.
Similarities between the sites bound by Ikaros and CSL/RBP-Jk, the Notch effector protein, which both recognize the core sequence TGGGAA, have been reported by Beverly and Capobianco (7). These authors hypothesized that Ikaros and CSL/RBP-Jk antagonize and compete for the same regulatory elements within the promoters of certain genes to differentially regulate transcription. However, Ikaros was not found to bind to the CSL/RBP-Jk binding sites from the promoters of Notch target genes such as Hes-1, pTα, and cyclin D1.
We noticed that the Hes-1 sequence previously used in the Beverly and Capobianco assays was incomplete (7) and contained only the most 5′ of two consecutive TGGGAA motifs known to be recognized by CSL/RBP-Jk (22). Therefore, we reexamined the capacity of Ikaros to bind to the Hes-1 regulatory sequence, using the complete site of the Hes-1 promoter in an EMSA. We compared the binding activity of the wild-type sequence with those containing altered sites, in which drastic mutations were introduced into one or both repeats (Fig. 7A). Both the Ik1 and Ik1* proteins efficiently bound to the intact Hes-1 site. The complexes were identical to those formed on a high-affinity Ikaros binding site (IkBS4), and their mobility was increased when extracts containing the smaller Ik1* were employed, indicating specific binding. Importantly, mutations in either site A or B reduced binding efficiency, and binding was abolished by the simultaneous disruption of both repeats. Thus, both repeats are involved in the high-affinity binding of Ikaros to the Hes-1 regulatory sequence.
FIG. 7.
Ik1 and Ik1* isoforms bind to the CSL/RBP-Jk binding site of the Hes-1 promoter. (A) EMSAs using the fragment of the Hes-1 promoter (AB) from −91 to −57 (22). The two core sequences of the CSL/RBP-Jk binding sites are marked in boldface type. AmB, site A mutated; ABm, site B mutated; AmBm, sites A and B mutated. Arrows show the different Ikaros complexes. Mutated binding sites are shown in italics. The control IkBS4 probe contains a high-affinity Ikaros binding site (37). All assays were performed using 3 μg of nuclear extracts from Cos cells transfected with Ik1- or Ik1*-expressing vectors or pBlueScript (mock) and repeated 3 times with similar results. The multiple bands on the gels are likely to correspond to monomeric, dimeric, and multimeric Ikaros complexes (52). Note the slightly higher electrophoretic mobility of the complexes generated with Ik1*, which reflects the smaller size of the protein compared to Ik1. (B) Luciferase reporter gene assay using a Hes-1 luc reporter that contains two CSL/RBP-Jk binding sites (A and B) or a Hes-1ΔAB luc reporter with the A and B sites deleted. HeLa cells were cotransfected with the indicated plasmids (+, present; −, absent) as well as a Renilla luciferase plasmid for normalization. The total DNA content was held constant by the addition of an empty vector. The black wedge depicts increasing concentrations of the Ik1 expression vector in these samples: 1 μg, 1.5 μg, 2 μg. Error bars show the standard deviations of one experiment performed in triplicate. Results are representative of 3 experiments.
To determine how Ikaros binding affects transcription from the Hes-1 promoter, Notch-sensitive reporter gene assays were performed in human HeLa cells using a Hes-1 luciferase reporter that contains 2 physiological CSL/RBP-Jk binding sites (Fig. 7B). The addition of Ikaros repressed NIC-dependent transcriptional activity in a dose-dependent manner. No luciferase activity was detected in samples using a Hes-1ΔAB luciferase reporter lacking both CSL/RBP-Jk binding sites. These results indicate that Ikaros represses transcription of Notch target genes in the presence of activating NIC.
DISCUSSION
In this report, we provide clear evidence that Ikaros functions as a bona fide tumor suppressor in T cells and point to the oncogenic activation of the Notch pathway as a major mechanism for Ikaros-induced tumorigenesis. This critical role of Notch is supported by three observations. (i) The Notch pathway is activated in all IkL/L tumors studied (n > 30). Furthermore, this activation is detected early and consistently in thymocytes of healthy IkL/L mice. (ii) Notch activation is required for tumor cell proliferation in vitro. (iii) Notch 1 is the frequent target of secondary mutations that lead to the expression of truncated intracellular forms. Convergence of the Notch- and Ikaros-dependent pathways in T-cell leukemogenesis was previously proposed by Beverly and Capobianco (7), who observed a strong synergy between dominant-negative Ikaros mutations and activated Notch 1, and by Lopez-Nieva et al. (35), who reported that loss-of-function mutations in the Ikaros gene and Notch 1 activation often occur together in radiation-induced thymic lymphomas. Our study further highlights this convergence, and we show here that Notch activation is a mandatory step downstream of Ikaros deficiency in T-cell tumorigenesis.
Since IkL/L thymocytes and tumor cells express low levels of truncated Ikaros (Ik1*) (see Kirstetter et al. [29]), an important issue is whether these mutant proteins function similarly to WT proteins during tumorigenesis. We think so, as Ik1* and Ik1 proteins are indistinguishable in their capacity to inhibit tumor cell proliferation, downregulate Notch target gene expression, and bind to the Hes-1 regulatory sequence. Thus, the residual Ik* proteins probably function normally but are present at levels too low to fully exert their tumor suppressor activity in IkL/L thymocytes. In this respect, it is noteworthy that T lymphomas develop faster in mice expressing dominant-negative Ikaros than in IkL/L mutants (57), consistent with the concept of a hypomorphic phenotype in IkL/L animals.
What is the link between Ikaros deficiency and Notch activation? Our data suggest that Ikaros acts as a repressor of Notch pathway genes. First, Notch target genes such as Deltex-1 and Hes-1 are ectopically expressed in thymocytes of young IkL/L mice showing no phenotypic signs of cancer (33) (Fig. 3). Second, reexpressing Ikaros in IkL/L tumor cell lines results in a rapid downregulation of all of the Notch target genes analyzed. Thus, both loss- and gain-of-function systems show that Ikaros negatively regulates Notch target genes by repressing their expression. Beverly and Capobianco (7) suggested one mechanism by which Ikaros may regulate Notch target genes, reporting in transient-transfection experiments that Ikaros can antagonize Notch by binding to a CSL/RBP-Jk consensus site. This mechanism, however, lacked direct experimental validation, since they did not show that Ikaros could bind to natural Notch-responsive DNA sequences. Our present data support the relevance of this model in the case of the Hes-1 gene. Ikaros binds to the CSL/RBP-Jk site from the Hes-1 promoter in vitro, and Ikaros represses NIC-mediated Hes-1 reporter activation. Together with our observations that Hes-1 is upregulated in IkL/L thymocytes and downregulated in cell lines reexpressing Ikaros, our experiments indicate that Ikaros may repress Hes-1 transcription by competing with the CSL/NIC complex for a common regulatory element.
Ikaros deficiency may therefore directly destabilize Notch target gene repression in nontransformed thymocytes. Indeed, we have found that approximately 5% of IkL/L DP thymocytes from young mice express Deltex-1 or Hes-1, while <1% of WT DP cells do (M. Sellars, unpublished data), suggesting a stochastic derepression of Notch target genes in a small percentage of IkL/L thymocytes. Early upregulation of some Notch targets upon Notch signaling may reinforce this signaling in mutant cells, ultimately driving the activation of the entire Notch pathway. This “snowball” effect is facilitated by the lack of Ikaros and reinforced via extracellular binding to Notch ligand(s), expressed in the thymus by stromal cells or the thymocytes themselves. Ultimately, transformation may result from the preferential outgrowth of cells exhibiting the strongest activation of the Notch pathway, a process that may require subsequent secondary genetic alterations. This scenario for tumorigenesis is supported by observations that Notch pathway genes are activated in IkL/L thymocytes, but not in peripheral IkL/L T cells (A. Dumortier, unpublished data), and may also account for the thymus dependency of the tumors. Unambiguous demonstration that Ikaros directly represses Notch target gene expression will be required to validate this model, however, as Notch activation might also result indirectly from widespread gene hyperacetylation or chromosome instability (3, 27).
Indeed, Ikaros deficiency lowers the activation threshold of T and B lymphocytes, making them hyperproliferative to TCR and B-cell receptor stimulation, respectively (29, 57). Although our data indicate that hyperproliferation cannot be the sole cause of tumorigenesis (IkL/L B cells and peripheral T cells, as well as Ik+/L thymocytes, also hyperproliferate to stimulation but do not become transformed) (29) (Fig. 2A), a reduced threshold of activation may favor the outgrowth of transformed thymocytes upon pre-TCR/TCR signaling. This possibility is consistent with our observations that IkL/L tumor cells express low to intermediate levels of TCR αβ (Fig. 1 and our unpublished data) and that the additional loss of RAG gene function prevents tumor development, suggesting that pre-TCR or TCR signaling is required (56; our unpublished results). Similarly, deletion of pTα function in Notch 3 transgenic mice abrogates T-cell leukemogenesis (6), and many tumors developing from cells with activated NIC are CD4+ CD8+ in phenotype (58). Thus, higher susceptibility to TCR or pre-TCR activation in Ikaros mutant mice may enhance the Notch-dependent effect in thymocytes, providing a basis for Ikaros/Notch synergy.
One of the most striking aspects of the IkL/L tumors is the appearance of smaller NIC-1 isoforms in a significant proportion of tumors. Our studies show that these smaller proteins harbor deletions in the C-terminal PEST domain, which may make them more stable and resistant to degradation, thereby prolonging the Notch signal in these cells. It will be important to determine if IkL/L tumor cells have a mutator phenotype (mutate at a high rate) that favors the appearance of secondary mutations or whether these mutations occur at a normal rate and dominate the tumor because of their strong selective advantage. Similar C-terminal Notch 1 truncations have previously been described in mouse and human T-ALL. In mice, they also occur following secondary retroviral insertions in tumors initiated by overexpression of the c-myc or E2A-Pbx oncogenes (16, 18). More recently, activating PEST domain mutations in Notch 1 have been found in a significant fraction of T-ALL patients (55). The IkL/L tumors may thus fit into a subgroup of Notch-dependent T-cell tumors, where activating C-terminal mutations play a critical role in enhancing the transforming capacity of Notch proteins. Importantly, our data also suggest that the IkL/L mouse line might serve as a good model to examine the efficacy of γ-secretase treatment as a therapeutic option for T-ALL.
In conclusion, there is mounting evidence that Ikaros plays a role in the pathogenesis of T- and B-ALL. Our data from a murine system indicate that Ikaros may exert its tumorigenic influence by regulating the Notch pathway. It would be tempting to speculate that the Ikaros/Notch axis also applies to the normal differentiation and function of lymphocytes and other hematopoietic lineages.
Acknowledgments
We thank F. Radtke, W. Pear, C. Benoist, and D. Mathis for discussions and critical reading; W. Pear for the MigR1 vector system; A. Israel for the Hes-1 luciferase plasmids; G. Nolan for the Eco-Phoenix cell line; and D. Dembélé for help in the microarray analyses. We thank S. Duhautbois-Boine, G. Kimmich, F. Diemunsch, and A. Lavaux for technical assistance; C. Ebel for help with flow cytometry; and F. Memedov and M. Gendron for animal husbandry.
This work was supported by institute funds from the Institut National de la Santé et de la Recherche Médicale, the Centre National pour la Recherche Scientifique, and the Hôpital Universitaire de Strasbourg and by grants from the Ligue Nationale contre le Cancer (LNCC—National, Haut-Rhin, and Bas-Rhin chapters), the Association pour la Recherche sur le Cancer (ARC), Fondation de France, the Ministère de la Recherche et de la Technologie (MRT, appel d'offre Affymetrix), and the Association Régionale pour L'Enseignement et la Recherche Scientifique et Technologie. A.D., E.K., P. Kirstetter, and R.J. received fellowships from the MRT, LNCC (E.K., P. Kirstetter, R.J.), ARC (A.D.), and the Fondation pour la Recherche Médicale (A.D.).
J.A.P. is on leave from Haverford College, Haverford, PA 19041.
REFERENCES
- 1.Adler, S. H., E. Chiffoleau, L. Xu, N. M. Dalton, J. M. Burg, A. D. Wells, M. S. Wolfe, L. A. Turka, and W. S. Pear. 2003. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J. Immunol. 171:2896-2903. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, N. C., and D. V. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avitahl, N., S. Winandy, C. Friedrich, B. Jones, Y. Ge, and K. Georgopoulos. 1999. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity 10:333-343. [DOI] [PubMed] [Google Scholar]
- 4.Back, J., A. Dierich, C. Bronn, P. Kastner, and S. Chan. 2004. PU.1 determines the self-renewal capacity of erythroid progenitor cells. Blood 103:3615-3623. [DOI] [PubMed] [Google Scholar]
- 5.Bellavia, D., A. F. Campese, E. Alesse, A. Vacca, M. P. Felli, A. Balestri, A. Stoppacciaro, C. Tiveron, L. Tatangelo, M. Giovarelli, C. Gaetano, L. Ruco, E. S. Hoffman, A. C. Hayday, U. Lendahl, L. Frati, A. Gulino, and I. Screpanti. 2000. Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J. 19:3337-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellavia, D., A. F. Campese, S. Checquolo, A. Balestri, A. Biondi, G. Cazzaniga, U. Lendahl, H. J. Fehling, A. C. Hayday, L. Frati, H. von Boehmer, A. Gulino, and I. Screpanti. 2002. Combined expression of pTalpha and Notch3 in T cell leukemia identifies the requirement of preTCR for leukemogenesis. Proc. Natl. Acad. Sci. USA 99:3788-3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beverly, L. J., and A. J. Capobianco. 2003. Perturbation of Ikaros isoform selection by MLV integration is a cooperative event in Notch(IC)-induced T cell leukemogenesis. Cancer Cell 3:551-564. [DOI] [PubMed] [Google Scholar]
- 8.Brown, K. E., S. S. Guest, S. T. Smale, K. Hahm, M. Merkenschlager, and A. G. Fisher. 1997. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell 91:845-854. [DOI] [PubMed] [Google Scholar]
- 9.Capobianco, A. J., P. Zagouras, C. M. Blaumueller, S. Artavanis-Tsakonas, and J. M. Bishop. 1997. Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol. Cell. Biol. 17:6265-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carron, C., F. Cormier, A. Janin, V. Lacronique, M. Giovannini, M. T. Daniel, O. Bernard, and J. Ghysdael. 2000. TEL-JAK2 transgenic mice develop T-cell leukemia. Blood 95:3891-3899. [PubMed] [Google Scholar]
- 11.Chiaramonte, R., A. Basile, E. Tassi, E. Calzavara, V. Cecchinato, V. Rossi, A. Biondi, and P. Comi. 2005. A wide role for NOTCH1 signaling in acute leukemia. Cancer Lett. 219:113-120. [DOI] [PubMed] [Google Scholar]
- 12.Choi, J. W., C. Pampeno, S. Vukmanovic, and D. Meruelo. 2002. Characterization of the transcriptional expression of Notch-1 signaling pathway members, Deltex and HES-1, in developing mouse thymocytes. Dev. Comp. Immunol. 26:575-588. [DOI] [PubMed] [Google Scholar]
- 13.Deftos, M. L., E. Huang, E. W. Ojala, K. A. Forbush, and M. J. Bevan. 2000. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity 13:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellisen, L. W., J. Bird, D. C. West, A. L. Soreng, T. C. Reynolds, S. D. Smith, and J. Sklar. 1991. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66:649-661. [DOI] [PubMed] [Google Scholar]
- 16.Feldman, B. J., T. Hampton, and M. L. Cleary. 2000. A carboxy-terminal deletion mutant of Notch1 accelerates lymphoid oncogenesis in E2A-PBX1 transgenic mice. Blood 96:1906-1913. [PubMed] [Google Scholar]
- 17.Felli, M. P., M. Maroder, T. A. Mitsiadis, A. F. Campese, D. Bellavia, A. Vacca, R. S. Mann, L. Frati, U. Lendahl, A. Gulino, and I. Screpanti. 1999. Expression pattern of notch1, 2 and 3 and Jagged1 and 2 in lymphoid and stromal thymus components: distinct ligand-receptor interactions in intrathymic T cell development. Int. Immunol. 11:1017-1025. [DOI] [PubMed] [Google Scholar]
- 18.Girard, L., Z. Hanna, N. Beaulieu, C. D. Hoemann, C. Simard, C. A. Kozak, and P. Jolicoeur. 1996. Frequent provirus insertional mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes Dev. 10:1930-1944. [DOI] [PubMed] [Google Scholar]
- 19.Harker, N., T. Naito, M. Cortes, A. Hostert, S. Hirschberg, M. Tolaini, K. Roderick, K. Georgopoulos, and D. Kioussis. 2002. The CD8alpha gene locus is regulated by the Ikaros family of proteins. Mol. Cell 10:1403-1415. [DOI] [PubMed] [Google Scholar]
- 20.Hoemann, C. D., N. Beaulieu, L. Girard, N. Rebai, and P. Jolicoeur. 2000. Two distinct Notch1 mutant alleles are involved in the induction of T-cell leukemia in c-myc transgenic mice. Mol. Cell. Biol. 20:3831-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, Y. H., D. Li, A. Winoto, and E. A. Robey. 2004. Distinct transcriptional programs in thymocytes responding to T cell receptor, Notch, and positive selection signals. Proc. Natl. Acad. Sci. USA 101:4936-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarriault, S., C. Brou, F. Logeat, E. H. Schroeter, R. Kopan, and A. Israel. 1995. Signalling downstream of activated mammalian Notch. Nature 377:355-358. [DOI] [PubMed] [Google Scholar]
- 23.Jeffries, S., and A. J. Capobianco. 2000. Neoplastic transformation by Notch requires nuclear localization. Mol. Cell. Biol. 20:3928-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakinuma, S., M. Nishimura, A. Kubo, J. Y. Nagai, Y. Amasaki, H. J. Majima, T. Sado, and Y. Shimada. 2005. Frequent retention of heterozygosity for point mutations in p53 and Ikaros in N-ethyl-N-nitrosourea-induced mouse thymic lymphomas. Mutat. Res. 572:132-141. [DOI] [PubMed] [Google Scholar]
- 25.Kaneta, M., M. Osawa, K. Sudo, H. Nakauchi, A. G. Farr, and Y. Takahama. 2000. A role for pref-1 and HES-1 in thymocyte development. J. Immunol. 164:256-264. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson, A., P. Soderkvist, and S. M. Zhuang. 2002. Point mutations and deletions in the znfn1a1/ikaros gene in chemically induced murine lymphomas. Cancer Res. 62:2650-2653. [PubMed] [Google Scholar]
- 27.Kathrein, K. L., R. Lorenz, A. M. Innes, E. Griffiths, and S. Winandy. 2005. Ikaros induces quiescence and T-cell differentiation in a leukemia cell line. Mol. Cell. Biol. 25:1645-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, J., S. Sif, B. Jones, A. Jackson, J. Koipally, E. Heller, S. Winandy, A. Viel, A. Sawyer, T. Ikeda, R. Kingston, and K. Georgopoulos. 1999. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 10:345-355. [DOI] [PubMed] [Google Scholar]
- 29.Kirstetter, P., M. Thomas, A. Dierich, P. Kastner, and S. Chan. 2002. Ikaros is critical for B cell differentiation and function. Eur. J. Immunol. 32:720-730. [DOI] [PubMed] [Google Scholar]
- 30.Klug, C. A., S. J. Morrison, M. Masek, K. Hahm, S. T. Smale, and I. L. Weissman. 1998. Hematopoietic stem cells and lymphoid progenitors express different Ikaros isoforms, and Ikaros is localized to heterochromatin in immature lymphocytes. Proc. Natl. Acad. Sci. USA 95:657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koipally, J., and K. Georgopoulos. 2000. Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J. Biol. Chem. 275:19594-19602. [DOI] [PubMed] [Google Scholar]
- 32.Koipally, J., A. Renold, J. Kim, and K. Georgopoulos. 1999. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 18:3090-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laws, A. M., and B. A. Osborne. 2004. p53 regulates thymic Notch1 activation. Eur. J. Immunol. 34:726-734. [DOI] [PubMed] [Google Scholar]
- 34.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Nieva, P., J. Santos, and J. Fernandez-Piqueras. 2004. Defective expression of Notch1 and Notch2 in connection to alterations of c-Myc and Ikaros in gamma-radiation-induced mouse thymic lymphomas. Carcinogenesis 25:1299-1304. [DOI] [PubMed] [Google Scholar]
- 36.Maillard, I., S. H. Adler, and W. S. Pear. 2003. Notch and the immune system. Immunity 19:781-791. [DOI] [PubMed] [Google Scholar]
- 37.Molnar, A., and K. Georgopoulos. 1994. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol. Cell. Biol. 14:8292-8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakase, K., F. Ishimaru, N. Avitahl, H. Dansako, K. Matsuo, K. Fujii, N. Sezaki, H. Nakayama, T. Yano, S. Fukuda, K. Imajoh, M. Takeuchi, A. Miyata, M. Hara, M. Yasukawa, I. Takahashi, H. Taguchi, K. Matsue, S. Nakao, Y. Niho, K. Takenaka, K. Shinagawa, K. Ikeda, K. Niiya, and M. Harada. 2000. Dominant negative isoform of the Ikaros gene in patients with adult B-cell acute lymphoblastic leukemia. Cancer Res. 60:4062-4065. [PubMed] [Google Scholar]
- 39.Nishii, K., N. Katayama, H. Miwa, M. Shikami, E. Usui, M. Masuya, H. Araki, F. Lorenzo, T. Ogawa, T. Kyo, K. Nasu, H. Shiku, and K. Kita. 2002. Non-DNA-binding Ikaros isoform gene expressed in adult B-precursor acute lymphoblastic leukemia. Leukemia 16:1285-1292. [DOI] [PubMed] [Google Scholar]
- 40.Okano, H., Y. Saito, T. Miyazawa, T. Shinbo, D. Chou, S. Kosugi, Y. Takahashi, S. Odani, O. Niwa, and R. Kominami. 1999. Homozygous deletions and point mutations of the Ikaros gene in gamma-ray-induced mouse thymic lymphomas. Oncogene 18:6677-6683. [DOI] [PubMed] [Google Scholar]
- 41.Papathanasiou, P., A. C. Perkins, B. S. Cobb, R. Ferrini, R. Sridharan, G. F. Hoyne, K. A. Nelms, S. T. Smale, and C. C. Goodnow. 2003. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity 19:131-144. [DOI] [PubMed] [Google Scholar]
- 42.Pear, W. S., J. C. Aster, M. L. Scott, R. P. Hasserjian, B. Soffer, J. Sklar, and D. Baltimore. 1996. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J. Exp. Med. 183:2283-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pear, W. S., J. P. Miller, L. Xu, J. C. Pui, B. Soffer, R. C. Quackenbush, A. M. Pendergast, R. Bronson, J. C. Aster, M. L. Scott, and D. Baltimore. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92:3780-3792. [PubMed] [Google Scholar]
- 44.Radtke, F., A. Wilson, S. J. Mancini, and H. R. MacDonald. 2004. Notch regulation of lymphocyte development and function. Nat. Immunol. 5:247-253. [DOI] [PubMed] [Google Scholar]
- 45.Reizis, B., and P. Leder. 2002. Direct induction of T lymphocyte-specific gene expression by the mammalian Notch signaling pathway. Genes Dev. 16:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ronchini, C., and A. J. Capobianco. 2001. Induction of cyclin D1 transcription and CDK2 activity by Notchic: implication for cell cycle disruption in transformation by Notchic. Mol. Cell. Biol. 21:5925-5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeter, E. H., J. A. Kisslinger, and R. Kopan. 1998. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393:382-386. [DOI] [PubMed] [Google Scholar]
- 48.Shimada, Y., M. Nishimura, S. Kakinuma, M. Okumoto, T. Shiroishi, K. H. Clifton, and S. Wakana. 2000. Radiation-associated loss of heterozygosity at the Znfn1a1 (Ikaros) locus on chromosome 11 in murine thymic lymphomas. Radiat. Res. 154:293-300. [DOI] [PubMed] [Google Scholar]
- 49.Sun, L., M. L. Crotty, M. Sensel, H. Sather, C. Navara, J. Nachman, P. G. Steinherz, P. S. Gaynon, N. Seibel, C. Mao, A. Vassilev, G. H. Reaman, and F. M. Uckun. 1999. Expression of dominant-negative Ikaros isoforms in T-cell acute lymphoblastic leukemia. Clin. Cancer Res. 5:2112-2120. [PubMed] [Google Scholar]
- 50.Sun, L., P. A. Goodman, C. M. Wood, M. L. Crotty, M. Sensel, H. Sather, C. Navara, J. Nachman, P. G. Steinherz, P. S. Gaynon, N. Seibel, A. Vassilev, B. D. Juran, G. H. Reaman, and F. M. Uckun. 1999. Expression of aberrantly spliced oncogenic ikaros isoforms in childhood acute lymphoblastic leukemia. J. Clin. Oncol. 17:3753-3766. [DOI] [PubMed] [Google Scholar]
- 51.Sun, L., N. Heerema, L. Crotty, X. Wu, C. Navara, A. Vassilev, M. Sensel, G. H. Reaman, and F. M. Uckun. 1999. Expression of dominant-negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 96:680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun, L., A. Liu, and K. Georgopoulos. 1996. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 15:5358-5369. [PMC free article] [PubMed] [Google Scholar]
- 53.Takanashi, M., T. Yagi, T. Imamura, Y. Tabata, A. Morimoto, S. Hibi, E. Ishii, and S. Imashuku. 2002. Expression of the Ikaros gene family in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 117:525-530. [DOI] [PubMed] [Google Scholar]
- 54.Trinh, L. A., R. Ferrini, B. S. Cobb, A. S. Weinmann, K. Hahm, P. Ernst, I. P. Garraway, M. Merkenschlager, and S. T. Smale. 2001. Down-regulation of TDT transcription in CD4(+)CD8(+) thymocytes by Ikaros proteins in direct competition with an Ets activator. Genes Dev. 15:1817-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weng, A. P., A. A. Ferrando, W. Lee, J. P. T. Morris, L. B. Silverman, C. Sanchez-Irizarry, S. C. Blacklow, A. T. Look, and J. C. Aster. 2004. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306:269-271. [DOI] [PubMed] [Google Scholar]
- 56.Winandy, S., L. Wu, J. H. Wang, and K. Georgopoulos. 1999. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. J. Exp. Med. 190:1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winandy, S., P. Wu, and K. Georgopoulos. 1995. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 83:289-299. [DOI] [PubMed] [Google Scholar]
- 58.Zweidler-McKay, P. A., and W. S. Pear. 2004. Notch and T cell malignancy. Semin. Cancer Biol. 14:329-340. [DOI] [PubMed] [Google Scholar]